Abstract
UNC93B1 associates with Toll-Like Receptor (TLR) 3, 7 and 9, mediating their translocation from the endoplasmic reticulum to the endolysosome, thus allowing proper activation by microbial nucleic acids. We found that the triple deficient ‘3d’ mice, which lack functional UNC93B1 as well as functional endossomal TLRs, are highly susceptible to infection with Trypanosoma cruzi. The enhanced parasitemia and mortality in 3d animals were associated with impaired pro-inflammatory response, including reduced levels of IL-12p40 and IFN-γ. Importantly, the phenotype of 3d mice was intermediary between MyD88−/− (highly susceptible) and TLR9−/− (less susceptible), indicating the involvement of an additional UN93B1-dependent-TLR(s) on host resistance to T. cruzi. Hence, our experiments also revealed that TLR7 is a critical innate immune receptor involved in recognition of parasite RNA, induction of IL-12p40 by dendritic cells, and consequent IFN-γ by T lymphocytes. Furthermore, we show that upon T. cruzi infection triple TLR3/7/9−/− mice had similar phenotype than 3d mice. These data imply that the nucleic acid-sensing TLRs are critical determinants of host resistance to primary infection with T. cruzi.
INTRODUCTION
Trypanosoma cruzi, the causative agent of Chagas disease, is an intracellular protozoan parasite (1). Control of T. cruzi replication during early stages of infection in mice is highly dependent on both innate and acquired T cell-mediated immune responses (2, 3). The mammalian Toll-like receptors (TLRs) sense conserved molecules from all classes of microorganisms, including those from protozoan parasites (4). The activation of the innate immune system by microbial products leads to the induction of antimicrobial effector mechanisms, and gives way, over time, to the development of T helper 1 (Th1) lymphocytes (4). Importantly, mice deficient in the myeloid differentiation gene 88 (MyD88), an adaptor molecule required for signaling events by most TLRs, except TLR3, show greatly enhanced susceptibility to infection with different protozoan parasites (3, 5–7), including T. cruzi (8).
Glycosylphosphatidylinositol (GPI) anchors have been previously defined as a major class of T. cruzi glycolipids that are recognized by TLRs. Purified GPI anchors derived from mucin-like glycoproteins of trypomastigotes contain unsaturated fatty acid chains and are potent agonists of TLR2 (9). In addition, a particular subset of free glycoinositolphospholipid-containing ceramides (GPL-ceramide) stimulates the production of proinflammatory cytokines by macrophages via TLR4 (10). Until recently, dogma in the field suggested that recognition of parasite surface GPI anchors was the critical component of the host innate immune response, analogous to the role of surface LPS in the genesis of fever and inflammation in Gram-negative sepsis. However, more recent studies showed that T. cruzi genomic DNA contains abundant oligodeoxynucleotide unmethylated CpG motifs (11) that promote host cell activation via TLR9 and stimulate cytokine response from macrophages and dendritic cells (DCs), triggering effector mechanisms that are essential for protection against acute infection (12, 13). Whereas TLR9 has been shown to be involved in host resistance to infection with T. cruzi, the contribution of other nucleotide-sensing TLRs, i.e. TLR3, TLR7 and TLR8 (14–16) have not been explored.
Tabeta and colleagues identified a mutant mouse line by forward genetic screening that is unresponsive to TLR3, TLR7 and TLR9 ligands (there is no known agonist for mouse TLR8) (17). These animals, named “3d” after their triple defect in TLR response, have altered function of UNC93B1, an endoplasmic reticulum (ER) resident protein that mediates the translocation of the nucleotide-sensing TLRs from the ER to the endolysosomes, allowing their proper activation by microbial RNA and DNA (18, 19). The 3d mouse has a point mutation in a transmembrane domain of UNC93B1, which renders the protein incapable of interaction with and translocation of intracellular TLRs. Consequently, 3d animals are unable to respond to nucleic acids of pathogens and to produce proinflammatory cytokines after infection, which culminates with enhanced susceptibility to many intracellular agents (17).
Here, we show that the 3d mice are extremely susceptible to infection with T. cruzi. We provide evidences for a critical role of UNC93B1 in mediating IL-12p40 as well as early IFN- γ production during acute infection with T. cruzi. Furthermore, we provide for the first time evidences indicating that TLR7 is critical on recognition of parasite RNA, induction of early cytokine response, and host resistance to primary T. cruzi infection. Altogether, our experiments reveal that UNC93B1 is an essential element in host resistance to T. cruzi infection, by mediating the translocation and subsequent activation of TLR7 and TLR9 by parasite nucleic acids in the endolysosomal sub-cellular compartment.
MATERIAL AND METHODS
Ethics Statement
Experiments involving animals were performed in accordance to guidelines set forth by the Institutional Animal Care and Use Committee (IACUC) and the Department of Animal Medicine from the University of Massachusetts Medical School (IACUC protocol A-1817-09).
Reagents
Cell culture reagents were obtained from Mediatech (Manassas, VA). Chicken egg albumin (OVA) and LPS derived from Escherichia coli strain 0111:B4 were purchased from Sigma (Saint Louis, MO). LPS was re-extracted by phenol chloroform to remove lipopeptides as described (20). Imiquimod (R837) was purchased from Invivogen (San Diego, CA). Phosphorothioate-stabilized unmethylated CpG-containing oligonucleotide (ODN 1826, 5’-TCCATGACGTTCCTGACGTT-3’) was purchased from IDT Technologies (Coralville, IA). The transfection reagent Gene Juice™ was obtained from Novagen (Madison, WI). TsKb20 peptide, a CD8+ T cell epitope derived from T. cruzi Transialidase, as well as the tetramer used to identify TsKb20 specific CD8+ T cells (21) were synthesized by Dr. Immanuel Luescher from the tetramer facility from the Ludwig Institute for Cancer Research (Lausanne, Switzerland). Alum (Imject®, Pierce) was obtained from Thermo Scientific (Rockford, IL).
Mice
C57BL/6 mice were obtained from Charles River Breeding Laboratories (Wilmington, MA). The 3d mice, (C57BL/6 mice bearing a non functional mutant UNC93B1 molecule), were generated by Dr. Bruce Beutler at The Scripps Research Institute in La Jolla, California (17). Mice deficient of TLR7, TLR9 and MyD88 were provided by Dr. Shizuo Akira (Department of Host Defense, Osaka University, Osaka, Japan). Mice deficient of TLR3 were generated by Dr. Richard Flavell (Yale University, New Haven, CN). Mice with triple deficiency of TLR3, TLR7 and TLR9 (TLR3/7/9−/− ) were generated through interbreeding of single knockout animals. All mice used were backcrossed to C57BL/6 background at least for 8 generations. Age (5–8 weeks old) and sex matched groups of wild type (WT) and knockout mice were used in all experiments.
T. cruzi parasites, experimental infections and vaccinations in mice
Experimental infection were performed with blood trypomastigotes of the virulent CL-Brener strain, which were maintained by continuous passage in RAG−/− mice by intra-peritoneal (i.p.) injection. Blood trypomastigotes were recovered from infected RAG−/− mice at the peak of parasitemia and inoculated by i.p. route at a dose of 100 trypomastigotes per mouse. Mice were then monitored daily for survival and every other day for parasitemia, or sacrificed at 13 days post-infection in order to collect blood and spleens for ex vivo immunological assays. Vaccination experiments were performed with the attenuated CL-14 strain, which was maintained as epimastigotes in liver infusion-triptose (LIT) medium, at 28° C. Epimastigotes were harvested from log phase cultures, washed once in PBS and suspended at 108 parasites/ml. For vaccination, two doses of 100 µl (107 parasites) were inoculated i.p., eight weeks apart. Four weeks after the last dose, animals were challenged with an i.p. injection of the virulent CL-Brener, as described above, or sacrificed for serum and spleen collection. Some animals were sacrificed after the first dose of CL-14, and spleen as well as serum samples were harvested for analysis of cellular and humoral immune responses, respectively. OT-I mice were vaccinated with 3 subcutaneous doses, one week apart, containing 25µg of OVA and 18 µg of ODN 1826 prepared in alum v/v.
T. cruzi lysates, RNA and DNA extraction from CL-Brener parasites
T. cruzi total lysate antigen (TLA) was obtained from epimastigotes grown in LIT medium. Epimastigotes obtained from log phase cultures were washed, suspended in PBS at 108 parasites/ml, and disrupted by repetitive cycles of freezing and thawing. After centrifugation of cellular debris, aqueous phase was recovered and protein content was determined by Bradford method (DC Protein Assay™, Bio-Rad, Hercules, CA). Genomic DNA was purified from freshly harversted epimastigotes (108 parasites), using the GFXTM genomic DNA purification kit (GE Healthcare, Piscataway, NJ). Total RNA was purified from trypomastigotes forms, which were obtained from infected L6 cells grown in RPMI medium supplemented with 10% fetal bovine serum, at 37°C and 5%CO2. Trypomastigotes were purified from cells as previously described (22), washed in PBS and pelleted. Extraction of total RNA from parasite pellets (5×107 – 108 trypomastigotes) was performed using RNeasy kit (Qiagen, Valencia, CA). Purified RNA samples were treated with DNase I (Fermentas, Glen Burnie, MD) and cleaned up using the RNeasy kit (Qiagen). RNA samples were assayed in PCR with two sets of T. cruzi specific primers: aromatic L-alpha-hydroxy acid dehydrogenase, (AHADH2, CCAAATGTTTCGCCACTCG and CACGCTGCGGAGGGATCTC) and mucinassociated surface protein (MASP, CAGGAGTGATGGCGATGATGAT and GTGTGCTTCGTGGGGTGAGGTG). Approximately 500 ng of total RNA were used in PCR reactions, with 200 µM dNTPs, 2 µM primers and 1.25U of Taq polymerase in 50 mM KCl, 10 mM Tris-HCl (pH=8.4), 1% Triton X-100 and 1.5 mM MgCl2.
Spleen cell culture
Spleens were disrupted in cell strainers to obtain single cell suspensions. Red blood cells were lysed in ACK Lysis Buffer™ (Sigma), splenocytes washed twice and re-suspended in complete RPMI medium (RPMI 1640 supplemented with 10% fetal bovine serum, 25 mM HEPES, 1 mM L-glutamine, 2 mM sodium piruvate, 50 µM β-mercaptoethanol and 100 U/ml of penicillin-streptomycin). Cells were cultured at 5x106/well in 24-well tissue culture plates in absence or presence of exogenous stimuli, as indicated in figure legends, and cell-free supernatants collected 24 hours later for cytokine measurement. Alternatively, purified CD11c+, CD4+ T cells and CD8+ T cells were obtained from mouse spleens using EasySep® cell separation kits from Stemcell Technologies (Vancouver, Canada), according to manufacturer’s instructions. The CD11c+ cells were then incubated in presence or absence of exogenous stimuli (as indicated in figure legends) and used as antigen presenting cells. The CD11c+ APC cells (5×104) were co-cultured with purified CD4+ T cells or CD8+ T cells (2×105 cells) in 96 well plates. Cell-free culture supernatants were collected for IFN-γ measurement.
Flow cytometry
Total spleen cells (2×106) were stained with fluorochrome-conjugated antibodies (eBioscience, San Diego, CA) specific for T cell surface markers (CD3, CD4 and CD8), during 30 minutes on ice. Following two washes with PBS supplemented with 1% bovine serum albumine (FACS buffer), spleen cells were stained for 45 minutes on ice with a Phycoerythrin-conjugated (PE) H-2Kb tetramer containing the TsKb20 epitope, for identification of anti-Transialidase specific T CD8+ cells. Cells were then washed in FACS buffer and fixed in 2% paraformaldehyde for 15 minutes on ice. After two washes in FACS buffer, cells were analyzed by flow cytometry in an LSRII cytometer (BD Bioscience, San Jose, CA). Data were acquired with DIVA software (BD Bioscience) and analyzed with FlowJo (Tree Star, Ashland, OR).
Cell culture
immortalized bone marrow macrophages from C57BL/6, 3d and TLR7 deficient mice were maintained in complete DMEM medium supplemented with 10% fetal bovine serum, 20mM HEPES and 100U/ml penicillin-streptomycin. Cells were seeded in 96-well plates at a density of 105 cells/well and treated with different stimuli, as indicated in figure legends. Supernatants were collected 24h later for cytokine measurement.
Cytokine measurement
Supernatants of splenocyte or macrophage cultures were assayed for pro-inflammatory cytokines with DuoSet ELISA kits from R&D Systems (Minneapolis, MN). Cytokine levels in mouse serum were assayed using the BD Cytometric Bead Array Mouse Inflammation kit (CBA, BD Biosciences).
Statistics
Comparisons of cumulative survival curves were performed by Long-rank (Mantel Cox) test. Analysis of parasitemia points after infection, as well as data obtained in the in vitro experiments was performed by one way or two-way ANOVA test, with Bonferroni post-test. All statistic tests were performed using GraphPad Prism 5.0 Software (GraphPad Softwares Inc., La Jolla, CA).
RESULTS
Enhanced susceptibility of 3d mice to T. cruzi infection
A body of evidence indicates that innate response to acute T. cruzi infection is mediated by a combined action of different TLRs (2, 3). To assess the effect of the combined deficiency of intracellular TLRs in protection against trypanosomiasis, we used the 3d mice that express a non-functional form of UNC93B1 and are non-responsive to agonists of nucleic acid-sensing TLRs (18, 19). We infected 3d mice in parallel with TLR9 and MyD88 deficient (indicated by −/−) with the CL-Brener clone of T. cruzi. As we have previously observed, although not as susceptible as the MyD88−/− mice, TLR9−/− mice showed enhanced parasitemia and accelerated mortality upon T. cruzi infection (12, 13). These results were confirmed and are presented in figure 1. Importantly, our experiments also show that 3d mice are highly susceptible to infection with T. cruzi, displaying an intermediary phenotype between MyD88−/− and TLR9−/− mice. We anticipated that in 3d mice, the lack of endosomal TLR function would also result in impaired cytokine production during T. cruzi infection. In fact, 3d mice infected with T. cruzi presented impaired production of pro-inflammatory cytokines, i.e. IL-12p40 as well as IFN-γ (Fig. 1B), which are critical mediators of host resistance to infection with protozoan parasites, including T. cruzi.
Figure 1.
Enhanced susceptibility and impaired proinflammatory cytokine production in 3d mice infected with T. cruzi. A, wild type (WT, C57BL/6), MyD88−/− 3d, and TLR9−/− mice (five animals per group) were infected by i.p. injection of 100 blood trypomastigote forms of T. cruzi. Parasitemia (left panel) was evaluated every other day, and cumulative mortality (right panel) followed daily. Statistical analysis conducted at day 17 post-infection shows significant difference of parasitemia and mortality (p<0.001) between MyD88−/− and remaining mouse groups. Analysis at later points (23 and 25 days post infection) shows significant differences in parasitemia and mortality (p<0.05 and p<0.001 at 23 and 25 days, respectively) when comparing, 3d to the groups of TLR9−/− and WT mice. Finally, from 29 to 35 days after infection, significant difference is observed between the mortality and parasitemia (p<0.001) of WT and TLR9−/− mouse groups. B, serum samples were obtained from 8 animals (WT and 3d infected groups), 6 animals (TLR9−/− and MyD88−/− infected groups) sacrificed at 13 days post-infection or 4 non-infected animals from different groups. Both IL-12 (left panel) and IFN-γ (right panel) levels were quantified. Asterisks indicate that cytokine levels in sera from WT mice are significantly higher than in sera from 3d, TLR9−/− and MyD88−/− animals (p<.05). Results are representative of one out of two experiments, performed independently, and yielding similar results.
Impaired T cell responses in 3d mice infected with T. cruzi
T cells are an important source of IFN-γ and essential effectors for protection against infections with T. cruzi. Thus, we investigated whether anti-T. cruzi specific T lymphocytes were being properly activated in 3d mice. Our results show that IFN-γ produced by T lymphocytes was significantly impaired in 3d mice infected with T. cruzi. Wild type (WT), 3d, TLR9−/− and MyD88−/− mice were infected with T. cruzi and had their antigen-driven IFN-γ responses as well as the frequency of parasite specific CD8+ T cells evaluated (Fig. 2). At 13 days post-infection, we observed an impaired IFN-γ production in response to T. cruzi total lysate antigens (TLA), in all mutant or TLR-deficient mouse strains. In addition, in the same experiment, we observed a decreased IFN-γ response to the immunodominant CD8+ T cell epitope TsKb20 of T. cruzi (21) by spleen cells from MyD88−/− and 3d, but not by cells from TLR9−/− mice (Fig. 2A). In contrast, the frequency of TsKb20-specific CD8+ T cells was not affected, in MyD88−/−, 3d, or TLR9−/− mice infected with T. cruzi (Fig. 2B).
Figure 2.
Impaired IFN-γ responses by T cells from 3d mice infected with T. cruzi. A, spleen cells obtained at day 13 after infection (+ T. cruzi infection) or non-infected (− T. cruzi infection) wild type (WT), 3d, TLR9−/− and MyD88−/− mice (three animals per group) were cultured in absence or presence of trypomastigote lysate antigen (TLA, 10 µg/ml), a CD8+ T cell epitope derived from T. cruzi Transialidase (TsKb20, 10 µM), a CD8+ T control epitope derived from Leishmania A2 antigen (Ctrl pep, 10 µM) or the mitogen Concanavalin A (Con A, 10 µg/ml). Supernatants from spleen cultures were harvested 24 hours after antigen stimulation for measurements of IFN-γ levels. Asterisks indicate that IFN-γ levels produced by splenocytes from WT mice were significantly higher (p<0.001) than from 3d, TLR9−/− and MyD88−/− mice. B, Spleen cells obtained at 13 days post-infection and non-infected mice (3 animals per group) were stained for CD8 marker along with a H2-Kb tetramer containing the TsKb20 epitope. Frequency of CD8+ T cells positively stained with the tetramer was quantified by flow cytometry. Results are representative of one out of two experiments that were performed independently and yielded similar results.
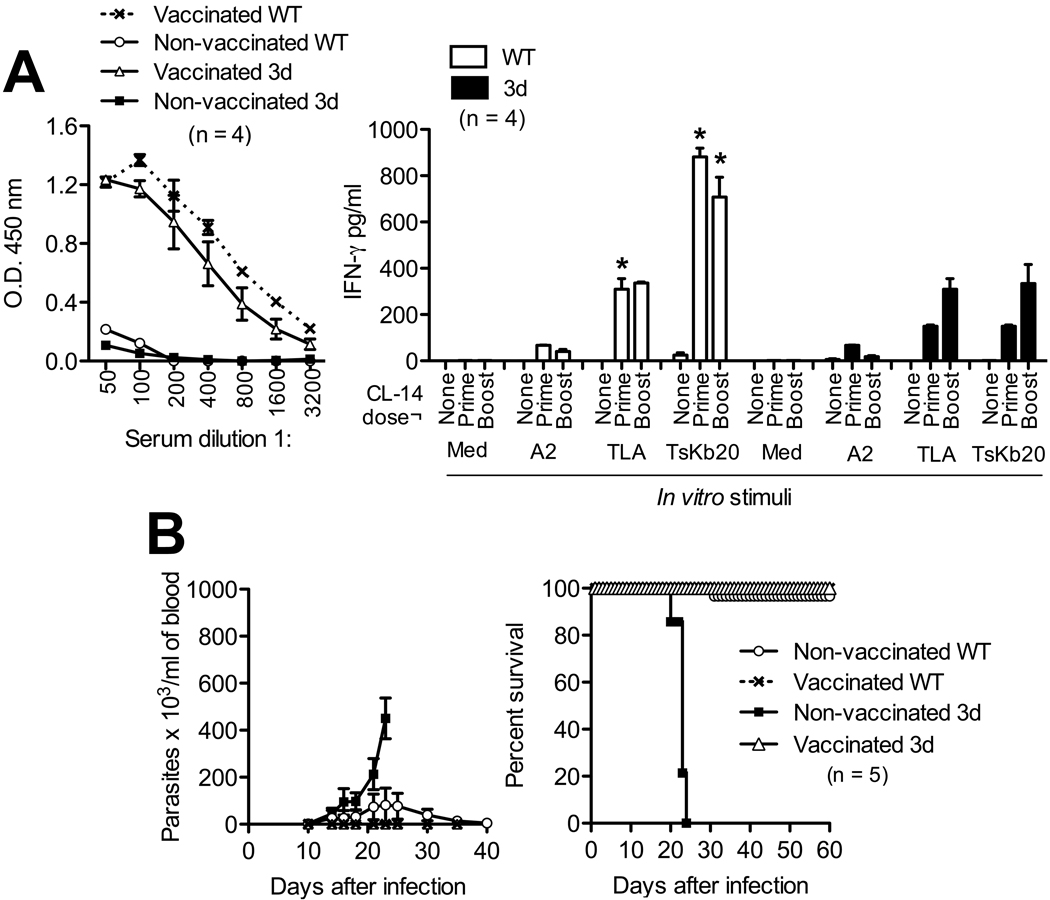
We also evaluated in vivo the essential requirement of UNC93B1, on development of acquired immunity and immunological protection against T. cruzi infection. WT and 3d mice were vaccinated with the highly attenuated CL-14 strain of T. cruzi (23, 24). The humoral response to T. cruzi antigens was identical when comparing 3d and WT after priming (Fig. 3A) and boost (not shown) vaccination doses. In contrast, after both prime and boost vaccination doses, the cellular responses, i.e. IFN-γ responses to TLA and to TsKb20, were significantly reduced in the 3d mice (Fig. 3A). Nevertheless, the humoral and cellular immune responses obtained with two doses of CL-14 were sufficient to protect 3d mice against a challenge with the highly virulent CL-Brener strain of T. cruzi (Fig. 3B).
Figure 3.
Impairment of IFN-γ response in 3d mice is overcome by vaccination with an attenuated strain of T. cruzi. Wild type (WT) and 3d mice were vaccinated with one (prime) or two (boost) doses of the CL-14 attenuated strain of T. cruzi. As controls, animals were left without any immunizations (none). A, serum samples were obtained 30 days after prime dose with CL-14 and quantification of anti-T. cruzi IgG performed by ELISA employing TLA, to confirm that all animals were successfully immunized (left panel). Spleen cells obtained 30 days after prime or boost dose of vaccine were re-stimulated in vitro with T. cruzi antigens (TLA at 10 µg/ml or TsKb20 peptide at 10 µM) or a control peptide (A2, 10 µM) (right panel). IFN-γ levels were evaluated in supernatants of spleen cultures by ELISA at 24 hours after antigen stimulation. Asterisks indicate significant difference between WT and 3d mice. B, prime-boost vaccinated animals were challenged with an i.p. dose of 100 trypomastigotes of virulent CL-Brener strain, 30 days after the boost dose with CL-14 clone. Parasitemia (left panel) and mortality (right panel) were followed for 60 days after challenge. Non-vaccinated 3d mice showed high susceptibility, with significantly higher parasitemia (p<0.001) starting at 21 days after infection and 100% mortality by 25 days after infection. On the other hand, vaccinated 3d were as resistant to infection as the vaccinated WT animals. The representative results are from one out of two experiments, which yielded similar results.
Enhanced susceptibility of TLR7−/− mice to T. cruzi infection
In addition to TLR9, UNC93B1 mediates TLR3 and TLR7 translocation and function (18, 19). Altogether, our results suggest that the combined action of TLR9 with TLR3 and/or TLR7 would account for the UNC93B1 role on innate recognition and host resistance to infection with T. cruzi. To test this hypothesis, we evaluated the ability of TLR3−/−, or TLR7−/− mice to control the infection with T. cruzi. The results shown in figure 4A demonstrate that TLR7−/− mice are more susceptible to T. cruzi infection, yielding a phenotype similar to that of TLR9−/− mice. In contrast, TLR3−/− mice were able to control parasitemia and survive infection. Importantly, the serum levels IL-12p40 and IFN-γ were greatly diminished in 3d and TLR7−/−, but not in the TLR3−/− mice (Fig. 4B, left and middle panels). Furthermore, the levels of IL-12p40 produced by spleen cells from 3d, TLR7−/− or TLR9−/− (but not TLR3−/−) mice were severely impaired (Fig. 4B, right panel). Consistently, the IFN-γ response by T cells after infection with T. cruzi was similarly affected in 3d, TLR9−/− and TLR7−/− but not in TLR3−/− mice (Fig. 4C).
Figure 4.
Enhanced susceptibility and impaired cytokine production by TLR7−/− mice infected with T. cruzi. A, wild type (WT), TLR3−/− TLR7−/− and TLR9−/− mice were infected by i.p. injection of 100 blood trypomastigote forms of T. cruzi. Parasitemia (left panel) and cumulative mortality (right panel) were evaluated every other day and daily, respectively. Statistical analysis conducted at days 20, 23, 25 and 27 post-infection denotes that the differences in parasitemia and survival curves are significant when comparing TLR7−/− TLR3/TLR7−/− and TLR9−/− to WT mice (p<0.05 on day 20 and p<0.001 on 23 through 27). B, serum samples were obtained from WT, 3d, TLR3−/− and TLR7−/− non-infected mice as well as at 13 days post-infection with 100 blood trypomastigote forms. Both IL-12 (left panel) and IFN-γ (middle panel) levels were measured. B - right panel, total spleen cells obtained from WT, 3d, TLR3−/− TLR7−/− and TLR9−/− at 13 days post-infection were cultured for 24 hours, without any addition of exogenous antigen, for measurement of IL-12 levels in culture supernatants. Asterisks indicate significant differences (p<0.05), when comparing cytokine levels in sera/supernatants derived from WT and TLR3−/− to 3d, TLR7−/− and TLR9−/− mice. C, spleen cells from infected and non-infected WT, 3d, TLR3−/− and TLR7−/− were submitted to stimulation with T. cruzi antigens (TLA, 10 µg/ml or TsKb20, 10 µM), control peptide (Leishmania A2, 10 µM) or Concanavalin A (10 µg/ml). The levels of IFN-γ in culture supernatants were measured by ELISA, 24 hours later. Asterisks indicate significant differences (p<0.05), when comparing IFN-γ levels in sera/supernatants derived from WT and TLR3−/− to 3d and TLR7−/− mice. Results are representative of one experiment. Each experiment was performed two independent times with similar results.
We also tested the ability of total RNA purified from T. cruzi to stimulate immortalized macrophages derived from WT, 3d and TLR7−/− mice. Parasite RNA induced a low TNF-αresponse, which was enhanced when RNA was delivered with a transfection reagent, in cells from WT, but not from 3d or TLR7−/− mice. As expected, Imiquimod (R837) and an unmethylated CpG-containing oligonucleotide (Oligo 1826) did not activate macrophages from 3d mice, and R837 did not induce TNF-αproduction by TLR7−/− macrophages (Fig. 5A). To avoid RNA contamination with DNA, all RNA preparations were treated with DNAse I. Absence of DNA was confirmed by PCR reactions employing two different sets of T. cruzi specific primers, e.g. AHADH2 and MASP. No amplification of parasite specific genes was observed when RNA was used as template, contrasting with the results obtained, when we used parasite DNA as template (Fig. 5B).
Figure 5.
In vitro stimulation of macrophage cell lines with T. cruzi RNA. A, immortalized macrophages (105 cells) derived from WT, 3d or TLR7−/− mice were treated for 24 hours with 2 µg of T. cruzi RNA with (transfected) or without (non-transfected) transfection reagent. As controls, cells were kept without any stimulation (Medium) or were incubated with transfection reagent only, 1 µM of a synthetic TLR7 agonist (R837), 1 µM of an unmethylated CpG-containg oligonucleotide (Oligo 1826), or 100 ng/ml of LPS. TNF-α levels were measured in culture supernatant by ELISA. Asterisk indicates significant difference (p<0.05), when comparing TNF-α levels in supernatants derived from macrophages from WT to 3d and TLR7−/− mice. B, total T. cruzi RNA (3 µg) was separated in 1.2% agarose/MOPS/formaldehyde gel (left panel). Genomic DNA and DNase I- treated and non-treated RNA samples were tested by PCR using specific primers for the T. cruzi genes AHADH2 (aromatic L-alpha-hydroxy acid dehydrogenase) and MASP (mucin- associated surface protein) (right panel).
Nucleic acid-sensing TLRs account for the immunological defect found in 3d mice infected with T. cruzi
It has also been raised the possibility that in addition to translocation of TLRs, UNC93B1 may also mediate other key functions in the immune system (17, 19), such as antigen presentation. With this in mind, we generated the TLR3/7/9−/− mice and compared the results with 3d mice, when both were challenged with T. cruzi in parallel infections. Importantly, the combination of TLR3, TLR7 and TLR9 deficiencies yielded a susceptibility phenotype identical to that observed in the 3d mice, as measured by mortality and parasitemia (Fig. 6A). Since TLR3−/− mice did not display an altered phenotype (Fig. 4A), we believe that the combination of TLR7 and TLR9 deficiency accounts for the enhanced susceptibility observed both in the TLR3/7/9−/− and 3d infected with T. cruzi. We also evaluated the IFN-γ production by spleen cells from WT, 3d and TLR3/7/9−/− mice infected with T. cruzi. Again, our results indicate a similar and significant impairment of IFN-γ production, when spleen cells from 3d or TLR3/7/9−/− mice were stimulated with either TLA or CD8+ T cell epitope (Fig. 6B).
Figure 6.
Combined TLR7 and TLR9 deficiency contributes to enhanced susceptibility and impaired cytokine production of 3d mice infected with T. cruzi. A, wild type (WT), 3d, TLR7−/−, TLR9−/− and TLR3/7/9−/− mice were infected by i.p. injection of 100 blood trypomastigote forms of T. cruzi. Parasitemia (left panel) and cumulative mortality (right panel) were evaluated every other day and daily, respectively. Statistical analysis denotes that the differences in parasitemia and survival curves are significant when comparing WT, TLR7−/− and TLR9−/− to TLR3/7/9−/− and 3d mice (p<0.05 on day 20 and p<0.001 on day 23); and when comparing WT to TLR7−/− and TLR9−/− mice (p<0.001 on day 28 through 32). No statistical differences were observed when comparing 3d and TLR3/7/9−/− mice. B, total spleen cells obtained from non-infected or WT, 3d and TLR3/7/9−/− mice at 13 days post-infection, were submitted to stimulation with T. cruzi antigens (TLA, 10 µg/ml or TsKb20, 10 µM), control peptide (Leishmania A2, 10 µM) or Concanavalin A (10 µg/ml). The levels of IFN-γ in tissue culture supernatants were measured by ELISA. Asterisks indicate significant differences (p<0.05), when comparing IFN-γ levels in supernatants derived from WT to 3d and TLR3/7/9−/− mice. C, CD4+ T cells were purified from spleens of naive or T. cruzi-infected WT, 3d and TLR3/7/9−/− mice. These cells were co-cultured for 48 hours with purified WT spleen CD11c+ APC previously pulsed with TLA (10 µg/ml). Non-treated CD11c+ APCs (Medium) were used as control. IFN-γ was measured in cell-free culture supernatants by ELISA. The asterisk indicate significant difference (p<0.001), when comparing IFN-γ production of WT CD4+ T cells to 3d and TLR3/7/9−/− CD4+ T cells. D, CD11c+ APCs were isolated from spleens of WT, 3d or TLR3/7/9−/− mice. The purified APC were pulsed with TLA (10 µg/ml) or particulate ovalbumin (OVA, 3 µg/ml). As controls, the APC were kept without any treatment (Medium). CD8+ T cells were purified from spleens of naïve or T. cruzi-infected WT mice. In parallel, CD8+ T cells were purified from spleens of naïve or OVA-vaccinated OT-I mice. APCs and CD8+ T cells were co-cultured for 96 hours for induction of IFN-γ that was measured in cell-free culture supernatants by ELISA. All results are representative of one experiment. Each experiment was performed two independent times with similar results.
The defect of IFN-γ production by CD4+ T cells from 3d was directly demonstrated by employing CD4+ T lymphocytes obtained from infected WT, 3d and TLR3/7/9−/− mice. Highly purified CD4+ T lymphocytes were stimulated with antigen presenting cells from WT mice pulsed with TLA (Fig. 6C). CD4+ T lymphocytes derived from both 3d and TLR3/7/9−/− mice secreted significantly lower levels of IFN-γ, when compared to the CD4+ T cells derived from WT mice (Fig. 6C). These results indicate that in vivo priming of the CD4+ T cells and possibly differentiation of Th1 cells are compromised in the 3d and TLR3/7/9−/− mice.
Finally, our results indicated that the frequency of T. cruzi specific CD8+ T lymphocytes were similar between the WT and different mouse strains infected with T. cruzi. Nevertheless, we consistently observed reduction in IFN-γ secretion by CD8+ T cells from 3d and TLR3/7/9−/− mice in response to the MHC I binding epitope TsKb20 (Fig. 6B). Thus, we investigated whether an eventual defect in antigen cross-presentation contributed to the decreased IFN-γ production by CD8+ T cells from 3d and TLR3/7/9−/− mice. For that, we isolated CD11c+ APCs from spleens of WT, 3d and TLR3/7/9−/− mice. These cells were then pulsed with TLA, as an exogenous antigen, and then incubated with highly purified CD8+ T cells obtained from syngeneic C57BL/6 mice infected with T. cruzi (Fig. 6D). Parallel experiments were performed with highly purified CD8+ T cells obtained from ovalbumin (OVA)-vaccinated OT-I mice and CD11c+ APCs pulsed with exogenous particulate OVA. We observed that APCs from either WT, 3d and TLR3/7/9−/− mice were equally efficient in presenting either TLA or OVA, as measured by IFN-γ production by CD8+ T cells from WT mice (Fig. 6D). Thus, in our model, we were not able to observe differences in antigen cross-presentation, when comparing WT, 3d and TLR3/7/9−/− mice.
DISCUSSION
TLRs are important elements for host defense against every known category of human microbial pathogens (4), including protozoan parasites (3). The most convincing data indicating the importance of the Toll/IL-1 Receptor domain (TIR) in host resistance to protozoan infections are those obtained in MyD88−/− mice. Increased susceptibility associated with impaired production of the Th1 associated cytokines (e.g., IFN-γ and IL-12) (3) is observed in MyD88−/− mice infected with different protozoan parasites (3, 5–7), including T. cruzi (8). However, it has been difficult to assign specific TLRs that account for pronounced susceptibility to protozoan parasites observed in the MyD88−/− mice (8, 10, 12, 25). Here, we demonstrated that 3d mice, which lack functional UNC93B1 that is responsible for translocation of nucleic acid-sensing TLRs (18, 19), are highly susceptible to experimental infection with T. cruzi. Furthermore, we demonstrated that TLR7−/− mice are more susceptible to T. cruzi. Thus, we propose that the enhanced susceptibility of 3d mice is a result of combined deficiency of TLR7 and TLR9. Consistently, triple TLR3/7/9−/− mice present a phenotype that is identical to the 3d mice, in terms of impaired production of IL-12p40 by dendritic cells, IFNγ by CD4+ T cells as well as by CD8+ T lymphocytes and host resistance to primary infection with T. cruzi.
Our previous studies demonstrated that upon infection with T. cruzi, TLR9−/− mice present impaired production of IL-12 by dendritic cells, of IFN-γ by T lymphocytes, increased parasitemia, and accelerated mortality. We also demonstrated that TLR9 is recruited to the endolysosome compartment, where it probably interacts with DNA released from parasites that have been destroyed during the initial uptake by dendritic cells. Furthermore, we found that immunostimulatory CpG motifs are abundant and preferentially distributed in regions of the genome that contains T. cruzi-specific genes, like multigene families encoding the mucin-like proteins, and the retro-element VIPER (26).
Importantly, upon infection with T. cruzi, the phenotype of TLR9−/− mice is less pronounced than that observed in MyD88−/− mice (12). Thus, we propose that host defense to primary T. cruzi infection is simultaneously orchestrated by different TLRs. This hypothesis is supported by the present study, which demonstrates that 3d animals that have combined deficiency of TLR3, TLR7 and TLR9 functions, are highly susceptible to T. cruzi infection, with a phenotype that is intermediate between TLR9−/− and MyD88−/− mice. However, UNC93B1 was also demonstrated to be involved on activation of CD8+ T cells (17, 27), possibly by controlling translocation of elements of the cross-presentation machinery, similar to the way it mediates the movement of TLRs to endosomes (19). Thus, the enhanced susceptibility of 3d mice to T. cruzi infection could be explained by an impaired cross-antigen presentation in dendritic cells. To address this question, we generated the triple TLR3/7/9 deficient mice and performed parallel experiments with the 3d mice. Our results demonstrate identical phenotypes in terms parasitemia, survival curve, decreased IL-12 production by DCs as well as impairment of IFNγ responses by both CD4+ T and CD8+ T lymphocytes, when comparing the mutant and triple knockout to WT mice infected with T. cruzi. Furthermore, CD11c+ APCs from WT, 3d and TLR3/7/9−/− were equally efficient in presenting T. cruzi soluble antigens to syngeneic CD8+ T cells derived from infected C57BL/6 mice. Thus, we favor the hypothesis that the primary defect associated with enhanced susceptibility of 3d mice to T. cruzi infection is the altered translocation of nucleic-acid TLRs and not of the “cross presentation machinery”.
Similar to the MyD88−/− mice, the enhanced susceptibility of both 3d and TLR3/7/9−/− mice to T. cruzi infection is associated to impaired production of IL-12 (8, 12). IL-12 is a critical cytokine for host resistance to T. cruzi infection (28–30) and induction of IFN-γ production by T CD4+ T as well as CD8+ T lymphocytes (31). We also proved in sequential experiments that TLR7 is a critical receptor in mediating activation of innate immune cells and host resistance to primary infection with T. cruzi. Since the TLR3 single knockout mice did not display altered immune responses and resistance to T. cruzi infection, we assume that the combined lack of TLR7 and TLR9 was the main defect underlying the 3d susceptibility to the parasite. Thus, despite of the redundant role of TLR7 and TLR9, the results obtained with the 3d and TLR3/7/9−/− mice indicate that their combined action accounts for the role UNC93B1 on IL-12 production by myeloid cells (e.g. DCs or macrophages) and, consequently the optimal IFN-γ production by T lymphocytes, and host resistance to T. cruzi infection.
Altogether, our results demonstrate that UNC93B1 is critical for host resistance to T. cruzi. These data are highly reminiscent of a recent report showing that UNC93B1 is an essential element of host resistance to another intracellular protozoan, namely Toxoplasma gondii (32). However, in this particular case, a single deficiency on either TLR7 or TLR9 did not affect host resistance to infection. Thus, either the combined TLR7/TLR9 deficiency or a TLR-independent mechanism is required to explain the enhanced susceptibility of 3d mice to T. gondii. In contrast, in the case of T. cruzi infection, the enhanced susceptibility of 3d mice can be explained solely on the basis of predicted role of UNC93B1, which is the translocation of nucleic acid-sensing TLRs (18, 19).
Whereas TLR7 has been implicated in the recognition and host resistance to viral and bacterial infection (16, 33, 34), we demonstrate for the first time that TLR7 is a critical innate immune receptor involved on recognition and host resistance to a protozoan infection. To date, TLR7 has been exclusively recognized as a receptor for single stranded RNA. By performing an in silico analysis, we found several potential TLR7 agonists, e.g. guanosine- or uridine-rich single-strand in the predicted parasite transcriptome. To this end, 27,752 potential transcribed elements of the genome were searched for GU-motifs with 20 or more G and/or U residues allowing only one mismatch. The dataset include 23,218 protein coding-regions, 2,424 retroelements and 2,110 structural RNAs. A total of 1,338 GU-motifs were found in 833 potential transcribed elements. Of these, 344 GU-motifs were found in genes encoding surface proteins, such as gp85/trans-sialidase, MASP, mucin TcMUC, DGF-1, GP63 and serine-alanine proline rich protein, all known to be highly expressed in the mammalian stages of the parasite (22, 35–38). However, this number of putative GU-motifs is likely to be underestimated since a small proportion of T. cruzi untranslated regions were already mapped (39).
Importantly, we found a critical the role for TLR7 on induction of IL-12p40 and consequent induction of IFN-γ by T cells during T. cruzi infection. Thus, we assume that the main source of IL-12 during T. cruzi are DCs and/or macrophages, which express TLR7. We believe that parasites that have been internalized by these professional phagocytic cells are destroyed, releasing RNA, which activates TLR7 in the phagolysosomes. As previously demonstrated in an experimental model of bacterial infection (34), TLR7 maybe also involved on the induction of Type I IFN, which is prominent during early stages of T. cruzi infection (40, 41).
It is noteworthy, that 3d mice are consistently less susceptible than MyD88−/− mice, indicating that an additional TLR(s) or cytokine receptors are necessary to explain the hyper susceptibility of MyD88−/− mice to T. cruzi infection. Indeed, early studies indicate that GPI anchors and GIPL-ceramide from T. cruzi parasites activate primarily TLR2 and TLR4, respectively (9, 10). Furthermore, double TLR2/TLR9−/− are slightly more susceptible than TLR9−/− to T. cruzi infection (12). Thus, although deficiency of either TLR2 or TLR4 in C57BL/6 does not enhance susceptibility, it is possible that the lack of TLR2 (and/or TLR4) functions associated with combined TLR7/TLR9 deficiencies may account for the complete role of MyD88 on host resistance to T. cruzi infection. Alternatively, the IL-1, IL-33 and/or IL-18 receptors, whose functions are also dependent on MyD88 (30, 42, 43), may contribute to enhanced susceptibility to T. cruzi infection in MyD88−/− although mice deficient in either IL-1R (data not shown) or IL-18R (30) are not more susceptible to experimental infection with T. cruzi.
In conclusion, our results indicate that the critical anti-parasitic role for UNC93B1 is the control of IL-12 production of APCs and early IFN-γ responses by both CD4+ T as well as CD8+ T lymphocytes. The combined action of TLR7/TLR9 appears to mediate the UNC93B1 role during T. cruzi infection. Thus, our results indicate that UNC93B1 as well as TLR7/TLR9 nucleic acid-sensing TLRs play a fundamental role on activation of innate immunity, initiation of T cell responses and host resistance to T. cruzi infection.
ACKNOWLEDGMENTS
We are thankful to Dr. Kenneth Rock for advice with T cell experiments performed in this study; Dr. Bruce Beutler (Scripps, San Diego, CA) for providing 3d mice; Dr. Shizuo Akira (Osaka University, Osaka, Japan) for providing TLR7−/− and TLR9−/− and Dr. Richard Flavell (Yale University, New Haven, CN) for providing TLR3−/− mice.
Footnotes
This work was supported by grants from Atlantic Philanthropies/Ludwig Institute for Cancer Research/ Program of Clinical Discovery (USA); Conselho Nacional de Ciência e Tecnologia (CNPq)/Instituto do Milenio para Desenvolvimento de Vacinas (Brazil); Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG)/Rede de Mineira de Biomoleculas and National Institutes of Health (USA)/R21 AI08097 and AI071319-01. BCC was a post-doctoral fellow of CNPq; DCB is supported by the World Health Organization/Special Program for Research and Training in Tropical Diseases and Fundacão de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil); RTG and DCB are research fellows of CNPq; DTG is supported by RO1 GM54060 and RO1 AI079293. The authors have no conflicting financial interests.
Abbreviations: UNC93B1, a protein that translocates TLR3, TLR7 and TLR9 from endoplasmic reticulum to the endolysosome compartment; 3d, a mouse that express non-functional UNC93B1 and has triple-defect (triple d) in nucleotide sensing TLR3, TLR7 and TLR9; MyD88, myeloid differentiation primary response gene; GPI, glycosylphospatidylinositol; GPL-ceramide, glycoinositolphospholipid-containing ceramide; DCs, dendritic cells; TIR, Toll/IL-1 receptor domain; Th1, T helper 1 lymphocytes; TLA, T. cruzi lysate antigen; TSKb20, a immunodominant CD8+ T cell epitope derived from T. cruzi transialidase; CL-14, an avirulent T. cruzi strain; CLBrener, a virulent T. cruzi strain.
REFERENCES
- 1.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 2.Golgher D, Gazzinelli RT. Innate and acquired immunity in the pathogenesis of Chagas disease. Autoimmunity. 2004;37:399–409. doi: 10.1080/08916930410001713115. [DOI] [PubMed] [Google Scholar]
- 3.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 6.Muraille E, De Trez C, Brait M, De Baetselier P, Leo O, Carlier Y. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J Immunol. 2003;170:4237–4241. doi: 10.4049/jimmunol.170.8.4237. [DOI] [PubMed] [Google Scholar]
- 7.Drennan MB, Stijlemans B, Van den Abbeele J, Quesniaux VJ, Barkhuizen M, Brombacher F, De Baetselier P, Ryffel B, Magez S. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J Immunol. 2005;175:2501–2509. doi: 10.4049/jimmunol.175.4.2501. [DOI] [PubMed] [Google Scholar]
- 8.Campos MA, Closel M, Valente EP, Cardoso JE, Akira S, Alvarez-Leite JI, Ropert C, Gazzinelli RT. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J Immunol. 2004;172:1711–1718. doi: 10.4049/jimmunol.172.3.1711. [DOI] [PubMed] [Google Scholar]
- 9.Campos MA, Almeida IC, Takeuchi O, Akira S, Valente EP, Procopio DO, Travassos LR, Smith JA, Golenbock DT, Gazzinelli RT. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167:416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira AC, Peixoto JR, de Arruda LB, Campos MA, Gazzinelli RT, Golenbock DT, Akira S, Previato JO, Mendonca-Previato L, Nobrega A, Bellio M. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J Immunol. 2004;173:5688–5696. doi: 10.4049/jimmunol.173.9.5688. [DOI] [PubMed] [Google Scholar]
- 11.Shoda LK, Kegerreis KA, Suarez CE, Roditi I, Corral RS, Bertot GM, Norimine J, Brown WC. DNA from protozoan parasites Babesia bovis, Trypanosoma cruzi, and T. brucei is mitogenic for B lymphocytes and stimulates macrophage expression of interleukin-12, tumor necrosis factor alpha, and nitric oxide. Infect Immun. 2001;69:2162–2171. doi: 10.1128/IAI.69.4.2162-2171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–3519. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 13.Bartholomeu DC, Ropert C, Melo MB, Parroche P, Junqueira CF, Teixeira SM, Sirois C, Kasperkovitz P, Knetter CF, Lien E, Latz E, Golenbock DT, Gazzinelli RT. Recruitment and endo-lysosomal activation of TLR9 in dendritic cells infected with Trypanosoma cruzi. J Immunol. 2008;181:1333–1344. doi: 10.4049/jimmunol.181.2.1333. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 16.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 17.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 18.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 20.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 21.Araujo AF, de Alencar BC, Vasconcelos JR, Hiyane MI, Marinho CR, Penido ML, Boscardin SB, Hoft DF, Gazzinelli RT, Rodrigues MM. CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2. Infect Immun. 2005;73:6017–6025. doi: 10.1128/IAI.73.9.6017-6025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartholomeu DC, Cerqueira GC, Leao AC, daRocha WD, Pais FS, Macedo C, Djikeng A, Teixeira SM, El-Sayed NM. Genomic organization and expression profile of the mucin-associated surface protein (masp) family of the human pathogen Trypanosoma cruzi. Nucleic Acids Res. 2009;37:3407–3417. doi: 10.1093/nar/gkp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paiva CN, Castelo-Branco MT, Lannes-Vieira J, Gattass CR. Trypanosoma cruzi: protective response of vaccinated mice is mediated by CD8+ cells, prevents signs of polyclonal T lymphocyte activation, and allows restoration of a resting immune state after challenge. Exp Parasitol. 1999;91:7–19. doi: 10.1006/expr.1999.4356. [DOI] [PubMed] [Google Scholar]
- 24.Lima MT, Lenzi HL, Gattass CR. Negative tissue parasitism in mice injected with a noninfective clone of Trypanosoma cruzi. Parasitol Res. 1995;81:6–12. doi: 10.1007/BF00932410. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira AC, de Alencar BC, Tzelepis F, Klezewsky W, da Silva RN, Neves FS, Cavalcanti GS, Boscardin S, Nunes MP, Santiago MF, Nobrega A, Rodrigues MM, Bellio M. Impaired innate immunity in Tlr4(−/−) mice but preserved CD8+ T cell responses against Trypanosoma cruzi in Tlr4−, Tlr2−, Tlr9− or Myd88-deficient mice. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000870. e1000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vazquez M, Ben-Dov C, Lorenzi H, Moore T, Schijman A, Levin MJ. The short interspersed repetitive element of Trypanosoma cruzi, SIRE, is part of VIPER, an unusual retroelement related to long terminal repeat retrotransposons. Proc Natl Acad Sci U S A. 2000;97:2128–2133. doi: 10.1073/pnas.050578397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cockburn IA, Tse SW, Radtke AJ, Srinivasan P, Chen YC, Sinnis P, Zavala F. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from plasmodium in vivo. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001318. e1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aliberti JC, Cardoso MA, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159:1723–1733. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graefe SE, Jacobs T, Gaworski I, Klauenberg U, Steeg C, Fleischer B. Interleukin-12 but not interleukin-18 is required for immunity to Trypanosoma cruzi in mice. Microbes Infect. 2003;5:833–839. doi: 10.1016/s1286-4579(03)00176-x. [DOI] [PubMed] [Google Scholar]
- 31.O'Garra A, Murphy KM. From IL-10 to IL-12: how pathogens and their products stimulate APCs to induce T(H)1 development. Nat Immunol. 2009;10:929–932. doi: 10.1038/ni0909-929. [DOI] [PubMed] [Google Scholar]
- 32.Melo MB, Kasperkovitz P, Cerny A, Konen-Waisman S, Kurt-Jones EA, Lien E, Beutler B, Howard JC, Golenbock DT, Gazzinelli RT. UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 34.Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 35.Abuin G, Freitas-Junior LH, Colli W, Alves MJ, Schenkman S. Expression of trans-sialidase and 85-kDa glycoprotein genes in Trypanosoma cruzi is differentially regulated at the post-transcriptional level by labile protein factors. J Biol Chem. 1999;274:13041–13047. doi: 10.1074/jbc.274.19.13041. [DOI] [PubMed] [Google Scholar]
- 36.Buscaglia CA, Campo VA, Frasch AC, Di Noia JM. Trypanosoma cruzi surface mucins: host-dependent coat diversity. Nat Rev Microbiol. 2006;4:229–236. doi: 10.1038/nrmicro1351. [DOI] [PubMed] [Google Scholar]
- 37.Baida RC, Santos MR, Carmo MS, Yoshida N, Ferreira D, Ferreira AT, El Sayed NM, Andersson B, da Silveira JF. Molecular characterization of serine-, alanine-, and proline-rich proteins of Trypanosoma cruzi and their possible role in host cell infection. Infect Immun. 2006;74:1537–1546. doi: 10.1128/IAI.74.3.1537-1546.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atwood JA, 3rd, Minning T, Ludolf F, Nuccio A, Weatherly DB, Alvarez-Manilla G, Tarleton R, Orlando R. Glycoproteomics of Trypanosoma cruzi trypomastigotes using subcellular fractionation, lectin affinity, and stable isotope labeling. J Proteome Res. 2006;5:3376–3384. doi: 10.1021/pr060364b. [DOI] [PubMed] [Google Scholar]
- 39.Campos PC, Bartholomeu DC, DaRocha WD, Cerqueira GC, Teixeira SM. Sequences involved in mRNA processing in Trypanosoma cruzi. Int J Parasitol. 2008;38:1383–1389. doi: 10.1016/j.ijpara.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Chessler AD, Ferreira LR, Chang TH, Fitzgerald KA, Burleigh BA. A novel IFN regulatory factor 3-dependent pathway activated by trypanosomes triggers IFN-beta in macrophages and fibroblasts. J Immunol. 2008;181:7917–7924. doi: 10.4049/jimmunol.181.11.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koga R, Hamano S, Kuwata H, Atarashi K, Ogawa M, Hisaeda H, Yamamoto M, Akira S, Himeno K, Matsumoto M, Takeda K. TLR-dependent induction of IFN-beta mediates host defense against Trypanosoma cruzi. J Immunol. 2006;177:7059–7066. doi: 10.4049/jimmunol.177.10.7059. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi O, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol. 2002;270:155–167. doi: 10.1007/978-3-642-59430-4_10. [DOI] [PubMed] [Google Scholar]
- 43.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]