Abstract
Through the design and synthesis of a new chiral phosphepine, the first catalytic asymmetric method for the [3+2] cycloaddition of allenes with olefins has been developed that generates cyclopentenes that bear nitrogen-, phosphorus-, oxygen-, and sulfur-substituted quaternary stereocenters. A wide array of racemic γ-substituted allenes can be employed in this stereoconvergent process, which occurs with good enantioselectivity, diastereoselectivity, regioselectivity, and yield. Mechanistic studies, including a unique observation of a (modest) kinetic resolution of a racemic allene, are consistent with addition of the phosphepine to the allene being the turnover-limiting step of the catalytic cycle.
INTRODUCTION
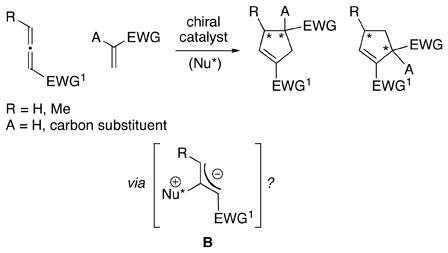
Interest in the use of tertiary phosphines as nucleophilic catalysts,1 particularly for enantioselective processes,2 has increased substantially in recent years. One especially powerful method, initially reported by Lu, is a formal [3+2] cycloaddition reaction between electron-poor allenes (or alkynes) and olefins,3 which generates cyclopentenes that are useful as endpoints or as intermediates for further functionalization. 4 Zhang described the first asymmetric variant of this process catalyzed by a chiral phosphine,5 and we6 and others7 have subsequently expanded the scope of this valuable transformation (eq 1).
 |
(1) |
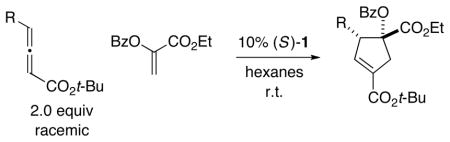
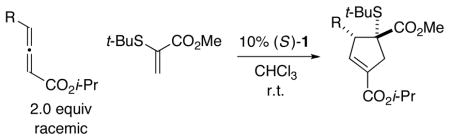
The presence of a heteroatom, rather than a hydrogen or a carbon, substituent on the olefin (A in eq 1) could be expected to have a significant impact on both reactivity and stereoselectivity in these [3+2] cycloadditions. Indeed, to the best of our know ledge, there have been few reports of the use of heteroatom-substituted olefins as partners in Lu annulations,8, 9 and there have been no descript ions of catalytic asymmetric processes that generate heteroatom-substituted quaternary stereocenters. 10 Nevertheless, the ability to accomplish bond construct ions of this type with good enantioselectivity 11 through the use of such olefins would substantially enhance the utility of these cyclopentene-forming reactions. In this report, we establish that this objective can be achieved with the aid of a new chiral phosphine catalyst (1) (eq 2).
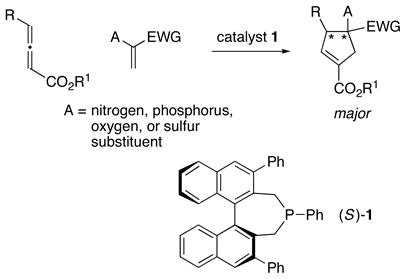 |
(2) |
RESULTS AND DISCUSSION
The most well-studied examples of phosphine-catalyzed [3+2] cycloadditions of electron-poor allenes/alkynes with heteroatom-substituted olefins involve nitrogen substituents.8 Consequently, as the starting point for our effort to develop the first such catalyticenantioselective annulations to generate heteroatom-bearing quaternary stereocenters, we chose to examine the reaction of a 5-methylenehydantoin8c,e (Table 1; product C can be transformed into a bioactive analogue of hydantocidin8e).
Table 1.
Enantioselective [3+2] Cycloadditions with a Heteroatom-Substituted Olefin: Effect of the Choice of Phosphine Catalysta
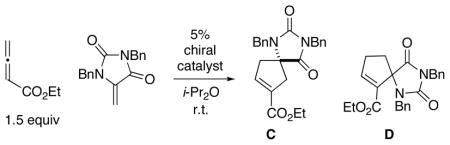 | ||||
|---|---|---|---|---|
| entry | chiral catalyst | ee (%)b | C: D | yield (%)c |
| 1 | (S,S)-DIPAMP | <2 | 1.0: 1 | 59 |
| 2 | (S,S)-Ph-BPE | <2 | 1.0: 1 | 66 |
| 3 | (S,S′, R,R′)-TangPhos | 11 | 0.8: 1 | 51 |
| 4 | (S)-2 | −51 | 0.8: 1 | 40 |
| 5 | (S)-3 | 59 | 1.1: 1 | 35 |
| 6 | (S)-4 | 76 | 1.2: 1 | 88 |
| 7 | (S)-1 | 97 | 1.3: 1 | 92 |
All data are the average of two experiments.
Enantiomeric excess of C. A negative value signifies that the R enantiomer was formed predominantly.
The yield was determined by HPLC analysis with the aid of an internal standard.
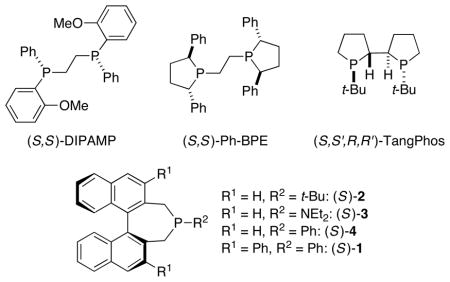
We surveyed a range of phosphines that have been useful in asymmetric catalysis,12 including nucleophilic catalysis. Several bidentate phosphines, such as DIPAMP,7g Ph-BPE, 13 and TangPhos,14 were ineffective, affording little or no ee (Table 1, entries 1–3), whereas phosphepines 2–415 provided somewhat promising enantioselectivity and yield (entries 4–6).
The addition of substituents to the 3,3′ positions of the 1,1′-binaphthyl framework has proved to be a valuable approach to enhancing the enantioselectivity afforded by an array of chiral catalysts.16 However, this strategy has not yet been exploited in the context of asymmetric nucleophilic catalysis by chiral phosphepines. We were therefore pleased to determine that replacement of the 3,3′ hydrogens of phosphepine 4 with phenyl groups leads to improved ee (Table 1, entry 7 vs. entry 6).17,18 An examination of the X-ray crystal structure of this new phosphepine catalyst reveals how the 3,3′ phenyl substituents extend the chiral environment of the binaphthyl unit in the direction of the nucleophilic phosphorus (Figure 1).
Figure 1.
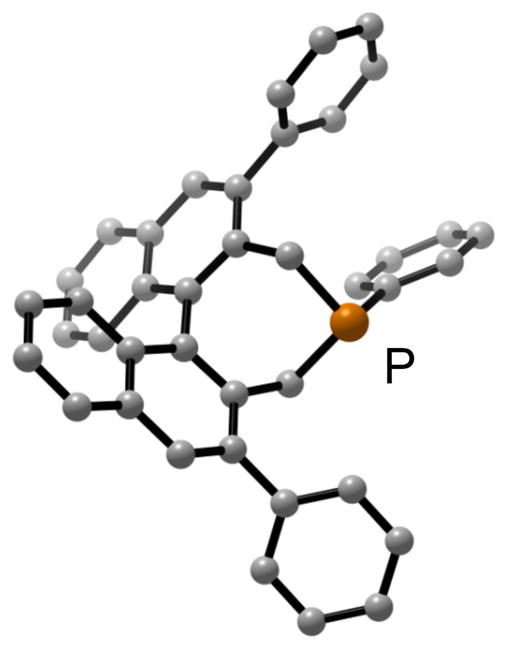
X-ray crystal structure of phosphepine (R)-1 (for simplicity, a solvent molecule (CH2Cl2) has been omitted).
All studies to date of nucleophile-catalyzed enantioselective cycloadditions of allenes with olefins have focused primarily on the use of allenes that cannot undergo isomerization to 1,3-dienes (i.e., R=H in eq 1; the 1,3-dienes are unreactive toward cyclopentene formation).19,20 On the other hand, the ability to employ γ-substituted allenes as reaction partners would markedly increase the structural as well as the stereochemical (two stereocenters rather than one) diversity of the products generated through this powerful cycloaddition process.
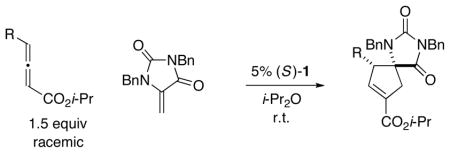
We have established that the conditions described in entry 7 of Table 1 can in fact be applied directly to [3+2] annulations of a wide array of γ-substituted racemic allenes with a 5-methylene hydantoin, furnishing the desired highly functionalized cyclopentenes, which bear adjacent quaternary and tertiary stereocenters, in good stereoselectivity, regioselectivity, and yield (Table 2).21 With γ substituents that range in steric demand from Me to CH2 cyclopentyl, the cycloadditions proceed cleanly with excel lent enantioselectivity in the presence of 5% of new phosphepine 1 (entries 1–3); in the case of a bulky i-Pr group, an increased catalyst loading (10%) is required in order to obtain a high yield (92%), and the cyclopentene is formed with moderate ee (77%) but excellent regioselectivity (50:1; entry 4). Functionalized γ substituents are compatible with the reaction conditions, including a phthalimide-containing group (entries 5–7). These are stereoconvergent processes where in both enantiomers of the racemic allene are being converted by the chiral catalyst into the same stereoisomer of the product with good selectivity.
Table 2.
Catalytic Enantioselective [3+2] Cycloadditions of γ-Substituted Allenes with a Nitrogen-Substituted Olefina
 | ||||
|---|---|---|---|---|
| entry | R | ee (%) | rrb | yield (%)c |
| 1 | Me | 98 | 8: 1 | 98 |
| 2 | n-Pr | 97 | 15: 1 | 97 |
| 3 |
|
92 | 17: 1 | 97 |
| 4d | i-Pr | 77 | 50: 1 | 92 |
| 5 | (CH2)4OBn | 96 | 11: 1 | 92 |
| 6 | (CH2)2CO2Me | 95 | 13: 1 | 88 |
| 7 |
|
98 | 9: 1 | 83 |
All data are the average of two experiments. For the determination of structure, including stereochemistry, see the Supporting Information.
Ratio of regioisomers (determined by 1H NMR analysis of the unpurified reaction mixture); the ratio of diastereomers is ≥20:1.
Yield of purified product (rr = 9–>20: 1)
Catalyst loading: 10%.
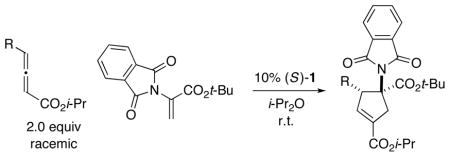
With the first method in hand for catalytic enantioselective [3+2] cycloadditions of allenes with a heteroatom-substituted olefin to produce quaternary stereo-centers, we turned our attention to determining the scope of this process with respect to other substituents, with the goal of providing a versatile approach. Indeed, we have established that not only 5-methylene hydantoin, but also other pnictogen-bearing olefins, are suitable cycloaddition partners in the presence of phosphepine catalyst 1 (Tables 3 and 4).22 Thus, an array of annulations of racemic γ-substituted allenes with nitrogen- and phosphorus-substituted olefins proceed in excel lent ee, regioselectivity, and diastereoselectivity (>20:1). We are not aware of a prior example of the use of a phosphorus-substituted alkene in such [3+2] cycloadditions (including non-asymmetric processes).
Table 3.
Catalytic Enantioselective [3+2] Cycloadditions of γ-Substituted Allenes with a Phthalimide-Substituted Olefina
 | |||
|---|---|---|---|
| entry | R | ee (%) | yield (%)b |
| 1 | Me | 98 | 81 |
| 2 |
|
98 | 72 |
| 3 |
|
98 | 86 |
All data are the average of two experiments. For the determination of structure, including stereochemistry, see the Supporting Information.
Yield of purified product; for each cycloaddition, the ratio of regioisomers and diastereomers is ≥20:1 (determined by 1H NMR analysis of the unpurified reaction mixture).
Table 4.
Catalytic Enantioselective [3+2] Cycloadditions of γ-Substituted Allenes with a Phosphorus-Substituted Olefina
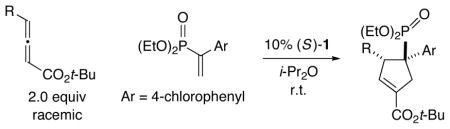 | |||
|---|---|---|---|
| entry | R | ee (%) | yield (%)b |
| 1 | Me | 99 | 82 |
| 2 | CH2CH2Ph | 97 | 79 |
| 3 |
|
97 | 87 |
All data are the average of two experiments. For the determination of structure, including stereochemistry, see the Supporting Information.
Yield of purified product; for each cycloaddition, the ratio of regioisomers and diastereomers is ≥20:1 (determined by 1H NMR analysis of the unpurified reaction mixture).
We have explored the possibility that the olefin coupling partner can bear other families of heteroatoms, e.g., chalcogens. In fact, C2-symmetric phosphepine 1 catalyzes [3+2] cyclo-additions of both oxygen- and sulfur-substituted olefins with γ-substituted allenes to generate highly functionalized cyclopentenes with good-to-excel lent stereoselectivity, regioselectivity (≥20:1), and yield (Tables 5 and 6). To the best of our know ledge, oxygen-substituted alkenes have not previously been employed in annulations of this type.
Table 5.
Catalytic Enantioselective [3+2] Cycloadditions of γ-Substituted Allenes with an Oxygen-Substituted Olefina
All data are the average of two experiments. For the determination of structure, including stereochemistry, see the Supporting Information.
Determined by 1H NMR analysis of the unpurified reaction mixture; for each cycloaddition, the ratio of regioisomers is ≥20:1.
Yield of purified product (dr ≥ 12: 1).
Table 6.
Catalytic Enantioselective [3+2] Cycloadditions of γ-Substituted Allenes with a Sulfur-Substituted Olefina
All data are the average of two experiments. The absolute stereochemistry is tentatively assigned by analogy with Table 5.
Determined by 1H NMR analysis of the unpurified reaction mixture; for each cycloaddition, the ratio of regioisomers is ≥20:1.
Yield of purified product (dr ≥ 8: 1).
These catalytic asymmetric cycloadditions of heteroatom-substituted olefins are not limited to couplings with allenoates. For example, allenamides are also suitable partners (eq 3).23 As far as we are aware, allenamides have not been reported by others to serve as substrates in phosphine-catalyzed [3+2] reactions with olefins.
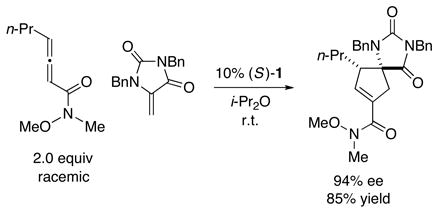 |
(3) |
The products of these catalytic asymmetric [3+2] cycloadditions can be converted into other potentially useful compounds. For example, the phthalimide can be deprotected to reveal a quaternary amino-acid ester (eq 4),24 the thioether can be unmasked to a tertiary thiol (eq 5), and the double bond of the cyclopentene can be functionalized with high diastereoselectivity to afford a cyclopentane that bears four contiguous stereocenters (two tertiary and two quaternary; eq 6).
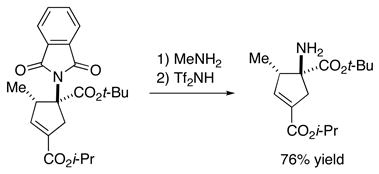 |
(4) |
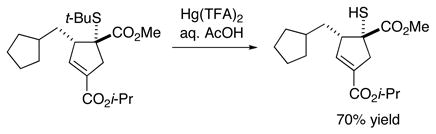 |
(5) |
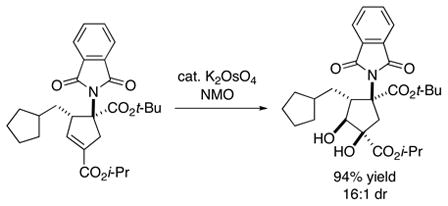 |
(6) |
Detailed mechanistic experiments have not yet been described for catalytic asymmetric [3+2] cycloadditions of allenes with olefins.25 For the process catalyzed by phosphepine 1, specifically, the reaction illustrated in eq 7, the rate is dependent upon the concentration of the allene and the catalyst, but not the olefin. During the cycloaddition, the predominant phosphorus-containing species (>90% according to 31P NMR spectroscopy) is the phosphepine itself, not a covalent adduct (e.g., B in eq 1). Furthermore, at partial conversion, we observe modest enantiomeric enrichment of the unreacted allene (30% ee at 93% conversion); to the best of our know ledge, this is the first example of a kinetic resolution in a phosphine-catalyzed allene/olefin [3+2] cycloaddition. Finally, we do not detect a nonlinear effect: the ee of the product correlates linearly with the ee of the catalyst. All of these data can be reconciled with a mechanism in which the rate-determining step of the catalytic cycle is the addition of the phosphine to the allene.
 |
(7) |
CONCLUSIONS
Although there have been numerous reports of phosphine-catalyzed [3+2] cycloadditions of allenes with olefins, a variety of noteworthy challenges had not been addressed. For example, with respect to non-asymmetric reactions, no processes had been described that employ phosphorus- or oxygen-substituted olefins to provide heteroatom-substituted quaternary centers; with regard to enantioselective transformations, there had been no success with any heteroatom-bearing olefin to produce a quaternary carbon, and there had been only limited progress (R=Me in eq 1) with γ-substituted allenes. By synthesizing and applying a new chiral phosphepine (1), we have developed a versatile method that furnishes cyclopentenes with an array of heteroatom-substituted (N, P, O, and S) quaternary stereocenters in good ee, diastereoselectivity, regioselectivity, and yield. We have used catalyst 1 in stereoconvergent reactions of racemic γ-substituted allenes, which afford products with greater structural and stereochemical diversity as compared with unsubstituted allenes. Furthermore, for the first time, we have employed allenamides in phosphine-catalyzed [3+2] cyclodditions. The highly functionalized cyclopentenes that are generated in these catalytic asymmetric annulations are poised for transformation into a variety of other target molecules. Mechanistic studies, including a unique observation of a (modest) kinetic resolution of the racemic allene, are consistent with addition of phosphepine 1 to the allene being the turnover-limiting step of the catalytic cycle. This is the first report in which modification of the 3,3′ positions of the binaphthyl unit of a phosphepine has been exploited in the context of asymmetric nucleophilic catalysis, and we anticipate that an array of other processes may benefit from this approach.
Supplementary Material
Acknowledgments
Support has been provided by the National Institutes of Health (Nat ional Institute of General Medical Sciences, grant R01–GM57034) and Dainippon Sumitomo Pharma Co., Ltd. (fellowship for Y. F.). We thank Dr. Jonathan E. Wilson for a preliminary study.
Footnotes
Supporting Information. Experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For some leading references to nucleophilic catalysis by phosphines, see: Ye LW, Zhou J, Tang Y. Chem Soc Rev. 2008;37:1140–1152. doi: 10.1039/b717758e.Methot JL, Roush WR. Adv Synth Catal. 2004;346:1035–1050.
- 2.For reviews of asymmetric catalysis by chiral phosphines, see: Marinetti A, Voituriez A. Synlett. 2010:174–194.Gröger H, Burda E. In: Phosphorus Ligands in Asymmetric Catalysis. Börner A, editor. Vol. 3. Wiley–VCH; New York: 2008. pp. 1175–1197.For an overview of catalytic enantioselective reactions of allenoates, see: Cowen BJ, Miller SJ. Chem Soc Rev. 2009;38:3102–3116. doi: 10.1039/b816700c.
- 3.(a) Zhang C, Lu X. J Org Chem. 1995;60:2906–2908. [Google Scholar]; (b) Lu X, Zhang C, Xu Z. Acc Chem Res. 2001;34:535–544. doi: 10.1021/ar000253x. [DOI] [PubMed] [Google Scholar]
- 4.Prostaglandins and furanoid carbasugars are two examples of bioactive compounds that contain five-membered carbocycles. For some leading references, see: Bazinet RP, editor. Prostaglandins, Leukotrienes and Essential Fatty Acids. Rassu G, Auzzas L, Pinna L, Battistini L, Curti C. Stud Nat Prod Chem. 2003;29:449–520.
- 5.Zhu G, Chen Z, Jiang Q, Xiao D, Cao P, Zhang X. J Am Chem Soc. 1997;119:3836–3837. [Google Scholar]
- 6.Wilson JE, Fu GC. Angew Chem, Int Ed. 2006;45:1426–1429. doi: 10.1002/anie.200503312. [DOI] [PubMed] [Google Scholar]
- 7.(a) Wallace DJ, Sidda RL, Reamer RA. J Org Chem. 2007;72:1051–1054. doi: 10.1021/jo062170l. [DOI] [PubMed] [Google Scholar]; (b) Cowen BJ, Miller SJ. J Am Chem Soc. 2007;129:10988–10989. doi: 10.1021/ja0734243. [DOI] [PubMed] [Google Scholar]; (c) Voituriez A, Panossian A, Fleury-Bregeot N, Retailleau P, Marinetti A. J Am Chem Soc. 2008;130:14030–14031. doi: 10.1021/ja806060a. [DOI] [PubMed] [Google Scholar]; (d) Panossian A, Fleury-Brégeot N, Marinetti A. Eur J Org Chem. 2008:3826–3833. [Google Scholar]; (e) Voituriez A, Panossian A, Fleury-Brégeot N, Retailleau P, Marinetti A. Adv Synth Catal. 2009;351:1968–1976. [Google Scholar]; (f) Sampath M, Loh TP. Chem Commun. 2009:1568–1570. doi: 10.1039/b819959k. [DOI] [PubMed] [Google Scholar]; (g) Sampath M, Loh T-P. Chem Sci. 2010;1:739–742. [Google Scholar]; (h) Pinto N, Neel M, Panossian A, Retailleau P, Frison G, Voituriez A, Marinetti A. Chem Eur J. 2010;16:1033–1045. doi: 10.1002/chem.200901893. [DOI] [PubMed] [Google Scholar]; (i) Voituriez A, Pinto N, Neel M, Retailleau P, Marinetti A. Chem Eur J. 2010;16:12541–12544. doi: 10.1002/chem.201001791. [DOI] [PubMed] [Google Scholar]; (j) Xiao H, Chai Z, Zheng CW, Yang YQ, Liu W, Zhang JK, Zhao G. Angew Chem, Int Ed. 2010;49:4467–4470. doi: 10.1002/anie.201000446. [DOI] [PubMed] [Google Scholar]; (k) Schuler M, Voituriez A, Marinetti A. Tetrahedron: Asymmetry. 2010;21:1569–1573. [Google Scholar]; (l) Han X, Wang Y, Zhong F, Lu Y. J Am Chem Soc. 2011;133:1726–1729. doi: 10.1021/ja1106282. [DOI] [PubMed] [Google Scholar]; (m) Pinto N, Retailleau P, Voituriez A, Marinetti A. Chem Commun. 2011:1015–1017. doi: 10.1039/c0cc03164j. [DOI] [PubMed] [Google Scholar]
- 8.Nitrogen-substituted olefins: Pyne SG, Schafer K, Skelton BW, White AH. Chem Commun. 1997:2267–2268.Ung AT, Schafer K, Lindsay KB, Pyne SG, Amornraksa K, Wouters R, Van der Linden I, Biesmans I, Lesage ASJ, Skelton BW, White AH. J Org Chem. 2002;67:227–233. doi: 10.1021/jo010864i.Pham TQ, Pyne SG, Skelton BW, White AH. Tetrahedron Lett. 2002;43:5953–5956.Du Y, Lu X, Yu Y. J Org Chem. 2002;67:8901–8905. doi: 10.1021/jo026111t.Pham TQ, Pyne SG, Skelton BW, White AH. J Org Chem. 2005;70:6369–6377. doi: 10.1021/jo050827h.Henry CE, Kwon O. Org Lett. 2007;9:3069–3072. doi: 10.1021/ol071181d.Guan XY, Wei Y, Shi M. Org Lett. 2010;12:5024–5027. doi: 10.1021/ol102191p.
- 9.Sulfur-substituted olefins: Ruano JLG, Nunez A, Jr, Martin MR, Fraile A. J Org Chem. 2008;73:9366–9371. doi: 10.1021/jo801896a.Nunez A, Jr, Martin MR, Fraile A, Ruano JLG. Chem Eur J. 2010;16:5443–5453. doi: 10.1002/chem.200903185.
- 10.For an example of a process that generates a tertiary sulfur-substituted stereocenter in low ee (<35%), see: Pakulski Z, Demchuk OM, Frelek J, Luboradzki R, Pietrusiewicz KM. Eur J Org Chem. 2004:3913–3918.
- 11.For leading references, see: Christoffers J, Baro A, editors. Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis. Wiley–VCH; New York: 2005.
- 12.Börner A, editor. Phosphorus Ligands in Asymmetric Catalysis. Wiley–VCH; New York: 2008. [Google Scholar]
- 13.For an application of Ph-BPE as a chiral nucleophilic catalyst, see: Tan B, Candeias NR, Barbas CF., III J Am Chem Soc. 2011;133:4672–4675. doi: 10.1021/ja110147w.
- 14.For an application of TangPhos as a chiral nucleophilic catalyst, see: Sun J, Fu GC. J Am Chem Soc. 2010;132:4568–4569. doi: 10.1021/ja101251d.
- 15.For the initial report of the use of this family of phosphepines in asymmetric nucleophilic catalysis, see: Wurz RP, Fu GC. J Am Chem Soc. 2005;127:12234–12235. doi: 10.1021/ja053277d.Such phosphepines were originally developed as chiral ligands for transition-metal catalyzed reactions. For an overview, see: Gladiali S, Alberico E. In: Phosphorus Ligands in Asymmetric Catalysis. Börner A, editor. Vol. 1. Wiley–VCH; New York: 2008. pp. 177–206.
- 16.For example, see: Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew Chem, Int Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240.Uraguchi D, Terada M. J Am Chem Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533.
- 17.Similar results are observed when chloroform is employed as the solvent and when the allene bears an isopropyl, rather than an ethyl, ester.
- 18.Phosphepine 1 can be handled conveniently in the air. After exposure of the solid phosphine to air for two months, no phosphine oxide was detected by 31P NMR spectroscopy. On the other hand, phosphepine 1 is susceptible to slow oxidation in solution (e.g., after exposure of a solution in CDCl3 to air for 3 days, 15% of the phosphine had been converted into the phosphine oxide).
- 19.For phosphine-catalyzed isomerization reactions to form 1,3-dienes, see: Trost BM, Kazmaier U. J Am Chem Soc. 1992;114:7933–7935.Kwong CKW, Fu MY, Lam CSL, Toy PH. Synthesis. 2008:2307–2317.
- 20.For isolated examples of catalytic asymmetric [3+2] cycloadditions of olefins with γ-substituted allenes that are prone to isomerize, see References 7b, g, and j. In each case, the only substituent that was investigated was a γ-methyl group.
- 21.Notes: (a) Under our standard reaction conditions: if the temperature of the reaction is lowered, there is a substantial decrease in rate and only a small improvement in ee; the cycloaddition proceeds more slowly in the presence of protic additives such as pivalic acid or phenol; on a gram scale, the reaction illustrated in entry 3 of Table 2 proceeds in 92% ee and 99% yield (1.74 g of product; 17:1 regioselectivity); the ee of the product is constant throughout the cycloaddition; the phosphine oxide of phosphepine 1 does not catalyze the reaction; the addition of water (up to 5 equiv) leads to a decrease in yield (~20%), but no erosion in enantioselectivity. (b) Allenoates can be generated in one step via the treatment of an acid chloride with a commercially available olefinating agent such as (carbethoxymethylene)triphenylphosphorane.
- 22.We do not currently have a satisfactory understanding of the origin of the observed stereoselection, particularly with respect to the new stereocenter that is derived from the olefin.
- 23.For a review of the utility of Weinreb amides, see: Balasubramaniam S, Aidhen IS. Synthesis. 2008:3707–3738.
- 24.For a review and leading references on the stereo-selective synthesis and the utility of cyclic quaternary α-amino acids, see: Cativiela C, Ordonez M. Tetrahedron: Asymmetry. 2009;20:1–63. doi: 10.1016/j.tetasy.2009.01.002.
- 25.For mechanistic studies of phosphine-catalyzed [3+2] cycloadditions of allenes with olefins, see: non-asymmetric: Xia Y, Liang Y, Chen Y, Wang M, Jiao L, Huang F, Liu S, Li Y, Yu ZX. J Am Chem Soc. 2007;129:3470–3471. doi: 10.1021/ja068215h.Liang Y, Liu S, Xia Y, Li Y, Yu Z-X. Chem Eur J. 2008;14:4361–4373. doi: 10.1002/chem.200701725.Mercier E, Fonovic B, Henry C, Kwon O, Dudding T. Tetrahedron Lett. 2007;48:3617–3620.Zhang X-C, Cao S-H, Wei Y, Shi M. Chem Commun. 2011;47:1548–1550. doi: 10.1039/c0cc04289g.(b) asymmetric (model for stereoselection): Reference 7h.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.