Abstract
Herpes Simplex Virus-1 (HSV) infection of the cornea leads to a blinding immuno-inflammatory lesion of the eye termed stromal keratitis (SK). Recently, IL-17 producing CD4+ T cells (Th17) were shown to play a prominent role in many autoimmune conditions, but the role of IL-17 and/or of Th17 cells in virus immunopathology is unclear. Here we show that, after HSV infection of the cornea, IL-17 is upregulated in a biphasic manner with an initial peak production around day 2 pi and a second wave starting from day 7 pi with a steady increase until day 21 pi, a time point when clinical lesions are fully evident. Further studies demonstrated that innate cells particularly, γδ T cells, were major producers of IL-17 early after HSV infection. However, during the clinical phase of SK, the predominant source of IL-17 was Th17 cells which infiltrated the cornea only after the entry of Th1 cells. By ex-vivo stimulation, the half fraction of IFN-γ producing CD4+ T (Th1) cells were HSV specific, whereas very few Th17 cells responded to HSV stimulation. The delayed influx of Th17 cells in the cornea was attributed to the local chemokine and cytokine milieu. Finally, HSV infection of IL-17 receptor knockout mice, as well as IL-17 neutralization in WT mice showed diminished SK severity. In conclusion, our results show that IL-17 and Th17 cells contribute to the pathogenesis of SK, the most common cause of infectious blindness in the western world.
Introduction
Ocular infection with herpes simplex virus (HSV) can cause a chronic inflammatory reaction in the corneal stroma that may culminate in blindness (1, 2). This stromal keratitis (SK) lesion in humans is suspected to represent an immunopathological reaction, a notion well supported by studies with animal models of SK (2–5). An inevitable consequence of ocular HSV infection is life long latency in neuronal cells of the trigeminal ganglion (6). In humans, periodic reactivation from some latently infected cells give rise to replicating virus that acts as the most common stimulus for SK lesion development (2–5). A major objective of SK research is to identify the role of cellular and molecular events involved in tissue damage and its resolution with a view to improving current means of management of this often distressing disease. Studies in the mouse models of SK have firmly established an essential role for T cells as the principal orchestrators of SK lesions (7, 8). However, the actual tissue damage appearance to be the consequence of inflammatory events that derive primarily from neutrophils that represent the major cellular component of lesions at all phases of SK pathogenesis (9, 10). The prominence of neutrophils in SK lesions could indicate that the recently identified proinflammatory cytokine IL-17 is a significant participant in the pathogenesis of lesion development. Accordingly, IL-17 functions indirectly to cause tissue infiltration by neutrophils, acts as a neutrophil survival factor and also may drive the cells to produce and release tissue damaging molecules such as MMPs and oxyradicals (11–15).
In human SK, the presence of IL-17 has been reported (16). Additionally, the Lausch group showed that the severity of early SK lesions were diminished in mice unable to respond to IL-17 because they lacked the IL-17 receptor (17). However, the cellular source of IL-17 as well as the role this cytokine plays compared to other inflammatory mediators remains to be further defined. This is the topic of the present communication.
We show that HSV infection of the cornea leads to the biphasic upregulation of IL-17. Initially its source was innate cells that included γδ T cells, whereas later during the clinical phase Th17 cells were the predominant producer. The CD4+ T cell subset responsible for orchestrating SK appeared to be mainly Th1 cells at all stages of SK. On the other hand, very few Th17 cells infiltrated into the cornea during early stages of SK (day 8 and 15 pi) but became more prominent during very late stage of SK (day 21 pi), when SK lesions were fully evident. The late entry of Th17 cells was partly explained by the delayed upregulation of IL-6 and TGF-β, cytokines responsible for Th17 generation, as well as CCL20 expression in the cornea, a chemokine responsible for the migration of Th17 cells at the site of inflammation. On the basis of anti-cytokine suppression and comparison of lesion severity between WT and IL-17 receptor knock out (IL-17RKO) mice, our result show that IL-17 contributes to inflammatory events during the pathogenesis of SK. Future therapies targeting the IL-17 response could be useful to alleviate the SK lesion severity, an important cause of infectious blindness in humans.
Material and Methods
Mice, Virus and cell lines
IL-17RA −/− mice on C57B1/6 background were obtained from Amgen Inc. (Thousand Oaks, CA, USA). C57BL/6 mice were purchased from Harlan Sprague Dawley, Indianapolis, IN. Animals were housed in the animal facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) at The University of Tennessee and all experimental procedures were in complete agreement with the Association for research in Vision and Ophthalmology resolution on the use of animals in research. HSV-1 RE Tumpey and HSV-KOS viruses were grown in Vero Cell monolayers (ATCC no. CCL81, American Type Culture Collection). The virus was concentrated, titrated, and stored in aliquots at −80° C until use.
Corneal HSV Infection and Clinical Scoring
Corneal infections of mice were conducted under deep anaesthesia induced by i.p. injection of avertin as previously described (18). Mice were scarified on their corneas with 27-gauge needle, and a 3-µl drop containing 1×104 PFU of virus was applied to the eye. The eyes were examined on different days pi for the development and progression of clinical lesion by slit-lamp biomicroscope (Kowa Company, Nagoya, Japan). The progression of SK lesion severity and angiogenesis of individually scored mice was recorded. The scoring system was as follows: 0, normal cornea; +1, mild corneal haze; +2, moderate corneal opacity or scarring; +3, severe corneal opacity but iris visible; +4, opaque cornea and corneal ulcer; +5, corneal rupture and necrotizing keratitis. The severity of angiogenesis was recorded as described previously (19). According to this system, a grade of 4 for a given quadrant of the circle represents a centripetal growth of 1.5 mm towards the corneal center. The score of the four quadrants of the eye were then summed to derive the neo vessel index (range 0–16) for each eye at a given time point.
Reagents and Antibodies
CD4-allophycocyanin (RM4-5), IL-17-PE (TC11-18H10), IFN- γ-FITC (XMG1.2), γδ TCR-FITC (GL3), anti-CD3 (145.2C11), anti-CD28 (37.51), purified anti-IL-17, purified anti-IFN- γ and GolgiStop (Brefeldin A) were purchased from BD Bioscience. Foxp3-PE, IL-17 and IFN- γ ELISA kit were purchased from eBioscience. Anti γδ TCR (UC3-10A6) and Hamster IgG isotype control Ab were purchased from Bio-X-Cell. PMA and ionomycin were purchased from Sigma-Aldrich.
γδ T cell Depletion
C57BL/6 mice were injected on day 2 before infection with 500 µg/mouse anti-mouse γδ TCR (clone UC3-10A6) followed by 250 µg/mouse on day of infection.
Subconjunctival Injection
Subconjunctival injections of anti-IL-17 were performed as described previously (20). Briefly, subconjunctival injections were done using a 2-cm, 32-gauge needle and syringe (Hamilton, Reno, NV) to penetrate the perivascular region of conjunctiva, and the required dose of anti-IL-17 mAb was delivered into subconjunctival space. Control mice received isotype mAb.
IL-17 and IFN-γ Neutralization
C57BL/6 mice were ocularly infected and 0.05 mg of anti-IL-17 or anti-IFN- γ was given intraperitoneally (i.p.) starting from day -1 followed by day 2 and day 5 pi or starting from day 7 followed by day 10 and 13 pi. For local depletion, 5 µg anti-IL-17 mAb was administered by sub-conjunctival injection following same regimes as described above.
Isolation of corneal-infiltrating cells and flow cytometry
HSV infected corneas were harvested from different group of mice at indicated time point pi. Six to eighth corneas per group were excised, pooled group wise, and digested with 60 U/ml Liberase for 35 minutes at 37°C in a humified atmosphere of 5% CO2. After incubation, the corneas were disrupted by grinding with a syringe plunger on a cell strainer and single-cell suspensions were made in complete RPMI 1640 medium. The single cell suspensions obtained from corneas, DLNs and spleen were stained for different cell surface molecules for FACS. All steps performed at 4°C. Briefly, cell suspension were first blocked with an unconjugated anti-CD32/CD16 mAb for 30 min in FACS buffer. After washing with FACS buffer, samples were incubated with CD4-APC (RM4-5) for 30 min on ice. Finally, the cells were washed three times and re-suspended in 1% para-formaldehyde.
To measure the number of IFN-γ, IL-17 and IL-2 producing CD4+ T cells, intracellular cytokine staining was performed. Briefly, 106 freshly isolated cells from DLNs, spleens or corneas were left untreated or stimulated with PMA plus ionomycin along with GolgiStop for 4 hrs at 37°C in 5% CO2. To quantify IL-2 producing CD4+ T cells, cells were stimulated with anti-CD3 (1ug/ml) and anti-CD28 (0.5 ug/ml) for 5 hrs in the presence of GolgiStop at 37°C in 5% CO2. To enumerate the HSV-specific Th1 and Th17 cells, cells were stimulated with 2 multiplicity of UV-inactivated HSV and incubated overnight at 37°C in 5% CO2. GolgiStop (10mg/ml) was added for the last 5 hrs of the stimulation. At the end of stimulation period, cell surface staining was performed as described above, followed by intracellular cytokine staining using Cytofix/Cytoperm kit (BD Pharmingen) in accordance with the manufacturer’s recommendations. FITC labeled IFN-γ and PE labeled IL-17 antibodies were used. After final washing cells were resuspended in 1% para-formaldehyde. The stained samples were acquired with a FACS Calibur (BD Biosciences) and the data were analyzed using the FlowJo software.
Quantitative PCR (QPCR)
Corneal cells were lysed and total mRNA was extracted using TRIzol LS reagent (Invitrogen). Total cDNA was made with 500 ng of RNA using oligo(dT) primer. Quantitative PCR was performed using SYBR Green PCR Master Mix (Applied Biosystem, Foster City, CA) with iQ5 real-time PCR detection ssystme (Bio Rad, Hercules, CA). The expression levels of different molecules were normalized to β-actin using ΔCt calculation. Relative expression between control and experimental groups were calculated using the 2-ΔΔCt formula. The PCR primers used were as follows β ACTIN- For 5’-TCCGTAAAGAATCTCATGCC-3’, Rev 5'-ATCTTCATCCTCCTAGGAGC-3'; IFN-Υ For 5’-GGATGCATTCATGAGTATTGC-3’, Rev 5’-GCTTCCTGAGGCTGGATTC-3’; IL17A For 5’- GCTCCAGAAGGCCCTCAG-3’, Rev 5’- CTTTCCCTCCGCATTGACA-3’; IL6- For 5’- CGTGGAAATGAGAAAAGAGTTGTGC-3’, Rev 5’- ATGCTTAGGCATAACGCACTAGGT-3’ TGFβ-For 5'- TTGCTTCAGCTCCACAGAGA-3’, Rev 5’- TGGTTGTAGAGGGCAAGGAC-3’; CCL20 - For 5’-GCCTCTCGTACATACAGACGC-3’, Rev5’-CCAGTTCTGCTTTGGATCAGC-3’.
ELISA
The pooled corneal samples were homogenized using a tissue homogenizer and supernatant was used for analysis. The concentrations of nd IFN-γ were measured by using sandwich ELISA kits (eBioscience) as per the manufacturer’s instructions.
Statistics
Student’s t test was performed to determine statistical significance and data are expressed as mean ± SEM.
Results
IL-17 is up-regulated in the cornea of mice ocularly infected with HSV-1
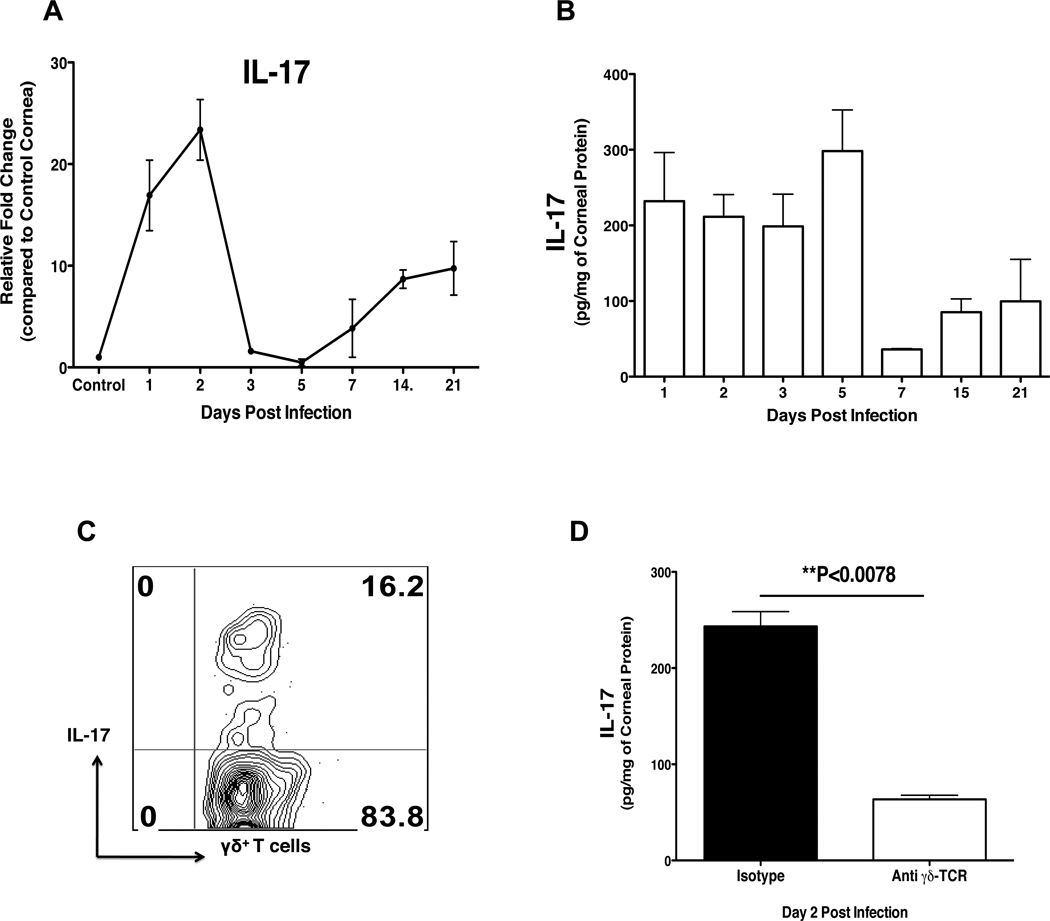
Total mRNA isolated from the corneas of HSV infected mice was analyzed for the relative fold changes in IL-17 mRNA expression by RT-PCR. As is evident in Fig1A, the expression profile revealed that IL-17 was upregulated in a biphasic manner. During the early phase, IL-17 expression was elevated at day 2 pi, but returned to near basal levels around day 5 pi. This was followed by a subsequent upregulation of IL-17 starting from day 7 pi with a steady increase until day 21 pi (last observed time point), which corresponds to the chronic phase of the disease. In addition, quantification of IL-17 by ELISA revealed a similar pattern of expression as observed with RT-PCR (Fig 1B). Early after HSV-1 infection, innate cells infiltrate the cornea, and CD4+ T cells start infiltrating the infected cornea around day 7p.i. (1). Recent studies have implicated innate cells, particularly γδ T cells as one of the major sources of IL-17 (21–25). Therefore to investigate this possibility in our system, IL-17 producing γδ T cells were enumerated by intracellular cytokine staining (ICCS) after stimulation with PMA/ionomycin. Around 16% of total γδ T cells produced IL-17 (Fig 1C), and depletion of γδ T cells using a specific monoclonal antibody diminished the levels of IL-17 as measured by ELISA (Fig 1D). Collectively, these data show that IL-17 expression is upregulated in a biphasic manner following HSV infection and innate γδ T cells contribute to the early source of IL-17.
Figure 1. IL-17 expression is upregulated in the HSV infected cornea and innate cells contribute to the early source of IL-17.
C57BL/6 mice were ocularly infected with 1× 104 PFU of HSV. Control mice were mock infected with PBS. At each time point 6 corneas were collected and pooled for measurement of IL-17 levels by RT-PCR, ELISA or ICCS assay. (A–B) The kinetic analysis for the expression of IL-17 in the HSV infected cornea as determined by RT-PCR (A) and ELISA (B). The expression levels of IL-17 by RT-PCR at various time points were quantified relative to β-actin using ΔΔCT method. (C) On day 2 pi, corneas were collected and analyzed by ICCS assay for the production of IL-17 by γδ T cells. Cells were stimulated with PMA/Ionomycin for 4 hrs in the presence of Brefeldin A. (D) The IL-17 levels at day 2 pi in γδ T cell depleted C57BL/6 mice as determined by ELISA. Animals in the control group received isotype antibody. Data are shown for one representative experiment out of two with 6 to 8 mice at each indicated time point pi. Statistical levels of significance were analyzed by Student’s t test. Error bars are SEM.
Th1 cells predominate during early stages of SK pathogenesis followed by Th17 cells in later stages
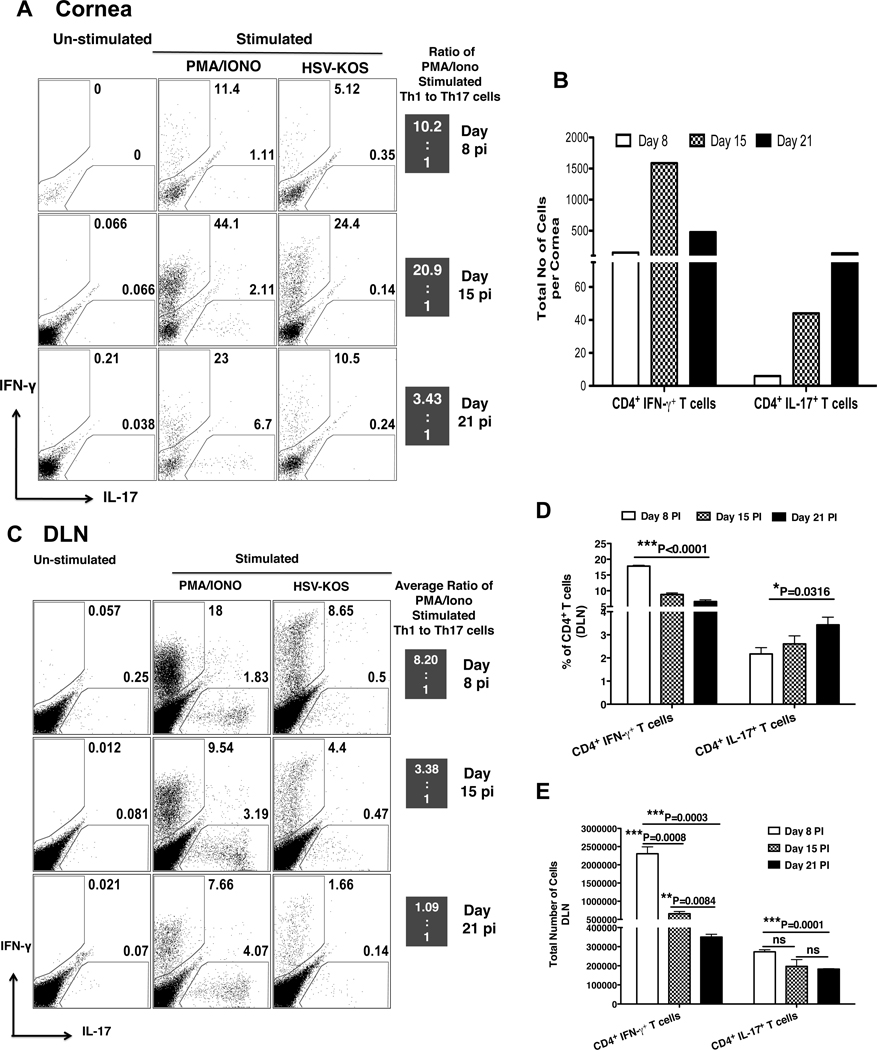
Past studies showed that CD4+ T cell infiltration of the corneal stroma becomes readily apparent by 7 day pi (1, 7, 8), but the relative composition of the CD4+ T effectors was not recorded. Using the intracellular cytokine staining approach with cells isolated from collagen digested pooled corneas, the relative frequencies of Th17 and Th1 cells were measured in the HSV infected corneas at day 8, 15 and 21 pi. As shown in Fig 2A, stimulation of corneal cells with PMA and ionomycin revealed a large proportion of CD4+ T cells producing either IFN-γ or IL-17 within the corneal infiltrate. However, the ratio of frequencies of Th1 to Th17 were higher at all tested time point pi. Additionally, Th1 cells showed an initial increase at day 15 over day 8 pi followed by reduced frequencies at day 21 pi. In contrast to Th1 cells, Th17 cells were scarcely detectable on day 8 pi and this was followed by a gradual increase in their frequency with the progression of SK (Figure 2A). The total cell numbers for each population revealed similar kinetics (Figure 2B, middle column). Furthermore, intracellular staining of corneal cells stimulated with UV inactivated viral antigen preparation revealed a high percentage (≤ 24.4 %) HSV specific Th1 cells as compared to a lower percentage (≤ 0.35 %) of HSV specific Th17 cells (Figure 2A right column). In addition, analysis of local draining lymph nodes (DLN) for Th1 and Th17 cells, revealed a similar pattern as observed for the cornea (Figure 2C, D). These results indicate Th1 cells initially infiltrate the site of infection followed by the increased infiltration of Th17 cells during late stages of SK.
Figure 2. Kinetic analysis of Th1 and Th17 cells in the cornea and DLN of HSV infected mice.
C57BL/6 mice were ocularly infected with 1× 104 PFU of HSV and each time point 6 to 8 corneas were collected, pooled and liberase digested. The corneal single cell suspensions were analyzed for the CD4+ T cell expression of IL-17 and IFN-γ by ICCS assay. (A) Representative FACS plots for the corneal Th1 and Th17 cells at each time point pi. The cells were either un-stimulated (left column) or stimulated with PMA/Ionomycin for 4 hrs (middle column) in the presence of Brefeldin A. For quantification of Ag-specific CD4+ T cells (right panel), cells were stimulated for 16 hrs with UV-inactivated HSV-KOS, with the addition of Brefeldin A for last 5 hrs of stimulation. FACS plots shown are gated on CD4+ T cells. The ratio shown on the right side is a comparison of PMA/Ionomycin stimulated Th1 to Th17 cells on respective day pi. (B) Total number Th1 and Th17 cells per cornea at day 8, 15 and 21 pi by ICCS assay post stimulation with PMA/Ionomycin. (C) Representative FACS plot for Th1 and Th17 cells from the local DLN of HSV infected mice at day 8, 15 and 21 pi. The cells were either un-stimulated or stimulated with PMA/Ionomycin for 4 hrs or with UV-HSV-KOS for 16 hrs. (D) Frequencies and total numbers (E) of Th1 and Th17 cells from DLN at day 8, 15 and 21 pi as determined by ICCS assay post stimulation with PMA/Ionomycin. Data are shown for one representative experiment out of two with 6 to 8 mice at each indicated time point pi. Statistical levels of significance were analyzed by Student’s t test. Error bars are SEM.
Local cytokine/chemokine directs delayed entry and/or differentiation of Th17 cells in the cornea
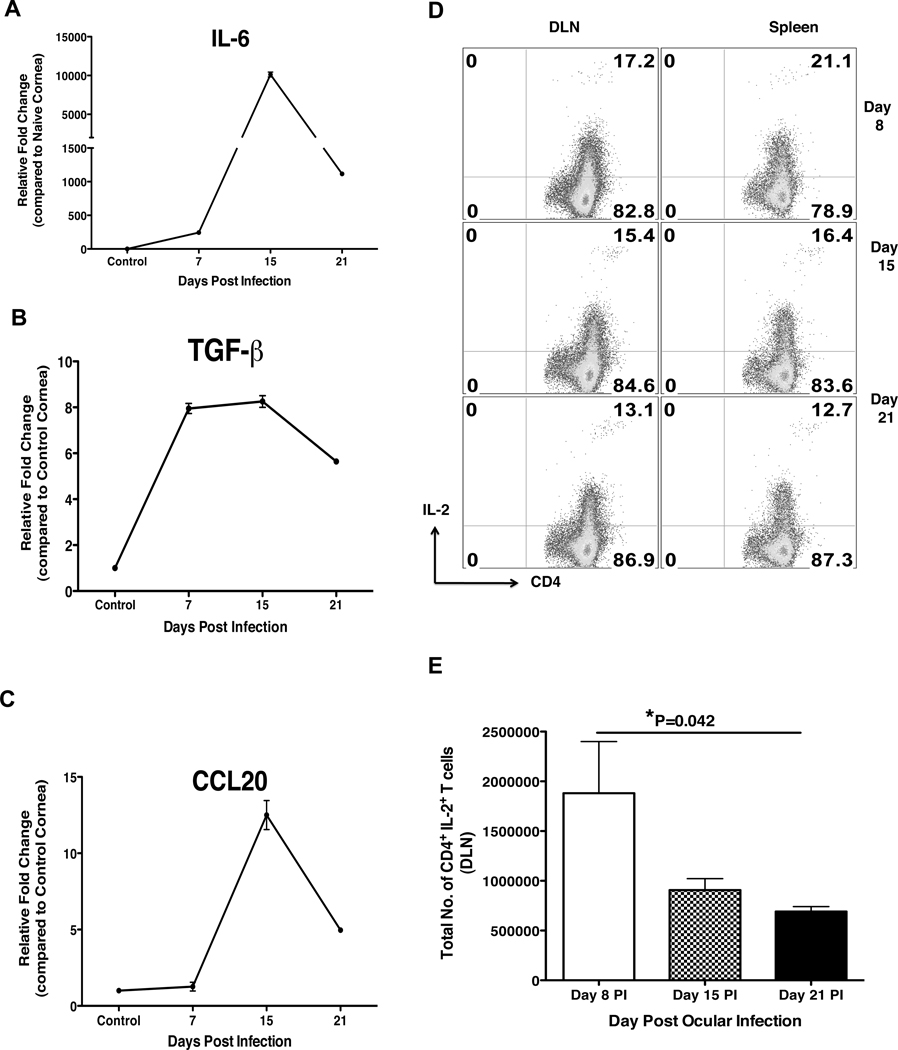
The delayed migration of Th17 cells in the cornea could be the result of the early conditions suppressing Th17 cells or conditions such as local cytokine and chemokine milieu favoring Th17 cell generation and/or migration during later stages of SK. To further investigate this issue, studies were carried out for the corneal quantification of different cytokines as well as chemokines, particularly those that are involved in the generation, migration and suppression of Th17 cell responses. Since Th17 cells differentiate from naïve CD4+ T cell in the presence of IL-6 and TGF-β with antigenic stimulation (26–28), we quantified the relative fold change of IL-6 and TGF-β at various days pi as compared to mock infected cornea (Figure 3A and 3B). The results showed relatively low levels of IL-6 at day 8 pi followed by a peak at day 15 pi. (Figure 3A). TGF-β levels were upregulated from day 8 pi and, peaked at day 15 pi. As compared to day 15 pi, both IL-6 and TGF-β levels were reduced by day 21 pi. Furthermore, quantification of CCL20 a known ligand for CCR6, which is preferentially, expressed by homeostatically proliferating Th17 cells as well as Treg cells (29, 30), was significantly expressed only after day 15 pi (Figure 3C). Furthermore, since a recent study has shown the suppressive role of IL-2 in the generation of Th17 cells (31), we quantified CD4 T cells producing IL-2 in the local DLN and spleen by ICCS assay. As shown in Figure 3D–E, IL-2 producing CD4+ T cells were significantly higher in frequency as well as numbers at day 8 pi followed by a reduction at day 15 and day 21 pi. This early and robust IL-2 production by CD4+ T cells could be responsible for the promotion of Th1 as well as the suppression of Th17 responses in lymphoid organs early after HSV infection. However, during the progression of SK, increased IL-6, TGF-β as well as chemokine CCL20 expression in the cornea could be responsible for either generation or migration of Th17 cells during the later stages of SK.
Figure 3. Delayed expression of IL-6, TGF-β and CCL20 along with reduced IL-2 expression by CD4+ T cells directs the delayed generation and/or entry of Th17 cells.
C57BL/6 mice were ocularly infected with 1× 104 PFU of HSV. Control mice were mock infected with PBS. Mice were sacrificed on day 8, 15 and 21 pi and corneas, local DLN and spleen were collected for the analysis by RT-PCR and ICCS assay. (A–C) At each time point 6 corneas were collected and pooled for the measurement of IL-6 (A), TGF-β (B) and CCL20 (C) levels by RT-PCR. The expression levels of different molecules were quantified relative to β-actin using ΔΔCT method. The IL-2 producing CD4+ T cells were analyzed by ICCS assay as described in materials and methods. Representative FACS plots (D) and total cell numbers (E) for IL-2 producing CD4+ T cells from the DLN and spleen at day 8, 15 and 21 day pi. Cells are gated on CD4+ T cells. Data are shown for one representative experiment out of two with 6 mice at each indicated time point pi. Statistical levels of significance were analyzed by Student’s t test. Error bars are SEM.
The Dual Role of IFN-γ in HSV induced immunopathology
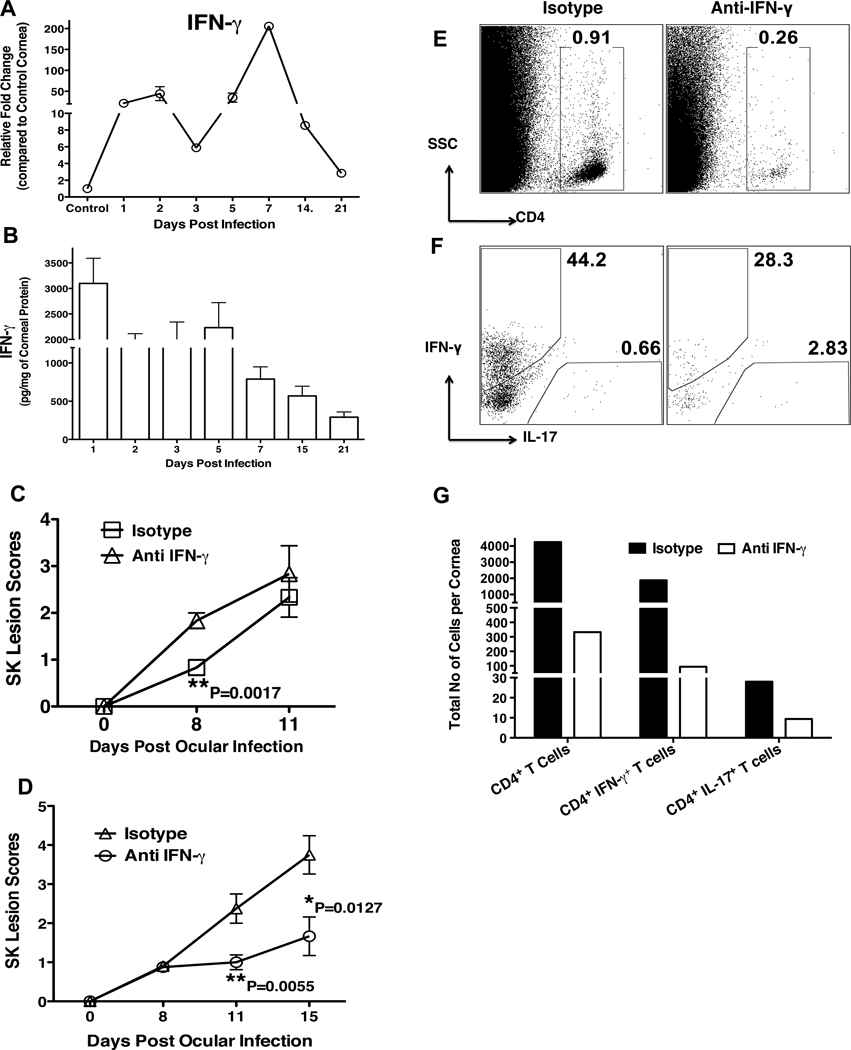
Since, IFN- γ suppresses the induction and expansion of Th17 cells (17, 32–34), we investigated the role of IFN-γ in relation to IL-17 as well as Th-17 cells. Similar to IL-17, IFN-γ showed biphasic upregulation. However, the second peak expression was observed around day 7 pi followed by a steady decline with the progression of SK (Figure 4A). Measurement of IFN-γ cytokine by ELISA revealed similar kinetics of expression as observed with QPCR analysis (Figure 4B). To demonstrate the role of IFN-γ in SK, IFN-γ depletion was carried out from day -1 followed by depletion on day 2 and day 5. The severity of SK lesions was scored on day 8 and day 11. As shown in figure 4C, early depletion of IFN-γ significantly increased the severity of SK on day 8. However, on day 11 it reached that of isotype antibody treated control mice group. Furthermore, all mice from IFN- γ depleted group showed signs of severe encephalitis, which necessitated termination of experiments. In contrast, depletion of the second peak of IFN-γ during later stages of SK (on day 7, 10 and 13) showed significantly diminished SK lesion severity on day 11 and day 15 pi (Figure 4D). The analysis of various cell types from the corneas showed reduced frequencies of total CD4+ T cells, the Th1 subset, but increased percentage of the Th17 subset (Figure 4E–F). However, the total numbers of total CD4+ T cells, Th1 and Th17 cells were reduced in IFN-γ depleted mice as compared to isotype treated antibody treated mice. These results demonstrate an early protective role of IFN-γ likely acting by controlling virus replication in cornea. However, IFN-γ exerts a pathologic role once virus is cleared from the cornea as occurs during the later stages of SK. Taken together, our data indicates that Th1 cells are the initial main orchestrators of SK lesions followed later on in the chronic phase by the contribution of both Th1 and Th17 cells. Early infiltration of Th1 cells initiates the inflammatory condition particularly cytokines and chemokine environment that promotes generation and/or migration of highly pathogenic Th17 cells. This late migration of Th17 cells maintains the SK pathology via their secretion of IL-17, which acts as a pro-inflammatory cytokine through various mechanisms.
Figure 4. Early protective and late pathologic role of IFN-γ in SK.
C57BL/6 mice were ocularly infected with 1× 104 PFU of HSV. Control mice were mock infected with PBS. At each time point 6 corneas were collected and pooled for measurement of IFN- γ levels by RT-PCR, ELISA or for analysis by ICCS assay. (A–B) The kinetic analysis for the expression of IFN-γ in the HSV infected cornea as determined by RT-PCR (A) and ELISA (B). The expression levels of IFN-γ by RT-PCR at various time points were quantified relative to β-actin using ΔΔCT method. (C–D) Diminished SK lesion severity in mice treated with anti-IFN- γ as compared to isotype Ab treated mice. Group of 2 to 4 mice were treated i.p. with anti-IFN- γ on either starting from day -1, followed by treatment on day 2 and day 5 pi (C); or either starting from day 7 pi followed by treatment on day 10 and 13 pi (D). Mice in control group received isotype control Ab. (E–H) Mice from experiment (D) were sacrificed on day 15 pi and corneas were collected for the analysis of different cell population by flow cytometry. Representative FACS plots for total corneal infiltrating CD4+ T cells (E) and Th1 and Th17 cells after stimulation with PMA/Ionomycin (F) from isotype and anti-IFN-γ treated groups. (G) Total cell number per cornea for CD4+ T cells, Th1 and Th17 cells shows reduced infiltration of cells in the cornea after IFN-γ neutralization. Data are a representative summary of two independent experiments. Statistical levels of significance were analyzed by Student’s t test. Error bars are SEM.
IL-17 neutralization diminishes SK lesion severity
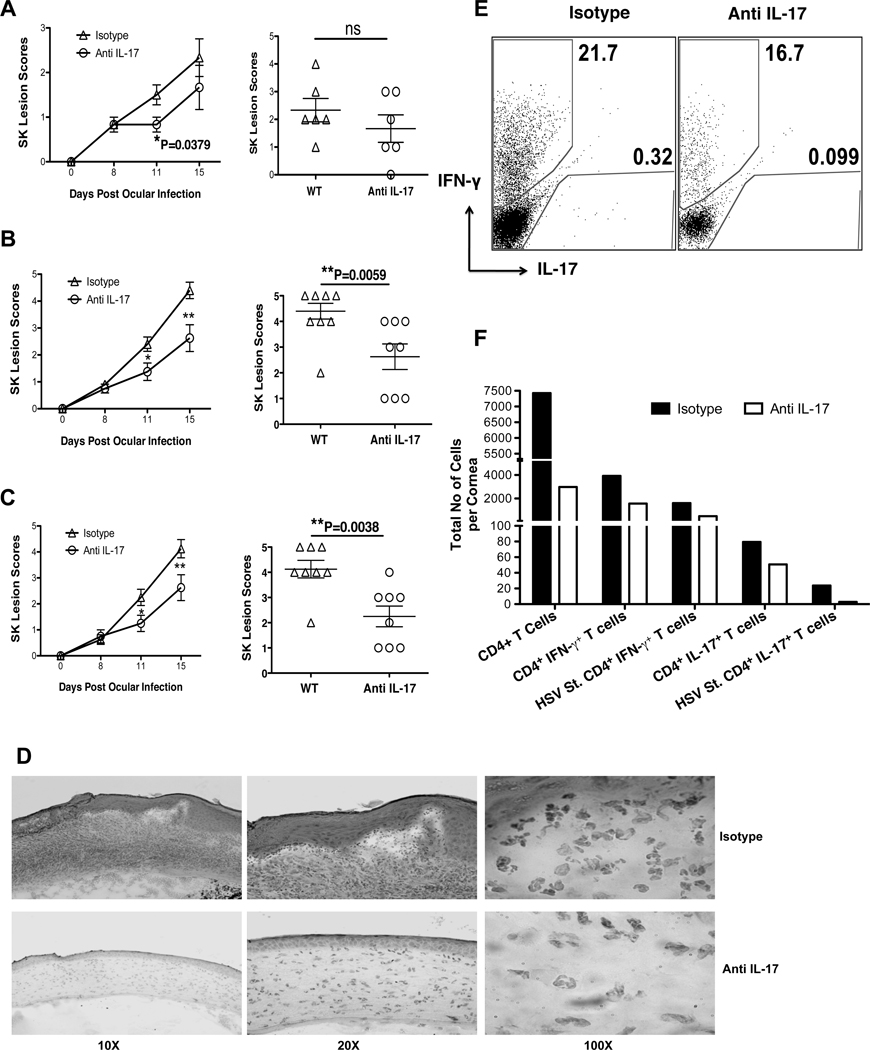
To further investigate the role of IL-17 in SK pathogenesis, the effects of neutralizing IL-17 with monoclonal Ab was studied over a 15 day observation period. Intraperitoneal Treatment was began on 1 day before infection followed by injections on day 2 and day 5 pi. The early neutralization of IL-17 reduced the SK lesion severity on day 11 pi (Fig 5A). To further confirm the role of IL-17 during later stages of SK, IL-17 neutralization was started from day 7 pi when SK lesions become evident, followed by depletion on day 10 and 13 pi. This therapeutic neutralization of IL-17 also reduced the severity of SK lesions further confirming the pathogenic role of IL-17 during the later stages of SK (Fig 5B). In addition, therapeutic local depletion of IL-17 by subconjunctival administration of anti-IL-17 antibody also resulted in significantly diminished SK lesion scores (Fig 5C). Examination of corneal sections by histopathological analysis revealed reduced inflammatory response in the IL-17 depleted corneas, with less fibrosis of corneal stroma, no or minimal hypertrophy of epithelial layers as well as diminished infiltration of leukocytes, particularly neutrophils (Fig 5D). Moreover, the frequencies as well as total numbers of CD4+ T cells, Th1 and Th17 cells were also reduced in the anti-IL-17 treated group (Fig 5E–F). The reduced numbers of Th1 cells could be the result of reduced infiltration of neutrophils, which may act as a source of CXC chemokines essential for Th1 infiltration (35). Taken together, these data indicated the pathogenic role of IL-17 during early as well as late phases of SK.
Figure 5. IL-17 neutralization diminishes SK lesion severity.
C57BL/6 mice ocularly infected with 1×104 PFU of HSV were divided into two groups each with 6 to 8 mice. One group of mice received antibody against IL-17 and mice in control group were administered with isotype antibody. (A–C) Diminished SK lesion severity in mice treated with anti-IL-17 mAb. SK lesion score for individual cornea on day 15 pi (right) are shown. (A) Early neutralization of IL-17 was carried by i.p. injection of anti-IL-17 mAb from day -1 followed by day 2 and 5 pi. (B) Late neutralization of IL-17 was carried by i.p. injection of anti-IL-17 mAb from day 7 followed by day 10 and 13 pi. (C) Local depletion of IL-17 was carried by sub-conjunctival delivery of anti-IL-17 using same strategy as that of late depletion. (D) Representative H&E stained corneal sections of isotype treated (top panel) and IL-17 depleted (bottom panel) mice collected on day 15 pi from experiment B. The figure shows the pictures of the section taken at X 10 (left), X 20 (middle) and X 100 (right) of original magnification. (E–F) Mice from late depleted groups (as shown in B) were sacrificed on day 15 pi and corneas were harvested and pooled group wise for the analysis of various cell types (n = 6–8 per group) by flow cytometry. Representative FACS plot for total corneal infiltrating Th1 and Th17 cells (E) from isotype and anti-IL-17 treated groups. (F) Total cell number per cornea for CD 4+ T cells, Th1, Th17 cells shows reduced infiltration of cells in the cornea after IL-17 neutralization. Data are a representative summary of two independent experiments. Statistical levels of significance were analyzed by Student’s t test. Error bars are SEM. Statistical levels of significance were analyzed by Student’s t test. Error bars are SEM.
IL-17R KO mice show reduced SK lesion severity
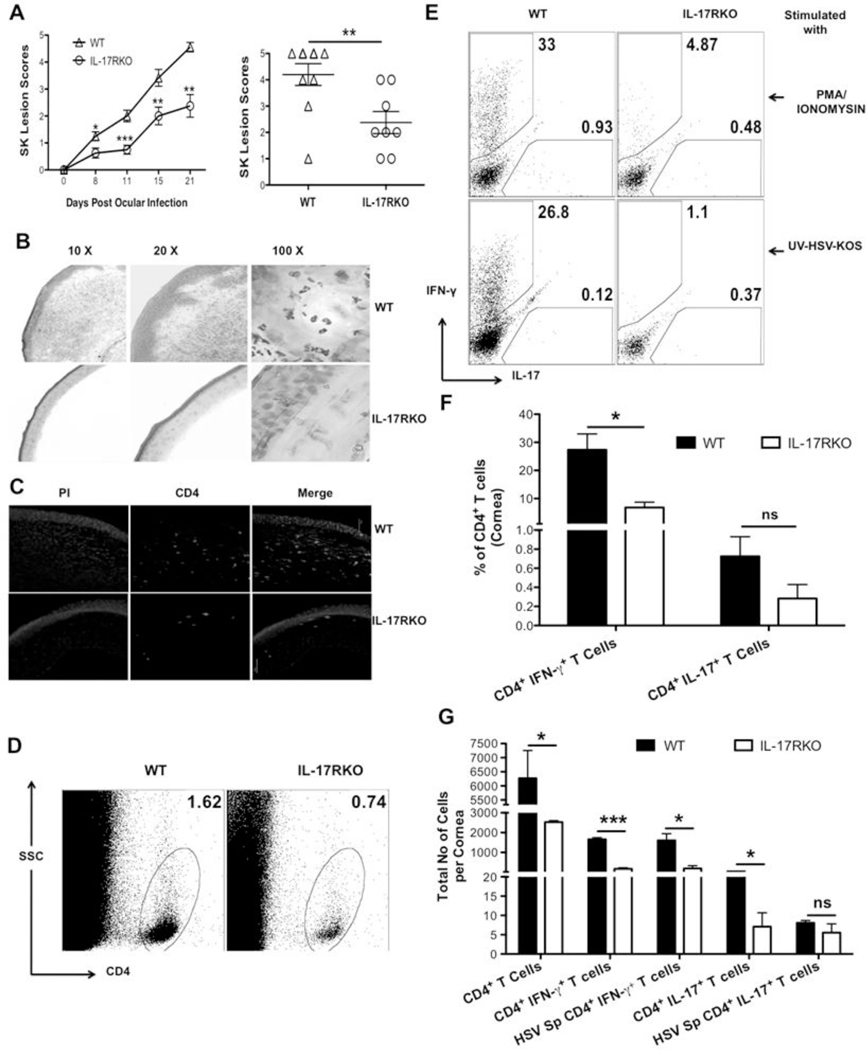
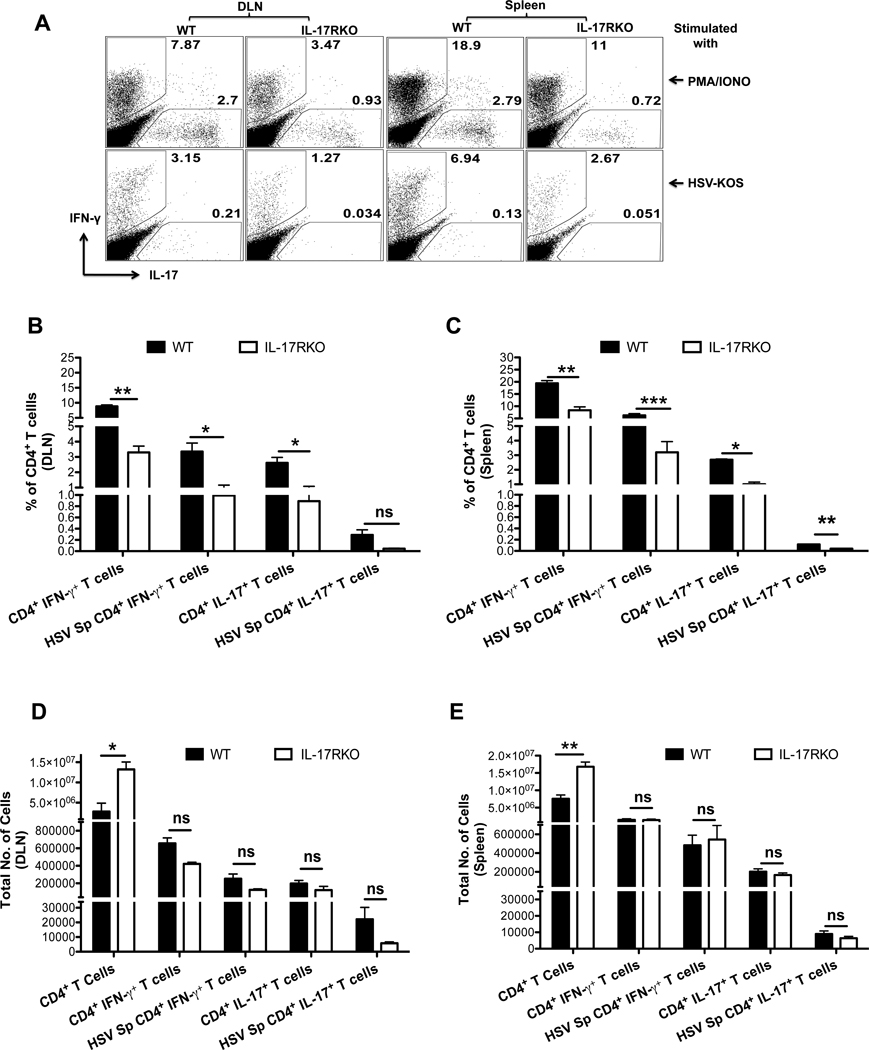
To further confirm the role of IL-17 in SK pathogenesis, mice lacking IL-17 receptor signaling were ocularly infected with HSV and SK lesion severity was monitored and compared with WT mice. The disease severity differed significantly at all tested time point pi between WT and IL-17R KO mice (Fig 6A). The onset of SK was delayed and SK lesions were less severe in IL-17R KO, as compared to their WT counterparts. The severity of SK lesions (scores of ≥3) was reduced in mice in the IL-17R KO group (3 of 8 eyes at day 21) compared to WT counterparts (7 of 8 eyes at day 21) (Fig 6A right panel). Furthermore, histopathological analysis of WT corneas at day 15 pi showed loss of the corneal epithelial layer, extensive stromal fibrosis, infiltration of large number of leukocytes in the stromal layers with the resultant thickening of corneal stroma (Fig 6B, upper panels). In contrast to WT corneas, section from IL-17R KO corneas showed no damage to the epithelial layer with minimal fibrosis as well as infiltration by inflammatory leukocytes at day 15 pi (Fig 6B lower panels). Moreover, immunofluorescence staining of corneal sections for CD4+ T cells at day 15 pi showed diminished infiltration of CD4+ T cells in IL-17R KO mice as compared to WT mice (Figure 6C). To further quantify the different cell types infiltrating the cornea, experiments were terminated on day 15 to study cellular parameters. As shown, the frequencies (Fig 6D–F) and absolute numbers (Fig 6G) of total CD4+ T cells, Th1 and Th17 cells were decreased in the IL-17R KO compared to WT mice. Additionally, the expression of pro-inflammatory cytokines such as IL-6, IL-17, IL-1β were diminished in IL-17R KO mice corneas as compared to the WT samples (data not shown). Analysis of DLN and spleen of WT and IL-17R KO, mice showed reduced frequencies, but not total cell numbers of each population (Fig 7). As shown in Figure 7A–D, the frequencies of Th1 and Th17 cells showed two fold reduction in DLNs and spleen from IL-17R KO mice as compared to WT mice. However, the total numbers of each subpopulation were equal in both IL-17R KO and WT mice DLNs and spleen (Fig 7E and F). These observations revealed that although, the total cell numbers were equal in DLNs and spleens from IL-17R KO and WT mice, the frequencies and total cell numbers were reduced at the inflammatory site (cornea) in IL-17R KO mice as compared to WT counterparts. Taken together, these results indicated the pro-inflammatory role of IL-17 in SK pathogenesis.
Figure 6. IL-17R KO mice are resistant to SK.
C57BL/6 and IL-17R KO mice were infected with 1×104 PFU of HSV. (A) The disease progression kinetics and SK severity was assessed on day 8, 11, 15 and 21 pi (left panel). Right panel shows the score of individual eye on day 15 pi
(B) Representative H&E stained corneal sections from WT (upper row) and IL-17R KO (lower row) mice collected on day 15 pi. The figure shows the pictures of the section taken at X 10 (left), X 20 (middle) and X 100 (right) original magnification.
(C) Representative immunofluorescence micrograph of corneas for CD4+ T cells (green) from WT and IL-17R KO mice on day 15 pi. The sections were counterstained with propidium iodide (red). Scale bars, 75µm. Mice were sacrificed on day 15 pi and corneas were harvested and pooled group wise for the analysis of various cell types (n = 6–8 per group). (D) Representative FACS plots for corneal infiltrating total CD4+ T cells. (E) Intracellular staining was conducted to quantify Th1 and Th17 cells by stimulating them with PMA/Ionomycin (top) and HSV-KOS virus (bottom). (F) The bar diagram shows the average frequencies of Th1 and Th17 cells after stimulation with PMA/ionomycin or UV inactivated HSV-KOS from two independent experiments. (G) The bar diagram is a summary for total numbers of corneal infiltrating CD4+ T cells, IFN-γ+ CD4+ T cells, HSV stimulated IFN- γ+ CD4+ T cells, IL-17+ CD4+ T cells, HSV-Stimulated IL-17+ CD4+ T cells in the corneas of WT and IL-17R KO mice. Data are shown as a summary of two independent experiments with 6 to 8 mice per group. Statistical levels of significance were analyzed by Student’s t test. *< 0.05; ***< 0.001. Error bars are SEM.
Figure 7. IL-17R KO mice exhibit reduced frequencies but not numbers of Th1 and Th17 cells in DLN and spleen post HSV infection.
C57BL/6 and IL-17R KO mice were infected with 1×104 PFU of HSV. Mice were sacrificed on day 15 pi and single cell suspensions of the individual spleen and draining cervical lymph nodes (DLN) were prepared. (n = 4). (A) Representative FACS plots for Th1 and Th17 cells stimulated with PMA/Ionomycin (top) or HSV-KOS virus (bottom). (B, C) Summary for average frequencies of different CD4+ T cell types as depicted in A from DLN (B) and spleen (C). (D, E) The total numbers of IFN-γ+ CD4+ T cells, HSV stimulated IFN- γ+ CD4+ T cells, IL-17+ CD4+ T cells, HSV-Stimulated IL-17+ CD4+ T cells and Foxp3+ CD4+ T cells from DLN (D) and spleen (E) of WT and IL-17RKO mice.
Discussion
In this study we show that HSV infection of the cornea leads to a biphasic increase in corneal expression of IL-17. Moreover, the subsequent HSV induced SK immunopathology is the outcome of the effector functions mediated by both Th1 and Th17 cells. The pro-inflammatory cytokine IL-17 drives initial as well as late events in SK pathogenesis and also contributes significantly to the pathology of SK. However, the sources of IL-17 during early and late stages of SK were different. Initially, innate cells such as γδ T cells were the main producers and later on in the chronic phase Th17 cells were the producers. Additionally, IFN-γ was shown important for the early protection from HSV infection, but cells producing IFN-γ also contributes to the pathology once the virus is absent. Furthermore, HSV induced immunopathological lesions involve both Th1 and Th17 cells with their respective importance changing with time. Accordingly, in line with previous published reports, our data demonstrates that Th1 cells mainly initiate and orchestrate SK lesions at least during the early stages followed by the entry of Th17 cells when the disease is at peak. Interestingly, significant proportion of Th1 cells secreted IFN- γ upon stimulation with UV inactivated HSV virus, but most Th17 cells failed to produce IL-17 under similar conditions, indicating that Th17 cells could be involved in a bystander fashion in SK pathogenesis. Furthermore, depletion of IL-17 during both early as well as during the chronic phase of SK pathogenesis and infection of IL-17R KO mice showed reduced SK lesion severity. The findings indicate an important role for IL-17 at all stages of SK pathogenesis. Accordingly targeting IL-17 production represents a logical target to control SK, an important cause of human blindness.
Innate cells such as γδ T cells act as one of the major early sources of IL-17, as shown in some other systems (21–25). Thus our data is in line with the previously published reports where γδ T cells were shown to be main source of innate IL-17 (21–25). IL-17 orchestrates the local inflammatory response by stimulating epithelial, endothelial and fibroblastic cells to produce and release various inflammatory cytokines (12, 36–39). Furthermore, IL-17 drives increased influx, as well as survival, of neutrophils at the site of inflammation by the induction of neutrophil chemoattracts, as well as GM-CSF mediated granulopoeisis (12, 39–41). In ocular HSV infection, an initial influx of neutrophils plays an important role in virus clearance (42). The diminished expression of neutrophil chemoattractants for neutrophils and subsequent reduced infiltration of neutrophils occurs in IL-17R KO mice post HSV infection (17). Similarly, we found a reduced infiltration of neutrophils in HSV infected IL-17R KO mice as well as post IL-17 neutralization during early as well as during late stages of SK pathogenesis (data not shown). Taken together, the data show that during HSV infection of the cornea, IL-17 contributes to the early innate cell response by promoting the migration of neutrophils.
Recent work has discovered a critical role of Th17 cells in various chronic and autoimmune inflammatory conditions (33, 34, 43–45), and our data show that Th17 cells do participate in SK pathogenesis. However, in contrast to autoimmune as well as some chronic inflammatory conditions, where Th17 cells may play a dominant role, HSV induced immunopathology results from the participation of both Th1 and Th17 cells with the relative importance changing as disease progresses. Accordingly, our data from IFN-γ and IL-17 neutralization as well as IL-17R KO mice shows that both Th1 and Th17 mediated immune response contributes to SK pathogenesis. Th1 cells were the first to infiltrate the cornea, and significant proportions of them were HSV-specific. They set the stage for the development of complex SK lesions that additionally involves Th7 cells. However, the Th17 cells could migrate only after the entry of Th1 cells, indicating their role in the later stages of SK. Our data accord with a recent study showing that only Th1 cells could gain entry in the non-inflamed tissue and initiate inflammatory response followed by entry of Th17 cells in the inflamed CNS (46). Furthermore, almost none of the Th17 cells responded to HSV specific antigen when stimulated ex-vivo which may indicate their bystander role in SK pathogenesis. However, diminished SK severity in the absence of IL-17 receptor signaling, as well as post IL-17 neutralization during early as well as late stages of SK, indicated that IL-17 does play a pro-inflammatory role in SK pathogenesis.
Although, presently we cannot detect the antigen specificity of these Th17 cells, they could be reactive against yet unidentified unmasked self-corneal antigens or be functioning in a bystander fashion. A recent study on an autoimmune condition of the retina using experimental autoimmune uveitis model, showed that both the retinal antigen specific Th1 and Th17 cells could drive autoimmunity depending upon the local conditions at the time of antigen exposure as well as type of T effector cell present (47). However, our data show that during viral immunopathology, higher levels of IFN-γ occur early after HSV infection could promote Th1 and suppress Th17 immune responses. During autoimmune conditions exposure of self-unmasked antigens occur that provides constant stimulation to the T effector cells at the site of inflammation (48). The body might sense this chronic persistence of antigen as the need arises for stronger and more pathogenic immune response for the removal of such persistent antigen. The highly pathogenic nature of IL-17 and or Th17 cells meets this criterion and might be the outcome of such persistent antigenic stimulation as observed in many autoimmune conditions. However, during acute infections, effective Th1 and Th2 responses could clear the antigen from the site of infection, avoiding the Th17 mediated immunopathology. In contrast, chronic persistence of antigen could be sensed by the body, with a resultant robust T effector response such as a Th17 cell mediated immune response, which can cause damage and subsequent immunopathology. SK, being a chronic disorder could mimic this condition, where initial acute viral infection is controlled by the Th1 cell response that initiates inflammatory milieu in the cornea. This initial damage to the corneal tissue could uncover self-antigens that help drive the subsequent Th17 mediated highly inflammatory reaction. Alternatively, one recent study showed that Th17 cells can be activated in the context of the local microenvironment and could cause tissue specific damage in an antigen non-specific way that depended upon the presence of IL-6 (49). Thus, it might be possible that these Th17 cells during late stages of SK could be non-specific to the corneal Ag and acting in a bystander fashion stimulated in the presence of the complex inflammatory cytokine milieu. However, this issue needs to be addressed further using adoptive transfer of non-specific Th17 cells in the context of HSV induced SK immunopathology. Such studies are currently undergoing in our laboratory.
Our data also demonstrates a dual role of IFN-γ during HSV mediated immunopathology. Innate cells contribute to the early source of IFN-γ after HSV infection and Th1 cells from day 7 pi (1, 50). Thus, IFN-γ neutralization during the pre-clinical phase when replication virus is still present in cornea (1), lead to the transient increased tissue damage presumably driven by virus. Furthermore, this early depletion caused severe encephalitis, possibly an effect of higher viral titers indicating the early protective role of IFN-γ as previously documented (1, 49, 51). Interestingly, neutralization of IFN-γ after day 7 pi resulted in significantly reduced SK severity indicating a crucial role of IFN-γ and/or Th1 cells as the initiators and main orchestrators of early immunopathological lesions of SK. Since, IL-2 and IFN-γ suppresses Th17 response (17, 31–34), conceivably during HSV induced immunopathology higher levels of IFN-γ in the cornea could be suppressing the early development of Th17 responses. Furthermore the chronic inflammatory situation in the cornea might be creating appropriate conditions for the generation and or migration of Th17 cells. Naïve CD4+ T cells differentiate into Th17 cells under appropriate antigenic stimulation in the presence of IL-6 and TGF-β (26–28). Furthermore, Th17 cells express the chemokine receptor, CCR6, and migrate to the site of inflammation through the specific chemokine, CCL20 (29, 30). Indeed, our data for the expression levels of IL-6, TGF-β and CCL20 indicated that conditions favored either migration and/or generation of Th17 cells occur only during the very late stage of SK pathogenesis. This might explain the delayed appearance of Th17 cells in the cornea.
Taken together, our results demonstrate the relative contribution of IL-17/Th17 and IFN-γ/Th1 cells in the pathogenesis of SK. Both cell types participate in the pathogenesis of SK depending on the stage of SK. The demonstration of the pathogenic role of IL-17/Th17 cells in later stages of SK is a novel finding and could have implication for future therapeutic intervention for HSV induced immunopathology, an important cause of infectious human blindness.
Acknowledgments
We thank Dr. John Dunlop for his assistance on confocal microscopy. We also thank Fernanda, Sachin Mulik and Greg Spencer for their invaluable assistance during research and manuscript preparation in many ways.
Footnotes
This study was supported by National Institute of Allergy and Infectious Diseases Grant AI 063365 and National Institutes of Health Grant EY 005093.
References
- 1.Biswas PS, Rouse BT. Early events in HSV keratitis--setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 3.Metcalf JF, Hamilton DS, Reichert RW. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979;26:1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell RG, Nasisse MP, Larsen HS, Rouse BT. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 1984;25:938–944. [PubMed] [Google Scholar]
- 5.Sarangi PS, Rouse BT. Herpetic Keratitis. In: Levin LA, Alberts DM, editors. ocular Disease Mechanisms and Management. Philadelphia, PA: Saunders Elsevier; 2010. pp. 91–97. [Google Scholar]
- 6.Knotts FB, Cook ML, Stevens JG. Pathogenesis of Herpetic Encephalitis in Mice after Ophthalmic Inoculation. J Infect Dis. 1974;130:16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- 8.Niemialtowski MG, Rouse BT. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J Immunol. 1992;148:1864–1870. [PubMed] [Google Scholar]
- 9.Suryawanshi A, Mulik S, Sharma S, Reddy PB, Sehrawat S, Rouse BT. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor A and its soluble receptor. J Immunol. 2011;186:3653–3665. doi: 10.4049/jimmunol.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 12.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovanovic DV, Martel-Pelletier J, Di Battista JA, Mineau F, Jolicoeur FC, Benderdour M, Pelletier JP. Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophages: a possible role in rheumatoid arthritis. Arthritis Rheum. 2000;43:1134–1144. doi: 10.1002/1529-0131(200005)43:5<1134::AID-ANR24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Koenders MI, Kolls JK, Oppers-Walgreen B, van den Bersselaar L, Joosten LA, Schurr JR, Schwarzenberger P, van den Berg WB, Lubberts E. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 2005;52:3239–3247. doi: 10.1002/art.21342. [DOI] [PubMed] [Google Scholar]
- 15.Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273:27467–27473. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- 16.Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- 17.Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008;83:401–408. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande SP, Lee S, Zheng M, Song B, Knipe D, Kapp JA, Rouse BT. Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis. J Virol. 2001;75:3077–3088. doi: 10.1128/JVI.75.7.3077-3088.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dana MR, Zhu SN, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea. 1998;17:403–409. doi: 10.1097/00003226-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002;43:737–743. [PubMed] [Google Scholar]
- 21.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 22.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 24.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 25.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 28.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 33.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 34.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 36.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 37.Pickens SR, Volin MV, Mandelin AM, 2nd, Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 2010;184:3233–3241. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 39.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 41.Laan M, Prause O, Miyamoto M, Sjostrand M, Hytonen AM, Kaneko T, Lotvall J, Linden A. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17 and TNF-alpha. Eur Respir J. 2003;21:387–393. doi: 10.1183/09031936.03.00303503. [DOI] [PubMed] [Google Scholar]
- 42.Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 44.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, Joosten LA, van den Berg WB. Induction of cartilage damage by overexpression of T cell interleukin-17A in experimental arthritis in mice deficient in interleukin-1. Arthritis Rheum. 2005;52:975–983. doi: 10.1002/art.20885. [DOI] [PubMed] [Google Scholar]
- 45.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 49.Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, Laube G, Luche H, Lehnardt S, Fehling HJ, Griesbeck O, Zipp F. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Yu Z, Manickan E, Rouse BT. Role of interferon-gamma in immunity to herpes simplex virus. J Leukoc Biol. 1996;60:528–532. doi: 10.1002/jlb.60.4.528. [DOI] [PubMed] [Google Scholar]
- 51.Bouley DM, Kanangat S, Wire W, Rouse BT. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]