Abstract
Although [18F]fluoro-L-dopa [FDOPA] positron emission tomography (PET) has been used as a surrogate outcome measure in Parkinson's disease therapeutic trials, this biomarker has not been proven to reflect clinical status longitudinally. We completed a retrospective analysis of relationships between computerized sampling of motor performance, FDOPA PET, and clinical outcome scales, repeated over 4 years, in 26 Parkinson's disease (PD) patients and 11 healthy controls. Mixed effects analyses showed that movement time and tongue strength best differentiated PD from control subjects. In the treated PD cohort, motor performance measures changed gradually in contrast to a steady decline in striatal FDOPA uptake. Prolonged reaction and movement time were related to lower caudate nucleus FDOPA uptake, and abnormalities in hand fine force control were related to mean striatal FDOPA uptake. These findings provide evidence that regional loss of nigrostriatal inputs to frontostriatal networks affects specific aspects of motor function.
Keywords: Fluorodopa, motor control, Parkinson's disease, positron emission tomography, ageing, Tongue/*physiopathology, Facial Muscles/*physiopathology
Introduction
Parkinson's disease (PD) is the second most common late-life neurodegenerative disease, with a lifetime risk of 4%(Elbaz, et al., 2002). In PD, the onset of motor symptoms (muscle rigidity, slow movements, and tremor) coincides with death of dopaminergic neurons in the substantia nigra pars compacta that project to the striatum (Braak, et al., 2003). The specific mechanism by which this lesion causes motor symptoms remains an area of active investigation through motor performance, functional imaging, and neurophysiologic studies. Asymmetric onset of motor symptoms is present in 85% of cases of idiopathic PD (Yust-Katz, et al., 2008). Progression from unilateral to bilateral symptoms is one basis for the clinical staging system (Hoehn & Yahr, 1967).
Motor performance testing has been used to quantify motor abnormalities in PD. Untreated PD patients have prolonged simple reaction time (Evarts, et al., 1981) that is shortened by anti-Parkinson medications (Montgomery, et al., 1991). Parkinson's disease patients are unable to adequately increase movement velocity with increasing reach distances, unlike healthy control subjects (Draper & Johns, 1964; Flowers, 1975). During force matching tasks, PD patients show a slower rate of force development, but similar ability to maintain isometric force, in comparison with elderly control subjects (Stelmach, et al., 1989). Aging also affects reaction time, movement velocity, and bulbar strength (Crow & Ship, 1996).
[18F]fluoro-L-dopa [FDOPA] positron emission tomography (PET), which measures uptake and trapping of dopamine precursors in nigrostriatal projections, has been used as a surrogate outcome measure for disease progression in clinical trials (Whone, et al., 2003). A number of cross-sectional analyses have correlated examiner-dependent ratings of clinical signs in PD with striatal uptake of dopamine transporter radiotracers (Seibyl, et al., 1995) or FDOPA (Morrish, et al., 1996a; Nagasawa, et al., 1993; Vingerhoets, et al., 1997). Pathological investigations have also shown that the severity of bradykinesia is correlated with the degree of dopamine depletion in the putamen (Bernheimer, et al., 1973). However, the rate of change in striatal radiotracer uptake does not correlate with clinical change in individual patients studies longitudinally with dopamine transporter ligands (Marek, et al., 2001; Pirker, 2003) or [18F]FDOPA (Morrish, et al., 1996b). In this study we used automated measurement systems to acquire measures of reaction time and movement velocity over different reach distances, and to measure maximum forces and isometric force control in both limb and bulbar muscles. The baseline motor testing was acquired while patients were off anti-Parkinson medication; subsequent testing was performed on medication. Therefore, the effects of medication were not controlled and therefore the measures we evaluated may represent optimized motor function in PD. However, few studies have measured as many parameters, have measured them serially, or have compared them with PET. We hypothesized that in spite of ongoing treatment, the effects of disease on motor performance and the rate of change in motor performance would be distinguishable from the effects of aging. We hypothesized that specific relationships would be discovered between the motor performance measures, striatal FDOPA uptake, and clinical disability as measured by the Unified Parkinson's Disease Rating Scale Score (UPDRS)(Fahn, et al., 1987).
Methods
Subjects
We performed a retrospective analysis of PET, motor performance, and clinical data gathered as research between 1993 and 1999. PD patients and age-matched normal controls were recruited through regional neurology clinics. Thirty patients who initially met UK Parkinson's Diseases Society Brain Bank criteria (Gibb & Lees, 1988) for idiopathic PD were originally enrolled. Data from four PD patients were subsequently excluded based on clinical or pathological findings of atypical Parkinsonism (1), early age of onset (1), missing data (1), or dropout (1). Twenty-six PD patients (age 56 ± 11 years; 15 male, 11 female) and 11 healthy control subjects (age 61 ± 12 years; 6 male, 5 female) had data sufficient for analysis. At enrollment, mean disease duration for the PD group was 3.2 years (SD 2.1), and mean duration of pharmacotherapy for Parkinson's disease was 1.0 year. All PD patients were treated: 18 with carbidopa/levodopa; 19 with selegiline; 2 with dopamine agonists (pergolide or pramipexole); and one with trihexyphenidyl. Six additional PD patients were started on carbidopa/levodopa during the study interval. Precise medication doses were not uniformly recorded. 14 subjects were Hoehn and Yahr (HY) stage I, 11 HY II, and 1 HY III. During the study, 6 PD patients experienced disease progression to a higher HY stage. Mean total UPDRS scores taken from the maximally affected limbs were 15.7 +/− 8.4, 16.4 +/− 7.7, and 20.3 +/− 11.8 for the three sessions consecutively. At enrollment, mean Mini Mental State Examination (MMSE) scores were 28/30 for PD patients and 29.7/30 for control subjects. The protocol was approved by the local Institutional Review Board, and written informed consent was obtained from all participants.
Procedures
Each session included administration of the UPDRS by a movement disorders neurologist, 6-L-[18F]-fluorodopa (FDOPA) PET scanning, and motor performance testing (S.D.). A total of 19 PD patients and 11 control subjects completed three study sessions; the remaining 7 PD patients completed two sessions. The mean interval between sessions was 1.8 years, and the mean interval between PET imaging and motor testing was 0.11 years. Baseline motor performance testing was conducted off anti-Parkinson medication; subsequent testing was conducted without alteration of PD patients' usual medication regimen.
PET acquisition and quantification
PET data consisted of 90-minute, three-dimensional dynamic PET images acquired on the same Advance scanner (General Electric Medical Systems, Waukesha, WI) after intravenous administration of 204–284 MBq (5.5–7.7 mCi) of FDOPA (Brown, et al., 1999). PD patients were off medication for 18 hours prior to scanning, and all subjects ingested 100 mg of carbidopa 30 minutes before radiotracer injection. A 124-section axial spoiled-gradient recalled (SPGR) volume (repetition time 29 ms, echo time 13 ms, flip angle = 35 degrees, FOV = 220 mm; slice thickness = 1.2 mm), obtained on a 1.5-T magnetic resonance (MRI) scanner (General Electric Medical Systems, Waukesha, WI), was available for coregistration to PET in all but four subjects.
Within-subject realignment of PET sum images (time frames from 5 to 30 minutes post-injection) to MRI using FSL/FLIRT (http://www.fmrib.ox.ac.uk/analysis/research/flirt/) was followed by spatial normalization of the MRI to Montreal Neurological Institute (MNI) space, and application of these spatial transforms to the PET sum image and its aligned dynamic frames. As part of the normalization process, the PET and MRI volumes were resampled to 2-mm cubic voxels, and then visually inspected for misregistration. Using an in-house software package (http://brainimaging.waisman.wisc.edu/~oakes/spam), five volumes of interest (VOIs), encompassing each putamen, and head and body of the caudate nucleus, and an occipital cortex reference region of 900–1000 voxels, were manually drawn over each subject's normalized MRI scan by one rater (C.G.). This technique allowed for individual differences in the location of subcortical structures, while applying the same subject-specific VOIs to repeat PET scans. For the four subjects with missing MRI data, VOIs were drawn on a normalized PET sum image. Once drawn, all VOI volumes were compared, and cases that represented outliers (±2 standard deviations from mean volume) were redrawn if inaccurate. Average radiotracer influx (Kocc) values for each VOI were computed from 30- to 90-minute PET frames using a standard multiple-time graphical analysis method (MTGA) with occipital cortex (tissue) input function (Patlak & Blasberg, 1985). For statistical analysis, caudate and putamen Kocc values were averaged between brain hemispheres.
Motor testing
The motor testing protocol used computer-cued tasks to measure simple reaction time, maximum instantaneous movement velocity, time from movement initiation to maximum movement velocity, pinch strength, and fine force control, for each hand. Intraoral force transducers were used to measure tongue strength.
Reaction and movement times
Subjects were seated comfortably, facing an apparatus with a depressible base and three elevated targets 3, 6, and 9 inches (approximately 7.6, 15.2, and 22.9 cm) from the base. An accelerometer was attached to the back of the active hand; the subject placed this hand on the base (which, when depressed, completed a circuit) and then, when cued by a tone, touched the first target as quickly as possible. The cuing tone, generated at random intervals by a computer, was repeated three times for each target. For each movement, simple reaction time was recorded from the cuing tone to interruption of the base circuit, and an average of these nine trials (RT) for each hand, was used for analysis. Accelerometer output was used to derive time from interruption of the base circuit to peak velocity (movement time, MT), and maximum instantaneous movement velocity. This method of measuring MT was chosen because at times tremor and overshoot made it difficult to reliably determine from the accelerometer signal when the target was reached. The average MT of the three trials reaching to the most distant target (MT9) was used for analysis. The VMx for the 3-inch target (~7.6 cm) was subtracted from that for the 9-inch (~22.9 cm) target to generate an index of peak velocity scaling (VMx93).
Because of bradykinesia, PD patients are expected to take a longer time to generate peak movement velocity (longer MT) and, because of abnormalities in motor planning, PD patients are expected to be unable to scale reach speed to anticipated movement length, and therefore to have smaller values for VMx93.
Maximum force measurements
Isometric pinch grip force (PinMx) was measured by asking the subject to grasp and hold with maximum effort a force transducer between the pad of the thumb and side of the index finger; at steady state, the force generated in grams was recorded (Wing, 1988). A lingual strain gauge was used to measure maximum tongue protrusion force (TongMx) according to techniques described previously (Barlow & Abbs, 1984).
Force control measures
While gripping the pinch force transducer, subjects were asked to generate and maintain a target force of 200 g for 5 seconds. A cursor representing the target force level was displayed on an oscilloscope at slow sweep speed (500 ms/division); a cursor reflecting the subject's force output was provided and subjects were directed to match the target line as closely as possible. Three seconds of force signal at steady state (after at least 1 second on task) was computer digitized (300 samples/s), and the mean and standard deviation of these 900 samples recorded as pinch force control (Pin200) and standard deviation (Pin200SD).
Statistical analysis
We analyzed the motor data according to side of hand dominance, rather than to side of symptom onset (in PD subjects), so that limbs with a similar level of dexterity were compared between groups. To evaluate the relationship between repeated PET, motor performance, and UPDRS measures, we used general linear mixed effects models that are explicitly designed for modeling longitudinal data (http://www.math.mcgill.ca/keith/surfstat). The main difference between these longitudinal and cross-sectional models is that they specifically incorporate the dependence of repeated measurements taken in the same individual. For each subject, the number of variables entered into the model is equal to the number of observations/sessions. Each model has both fixed effect (age, group, gender, motor variables) and a random effect (subject) terms, with associated error. Within-subject variability is typically smaller than the between-subject variability. Correction for multiple comparisons is not required in this statistical approach because each set of variables is evaluated in a separate model. The three types of such models that were used are described below.
Type 1: Motor Performance Variable= 1 + Group + Age + Gender + Random (subject) + I
To evaluate the effects of diagnostic group on motor performance, a separate mixed effects model was constructed for each motor measurement in which this measurement was regressed against group (PD versus control), age, and gender covariates. A contrast was then applied to yield a t-statistic and P-value describing the significance level for each covariate's contribution to the model.
Type 2: Motor or PET variable = 1 + Group + Gender + Time+ Time*Group + Random (subject) + I
We hypothesized that even in treated PD patients, motor variables would change at a greater rate than in control subjects. To test this hypothesis, we constructed a separate model for each motor performance and PET measurement, in which this dependent variable was regressed against group, gender, time from session 1, and a time-by-group interaction term. If the time-by-group interaction is significant, the rate of change in the disease group is different than would occur due to normal aging. Age was not included in these models because it is collinear with the time variable.
Type 3: PET variable or UPDRS total score = 1 + Motor variable + Age + Gender + Random (subject) + I
To test the hypothesis that the motor performance measures would be related to striatal FDOPA uptake in the PD group, three mixed effects models were constructed for each motor performance variable, with age and gender as covariates. The dependent variables for each of the three models were mean caudate nucleus Kocc, mean putamen Kocc, and total UPDRS score. Significant relationships were plotted in Matlab (version R2009a). If these relationships were based on outlying values, these values were replaced with the mean plus or minus twice the standard deviation.
Results
At baseline, the PD and control groups did not differ significantly in gender, hand dominance, or age, but did differ in years of education (PD mean, 14.3 years vs. control mean, 17.7 years; P = 0.02). Mean baseline Kocc (±SD) for caudate nuclei and putamina were 0.012 (±0.001)/0.014 (±0.001) min−1 in control subjects and 0.010 (±0.002)/0.008 (±0.002).
Group effects
Parkinson's disease patients had prolonged non-dominant hand reaction time (RT), prolonged bilateral hand movement time (MT9), and lower dominant hand force control (Pin200) than control subjects (Table 1). Tongue strength (TongMx) was lower in PD, while hand strength was equal to controls. Age had highly significant effects on several motor performance variables, including reaction time (t[df]>3.4[104], P <0.0001), peak velocity scaling (t[df]<−2.6[104], P <0.01), movement time (t[df]<2.0[104], P <0.05), maximum pinch (t[df]<−2.7[104], P <0.005) and tongue strength (t[df]<−3.5[104], P <0.0005). Age-by-group interactions were present for reaction time and movement time (|t|[df] > 1.8 [104], P < 0.05).
Table 1.
Group differences and baseline values
| RT (ms | VMx93 (m/s) | MT9 (ms) | Pin200 (g) | Pin200SD (g) | PinMx (kg) | TongMx (kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | ND | D | ND | D | ND | D | ND | D | ND | D | ND | ||
| Baseline mean (SD) | |||||||||||||
| Control | 246 (46) | 240 (47) | 0.63 (.22) | 0.56 (.14) | 180 (44) | 169 (70) | 206 (7.5) | 204 (5.4) | 7 | 5 | 6.1 (1.5) | 6.2 (1.6) | 1.3 (0.5) |
| PD | 236 (67) | 257 (73) | 0.62 (.21) | 0.61 (.21) | 222 (106) | 234 (79) | 199 (9) | 202 (13) | 11 | 8 | 6.9 (2.0) | 6.9 (2.0) | 0.9 (0.3) |
| Group Effect | |||||||||||||
| t | 1.20 | 2.34 | 0.12 | −1.00 | 2.70 | −3.20 | −2.40 | −0.70 | 0.80 | 1.00 | 0.53 | 0.37 | −3.31 |
| P | 0.12 | 0.01* | 0.45 | 0.15 | 0.004** | <0.0001*** | 0.01* | 0.24 | 0.20 | 0.13 | 0.47 | 0.35 | <0.0001*** |
Model type 1: Motor variable = 1 + Group + Age + Gender ± Age*Group + Random(Subject) + I. Outcome variables are listed across the top. After building each model, a contrast for the covariate of interest (group, age, or gender) was applied to derive the t-statistics and P-values; degrees of freedom = 104.
P = 0.01–0.05
P = 0.001–0.009
P <0.001.
Abbreviations and variables: D, dominant hand; MT9, time from movement onset to peak velocity when reaching to the 9-inch target; ND, non-dominant hand; Pin200, mean force accuracy for 200 g target; Pin200SD, measured standard deviation in Pin200; PinMx, maximum pinch strength; RT, reaction time; TongMx, maximum tongue protrusion strength; VMx93, maximum instantaneous movement velocity scaling to reach distance (peak velocity for 9-inch reach [~22.9 cm] – peak velocity for 3-inch reach [~7.6 cm]).
Time effects
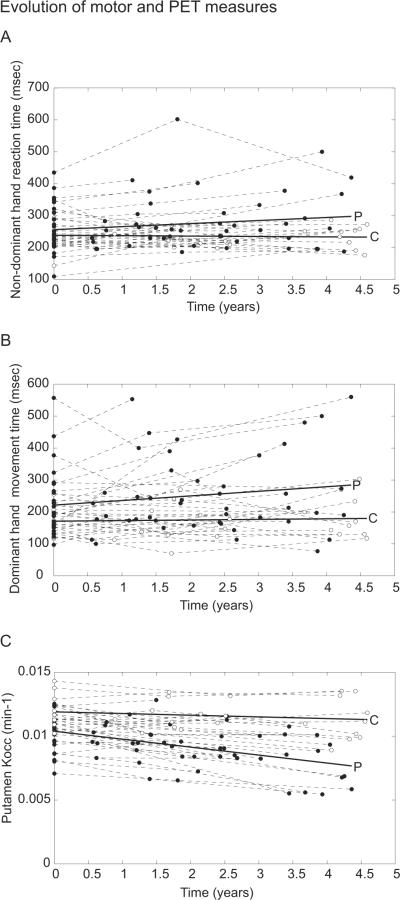
Time-by-group interactions, indicating that the rate of change in PD was significantly greater than would be expected due to aging, were present for non-dominant hand reaction time (t [df]=2.08 [104], P < 0.05) and dominant hand MT (t [df] = 1.8[104], P < 0.05). However, the significance of the interaction term was greatest when for striatal FDOPA Kocc was modeled as the dependent variable (t [df] < −4.0 [104], P < 3×10−5). Longitudinal changes in movement time, reaction time, and FDOPA uptake over the study interval are presented in Figure 1. The effect of time on UPDRS scores was not significant in the PD subject group (t [df] = 1.3[68], P = 0.13).
Figure 1. Evolution of motor and PET measures.
Non-dominant hand reaction time (A), dominant hand movement time (B), and mean putamen FDOPA Kocc (C) for PD patients (filled circles, regression line indicated by P) and control subjects (unfilled circles, regression line indicated by C) are plotted against timing of visits over the study interval. Within-subject measurements are connected by dashed lines. The time effect is of greatest significance for putamen Kocc (t [df] =-8.7 [104], P<10−13) in contrast to motor measures (t [df] >1.8 [104], P< 0.04).
Relationship of motor performance measures to PET
We then hypothesized that the mixed effects models, which are designed to evaluate repeated within-subject measurements, would show relationships between the PET and motor variables. Non-dominant hand reaction time (RT) and dominant hand movement time (MT) were inversely related to caudate nucleus FDOPA uptake (Table 2, Figure 2). Higher caudate nucleus and putamen Kocc was related to greater increases in dominant hand peak movement velocity in response to increasing reach distance (VMx93), and to higher mean target forces during the fine force control task. Greater variation in non-dominant hand fine force control (Pin200SD) was also related to lower striatal FDOPA uptake. The incorporation of disease duration instead of age into the model did not significantly improve the significance of these relationships, although striatal FDOPA Kocc, as expected, was strongly related to disease duration (t[df]<−7.8[67], P<1×10−11).
Table 2.
Relationship of imaging and clinical measures to motor performance in PD (model type 3)
| Motor variable: | RT (ms) | VMx93 (m/s) | MT9 (m/s) | Pin200 (g) | Pin200SD (g) | TongMx (kg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome: | D | ND | D | ND | D | ND | D | ND | D | ND | |
| Caudate Kocc (min−1) | |||||||||||
| t | −1.27 | −1.90 | −3.27 | −2.18 | −2.04 | −1.49 | 4.67 | 1.61 | −0.89 | −2.18 | 1.10 |
| P | 0.10 | 0.03* | 0.001** | 0.02* | 0.03* | 0.07 | 0.001** | 0.06 | 0.18 | 0.01* | 0.13 |
| Putamen Kocc (min−1) | |||||||||||
| t | −0.45 | −1.20 | −3.10 | −1.52 | 0.79 | −1.47 | 3.8 | 1.05 | −0.15 | −1.90 | 0.90 |
| P | 0.32 | 0.11 | 0.001** | 0.07 | 0.22 | 0.07 | 0.001** | 0.15 | 0.43 | 0.02* | 0.18 |
| UPDRS Total | |||||||||||
| t | 0.82 | −0.67 | 0.14 | −1.80 | 0.12 | 0.28 | 1.20 | 0.06 | 1.00 | 2.90 | −2.05 |
| P | 0.20 | 0.25 | 0.44 | 0.04* | 0.12 | 0.39 | 0.11 | 0.47 | 0.16 | 0.002** | 0.02* |
Model type 3: Outcome = 1 + Motor Variable + Age + Gender + random(Subject) + I; degrees of freedom = 67 (PD patients only). Dependent variables (left side of equation) are mean caudate FDOPA Kocc (averaged between brain hemispheres), mean putamen FDOPA Kocc, and total UPDRS scores. Motor variables (right side of the equation), modeled separately for the dominant (D) and non-dominant (ND) hand, were RT, VMx93, MT9, Pin200, and Pin200SD. The significance of contribution of each motor variable to the model is tested using a contrast that yields a t-statistic and P-value.
P = 0.01–0.05
P = 0.001–0.009
P < 0.001.
Abbreviations and variables: D, dominant hand; FDOPA, [18F]fluoro-L-dopa; Kocc, uptake of FDOPA; MT9, movement time to achieve peak velocity during the longest movement; ND, non-dominant hand; Pin200, mean force accuracy for 200 g target (Pin200); Pin200SD, standard deviation in force accuracy at 200 g; RT, simple reaction time; TongMx, maximum tongue protrusion strength; UPDRS, Unified Parkinson's Disease Rating Scale total score; VMx93, maximum instantaneous movement velocity scaling to reach distance (peak velocity for 9-inch reach [~22.9 cm] – peak velocity for 3-inch reach [~7.6 cm])
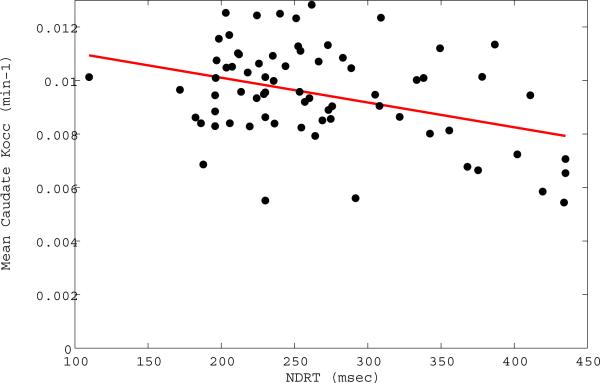
Figure 2. Reaction time versus caudate nucleus FDOPA uptake.
In the Parkinson's group, non-dominant hand reaction time (NDRT) was significantly related to lower caudate nucleus Kocc averaged between brain hemispheres (t [df] =−1.90 [67], P=0.03).
When UPDRS scores, rather than striatal FDOPA uptake, were modeled as the dependent variable, greater variability in force levels (Pin200SD), lower tongue strength (TongMx), and impaired velocity scaling (VMx93) predicted higher (more impaired) UPDRS scores (Figure 3). UPDRS scores were not significant contributors to models of caudate or putamen FDOPA Kocc. The effect of age was significant (t [df] >3 [67], P < 0.005) in all of the models, and tended to overwhelm the effects of other covariates.
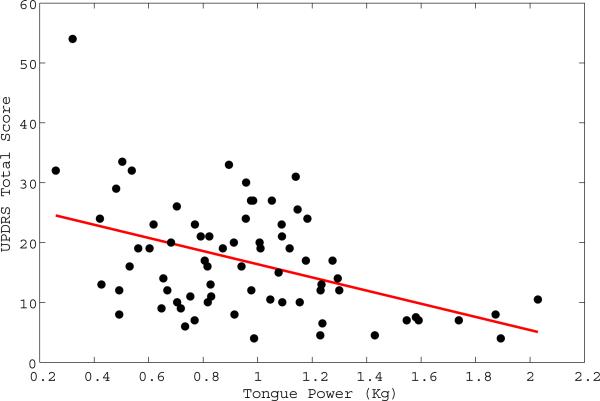
Figure 3. Tongue power versus UPDRS total score.
In the Parkinson's group, lower tongue strength was significantly related to higher (more impaired) UPDRS scores (t [df] =−2.05, [67], P=0.02).
Discussion
This study presents a retrospective analysis of longitudinal motor performance, clinical, and FDOPA PET data. We used mixed effects models to separate the effects of demographic covariates from disease effects, but could not control for the effects of pharmacotherapy since PD subjects' medication regimens were not altered for motor testing, and precise medication doses were not recorded. Consistent with previous investigations, we found disease (group) effects on reaction time (Hallett & Khoshbin, 1980; Montgomery, et al., 1991), movement time, hand fine force control (Stelmach, et al., 1989), and tongue strength (O'Day, et al., 2005; Solomon, et al., 1995). Among the motor performance variables tested, the most robust disease indicators were prolonged movement time and reduced tongue strength. Therefore, these measures may be useful as disease biomarkers.
Disease group differences were overshadowed by the significant effects of age on motor performance, which were accentuated in the PD group. Evidence of an interaction between aging and clinical symptom severity in PD is abundant, but has not been specifically quantified using motor performance testing. In PD, advanced age is a risk for faster progression of motor disability (Diederich, et al., 2003), gait and postural impairment (Levy, et al., 2005), dementia (Aarsland & Kurz, 2009), and failure to benefit from standard therapies (Russmann, et al., 2004).
Time-by-group interactions, indicating a greater rate of change in the PD group than would occur due to normal aging, were observed for simple reaction time and movement time. Rate of change in the motor performance measures was subtle in comparison to the rate of decline in striatal FDOPA uptake; however, ongoing treatment may have reduced the significance level for motor abnormalities in the PD group. Using a complex motor task during [15O]H2O PET, Carbon et al. showed movement onset and velocity to be relatively prolonged in PD and to lengthen over time, and to correspond to increased blood flow in the right dorsal premotor and dorsolateral prefrontal cortex (Carbon, et al., 2007).
Non-human primate studies have shown that parallel networks connect distinct striatal regions with frontal cortical regions, and prefrontal cortex with the cerebellum (Alexander, et al., 1986). These functional networks participate in motor planning, attention, motivation, timing, and adjustment of ongoing movements (Durston, et al.). They are essential to the initiation of accurate preprogrammed hand movements (Desmurget, et al., 2003), and to regulation of ongoing movements through submovements (Tunik, et al., 2009). PD subjects underestimate the required force to accomplish motor tasks, and require additional adjustments to ongoing movements in comparison to controls (Hallett & Khoshbin, 1980; Hallett & Marsden, 1979). We found that indices of motor planning (velocity scaling), force estimation (mean fine force accuracy), and submovements (standard deviation in fine force accuracy) were related to striatal FDOPA uptake. These findings help to confirm that insufficient nigrostriatal dopamine input contributes the diverse motor control problems observed in PD.
The caudate nucleus has been considered part of the “spatial” or “oculomotor” circuit, receiving projections for the dorsolateral prefrontal cortex and posterior parietal cortex. We found that prolonged reaction time and movement time were each related to lower caudate nucleus FDOPA uptake. In a study of healthy elderly subjects, lower dopamine transporter binding in either caudate nucleus or putamen was equally correlated with longer simple reaction time (van Dyck and Avery, 2008). Since FDOPA uptake declines throughout the striatum in PD, with relative preservation of anterior and ventral regions, caudate nucleus uptake may be an indicator of the overall severity of dopamine synthesis and storage insufficiency (Bruck, et al., 2006). However, animal studies suggest that lesions of the dorsomedial striatum selectively prolong simple reaction time, possibly due to effects on attentional control (Hauber & Schmidt, 1994). Caudate nucleus Kocc is correlated with performance in attention-demanding tasks such as the Stroop interference task (Rinne, et al., 2000). Huntington's disease patients, who show various oculomotor abnormalities attributed to this circuit, have prolonged saccadic latency (i.e. visual reaction time) (Lasker & Zee, 1997). FDOPA uptake in the right (non dominant hemisphere) caudate nucleus has also been correlated with performance of bimanual tasks (de la Fuente-Fernandez, et al., 2000).
In our data, greater standard deviation in non-dominant hand fine force control and reduced maximum tongue strength predicted greater impairment on the UPDRS scale. These results are particularly encouraging, because effective interventions are available to improve tongue and pharyngeal function both in aging and in PD (Connor, et al., 2009; El Sharkawi, et al., 2002), and exercise programs are known to improve UPDRS scores (Nocera, et al., 2009; Yousefi, et al., 2009); occupational therapy to improve fine motor control might also improve function in activities of daily living. Tongue strength can be improved by treatments that improve motor function in PD, specifically subthalamic nucleus deep brain stimulation (Gentil, et al., 1999).
Limitations
The retrospective aspect of this data analysis produced significant limitations. To determine the severity of disease-related motor changes, motor testing should have been conducted while subjects were off anti-Parkinson medication for at least 12 hours. Also, since the precise doses of medication were not known for all participants, levodopa-equivalent doses could not be incorporated as covariates into the statistical models. Therefore, any relationships between PET and motor function discovered in this exploratory analysis should be interpreted with caution. Because the disease group and control group were not ideally matched for years of education, we cannot exclude a contribution of education to motor performance differences between the groups. All cuing and recording of results from the motor performance testing was automated, but those administering the tests (S.D.) were not blinded to the clinical condition of research subjects. There was also variability in the frequency of administration of the motor test battery, with some PD patients being tested multiple times; control subject were tested a maximum of three times. Practice effects, however, would be expected to reduce the difference between groups.
Acknowledgements
This work was supported with use of facilities at the William S. Middleton Memorial Veterans Hospital Geriatric Research Education and Clinical Center and the Waisman Laboratory for Brain Imaging and Behavior, Madison, WI, USA. The manuscript was edited by Gundega Korsts, Science Editor, Madison, WI.
Funding This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development Service [grant to C.G]; National Institutes of Health [grant number 1R29NS31612 to D.B] and University of Wisconsin Institute for Clinical and Translational Research, funded through a National Institutes of Health Clinical and Translational Science Award, [grant number 1UL1RR025011 to C.G.].
Abbreviations
- D
dominant side
- FDOPA
[18F]fluoro-L-dopa
- MRI
magnetic resonance imaging
- ND
non-dominant side
- PD
Parkinson's disease
- PET
positron emission tomography
- UPDRS
Unified Parkinson's Disease Rating Scale
- VOI
volume of interest
References
- Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2009 doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Abbs JH. Orofacial fine motor control impairments in congenital spasticity: evidence against hypertonus-related performance deficits. Neurology. 1984;34(2):145–150. doi: 10.1212/wnl.34.2.145. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brown WD, Taylor MD, Roberts AD, Oakes TR, Schueller MJ, Holden JE, et al. FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology. 1999;53(6):1212–1218. doi: 10.1212/wnl.53.6.1212. [DOI] [PubMed] [Google Scholar]
- Bruck A, Aalto S, Nurmi E, Vahlberg T, Bergman J, Rinne JO. Striatal subregional 6-[18F]fluoro-L-dopa uptake in early Parkinson's disease: a two-year follow-up study. Mov Disord. 2006;21(7):958–963. doi: 10.1002/mds.20855. [DOI] [PubMed] [Google Scholar]
- Carbon M, Felice Ghilardi M, Dhawan V, Eidelberg D. Correlates of movement initiation and velocity in Parkinson's disease: A longitudinal PET study. Neuroimage. 2007;34(1):361–370. doi: 10.1016/j.neuroimage.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52(3):732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. J Gerontol A Biol Sci Med Sci. 1996;51(5):M247–250. doi: 10.1093/gerona/51a.5.m247. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Kishore A, Calne DB, Ruth TJ, Stoessl AJ. Nigrostriatal dopamine system and motor lateralization. Behav Brain Res. 2000;112(1–2):63–68. doi: 10.1016/s0166-4328(00)00165-0. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Exp Brain Res. 2003;153(2):197–209. doi: 10.1007/s00221-003-1593-3. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Moore CG, Leurgans SE, Chmura TA, Goetz CG. Parkinson disease with old-age onset: a comparative study with subjects with middle-age onset. Arch Neurol. 2003;60(4):529–533. doi: 10.1001/archneur.60.4.529. [DOI] [PubMed] [Google Scholar]
- Draper IT, Johns RJ. The Disordered Movement in Parkinsonism and the Effect of Drug Treatment. Bull Johns Hopkins Hosp. 1964;115:465–480. [PubMed] [Google Scholar]
- Durston S, Belle JV, Zeeuw PD. Differentiating Frontostriatal and Fronto-Cerebellar Circuits in Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- El Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, et al. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT): a pilot study. J Neurol Neurosurg Psychiatry. 2002;72(1):31–36. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Risk tables for parkinsonism and Parkinson's disease. J Clin Epidemiol. 2002;55(1):25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Teravainen H, Calne DB. Reaction time in Parkinson's disease. Brain. 1981;104(Pt 1):167–186. doi: 10.1093/brain/104.1.167. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Members UP. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinsons Disease. Vol. 2. McMillan Healthcare Information; Florham Park, N.J.: 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Flowers K. Ballistic and corrective movements on an aiming task. Intention tremor and parkinsonian movement disorders compared. Neurology. 1975;25(5):413–421. doi: 10.1212/wnl.25.5.413. [DOI] [PubMed] [Google Scholar]
- Gentil M, Tournier CL, Pollak P, Benabid AL. Effect of bilateral subthalamic nucleus stimulation and dopatherapy on oral control in Parkinson's disease. Eur Neurol. 1999;42(3):136–140. doi: 10.1159/000008087. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103(2):301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Hauber W, Schmidt WJ. Differential effects of lesions of the dorsomedial and dorsolateral caudate-putamen on reaction time performance in rats. Behav Brain Res. 1994;60(2):211–215. doi: 10.1016/0166-4328(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Lasker AG, Zee DS. Ocular motor abnormalities in Huntington's disease. Vision Res. 1997;37(24):3639–3645. doi: 10.1016/S0042-6989(96)00169-1. [DOI] [PubMed] [Google Scholar]
- Levy G, Louis ED, Cote L, Perez M, Mejia-Santana H, Andrews H, et al. Contribution of aging to the severity of different motor signs in Parkinson disease. Arch Neurol. 2005;62(3):467–472. doi: 10.1001/archneur.62.3.467. [DOI] [PubMed] [Google Scholar]
- Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, et al. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson's disease progression. Neurology. 2001;57(11):2089–2094. doi: 10.1212/wnl.57.11.2089. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Jr., Nuessen J, Gorman DS. Reaction time and movement velocity abnormalities in Parkinson's disease under different task conditions. Neurology. 1991;41(9):1476–1481. doi: 10.1212/wnl.41.9.1476. [DOI] [PubMed] [Google Scholar]
- Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson's disease. Brain. 1996a;119(Pt 2):585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- Morrish PK, Sawle GV, Brooks DJ. Regional changes in [18F]dopa metabolism in the striatum in Parkinson's disease. Brain. 1996b;119(Pt 6):2097–2103. doi: 10.1093/brain/119.6.2097. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Saito H, Kogure K, Hatazawa J, Itoh M, Fujiwara T, et al. 6-[18F]fluorodopa metabolism in patients with hemiparkinsonism studied by positron emission tomography. J Neurol Sci. 1993;115(2):136–143. doi: 10.1016/0022-510x(93)90216-l. [DOI] [PubMed] [Google Scholar]
- Nocera J, Horvat M, Ray CT. Effects of home-based exercise on postural control and sensory organization in individuals with Parkinson disease. Parkinsonism Relat Disord. 2009 doi: 10.1016/j.parkreldis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Day C, Frank E, Montgomery A, Nichols M, McDade H. Repeated tongue and hand strength measurements in normal adults and individuals with Parkinson's disease. Int J Orofacial Myology. 2005;31:15–25. [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Pirker W. Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord. 2003;18(Suppl 7):S43–51. doi: 10.1002/mds.10579. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M, et al. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]fluorodopa positron emission tomographic study. Arch Neurol. 2000;57(4):470–475. doi: 10.1001/archneur.57.4.470. [DOI] [PubMed] [Google Scholar]
- Russmann H, Ghika J, Villemure JG, Robert B, Bogousslavsky J, Burkhard PR, et al. Subthalamic nucleus deep brain stimulation in Parkinson disease patients over age 70 years. Neurology. 2004;63(10):1952–1954. doi: 10.1212/01.wnl.0000144198.26309.d8. [DOI] [PubMed] [Google Scholar]
- Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea-Ponce Y, et al. Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol. 1995;38(4):589–598. doi: 10.1002/ana.410380407. [DOI] [PubMed] [Google Scholar]
- Solomon N, Lorell D, Robin D, Rodnitzky R, Luscheni E. Tongue strength and endurance in mild to moderate Parkinson's disease. Journal of medical Speech-Language Pathology. 1995;3:15–26. [Google Scholar]
- Stelmach GE, Teasdale N, Phillips J, Worringham CJ. Force production characteristics in Parkinson's disease. Exp Brain Res. 1989;76(1):165–172. doi: 10.1007/BF00253633. [DOI] [PubMed] [Google Scholar]
- Tunik E, Houk JC, Grafton ST. Basal ganglia contribution to the initiation of corrective submovements. Neuroimage. 2009;47(4):1757–1766. doi: 10.1016/j.neuroimage.2009.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyck CH, Avery RA, MacAvoy MG, Marek KL, Quinlan DM, Baldwin RM, et al. Striatal dopamine transporters correlate with simple reaction time in elderly subjects. Neurobiology of Aging. 2008;29:1237–1246. doi: 10.1016/j.neurobiolaging.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets FJ, Schulzer M, Calne DB, Snow BJ. Which clinical sign of Parkinson's disease best reflects the nigrostriatal lesion? Ann Neurol. 1997;41(1):58–64. doi: 10.1002/ana.410410111. [DOI] [PubMed] [Google Scholar]
- Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54(1):93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- Yousefi B, Tadibi V, Khoei AF, Montazeri A. Exercise therapy, quality of life, and activities of daily living in patients with Parkinson disease: a small scale quasi-randomised trial. Trials. 2009;10:67. doi: 10.1186/1745-6215-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yust-Katz S, Tesler D, Treves TA, Melamed E, Djaldetti R. Handedness as a predictor of side of onset of Parkinson's disease. Parkinsonism Relat Disord. 2008;14(8):633–635. doi: 10.1016/j.parkreldis.2008.01.017. [DOI] [PubMed] [Google Scholar]