Abstract
Defects in the 'Bcl-2-regulated' apoptotic pathway inhibit the deletion of self-reactive T cells. What is unresolved, however, is the nature and fate of such self-reactive T cells escaping deletion. Here, we report that mice with such defects contained increased numbers of CD25lowFoxP3+ cellsin the thymus and peripheral lymph tissues. The increased CD25lowFoxP3+ population contained a large fraction of cells bearing self-reactive TCRs, evident from a prominent increase in self-superantigen specific FoxP3+Vβ5+ CD4+ T cells in BALB/c Bim−/− mice compared to control animals. The survival rate of the expanded CD25lowFoxP3+ cells was similar to that of CD25highFoxP3+ CD4 T cells in vitro and in vivo. IL-2R stimulation, but not TCR ligation, up-regulated CD25 on CD25lowFoxP3+ CD4+ T cells in vitro and in vivo. The expanded CD25lowFoxP3+ CD4+ T cells from Bim−/− mice were anergic but also had weaker regulatory function than CD25highFoxP3+ CD4+ T cells from the same mice. Analysis of Bim−/− mice that also lacked Fas showed that the peripheral homeostasis of this expanded population was in part regulated by this ‘death receptor’. In conclusion, these results show that self-reactive T cell escapees from thymic deletion in mice defective in the 'Bcl-2-regulated' apoptotic pathway up-regulate FoxP3 and become unresponsive upon encountering self-antigen without necessarily gaining potent regulatory function. This clonal ‘functional diversion’ may help to curtail auto-aggressiveness of escaped self-reactive CD4+ T cells and thereby safeguard immunological tolerance.
Introduction
The “Bcl-2-regulated” apoptotic pathway is critical for the deletion of self-reactive T cells during thymic development (1). Over-expression of anti-apoptotic Bcl-2 (2, 3) or deficiency of pro-apoptotic Bcl-2 family members, such as the BH3-only protein Bim (4, 5) or the multi-BH domain proteins Bax plus Bak (6) cause defects in the killing of self-reactive T cells by apoptosis during their development in the thymus. Accordingly, thymocytes from autoimmune diabetes-prone NOD mice have been reported to be defective in TCR stimulation-induced up-regulation of Bim (7).
Defects in the deletion of high-affinity self-reactive TCR expressing T cells can lead to rapid destruction of “self-antigen”-expressing tissues (8, 9). For example, humans and mice lacking AIRE, which is critical for presentation of auto-antigens in the thymus,have a severe defect in the deletion of auto-reactive thymocytes (10) and consequently develop multi-organ autoimmune disease (10, 11). In contrast, Bim−/−mice and transgenic mice over-expressing Bcl-2 in lymphoid cells do not develop multi-organ T cell-mediated autoimmune diseases, despite a severe defect in deleting auto-reactive T (3, 4) and B cells (12). There are at least four possible mechanisms that might restrain auto-aggression of self-reactive lymphocytes escaping deletion in mice defective in the ‘Bcl-2 regulated’ apoptotic pathway. First, escapees from deletion may become functionally unresponsive (anergic) upon encountering self-antigens (13, 14). In contrast, such induction of anergy will most likely not occur in AIRE−/− mice, due to impaired presentation of self-antigen. Second, “forbidden clones” (15) may become subject to regulation by CD25+FoxP3+ regulatory T cells (Treg cells). Third, escapees may undergo peripheral deletion by death mechanisms independent of the ‘Bcl-2-regulated’ apoptotic pathway, akin to the ‘death receptor’ mediated control of mature T cells that had been activated continuously through their TCR by persistent antigens in peripheral lymphoid organs (16–18). Finally, potentially dangerous T cell clones may be diverted to differentiate into an alternate, ‘harmless’ state to preserve tolerance (19).
To explore these possibilities, we studied the fate of self-reactive thymocytes in mice defective in the ‘Bcl-2 regulated’ apoptotic pathway, including Bim−/−, Bim−/−Puma−/−, vav-Bcl-2 transgenic and Bax−/−Bak−/− mice. We found that a subset of FoxP3+ CD4 T cells was preferentially and substantially increased in these mutant mice and investigated the underlying mechanisms for their selective increase and their functional attributes. These studies showed that ‘clonal diversion of differentiation’ of auto-reactive thymocytes can help maintain immunological tolerance, at least when the Bcl-2 regulated’ apoptotic pathway within these cells is impaired. Such impairment due to either failure to induce pro-apoptotic proteins or dysregulation of anti-apoptotic regulators can occur in autoimmune NOD mice (7) and in humans (20).
Materials and Methods
Mice
C57BL/6 Bim−/− (line 266), Puma−/−, Bim−/−Puma−/− and OT-II-Bim−/− mice were described previously (21, 22). BALB/c Bim−/− mice were generated by backcrossing C57BL/6 Bim−/− (line 266) mice for 10 generations onto a BALB/c background. FoxP3GFPKI/Bim−/− mice and FoxP3GFPKI/vavBcl-2 transgenic (Tg) mice were generated by mating FoxP3GFPKI mice (backcrossed for at least 6 generations with C57BL/6 mice) (23) with C57BL/6 Bim−/− mice. C57BL/6-Ly5.1 Rag-1−/− mice reconstituted with a Bax−/−Bak−/− hemopoietic system (using Bax−/−Bak−/− fetal liver cells as donors) after lethal irradiation (2x5.5 Gy, 4 h apart). Vav-Bcl-2 transgenic mice and FASlpr/lpr mutant mice were maintained at the Walter and Eliza Hall Institute animal facilities under specific pathogen free conditions. All experiments with mice were conducted in accordance with the rules of The Walter and Eliza Hall Institute’s Animal Ethics Committee.
Flow cytometry
Antibodies (Ab) used in this study were all purchased from BD Biosciences, (San Jose, CA) except where stated otherwise. PE-Cy7 or APC conjugated anti-mouse CD4 (clone RM4-5), APC-Cy7 conjugated anti-mouse CD8 (clone 53-6.7), FITC or PE-conjugated anti-mouse CD25 (clone PC61) Abs were used for surface staining. FITC conjugated Abs specific for individual TCR Vβ chains were supplied as a kit by BD Biosciences. To stain cell surface proteins, cells were incubated with optimally diluted Abs for 30 min on ice. Viable cells, identified by propidium iodide (PI) exclusion, were analyzed using a FACSaria (Becton Dickinson, San Jose, CA) and Cell-Quest software (San Carlos, CA). Intracellular FoxP3 protein was detected using a staining kit from eBioscience (San Diego, CA). Cells from the thymus, spleen and lymph node were stained for surface markers CD4, CD8 and CD25, and sorted in a FACSaria (Becton-Dickinson).
Cell survival assays
Cell survival assays were performed as previously reported (24). Purified cell subsets (>95% pure) were cultured at 2x104 in 200 μL medium in U-bottom 96-well plates in the presence or absence of 5 μg/mL anti-CD3 and 2μg/mL anti-CD28 Abs for 2 days. Cell survival was measured by flow cytometry with PE-conjugated FACS calibration beads (CaliBRITE, BD) and PI to determine numbers of viable cells. Recombinant human IL-7 (2 ng/mL, Peprotech, Rocky Hill, NJ, USA) or human IL-2 (20 U/mL, NCIBRB preclinical Repository, Rockville, MD) were used in some cultures.
In vivo tracking of transferred CD4 T cell subsets
CD4 T cell subsets were sorted. Each population was labelled with CellTrace violet dye according to the manufacturer’s instructions (Invitrogen, Eugene, OR). Labeled cells (2x105) were injected i.v. into C57BL/6-Ly5.1 mice. Day 3 and Day 7 post transfer, spleens were harvested and single cell suspensions were prepared. Spleen cells were then stained for Ly5.2, CD4, CD8 and CD25. Transferred cells were identified as Ly5.2+CellTrace violet+CD4+ cells. The total number of transferred cells was calculated by multiplying the total spleen cellularity with the percentage of transferred cells within the total spleen cell population.
Quantitative real-time PCR
RNA was extracted from pellets of purified cell populations using an RNeasy MiniKit (Qiagen, Melbourne, Victoria, Australia) as per manufacturer’s instructions. A total of 250 ng RNA was treated with DNase I recombinant, RNase-free (Roche, Mannheim, Germany) and reverse-transcribed to cDNA using random primers and Superscript III First-Strand Synthesis System RT-PCR kit (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was performed to determine the expression of FoxP3, CD25 and CTLA4 in CD4+ T cell subsets. The reactions consisted of GoTaq qPCR Master Mix (Promega, Madison, WI), 0.5 mM of specific primers, 2 μL cDNA, and carried out on a LightCycler (Roche). Specific primers (Sigma-Aldrich, Castle Hill, New South Wales, Australia) for quantitative real-time PCR were as follows: β-actin: F: GATCTGGCACCACACCTTCT, R: GGGGTGTTGAAGGTCTCAAA; FoxP3: F; ATGTTCGCCTACTTCAGAAACC, R: CAAATTCATCTACGGTCCACAC; CD25: F TTCCGAAGACTAAAGGAATTGG, R: TCTGTTGTGGTTTGTTGCTCTT; CTLA4: F: AGTTTCCTGGTCACTGCTGTTT, R: TTTTCACATTCTGGCTCTGTTG.
An initial activation step was carried out at 95°C for 15 min. Amplification was then carried out for 35 cycles with denaturation at 95°C for 15 s, annealing at 55°C for 30 s and extension at 72°C for 30 s, followed by melting point analysis. Data analysis was performed with SDS 2.2 software (Applied Biosystems). The expression level for each gene was determined using a standard curve prepared from 1 to 1026 pg of specific DNA fragment, then expressed as a ratio to the levels of β-actin mRNA.
Assays to measure T cell proliferation and Treg cell activity
CD25-FoxP3−, CD25lowFoxP3+ and CD25highFoxP3+ CD4+ T cell subpopulations were purified by FACS sorting from the thymi, spleens or lymph nodes of GFPKI/vavBcl-2 Tg mice, FoxP3-GFPKI/Bim−/− mice and FoxP3-GFPKI mice for the following functional assays. For cell proliferation assays, subsets of T cells (5x104 cells per well) were cultured in 96-well V-bottomed plates with 5 μg/mL anti-CD3 Ab together with 8x104γ-irradiated and T-cell depleted spleen cells as antigen presenting cells. For Treg cell functional assays, 5x104 CFSE-labeled CD25−CD4+ T cells from C57BL/6-Ly5.1 mice, used as effector cells were cultured either with 5x104 CD25lowFoxP3+ or CD25+FoxP3+ CD4+ T (from the mice listed above) in the presence of antigen presenting cells. Cells were stimulated for 72–96 h with 5 μg/mL soluble anti-CD3 Ab plus 2 μg/mL anti-CD28 Ab. Proliferation of effector cells was measured by flow cytometric analysis of CFSE dilution. PE-conjugated calibration beads (Becton Dickinson, San Jose, CA) were included to determine total cell numbers. In some experiments, unlabeled CD25-CD4+ T cells from WT mice were used as effector cells. Cultures were pulsed with 1 μCi per well of [3H]thymidine for the final 8 h of culture, harvested with a Tomtec Harvester96 (Tomtec, Hamden, CT) and cell incorporated radioactivity measured in a Packard TopCounter (Sterling, VA).
Cytokine production assays
Supernatants from cultured T cell subsets were investigated for cytokine content using the 23-plex on Bio-plex 2200 system according to the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA).
FasL-induced apoptosis assays
Spleen cells (2x105/well) from vavBcl-2 Tg/FoxP3GFPKI were cultured in RPMI+5% FCS and treated with serial dilutions of FasL-Fc (harvested from stable 293 cells expressing FasL-Fc (293-1117) clone H10, kind gift from Drs P. Schneider and J. Tschopp, University of Lausanne, Lausanne, Switzerland) in 96-well flat-bottom plates for 16 h. Cells were then stained for CD4, CD8 and CD25. The numbers of live cells were calculated by determining the ratio between PI-negative cells and FITC-coupled FACS calibration beads, which were added prior to FACS analysis.
Results
Defects in the ‘Bcl-2 regulated’ apoptotic pathway cause a preferential increase in FoxP3+ CD4 single positive (SP) thymocytes
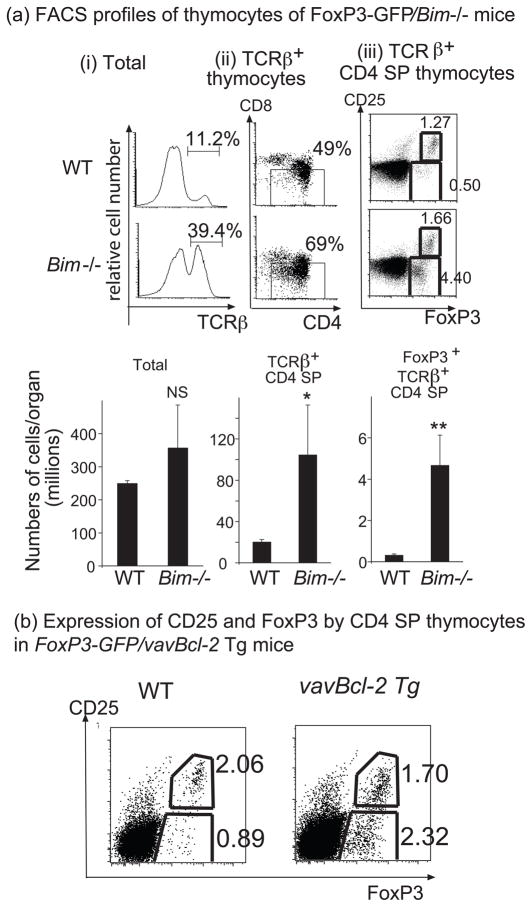
To explore the nature and fate of self-reactive T cells that escape thymic deletion due to a defect in the ‘Bcl-2 regulated’ apoptotic pathway, we first examined Bim−/− mice. Bim−/− mice had higher numbers of CD4 SP thymocytes (Mean±SEM: 24.9±2.7 million) than that of WT mice (Mean±SEM: 5.9±0.5 million; P<0.001) (Figure 1a-i). Furthermore, Bim−/− mice had a striking increase in FoxP3+ CD4 SP thymocytes (~14-fold) (1.38±0.15 million in Bim−/− mice vs 0.10±0.01 million in WT mice; P<0.0001). However, these cells did not express as high levels of CD25 as the corresponding cells from WT mice (Figure 1a-ii and iii). The CD25highFoxP3+ CD4 SP thymocytes were also increased in Bim−/− mice, albeit to a more moderate extent (2- to 6-fold) (0.35±0.01 million in Bim−/− mice vs 0.13±0.02 million in WT mice; P<0.04). Like Bim, Puma is also a potently pro-apoptotic BH3-only protein that binds with high affinity to all anti-apoptotic Bcl-2 family members (25). We observed an even greater increase in CD25lowFoxP3+ thymocytes in Puma−/−Bim−/− mice compared to WT mice (>40 fold) (2.44±0.22 million in Puma−/−Bim−/− mice vs 0.04±0.02 million in WT mice, P<0.01), although loss of Puma alone did not cause a significant increase in such cells (Figure 1a-iv).
Fig. 1. Inhibition of the ‘Bcl-2 regulated’ apoptotic pathway causes a marked increase in CD25lowFoxP3+CD4+ SP thymocytes.
Thymi were harvested from 6-10 week old mice. Thymocytes were first surface stained for CD4, CD8 and CD25 and then for intracellular FoxP3. (a) Comparison between Bim-/- and WT mice: (i) CD4 and CD8 expression on total thymocytes. (ii) Expression of CD25 and FoxP3 gated on CD4 SP thymocytes. (iii) Bar graphs show the mean numbers +/- SD of the indicated thymic sub-populations. Nine independent experiments were performed with similar results. (iv) Bar graphs show the mean numbers +/- SD of the indicated thymic sub-populations from the indicated genotypes of mice. Two independent experiments were performed with similar results. (b) Comparison between vavBcl-2 Tg and WT mice: (i) CD4 and CD8 expression on total thymocytes. (ii) Expression of CD25 and FoxP3 gated on CD4 SP thymocytes. (iii) Bar graphs show the mean numbers +/− SD of the indicated thymic sub-populations. Three independent experiments were performed. (c) Comparison between Bax−/−Bak−/− and control (WT) reconstituted mice: (i) CD4 and CD8 expression on total thymocytes from RAG-1−/− mice reconstituted with fetal liver cells from WT or Bax−/−Bak−/− mice. (ii) Expression of CD25 and FoxP3 gated on CD4 SP thymocytes. (iii) Bar graphs show the mean numbers +/−SD of the indicated thymic sub-populations. Three independent experiments were performed. Numbers in dot plots show the percentages of corresponding populations. Numbers in bar graphs indicate the fold-increase of a given population in the indicated mice compared to that in control WT animals. *P<0.05 compared to WT control.
Next, we examined vav-bcl-2 transgenic mice which over-express anti-apoptotic Bcl-2 in all hematopoietic cells (26). Similar to Bim−/− mice, the proportion and numbers of CD25lowFoxP3+ cells within the CD4 SP thymocytes from vav-Bcl-2 transgenic (vavBcl-2 Tg) mice were profoundly increased (~15-fold) compared to WT mice (3.44±0.58 million in vavBcl-2 Tg mice vs 0.18±0.02 million in WT mice; P<0.01) (Figure 1b). The increase in CD25highFoxP3+ CD4 SP thymocytes was again less pronounced but significant (~2-fold) (0.62±0.51 million in vavBcl-2 Tg mice vs 0.30±0.06 million in WT mice; P<0.01).
Finally, we examined C57BL/6-Ly5.1 Rag−/− mice reconstituted with Bax−/−Bak−/−fetal liver cells, this approach being taken because most Bax−/−Bak−/− mice die soon after birth (27). Bax and Bak activation is required for all “Bcl-2-regulated” apoptotic responses and their combined loss causes severe defects in intra-thymic T cell development (6). Accordingly, the cell subset composition of Bax−/−Bak−/− thymocytes (CD45.2+) was similar to that of Bim−/− and Puma−/−Bim-/− thymocytes. There was a great increase (>40-fold) in CD25lowFoxP3+ CD4 SP thymocytes in Bax−/−Bak−/− reconstituted mice compared to WT reconstituted mice (2.51±0.45 million of Bax−/−Bak−/− thymocytes vs 0.06±0.03 million in WT thymocytes; P<0.01) (Figure 1c). The numbers of total CD4 SP thymocytes and CD25highFoxP3+ CD4 SP thymocytes in Bax−/−Bak−/- reconstituted hosts were also increased compared to control WT reconstituted animals, albeit to a comparatively smaller extent (~4-fold) (Mean±SEM: 0.46±0.06 million of Bax−/−Bak−/− thymocytes vs 0.11±0.05 million in WT mice; P<0.01).
In addition to using intracellular staining of FoxP3 protein to characterize thymocytes from mice defective in the ‘Bcl-2 regulated’ apoptotic pathway, we also crossed Bim−/− mice and vavBcl-2 Tg mice with FoxP3GFPKI reporter mice, in which one FoxP3 allele has been replaced by GFP (23). Thus, live cells expressing FoxP3 can be tracked and their function analyzed. Compared to control FoxP3GFPKI mice, FoxP3GFPKI/Bim−/− mice had similar numbers of total thymocytes, but ~5-fold increase in total CD4 SP thymocytes (P<0.05) and greatly increased percentages and numbers of FoxP3+ (GFP+) CD4+ SP thymocytes (~15-fold; P<0.05) (Figure 2a). Similar to our observations in Bim−/− mice, the percentages of CD25lowFoxP3+ cells were higher in FoxP3GFPKI/Bim−/− mice compared to control FoxP3GFPKI mice (3.21%±0.74 in Bim−/− mice vs 0.45%±0.05 in WT mice; P<0.05), while the percentages of CD25highFoxP3+ cells were only moderately increased in FoxP3GFPKI/Bim−/− mice (1.68%±0.56 in Bim−/− mice vs 0.99%±0.16 in WT mice; P=0.293) (Figure 2a). Correspondingly, the increase in the numbers of CD25lowFoxP3+ thymocytes in FoxP3GFPKI/Bim−/− mice was more profound (~40-fold; P<0.01) compared to the increase in the numbers of CD25highFoxP3+ thymocytes (~6-fold, P<0.05). A disproportionate increase in CD25lowFoxp-GFP+ CD4 SP thymocytes was also observed in FoxP3GFPKI/vavBcl-2 Tg mice (Figure 2b).
Fig. 2. Inhibition of the ‘Bcl-2-regulated’ apoptotic pathway causes a marked increase in CD25lowFoxP3GFP+CD4+ thymocytes.
(a) Thymocytes from 6–8 week old FoxP3GFPKI or FoxP3GFPKI/Bim−/− mice were surface stained for CD4, CD8, TCRβ and CD25. (i) TCRβ expression on total thymocytes; (ii) CD4 and CD8 expression gated on TCRβ+ thymocytes; (iii) CD25 and FoxP3GFP expression gated on TCRβ+CD4+ SP thymocytes. Bar graphs show cell numbers +/−SD of the indicated thymic cell populations from 3 mice. NS, not significant; *P<0.05 compared to WT; **P<0.01 compared to WT. Four independent experiments were performed. (b) Thymocytes from 10 week-old FoxP3GFPKI or FoxP3GFPKI/vavBcl2 Tg mice were stained as in (a). Dot plots show expression of CD25 vs FoxP3 on gated CD4 SP thymocytes. Three independent experiments yielded similar results.
The increase in CD25lowFoxP3+ CD4+ T cells was also observed in secondary lymphoid organs (e.g. spleen and lymph nodes; Supplementary Figure 1) of mice with defective ‘Bcl-2-regulated’ apoptosis, suggesting that these cells are exported from the thymus. The increase in CD25highFoxP3+ CD4+ T cells in these mice was statistically significant but lesser in extent than the increase in the CD25lowFoxP3+ T cells.
Collectively, these results show that inhibition of the ‘Bcl-2-regulated’ apoptotic pathway causes a preferential expansion of FoxP3+ CD4+ T cells, particularly the CD25lowFoxP3+ population.
T cells bearing auto-reactive TCRs contribute preferentially to the increase in FoxP3+ CD4 thymocytes in mice with defects in the ‘Bcl-2 regulated’ apoptotic pathway
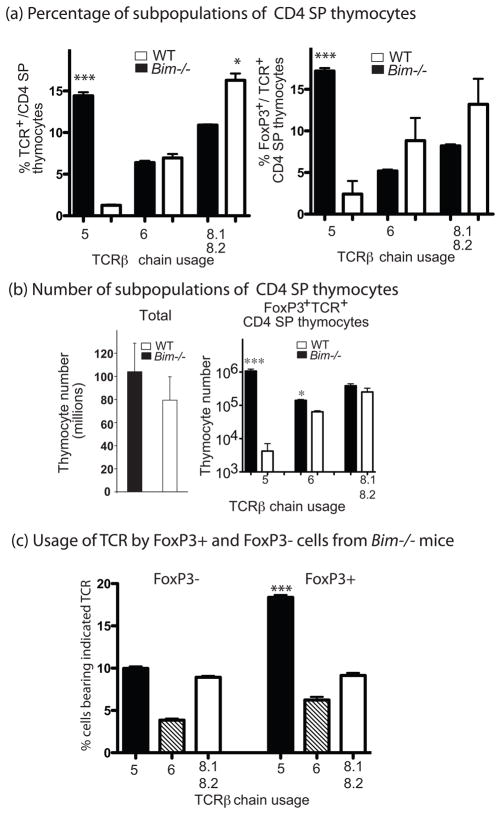
Next we examined whether the increase in FoxP3+ CD4 thymocytes in mice with defects in the ‘Bcl-2 regulated’ apoptotic pathway was linked to impaired deletion of self-reactive thymocytes. To address this question, we analyzed the selective deletion of thymocytes bearing specific TCR components (e.g. Vβ5) that confer specificity to endogenous retroviral super-antigens (a self-antigen) presented by IEd MHC molecules in BALB/c mice (28). Analysis of thymocytes revealed that the percentage of Vβ5+ CD4 SP thymocytes, but not Vβ6+ or Vβ8.1/8.2+ CD4 SP thymocytes (which do not confer reactivity to self super-antigens), was substantially increased in BALB/c Bim−/− mice (>10 fold; P<0.01 compared to WT; Figure 3a). Furthermore, the percentage of FoxP3+ cells was much higher amongst Vβ5+ CD4 SP thymocytes from BALB/c Bim−/− mice compared to the Vβ6+ thymocytes in the same mice (Figure 3a). In absolute numbers, FoxP3+Vβ5+ CD4 SP thymocytes were increased more than 250-fold, while the increase in FoxP3+ cells bearing TCRVβ6 or TCRVβ8.2 was less than 5-fold in BALB/c Bim−/− mice compared to WT mice (Figure 3b). Furthermore, when CD4+ T cells from Bim−/− mice were separated into FoxP3+ and FoxP3− populations, Vβ5+ cells were much more abundant in FoxP3+ fraction (Figure 3c).
Fig. 3. Loss of Bim causes a selective increase in self-superantigen specific TCR Vβ5+ FoxP3+CD4+ thymocytes on the BALB/c background.
Thymocytes from 3 individual 6–7 week old mice of the indicated genotypes were first surface stained for CD4, CD8, CD25 and individual TCR Vβ chains and then fixed, permeabilized and finally stained for intracellular FoxP3. (a) Percentages of subpopulations of CD4 SP thymocytes: [left panel] Mean +/− SEM of the percentages of CD4 SP thymocytes bearing the indicated TCR Vβ chain within total thymocytes. ***P<0.001 compared to WT mice. [right panel] Mean +/−SEM of the percentages of FoxP3+CD4+ cells within CD4+ SP thymocytes bearing the indicated TCR Vβ chain. *P<0.05, ***P<0.001 compared to WT mice. (b) Numbers of cells within the different sub-populations of CD4SP thymocytes: Mean +/− SD of the numbers of total thymocytes [left panel] and Mean +/− SEM of FoxP3+CD4 SP thymocytes bearing the indicated TCR Vβ chain [right panel]. *** P<0.001 compared to WT mice. (c) Usage of different vβ TCRs by FoxP3+ and FoxP3- CD4 SP thymocytes from Bim−/− mice: Bar graph shows mean +/−SEM of the percentages of FoxP3+ and FoxP3− CD4 SP thymocytes carrying different TCRvβ chains from 3 individual Bim−/−mice. Three experiments were performed with similar results.
Although C57BL/6 mice do not express MHC IE molecules, Vβ5 CD4+ T cells can still be reactive to endogenous retroviral antigens and are partially deleted via IA (6). When Vβ5 CD4+ T cells were compared between C57BL/6 and C57BL/6 Bim−/−mice, Vβ5 CD4+ T cells from WT mice contained a much large proportion of FoxP3+ cells (30%) and Vβ5 CD4+ T cells from C57BL/6 Bim−/− mice had an even higher proportion of FoxP3+ cells (>40%) (Supplement Fig 2a). On the other hand, cells bearing non-negative selecting Vβ6 contained ~10% FoxP3+ cells in WT and ~16% in C57BL/6 Bim−/− mice (Supplement Fig 2a). Thus, accumulation of FoxP3+ CD4+ T cells in Bim−/− mice is more prominent in cells expressing TCRs reactive to self-antigens.
A profound increase in CD25low FoxP3+ thymocytes was also observed in normal (i.e. Bim+/+) TCR transgenic mice in the presence of cognate selecting ligands (mOVA) (Supplement Fig 2b). Thymic expression of mOVA depleted most of the high-affinity DO11 TCR expressing thymocytes. However, for those remaining self-antigen specific TCR expressing cells, a significant proportion became CD25lowFoxP3+. Thus, the increase in CD25 low FoxP3+ thymocytes reflects self-reactivity, rather than a peculiarity of mice with defects in the 'Bcl-2-regulated' apoptotic pathway.
CD25lowFoxP3+CD4+ T cells and CD25highFoxP3+ have a similar survival rate
The preferential accumulation of CD25lowFoxP3+CD4+ cells in Bim−/− or Bcl-2 Tg mice described above is consistent with clonal diversion of self-reactive T lymphoid cells escaping deletion. However, the expansion could also reflect a survival advantage of CD25low FoxP3+CD4+ cells over the other CD4+ T cell subsets.
To examine this, sorted CD4 T cell subpopulations from vav-Bcl-2 Tg/FoxP3KI mice were labeled with CellTrace violet dye and adoptively transferred into C57BL/6-Ly5.1 recipient mice. Transferred cells (identified by CellTrace dye and CD45.2+) were analyzed 3 and 7 days after transfer. Several observations were made: 1) the phenotype of the transferred cells remained stable. Most of the CD25lowFoxP3+CD4+ T cells remained CD25low (Figure 4a); 2) recovery of CD25low and CD25high FoxP3+CD4+ T cells was similar, suggesting that they had similar survival capacity although both populations did not survive as well as FoxP3−CD4+ T cells (Figure 4b); 3) all three CD4+ T cell subpopulations did not divide substantially, at least within the first 7 days after transfer (Figure 4c).
Fig 4. Survival of CD25lowFoxP3+CD4+ T cells is similar to that of CD25highFoxP3+CD4+ T cells in vivo after adoptive transfer.
2x105 CellTrace-labeled CD4+ T cells were injected iv into C57BL/6-Ly5.1 recipient mice. 3 and 7 days after transfer, spleens were recovered. Transferred cells were identified as Ly5.2+CellTrace+ cells. (a) Dot plots show the expression of CD25 and FoxP3-GFP by CD4+ T cell sub-populations before and after transfer. (b) Bar graphs show the numbers of transferred cells (mean of 2–3 mice per group). (c) Histograms show Celltrace intensity of CD4+ T cell subpopulations. Two independent experiments were performed with similar results.
These in vivo data with transferred cells from vav-Bcl-2 Tg mice were also supported by in vitro survival analysis of CD4+ T cell subpopulations from Bim−/−mice. CD25−FoxP3− CD25lowFoxP3+ and CD25highFoxP3+ from lymph nodes of Bim−/-mice survived better than the corresponding cells from WT mice. However, CD25lowFoxP3+ cells and CD25highFoxP3+ cells from the same Bim−/− mice had a similar survival rate. With the same input number, the recovered number of viable CD25lowFoxP3+ cells was fewer than that of CD25−FoxP3− cells and comparable to CD25highFoxP3+ cells. Provision of IL-2 greatly enhanced the survival of FoxP3+ T cells from WT mice while IL-7 enhanced the survival of FoxP3− T cells from WT mice. Enhancement of survival of Bim−/− T cells by IL-2 and IL-7 was modest (because they already survived so well in their absence). Stimulation by anti-CD3 plus anti-CD28 antibodies also did not significantly alter the pattern of survival of these cell populations (data not shown).
Overall these results show that the selective increase in the CD25lowFoxP3+ CD4+ subset is unlikely to be a consequence of preferential survival of this cell subset.
CD25lowFoxP3+ CD4 T cells are hypo-responsive to TCR ligation
Functional analysis of FoxP3+CD4+ T cells relies on the use of reporter GFP since FoxP3 is an intracellular transcription factor. GFP expression in these reporter mice faithfully reflects FoxP3 transcription (Supplement Fig 3). Three populations of CD4+ T cells were purified according to CD25 and GFP expression by FACS sorting. Transcription of CD25 and FoxP3 by these three populations of CD4+ T cells was then analyzed by real-time qPCR. Not surprisingly, transcript levels of CD25 and FoxP3 were lower in CD25lowFoxP3+ cells compared to CD25highFoxP3+ cells (Supplement Fig 3). The expression of CTLA4, a molecule that is critical for the function of T regulatory cells (29), was also lower in CD25lowFoxP3+ cells, compared to CD25highGFP+ cells (Supplement Fig 3).
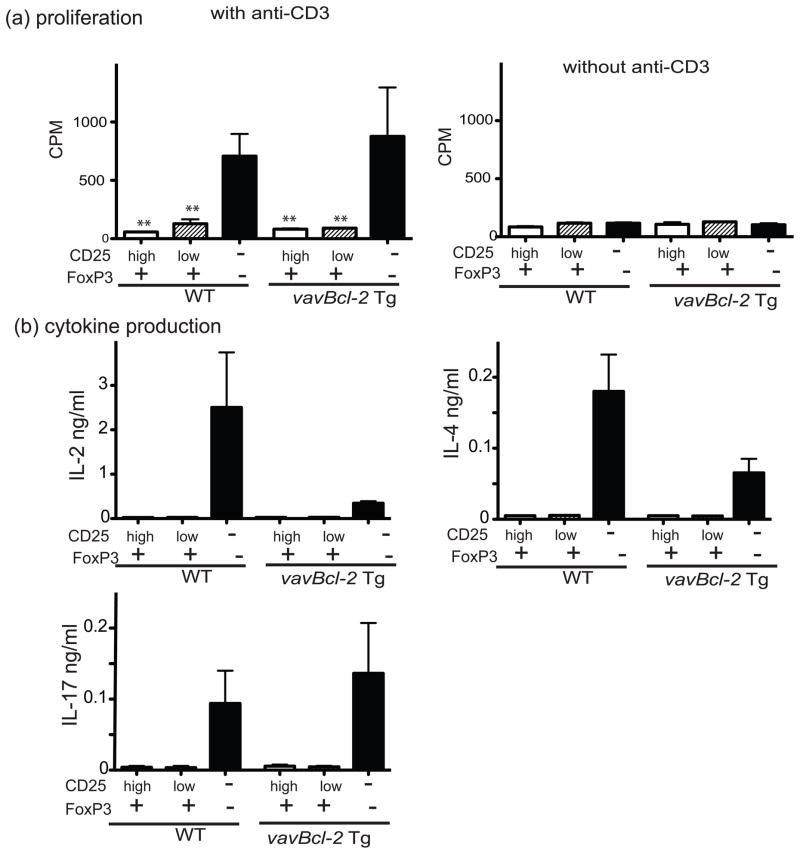
To examine the functional status of CD25lowFoxP3+ cells from mice with defects in the 'Bcl-2-regulated' apoptotic pathway, we measured the proliferative response and cytokine production of CD4+ T cell sub-populations to in vitro TCR (anti-CD3 plus anti-CD28 antibodies) stimulation comparing FoxP3-GFPKI/vav-Bcl-2 Tg mice and control FoxP3-GFPKI mice. CD4+ T cells were separated into three sub-populations based on FoxP3-GFP and CD25 expression. Compared to CD25−FoxP3− CD4+ cells, all FoxP3+CD4+ cells (either CD25low or CD25high) from both WT as well as vavBcl-2 Tg mice showed only poor proliferation in response to TCR ligation (Figure 5a). In addition, compared to CD25−FoxP3− CD4+ T cells, CD25lowFoxP3+ and CD25highFoxP3+ CD4+ T cells failed to produce appreciable amounts of IL-2, IL-4 and IL-17 (Figure 5b) as well as several other cytokines (e.g. IL-10 and IFN-γ; data not shown).
Fig. 5. CD25lowFoxP3+ cells are hypo-responsive to TCR stimulation.
(a) T cell proliferation: Distinct CD4+ T cell subpopulations from lymph nodes of FoxP3GFPKI or FoxP3GFPKI/vavBcl-2 Tg mice were sorted according to the levels of FoxP3-GFP and CD25 expression. These T cell subsets (2x104 cells per well) were cultured in 96-well round-bottomed plates with 5 μg/mL anti-CD3 plus 2 μg/mL anti-CD28 Abs in the presence of 8x104γ-irradiated and T cell depleted spleen cells (used as antigen presenting cells). The mean cpm +/− SD from 3–5 replicates per culture condition is shown. **P<0.01 compared to GFP-CD25−CD4+ lymph node cells. Three experiments were performed with similar results. (b) Cytokine production: supernatants from the above cultures harvested after 3 days of incubation were examined for cytokine content. Data represent the mean +/− SEM from three replicate cultures per cell type and genotype. Three independent experiments were performed and produced similar results.
Similarly, CD25lowFoxP3+ as well as CD25highFoxP3+ CD4+ T cells from FoxP3-GFPKI/Bim−/− mice also had a much lower proliferative response to TCR stimulation compared to the corresponding cell subsets from control FoxP3-GFPKI mice (data not shown).
Comparable results were obtained with the corresponding populations of T cells from either secondary lymphoid organs (spleen and lymph nodes) or thymus, although thymic T cells generally produced weaker responses (data not shown).
CD25lowFoxP3+CD4+ T cells have reduced regulatory potency compared to CD25highFoxP3+CD4+ T cells
Since FoxP3 expression is a hallmark of Treg cells, we tested the regulatory capacity of the expanded CD25lowFoxP3+CD4+ cells from FoxP3-GFPKI /vavBcl-2 Tg mice to suppress CD25−CD4+ T cell proliferation in a CFSE dilution based proliferation assay. We found that CD25highFoxP3+CD4+ cells from either vavBcl-2 Tg or WT mice readily suppressed the anti-CD3 Ab induced proliferation of effector T cells while CD25lowFoxP3+ CD4+ T cells from vavBcl-2 Tg were less potent (Figure 6).
Fig. 6. CD25lowFoxP3+CD4+ T cells are less potent suppressors of effector T cell activation compared to CD25highFoxp3+CD4+ T cells.
Suppression of effector T cell proliferation: CD25lowFoxP3+CD4+ and CD25highFoxP3+CD4+ cells (2x104 cells) from lymph nodes of mice of the indicated genotypes were cultured with 2x104 cells CFSE-labeled C57BL/6-Ly5.1 CD25-CD4+ T cells as effectors in 200 μL medium and left untreated or stimulated with 5 μg/mL anti-CD3 Abs in the presence of 8x104 spleen cells (used antigen presenting cells) for 3 days. After 3 days of culture, supernatants were harvested for measurement of cytokine content (Figure 8). Recovered cells were stained for Ly5.1, CD4 and CD25. Viable cells were enumerated by FACS analysis using PI exclusion and calibrating PE-beads. (a) FACS profile of CFSE dilution. Numbers in the plots indicate the percentages of proliferating cells. (b) Numbers of proliferating effector T cells. P values between samples that were compared are indicated. Three independent experiments were performed with similar results.
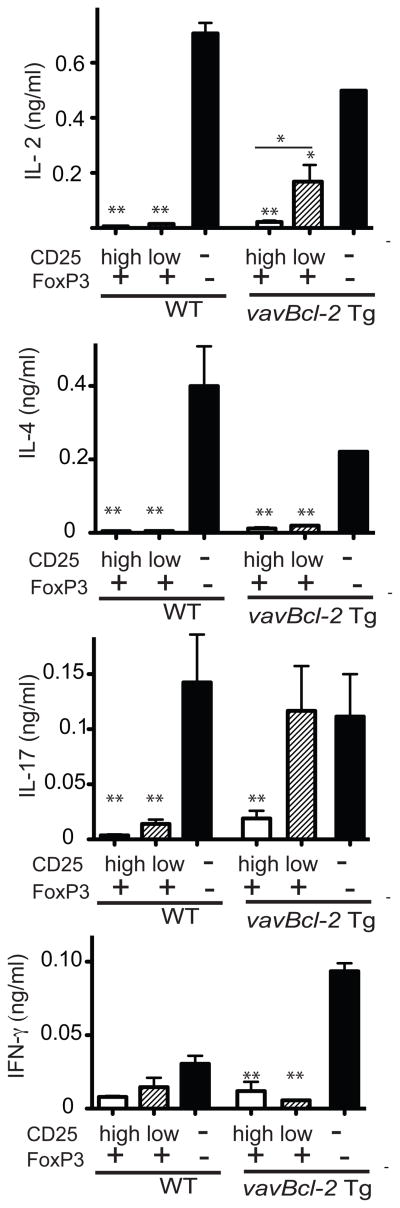
Cytokine production from co-cultures of effector cells with CD25highFoxP3+, CD25lowFoxP3+CD4+ T cells or FoxP3-CD4+ T cells from vavBcl-2 Tg mice were also measured. In accordance with the measurements of cell proliferation, the CD25lowFoxP3+CD4+ T cells from vavBcl-2 Tg mice were also less potent than CD25highFoxP3+ CD4+ T cells in suppressing IL-2 and IL-17 production (Figure 7). The two cell subsets were comparable in their ability to suppress IL-4 and IFN-γ production (Figure 7). CD25highFoxP3+CD4+ cells from both WT mice, as well as CD25lowFoxP3+CD4+ cells (though very few in number) from WT mice suppressed the production of suppress IL-2 and IL-17 production.
Fig 7. CD25lowFoxP3+CD4+ T cells are less capable of suppressing IL-17 production compared to CD25highFoxp3+CD4+ T cells.
Cultures were set up as described for Fig. 6. Supernatants from 3-day cultures were harvested to measure the content of the indicated cytokines. Data show the mean concentrations of triplicate cultures for selected cytokines. *P<0.05, **P<0.01 compared to cultures with CD25-FoxP3−CD4+ T cells. Two independent experiments produced similar results.
These results show that CD25lowFoxP3+CD4+ T cells were generally less efficacious than CD25highFoxP3+CD4+ T cells in inhibiting proliferation and cytokine production by conventional CD4 effector T cells.
CD25lowFoxP3+CD4+ T cells cannot be readily converted into CD25highFoxP3+CD4+ T cells by TCR stimulation
Next we investigated whether CD25lowFoxP3+CD4+ T cells were the precursors for CD25highFoxP3+CD4+ T cells. For this, CD4+ T cells from FoxP3-GFPKI/vavBcl-2 Tg mice and FoxP3-GFPKI/vavBcl-2 Tg mice were sorted into three sub-populations: CD25highFoxP3+, CD25lowFoxP3+, and CD25−FoxP3−CD4+ T cells from FoxP3-GFPKI/vavBcl-2 Tg mice. Cells were cultured with or without IL-2, and anti-CD3 plus anti-CD28 Abs. Expression of FoxP3 (reflected by expression of GFP) and CD25 by the three populations was measured after 36 h. In the presence of IL-2, we observed that only ~20% of the CD25low subset up-regulated their CD25 expression (Figure 8), compared to ~63% within the CD25high subset. In response to TCR stimulation, CD25 was up-regulated on a proportion of CD25−FoxP3− cells, but notably there was little change in the expression of CD25 in CD25low FoxP3+cells (Figure 8a). We also observed that IL-2 treatment resulted in a similar change in CD25 expression when CD25lowFoxP3+cells from FoxP3-GFPKI /vav-Bcl-2 Tg mice were transferred into B6 mice (Figure 8b). Changes in CD25 expression did not associate with cell expansion.
Fig. 8. IL-2R- but not TCR-stimulation can convert CD25lowFoxP3+CD4+ T cells into CD25highFoxP3+CD4+ T cells.
(a) in vitro assay. T cells from FoxP3GFPKI/vavBcl-2 Tg mice were sorted into three sub-populations according to the levels of CD25 and FoxP3 expression. Cells were cultured at a starting density of 2x104 cells/well in 200 μL medium in round-bottom 96-well plates in the presence or absence of IL-2 (20 U/mL), or anti-CD3 plus anti-CD28 Abs. Cells were harvested after 36 h and stained for CD25. Dot plots show expression of FoxP3 and CD25 on freshly sorted viable cells before culture and on viable cells after 36 h culture. (b) in vivo assay: B6 mice were iv injected with 105 CellTrace-labelled CD25lowFoxP3+ CD4+ T cells from Foxp3GFPKI/vavBcl-2 Tg mice. Half of mice were injected intra-peritoneally with 2 daily doses of IL-2 (1000U/dose). Mice were killed 60 h after the first dose of IL-2. Spleen cells from IL-2 treated and untreated mice were surface stained for CD4, CD8 and CD25. Contour plots show CD25 and Foxp3 expression on CellTrace-labelled CD4+ T cells. Histograms show for CellTrace violet intensity. Similar results were obtained from two independent experiments.
Overall, these results indicate that TCR-stimulation or culture in simple medium does not readily convert the CD25lowFoxP3+cells into CD25highFoxP3+ cells but that IL-2 can convert a fraction of the CD25lowFoxP3+cells into CD25high cells.
The ‘death receptor’ Fas has only a minor role in regulating thymic differentiation of FoxP3+ cells but is critical for peripheral homeostasis of FoxP3+CD4+ T cells
Although we focused our study on the 'Bcl-2-regulated' apoptotic pathway, we also extended our study to Fas ‘death receptor’-induced apoptosis. In contrast to mice with defects in the 'Bcl-2-regulated' apoptotic pathway, Fas deficient FASlpr/lpr mutant mice had no significant increase in CD4 SP thymocytes and had only a minor increase in FoxP3+CD4+ SP thymocytes (Supplement Fig 4a). The increase in FoxP3+ CD4+ thymocytes (both CD25low and CD25high) in mice that are defective in both Bim and Fas (Bim−/−FASlpr/lpr) was only marginally larger than the increase seen in Bim−/− mice (both in percentage and absolute cell number) (Supplement Fig 4a).
However, in mice that are defective in both Bim and Fas (Bim−/−FASlpr/lpr), we found that the accumulation of CD25lowFoxP3CD4+ T cells in secondary lymphoid organs (spleen and lymph nodes) was substantially accelerated and increased compared with either Bim−/− or FASlpr/lpr single mutant mice. This increase was a result of both an increased frequency of FoxP3+ cells within the CD4+ T cell subset (Supplement Fig 4b), and increased total cellularity of these lymphoid organs (Supplement Fig 4b). Although increased accumulation of FoxP3+CD4+ cells was also observed in older FASlpr/lpr mice, the majority of these cells were CD25highFoxP3+. These results show that Fas-mediated apoptosis contributes to the regulation of the numbers of FoxP3+CD4+ T cells in peripheral lymphoid organs although T regulatory cells are thought to be resistant to TCR stimulation induced apoptosis (AICD) (30), which is mediated by FasL/Fas signaling. As expected, FoxP3+CD4+ T cells were more sensitive to FasL-mediated killing, compared to FoxP3−CD4+ T cells in vitro (Supplement Fig 4c).
Discussion
In this study, we found that blocking deletion of auto-reactive thymocytes by inhibiting the 'Bcl-2-regulated' apoptotic pathway caused a large increase in FoxP3+CD4+ T cells in both the thymus and peripheral lymphoid organs. The increase was particularly striking within the CD25lowFoxP3+CD4+ population. The increase of this population is unlikely due to their preferential survival and is also unlikely due to their enhanced proliferation. Based on the finding that cells bearing self-antigen reactive TCR are highly over-represented in the population, we suggest that these cells represent a population that has undergone clonal diversion in the differentiation program towards a FoxP3+ T-cell fate. Induction of FoxP3, without high expression of CD25, might curtail the auto-aggressiveness of abnormally surviving self-reactive CD4+ T cells, but may also bestow upon them reduced potency as T regulatory cells. Signaling from IL-2R but not from the TCR allowed conversion of a fraction of these CD25low cells into CD25+ cells. Interestingly, Fas death receptor-mediated apoptosis was found to contribute to the regulation of the peripheral pool of CD25low FoxP3+ CD4+ T cells and thereby provides an additional check-point for cells that escaped from thymic deletion due to a defect in the 'Bcl-2-regulated' apoptotic pathway.
Here we investigated CD4+ T cell subsets in mice that are defective in different components of the “Bcl-2-regulated” apoptotic pathway. The increase in CD25lowFoxP3+CD4+ T cells was most prominent in mice reconstituted with Bax−/−/Bak−/− fetal liver cells. Deficiency in the BH3-only protein Bim causes a significant increase in FoxP3+CD4+ T cells in the thymus and peripheral lymphoid tissue. Bim−/−/Puma−/−mice showed a further increase in FoxP3+CD4+ T cells, although the loss of Puma alone has no appreciable impact on total thymic cellularity as well as the numbers of FoxP3+CD4+ cells. Like Bim, Puma is also a BH3-only protein that can bind with high affinity to all pro-survival Bcl-2-like proteins (25) (31). Puma has a role in regulating apoptosis of antigen stimulated T cells (32). Furthermore, Puma cooperates with Bim to regulate lymphocyte apoptosis (33). Findings from our study suggest that Bim and Puma have overlapping actions in controlling the pool of FoxP3+CD4+ T cells. Consistent with the findings from mice lacking pro-apoptotic proteins, transgenic mice over-expressing anti-apoptotic Bcl-2 also showed a profound increase in FoxP3+CD4+ T cells. However, this increase was lower compared to mice with a Bax/Bak doubly deficient hematopoietic system. Currently, the role of other anti-apoptotic molecules, such as Mcl-1 and A1, in regulating the FoxP3+CD4+ T cell pool remains unclear.
Given that an increase in the cellularity of overall T cells including FoxP3+ T cells in mice either lacking pro-apoptotic Bcl-2 family members or over-expressing anti-apoptotic Bcl-2-like proteins may not be surprising and has been documented (24), why is the increase in FoxP3+CD4+ T cells, particularly the CD25lowFoxP3+CD4+ subset, much more pronounced than the increase in conventional CD4+ T cells in these mice? Conceivably, there are several potential mechanisms leading to a preferential increase in FoxP3+CD4+ cells. Firstly, the survival rate of CD25lowFoxP3+ CD4+ T cells might be higher, compared to FoxP3−CD4+ and CD25highFoxP3+CD4+ T cells. However, CD25lowFoxP3+ CD4+ T cells from vavBcl-2 Tg mice and Bim−/− mice survived similarly to CD25highFoxP3+ cells in culture and did not survive as well as FoxP3−CD4+ T cells from the same apoptosis defective mice. Thus, the preferential increase in the CD25lowFoxP3+CD4+ population in mice with a defect in the ‘Bcl-2-regulated’ apoptotic pathway is unlikely due to preferential survival, although enhanced survival by T cells in these mice must contribute to the overall increase in all T cell subsets. Secondly, proliferation of CD25lowFoxP3+CD4+ T cells might be higher, compared to FoxP3−CD4+ and CD25highFoxP3+CD4 T cells. However, several results from our study do not support this possibility. Isolated CD25lowFoxP3+CD4+ T cells when adoptively transferred did not show cell division rates above the levels of FoxP3−CD4+ or CD25highFoxP3+CD4+ T cells. BrdU incorporation was also similar between FoxP3+CD4+ and the FoxP3−CD4+ T cell subsets (unpublished data); cell cycle analysis of the different CD4+ T cell subsets also did not show a higher percentage of cycling cells within CD25lowFoxP3+CD4+ T cells compared to the other subsets (unpublished data). Apart from increased cell survival and increased cell proliferation, we also exclude a major contribution of differential thymic location and thymic re-entry of FoxP3+CD4+ cells from the periphery as a cause of the preferential increase in FoxP3+CD4+ T cells in these mice (unpublished data). Thus, we favor a view that a preferential increase in CD25lowFoxP3+CD4 T cells is the consequence of increased differentiation/diversion, most likely originating from cells that are normally deleted by “Bcl-2 regulated” apoptosis after stimulation of their TCRs by self-antigens.
In support of the notion that the preferentially expanded population represents the self-reactive cells that escaped thymic deletion and have been diverted in their differentiation, we found an up to 500-fold increase in TCR Vβ5-bearing FoxP3+CD4+ T cells in BALB/c Bim−/− mice compared WT BALB/c mice. These T cells bear a TCR that is specific to endogenous super-antigens presented by IEd MHC molecules and are normally deleted on a BALB/c background (28), but loss of Bim allows their escape. Moreover, analysis of FoxP3 expression by CD4+ T cells bearing different TCR Vβ chains in BALB/c Bim−/− mice revealed that a much higher proportion of TCRVβ5-bearing CD4+ T cells express FoxP3 compared to cells bearing non-self-reactive TCRs, such as those containing TCR Vβ6. In fact, the percentage of Vβ6-bearing FoxP3+CD4+ T cells was comparable between WT and Bim−/− mice on a BALB/c background. When CD4+ T cells are separated into FoxP3+ and FoxP3− fractions, Vβ5-bearing cells are greatly over-represented within the FoxP3+ fraction. We therefore conclude that the increased FoxP3+CD4+ cells in mice with a defect in the 'Bcl-2-regulated' apoptotic pathway are derived from ‘forbidden clones’ (self-reactive) that are normally deleted in the thymus. A question raised by this study is whether a diversion into CD25lowFoxP3+CD4+ cells occurs in mice without a profound defect in the 'Bcl-2-regulated' apoptotic pathway. Importantly, we found that although in mice transgenic for both an OVA-specific TCR (KJ1-26+ DO11) and the cognate antigen (OVA) the majority of KJ1-26+CD4+ T cells were deleted, most of the residual KJ1-26+CD4+ thymocytes in these animals were FoxP3+ but CD25low. Hence even in mice with an intact ‘Bcl-2-regulated’ apoptotic pathway, auto-reactive thymocytes that have escaped deletion can be clonally diverted towards a CD25lowFoxP3+ state.
There are two unresolved issues relating to the increased differentiation of CD25lowFoxP3+CD4+ T cells. Firstly, which factors determine the differentiation of FoxP3+CD4+ T cells? Particularly, what signal differences exist between negative selection and positive selection of FoxP3+CD4+ cells? What has been revealed is that the strength of TCR engagement plays a critical role in determining FoxP3+CD4+ cell differentiation. Several elegant and detailed studies concluded that a TCR/MHC plus peptide avidity range somewhere between positive and negative is ideal for Treg differentiation, since very weak TCR interaction as well as very strong TCR stimulation impede generation of Treg cells (34–36). Notably, in in vitro as well as in vivo studies, not all CD4+ T cells became FoxP3+ even when all T cells expressed the same ‘Treg’ fate promoting (transgenic) TCR (37). This could be due to the fact that not all T cells experience the same dose of TCR stimulation. Alternatively, these findings might imply that the same signals emanating from TCR/MHC interaction might result in different cell fates, probably due to differences in intracellular “wiring” or the fact that different cells may experience different auxiliary stimuli from their micro-environment (38). It remains a challenge to delineate the ‘quantitative” or/and “qualitative’ signals directing FoxP3 transcription.
Secondly, unlike FoxP3+CD4+ T cells from WT mice, a large fraction of FoxP3+ CD4+ T cells from mice with defects in the ‘Bcl-2-regulated’ apoptotic pathway are CD25low. This raises the question of what controls CD25 expression in FoxP3+CD4+ T cells. In conventional CD4+ T cells, expression of CD25 is mainly regulated at the transcriptional level (39, 40) and three major transcription factors have been implicated: NFAT (41), NF-κB (42) and AP-1 (41). In the Treg lineage, the transcription factor FoxP3 has been shown to cooperate with NFAT to enhance CD25 expression while suppressing IL-2 transcription (43). Dissection of gene elements critical for CD25 expression revealed that TCR and IL-2 activate different elements of the CD25 gene to enhance transcription (39, 40, 44). In our study, we found that TCR ligation was not sufficient to up-regulate CD25 expression on CD25lowFoxP3+CD4+ T cells even though this stimulus could efficiently increase CD25 expression on FoxP3−CD4+ T cells (Figure 8). Conversely, provision of IL-2 was able to induce CD25 up-regulation on a proportion of CD25lowFoxP3+CD4+ T cells (Figure 8). This differential effect on CD25 regulation by TCR and IL-2R signaling in CD25lowFoxP3+ CD4+ T cells may reflect specific defects in TCR signaling in these cells. We predict that IL-2/IL-2R activation of Stat5 may proceed normally in these cells while the TCR-activated pathway may somehow be impaired. In vitro, IL-2 can partially convert CD25lowFoxP3+CD4+ to CD25highFoxP3+CD4+ T cells. Using an adoptive transfer system, we found that injection of IL-2 can indeed convert a fraction of CD25lowCD4+ cells into CD25highCD4+ cells in vivo. Whether these converted CD25highFoxP3+CD4+ cells would gain potent regulatory function as bona fide CD25highFoxP3+CD4+ cells warrants further investigation. This conversion in CD25 expression levels elicited by IL-2 may have implications for immune regulation. When a strong T-cell response is induced, the resultant high level IL-2 milieu may convert CD25lowFoxP3+CD4+ T cells into CD25highFoxP3+CD4+ T cells to reinforce immune regulation. When there is no such need for immune regulation (and IL-2 levels are low) during steady state, CD25lowFoxP3+CD4+ T cells may stay stable and serve as a reserve for potential future use.
Although we observed that an increase in CD25lowFoxP3+CD4+ T cells occurs both in the thymus and secondary lymphoid organs of mice with defective “Bcl-2 regulated” apoptosis, the relationship between the two pools of CD25lowFoxP3+CD4+ T cells warrants further investigation. There are many examples where self-reactive T cells can convert from a FoxP3−CD4+ into a FoxP3+CD4+ state in the periphery, particularly under the influence of TGF-β (45, 46). Furthermore, TGF-β can also cause an expansion of FoxP3+CD4+ T cells (47). Thymus derived FoxP3+CD4+ cells and TGF-β induced FoxP3+CD4+ cells differ in several requirements such as TCR signal strength and co-stimulation for their differentiation (48). The nature of the increased FoxP3+CD4+ population in secondary lymphoid organs (cf. thymus) of mice with defective “Bcl-2 regulated” apoptosis might be dissected based on the aforementioned differences.
The abnormally increased CD25lowFoxP3+CD4+ cells from Bim−/− and Bcl-2 Tg mice were found to be hypo-responsive to TCR stimulation (anergic) in culture. This was not surprising given that hypo-responsiveness to TCR stimulation in vitro is a common feature of FoxP3+CD4+ T cells. Indeed, expression of the transcription factor FoxP3 was shown to be sufficient to render T cells hypo-responsive to TCR stimulation (49, 50), although there are also FoxP3-independent mechanisms for rendering T cells anergic (14, 51, 52). It is presently unclear whether FoxP3-dependent and/or -independent pathways contribute to the anergic status of CD25lowFoxP3+CD4+ cells.
Interestingly, the expanded population of CD25lowFoxP3+CD4+ T cells from vav-Bcl-2 Tg and Bim−/− mice had a lower capacity to suppress effector T cell proliferation than CD25highFoxP3+CD4+ Treg cells. Why do these CD25lowFoxP3+CD4+ cells lack potent suppressive function? First, it has been reported that only CD4+ T cells with high levels of FoxP3 possess regulatory function (53, 54). We noticed that the CD25lowFoxP3+CD4 T cells in mice with defects in the ‘Bcl-2 regulated’ apoptotic pathway express lower levels of FoxP3. Consistent with this finding, CD25lowFoxP3+CD4+ cells from Bim−/−/FoxP3GFPKI mice exhibited greatly reduced expression of GFP during in vitro culture (without exogenous stimuli), whereas the reduction in GFP intensity on CD25highFoxP3+CD4+ cells was only moderate, implying less stable FoxP3 expression by CD25lowCD4+ cells. Second, one of the proposed mechanisms by which Treg cells influence effector T cell function is via sequestration of IL-2 and possibly other cytokines (55). Since CD25 (IL-2Rα chain) functions as a component of the IL-2R, CD25lowFoxP3+CD4+ cells would be expected to be less potent at binding IL-2 and thereby less potent at depriving effector T cells of this cytokine. Third, FoxP3 alone is unable to induce the full range of Treg transcriptional and functional features (56). For example, although all T cells from FoxP3 transgenic mice show reduced TCR stimulation-induced IL-2 production and proliferation (49), they have less regulatory potency compared to naturally occurring CD25highCD4+ T cells (49). Moreover, we found that CD25lowFoxP3+CD4+ cells had lower expression of CTLA4 (Supplement Fig 3), a molecule critical for regulatory function (29).
It appears that CD25lowFoxP3+CD4+ T cells do not efficiently suppress IL-17 production by FoxP3−CD4+ effector cells. However, it is possible that CD25lowFoxP3+CD4 T cells themselves contribute to the IL-17 production detected in co-cultures. It has previously been shown that reducing the levels of FoxP3 in cells committed to become FoxP3+CD4+ T cells increases their ability to produce pro-inflammatory and disease inducing cytokines, including IL-17 (53, 54). Given that CD25lowFoxP3+CD4+ T cells from mice with defective “Bcl-2 regulated” apoptosis have lower levels of FoxP3 transcription (Supplement Fig 3), it could be argued that CD25lowFoxP3+CD4+ T cells can gain the ability to produce IL-17, at least under certain conditions (e.g. co-culture with effector cells). This issue may be resolved by using RORγt effector cells to distinguish the contribution of Bcl-2 Tg CD25lowFoxP3+CD4+ T cells to IL-17 production.
Although induction of FoxP3 blunts the auto-aggressiveness of self-reactive CD4+ T cells, additional tolerance mechanisms may be required to safeguard immune tolerance. One of these mechanisms is deleting self-reactive T cells escaping the censorship by ‘Bcl-2-regulated’ apoptosis by the ‘death receptor’ apoptotic pathway. Although Fas had only a very minor role in the differentiation of FoxP3+CD4+ T cells in the thymus in our studies (supplement Fig 4a), this ‘death receptor’ exerted a prominent role in controlling the peripheral pool of FoxP3+CD4+ (particularly CD25low) T cells. There was a marked increase in the numbers of CD25lowFoxP3+CD4+ T cells in mice doubly deficient for Bim and Fas, compared to WT or single mutant mice. It has previously been reported that human CD25+FoxP3+CD4+ T cells (57) and mouse CD25+FoxP3+CD4+ T cells (58) are more sensitive to FasL-Fas induced apoptosis than effector CD4+ T cells. We also noticed that FoxP3+CD4+ T cells from mice with defective ‘Bcl-2 regulated’ apoptosis were more sensitive to FasL-induced apoptosis in vitro than FoxP3-CD4+ T cells (Supplement Fig 4c). Accordingly, FoxP3+ CD4+ T cells (either CD25low or CD25high) had higher levels of Fas than FoxP3−CD4+ T cells (data not shown). Taken together, these data show that Fas-mediated apoptosis can cooperate with the ‘Bcl-2 regulated’ apoptotic pathway to control self-reactive T cells in a manner similar to the process shown to control chronically activated effector T cells (16–18).
In conclusion, we used mice with defects in the ‘Bcl-2 regulated’ apoptotic pathway to reveal the clonal diversion in the differentiation program of self-reactive CD4+ T cells that escape the deletion process. Such abnormal survivors switch on FoxP3 transcription as a consequence of self-ligand/MHC induced TCR stimulation. Compared to classic Treg cells, these FoxP3+CD4+ T cells express lower levels of CD25 and FoxP3 and are less potent at suppressing proliferation and cytokine production by effector T cells. Moreover, the CD25low Foxp3+CD4+ T cells are hypo-responsive to TCR ligation. Moreover, IL-2R- but not TCR-stimulation, can convert some of these cells into CD25+ cells. The enlarged FoxP3+CD4+ population found in Bim−/− or vav-Bcl-2 Tg mice is subjected to control by Fas-mediated apoptosis, but only in peripheral lymphoid organs and not the thymus. These findings provide some explanation to why the escapees from thymic deletion in mice defective for the ‘Bcl-2 regulated’ apoptotic pathway do not always cause severe autoimmune disease.
Supplementary Material
Acknowledgments
This work was supported by grants and fellowships from the National Health and Medical Research Council of Australia (NH&MRC): Program grants (#516700 and #461221), Project grants (#575543 and #637324), NH&MRC Australia Fellowship #461299, NH&MRC Career Development Awards (#516754 and #637353), NH&MRC Independent Research Institutes Infrastructure Support Scheme grant #361646, NIH (CA 043540), Leukemia and Lymphoma Society (LLS SCOR 7413), the Juvenile Diabetes Research Foundation grant #466658 and a Victorian State Government Operational Infrastructure Support grant.
We thank N Ashman, L Mackiewicz, M Hancock, and J Gilbert for animal care and technical assistance. We thank Dr A Rudensky for permission to use FoxP3GFPKI mice. We thank J Brady for proofreading the manuscript.
References
- 1.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A, Harris AW, von Boehmer H, Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci U S A. 1994;91:1376–1380. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 4.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 5.Davey GM, Kurts C, Miller JF, Bouillet P, Strasser A, Brooks AG, Carbone FR, Heath WR. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J Exp Med. 2002;196:947–955. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 7.Liston A, Lesage S, Gray DH, O'Reilly LA, Strasser A, Fahrer AM, Boyd RL, Wilson J, Baxter AG, Gallo EM, Crabtree GR, Peng K, Wilson SR, Goodnow CC. Generalized resistance to thymic deletion in the NOD mouse; a polygenic trait characterized by defective induction of Bim. Immunity. 2004;21:817–830. doi: 10.1016/j.immuni.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 9.Degermann S, Reilly C, Scott B, Ogata L, von Boehmer H, Lo D. On the various manifestations of spontaneous autoimmune diabetes in rodent models. Eur J Immunol. 1994;24:3155–3160. doi: 10.1002/eji.1830241236. [DOI] [PubMed] [Google Scholar]
- 10.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. Epub 2002 Oct 1310. [DOI] [PubMed] [Google Scholar]
- 11.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DR. The suicide in the thymus, a twisted trail. Nat Immunol. 2003;4:207–208. doi: 10.1038/ni0303-207. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 15.Lesage S, Hartley SB, Akkaraju S, Wilson J, Townsend M, Goodnow CC. Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J Exp Med. 2002;196:1175–1188. doi: 10.1084/jem.20020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, 3rd, Wu T, Li QZ, Davis LS, Mohan C, Perlman H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 19.McCaughtry TM, Hogquist KA. Central tolerance: what have we learned from mice? Semin Immunopathol. 2008;30:399–409. doi: 10.1007/s00281-008-0137-0. [DOI] [PubMed] [Google Scholar]
- 20.Komaki S, Kohno M, Matsuura N, Shimadzu M, Adachi N, Hoshide R, Nishiyama S, Matsuda I. The polymorphic 43Thr bcl-2 protein confers relative resistance to autoimmunity: an analytical evaluation. Hum Genet. 1998;103:435–440. doi: 10.1007/s004390050847. [DOI] [PubMed] [Google Scholar]
- 21.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 22.Villunger A, V, Marsden S, Zhan Y, Erlacher M, Lew AM, Bouillet P, Berzins S, Godfrey DI, Heath WR, Strasser A. Negative selection of semimature CD4(+)8(−)HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc Natl Acad Sci U S A. 2004;101:7052–7057. doi: 10.1073/pnas.0305757101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Pandiyan P, Lenardo MJ. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 26.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci U S A. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bill J, Kanagawa O, Linten J, Utsunomiya Y, Palmer E. Class I and class II MHC gene products differentially affect the fate of V beta 5 bearing thymocytes. J Mol Cell Immunol. 1990;4:269–279. discussion 279–280. [PubMed] [Google Scholar]
- 29.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SR, Alexander DR, Cooper JC, Higgins CF, Elliott JI. Regulatory T cells are resistant to apoptosis via TCR but not P2X7. J Immunol. 2007;178:3474–3482. doi: 10.4049/jimmunol.178.6.3474. [DOI] [PubMed] [Google Scholar]
- 31.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Fischer SF, Belz GT, Strasser A. BH3-only protein Puma contributes to death of antigen-specific T cells during shutdown of an immune response to acute viral infection. Proc Natl Acad Sci U S A. 2008;105:3035–3040. doi: 10.1073/pnas.0706913105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, Michalak E, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 35.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 36.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2010 doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 39.Kim HP, Kelly J, Leonard WJ. The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: importance of two widely separated IL-2 response elements. Immunity. 2001;15:159–172. doi: 10.1016/s1074-7613(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 40.Kim HP, Leonard WJ. The basis for TCR-mediated regulation of the IL-2 receptor alpha chain gene: role of widely separated regulatory elements. EMBO J. 2002;21:3051–3059. doi: 10.1093/emboj/cdf321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuh K, Twardzik T, Kneitz B, Heyer J, Schimpl A, Serfling E. The interleukin 2 receptor alpha chain/CD25 promoter is a target for nuclear factor of activated T cells. J Exp Med. 1998;188:1369–1373. doi: 10.1084/jem.188.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan TH, Huang GP, Sica A, Ghosh P, Young HA, Longo DL, Rice NR. Kappa B site-dependent activation of the interleukin-2 receptor alpha-chain gene promoter by human c-Rel. Mol Cell Biol. 1992;12:4067–4075. doi: 10.1128/mcb.12.9.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 44.Soldaini E, Pla M, Beermann F, Espel E, Corthesy P, Barange S, Waanders GA, MacDonald HR, Nabholz M. Mouse interleukin-2 receptor alpha gene expression. Delimitation of cis-acting regulatory elements in transgenic mice and by mapping of DNase-I hypersensitive sites. J Biol Chem. 1995;270:10733–10742. doi: 10.1074/jbc.270.18.10733. [DOI] [PubMed] [Google Scholar]
- 45.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 47.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Khattri R, Kasprowicz D, Cox T, Mortrud M, Appleby MW, Brunkow ME, Ziegler SF, Ramsdell F. The amount of scurfin protein determines peripheral T cell number and responsiveness. J Immunol. 2001;167:6312–6320. doi: 10.4049/jimmunol.167.11.6312. [DOI] [PubMed] [Google Scholar]
- 50.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 51.Borde M, Barrington RA, Heissmeyer V, Carroll MC, Rao A. Transcriptional basis of lymphocyte tolerance. Immunol Rev. 2006;210:105–119. doi: 10.1111/j.0105-2896.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 52.Heissmeyer V, Rao A. E3 ligases in T cell anergy--turning immune responses into tolerance. Sci STKE. 2004;2004:pe29. doi: 10.1126/stke.2412004pe29. [DOI] [PubMed] [Google Scholar]
- 53.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 54.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 55.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 56.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Fritzsching B, Oberle N, Eberhardt N, Quick S, Haas J, Wildemann B, Krammer PH, Suri-Payer E. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand- but not to TCR-mediated cell death. J Immunol. 2005;175:32–36. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 58.Reardon C, Wang A, McKay DM. Transient local depletion of Foxp3+ regulatory T cells during recovery from colitis via Fas/Fas ligand-induced death. J Immunol. 2008;180:8316–8326. doi: 10.4049/jimmunol.180.12.8316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.