Abstract
Introduction
To quantify transmembrane transport of dipeptides by PepT1, passive uptake (non-PepT1 mediated) must be subtracted from total (measured) uptake. Three methods have been described to estimate passive uptake: perform experiments at cold temperatures, inhibit target dipeptide uptake with greater concentration of a second dipeptide, or use modified Michaelis-Menten kinetics. We hypothesized that performing uptake experiments at pH (8.0) would estimate passive uptake accurately, because PepT1 requires a proton gradient. Our aim was to determine the most accurate method to estimate passive uptake.
Methods
Caco-2 cells were incubated with various concentrations of glycyl-sarcosine (gly-sar) at pH 6.0 and at 37 °C to measure total uptake. Passive uptake was estimated: 1) by incubating Caco-2 cells with varying concentrations of gly-sar at 4 °C, 2) in the presence of 50 mM glycyl-leucine, 3) in solution at pH 8.0, or 4) using modified Michaelis-Menten kinetics. PepT1-mediated uptake was calculated by subtracting passive uptake from total uptake. Km, Vmax, and % gly-sar transported by PepT1 were calculated and compared.
Results
Km, Vmax, and % gly-sar transported by PepT1 varied from 0.7-2.4 mM, 8.4-21.0 nmol/mg protein/10 min, and 69%-87%, respectively. Uptakes calculated with cold, 50 mM gly-leu and using modified Michaelis-Menten kinetics were similar but differed significantly from uptake at pH 8.0 (p<0.001).
Conclusions
Estimating passive uptake at pH 8.0 does not appear to be accurate. Measuring uptake at cold temperatures or in the presence of a greater concentration of a second dipeptide, and confirming results with modified Michaelis-Menten kinetics is recommended.
Keywords: peptide, PepT1, Caco-2, dipeptide transport, passive dipeptide transport, transporter-mediated dipeptide transport
INTRODUCTION
In the study of intestinal nutrient absorption and transporter physiology, uptake of a specific substance by the transporter of interest can be difficult to measure accurately. For many substances, especially hexoses and short peptides, there is a transporter-mediated, saturable component (active) and a non-transporter-mediated, nonsaturable component of absorption (passive) (1, 2). The saturable component is determined by the kinetic parameters of the membrane transporter and represents the amount of absorption that is mediated by the transporter, either as an active, energy-requiring process or as a “facilitated” transport process. Curves corresponding to simple, saturable, transporter-mediated uptake exhibit asymptotic behavior and are described accurately by Michaelis-Menten kinetics (1, 3, 4).
The nonsaturable or “passive” component of absorption is directly proportional to the concentration of the substance and increases linearly without plateau as the extracellular concentration of a substance increases (1, 2, 5). In laboratory experiments, this nonsaturable component can be attributed not only to what is absorbed in a nontransporter-mediated manner (paracellular flow, solvent drag, etc.) across the epithelial lining but also to the amount of substance of interest that is adsorbed onto the tissue being tested or adherent to the tissue in which the assay is being performed. Therefore, it is imperative to account for passive uptake in order to study accurately the physiology of the transporter without the “noise” of passive uptake. Diagrammatic representations of nonsaturable or “passive” absorption are usually straight lines with an origin at zero that exhibit a linear increase in absorption as the concentration increases.
In most experiments, total uptake is the value that is measured, and the passive component is subtracted from this value resulting in the calculated, “transporter-mediated” component of absorption (transporter-mediated = total – passive) (1, 2, 4-6). To these transporter-mediated values, biologic formulas such as the Michaelis-Menten equation can be applied and important kinetic descriptors (i.e. Km and Vmax) determined.
In our laboratory we have studied previously the hexose transporters SGLT1 and GLUT2. Our method of determining specifically the active transport component of uptake exploits the stereospecificity of these transporters. We use [14C]d-glucose to determine total uptake and [3H]l-glucose, which is not transported by the stereospecific transporters SGLT1 and GLUT2, to determine passive uptake. Subtracting passive uptake from total uptake allows accurate calculation of the amount of glucose actually transported by SGLT1 and GLUT2 (7-11).
As we have expanded the scope of our laboratory to study peptide absorption, we found that determining the transporter-mediated proportion of peptide transport is not as straightforward. PepT1 (human gene SLC15A1) is the putative transporter of the bulk of dietary protein/nitrogen in the form of all 400 di- and all 8,000 tripeptides from the intestinal lumen across the apical membrane into the enterocyte (12, 13). PepT1 is also responsible for the absorption of many peptidimetic drugs, such as angiotensin-converting enzyme inhibitors (14, 15), ß-lactam antibiotics (16), and antivirals such as oseltamivir (17), acyclovir, valganciclovir (18), and azidothymidine (AZT) (19).
The wide variety of compounds transported by this protein presents a unique challenge to its study. Unlike the glucose transport system of SGLT1 and GLUT2, stereospecificity cannot be exploited, and thus, differentially radio-labeled stereoisomers cannot be used. Reports in the literature are not unanimous and employ typically one of three methods to correct total uptake for passive uptake in the study of PepT1-mediated transport: 1) perform the experiment at near-freezing temperatures in order to “inactivate” the transporter (20, 21), 2) inhibit competitively the uptake of the compound of interest with a markedly greater concentration of a second substrate for which the transporter has a greater affinity (lesser Km) (2, 6, 15, 22-24), and 3) use nonlinear regression analysis of total uptake with modified Michaelis-Menten kinetics (1, 6, 25-28). Because PepT1 relies on a proton gradient and functions most efficiently at a pH of 6.0, we hypothesized that passive uptake might also be estimated by increasing the pH of test solutions to a pH of 8.0 (29).
To our knowledge, there has not been a direct, rigorous evaluation and comparison of these four methods to estimate passive uptake of peptides. We devised a study using Caco-2 cells and the model dipeptide glycyl-sarcosine (gly-sar). The Caco-2 cell line is a well-characterized, human-derived, cell culture model of intestinal epithelium known to express PepT1; Caco-2 cells are used frequently in studies of nutrient absorption and pharmacokinetics (1-3, 6, 14, 15, 20-22, 24-28, 30-32). Gly-Sar is a model, hydrolysis-resistant dipeptide not found in nature and is used frequently as a substrate to study PepT1-mediated transport (13). The Km of PepT1 for gly-sar has been reported to be 0.35-3.8 mM depending on experimental conditions (3-6, 30-34). Additionally, in transfection studies comparing PepT1 and non-PepT1 containing cells, PepT1 is responsible for approximately 80% of the absorbed gly-sar at concentrations less than its Km (1, 4, 5, 29).
The aim of our study was to determine the best method to estimate passive uptake of dipeptides and thereby calculate accurately the PepT1-mediated transport in Caco-2 cells. An appropriate method per our criteria would produce a linear passive curve without a plateau that would correct total uptake resulting in an uptake curve consistent with a transporter-mediated process. Our hypothesis was that all four methods would be equivalent and yield similar results.
METHODS
Materials
Caco-2 cells were purchased from American Type Culture Collection at passage 18 (Manassas, VA), while the 24-well cell culture plates were from Corning Life Sciences (Lowell, MA) and T-75 culture flasks were from BD Biosciences (San Jose, CA). HEPES, MES, glycylsarcosine (gly-sar), and glycyl-leucine (gly-leu) were purchased from Sigma (St. Louis, MO). Hank’s balanced salt solution with calcium and magnesium (HBSS), minimum essential medium nonessential amino acids (NAA) (100x), and sodium pyruvate (100 mM) were purchased from Mediatech Cellgro Inc. (Manassas, VA). Phosphate-buffered saline (PBS), Dulbecco’s modified Eagle medium (DMEM), and penicillin10,000 units/ml-streptomycin 10,000 μg/ml (P-S) were purchased from Invitrogen Corp. (Carlsbad, CA). Fetal bovine serum (FBS) was obtained from PAA laboratories (Dartmouth, MA) and [14C]Glycyl-sarcosine ([14C]gly-sar) from Moravek Biochemicals (Brea, CA). BCA Protein Assay Kit (#23225) was purchased from Thermo Fisher Scientific Inc. (Rockford, IL). Solvable™ and Opti-Fluor were obtained from PerkinElmer (Waltham, MA) and high density polyethylene liquid scintillation vials (7 ml) from Research Products International Corp. (Mount Prospect, IL). Liquid scintillation counting was performed using a Beckman LS6000SC (Beckman Coulter Inc., Brea, CA).
Cell Culture
Caco-2 cells were maintained in DMEM (4.5 g/L d-glucose, 4 mM l-glutamine) supplemented with 20% v/v FBS, 1% sodium pyruvate, 1% NAA, and 1% P-S. Cells were cultured in T-75 flasks at 37°C in 95%O2/5%CO2 and 90% humidity and were replated in a 24 well plate when they reached >80% confluence. The culture medium was changed every 2-3 days as needed.
Uptake Studies
Caco-2 cells were plated at a density of 1 × 105 cells/cm2 in 24-well plates. After confluence was reached (~3 days), cells were maintained for 14 days to polarize under the conditions described previously. We performed scanning electron microscopy and showed that at 14 days, the cells had differentiated by forming tight junctions and microvilli at the apical membrane of the cells (unpublished data). Uptake studies were performed in triplicate on the same day and in the same passage and were repeated in triplicate (n=9 independent monolayers) on different days and in different passages. Passages 28-32 were used in this study. Media was always changed the day prior to the experiment.
Control monolayers (to measure total uptake) were washed gently with 500 μl of HBBS buffered with 10 mM HEPES and adjusted to pH 7.4 (HBSS 7.4) after which they were incubated in an additional 500 μl HBSS 7.4 for 10 min. The HBSS 7.4 was then aspirated, and 300 μl of HBSS buffered with 10 mM MES and adjusted to pH 6.0 containing 0.02, 0.1, 0.5, 1, 2.5, 5, 7.5, or 10 mM gly-sar labeled with 125 nCi/ml [14C]gly-sar was instilled. Monolayers were incubated with these test solutions for 10 min. Uptake of gly-sar was stopped by aspirating the test solution and rinsing and aspirating gently three times with 500 μl of ice-cold PBS (pH 7.4). All solutions and incubations were maintained at 37 °C unless noted otherwise. Cells were solubilized overnight with 300 μl/well of Solvable™; 200 μl was used for liquid scintillation counting in rigid polyethylene vials to which 4.5 ml of Opti-Fluor was added, and 10 μl × 2 was used for determining total protein/well in duplicate using the BCA Test Kit according to the manufacturer’s directions.
Passive uptake was determined at cold (~4 °C) temperature, in the presence of 50 mM gly-leu and in solution at pH 8.0. To test uptake at cold temperatures, monolayers were treated similar to control monolayers, except all solutions were ice-cold, and incubations took place on ice. To test uptake in the presence of a competitive inhibitor, 50 mM gly-leu was added to each gly-sar containing solution. Gly-Leu was chosen, because it is a hydrolysis-resistant dipeptide used frequently in PepT1 transport studies; moreover, gly-leu has a Km less than the Km of gly-sar (0.08 mM vs. 1.9 mM) (29). The gly-leu concentration of 50 mM was selected to saturate PepT1, so that it preferentially became saturated with gly-leu, and all [14C]gly-sar detected after lysing the cells was attributed to passive uptake. To test uptake at pH 8.0, the same procedure as for controls was followed except that the HBSS containing gly-sar was buffered at pH 8.0 with 10 mM HEPES (HBSS 8.0).
Data Analysis
Values for transporter-mediated uptake at each gly-sar concentration were determined by subtracting passive uptake (pH 8.0, 50 mM gly-leu, or cold temperature) from total (control) uptake. Using nonlinear regression (GraphPad Prism 4.03, GraphPad Software Inc., San Diego, CA), the best fit curve of the Michaelis-Menten equation was determined for transporter-mediated uptake values using equation 1:
| Equation 1. |
where Vo is the initial uptake velocity, Vmax is the maximal uptake velocity at saturating substrate concentrations, Km is a constant analogous to the Michaelis-Menten constant, and S is the substrate concentration.
To determine transporter-mediated and passive components of total uptake using only nonlinear regression, prior to subtracting passive uptake, the best fit curve for total uptake values was determined using a modified Michaelis-Menten equation reported previously (1, 6, 25-28):
| Equation 2. |
where Vo, Vmax, Km, and S are as described above, and Kd is the rate constant for the nonsaturable (passive) uptake. To display graphically the line for passive uptake and transporter-mediated uptake using this model, Kd was multiplied by the tested gly-sar concentrations. The resulting product was subtracted from the values of total absorption resulting in values for transporter-mediated transport. These values were then plotted using the traditional Michaelis-Menten equation and nonlinear regression. The Km and Vmax values reported, however, are those calculated using the modified Michaelis-Menten equation applied to total uptake.
First order polynomial nonlinear regression was used to fit the lines for passive uptake using the equation:
| Equation 3. |
where y is reaction velocity, x is substrate concentration, m is the slope of the line, and b is the y-intercept; b was not constrained to zero in order to fit the line most accurately (and to account for adsorption).
Statistical Analysis
Matched pairs ANOVA using the Tukey post hoc test (GraphPad Prism 4.03) was used to determine if differences in passive and transporter-mediated values existed between the various methods. All results are expressed as mean ± standard error of the mean (SEM) unless noted otherwise.
RESULTS
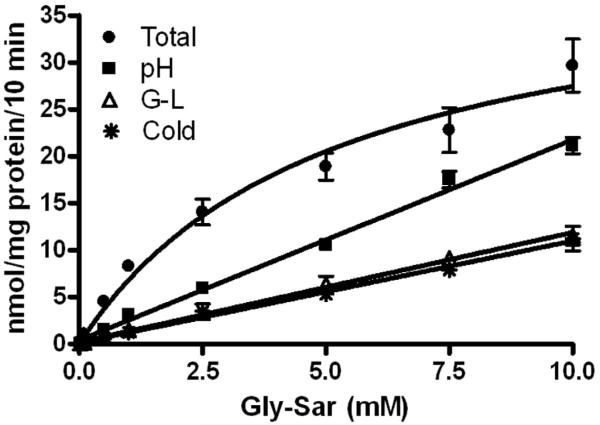
As expected, the total uptake of gly-sar, uncorrected for passive uptake, demonstrated a saturable component and nonsaturable component based on its appearance – an initial sharp increase in uptake followed by a gentle, constant, positive slope (fig 1). Analysis of total uptake by the Michaelis-Menton equation, without any correction for passive uptake, resulted in a Km and Vmax of 5.1±1.4 mM and 41.4±5.1 nmol/mg protein/10 min, respectively.
Figure 1.
Total and passive uptake (pH 8.0, 50 mM gly-leu, and 4 °C) of gly-sar into Caco-2 cells. Uptake values are presented as mean ± the standard error of the mean (n=9).
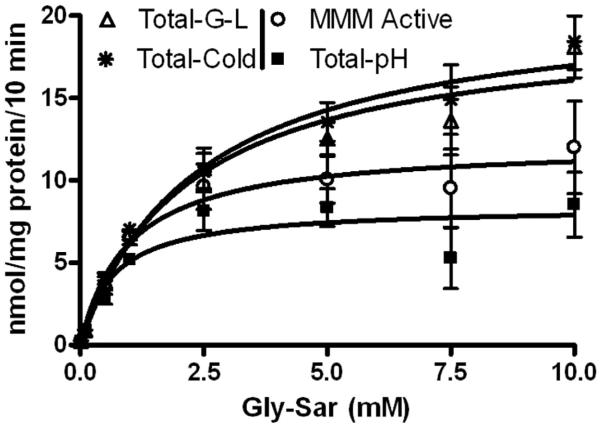
Each method of estimation of passive uptake of gly-sar showed appropriately a directly proportional, linear, concentration-dependent increase in uptake that did not plateau or become asymptotic (fig 1). The y-intercept and slopes ranged from 0.14-0.41 nmol/mg protein/10 min and 1.1-2.1 nmol/mg protein/10 min/mM, respectively (table 1). Values for passive uptake of gly-sar as estimated by testing gly-sar absorption at pH 8.0 were substantially greater than the values estimated at cold temperatures or using 50 mM gly-leu (p<0.05 respectively). There were no differences in the values for passive uptake of gly-sar estimated at cold temperature compared to 50 mM gly-leu (p>0.05) (table 2). Subtracting passive uptake from total uptake resulted in values of transporter-mediated uptake that were greater when corrected by the cold assay technique and by the 50 mM gly-leu technique than for values of transporter-mediated uptake as determined by correction for passive uptake estimated at pH 8.0 (p<0.001 respectively) (fig 2 and table 2). Values for Km and Vmax ranged from 0.7-2.4 mM and 8.4-21.0 nmol/mg protein/10 min respectively (table 3).
Table 1.
Methods of Estimation of Passive Uptake
| Method | Slope (SEM) |
y-intercept (SEM) |
|---|---|---|
| pH=8.0 | 2.1 (0.1) | 0.41 (0.23) |
| Gly-Leu | 1.2 (0.1) | 0.19 (0.23) |
| Cold | 1.1 (0.1) | 0.14 (0.26) |
Line parameters for passive uptake determined by nonlinear regression using the equation y=m(x) + b.
Table 2.
Comparison of Curves Estimating Transporter-mediated and Passive Uptake
| Comparison of Transporter-mediated Curves p |
Comparison of Passive Uptake Curves p |
|
|---|---|---|
| pH vs. Gly-Leu | < 0.01 | < 0.05 |
| pH vs. Cold | < 0.01 | < 0.05 |
| pH vs. Modified Michaelis-Menten Equation | > 0.05 | |
| Gly-Leu vs. Cold | > 0.05 | > 0.05 |
| Gly-Leu vs. Modified Michaelis-Menten Equation | > 0.05 | |
| Cold vs. Modified Michaelis-Menten Equation | > 0.05 |
p-values for the comparisons of active and passive uptake curves using different methods to estimate passive uptake.
Figure 2.
PepT1-mediated uptake of gly-sar into Caco-2 cells. Uptake values are presented as mean ± the standard error of the mean (n=9). MMM is Modified Michaelis-Menten Equation
Table 3.
Kinetic Parameters of Transporter-mediated Uptake
| Method of Correction* |
Uncorrected | pH | Gly-Leu | Cold | Michaelis- Menten Equation |
|---|---|---|---|---|---|
| Km (mM) | 5.1 ± 1.4 | 0.7 ± 0.3 | 2.2 ± 0.6 | 2.4 ± 0.6 | 1.0 ± 0.8 |
| Km 95% CI | 2.4-7.8 | 0.02-1.3 | 1.0-3.4 | 1.1-3.6 | 0.1-1.8 |
| Vmax (nmol/mg protein/10min) |
41.4 ± 5.1 | 8.4 ± 0.9 | 19.6 ± 1.7 | 21.0 ± 1.8 | 12.2 ± 5.3 |
| Vmax 95% CI | 31.2-51.5- | 6.6-10.2 | 16.1-23.1 | 17.4-24.6 | 9.5-14.9 |
| Kd (nmol\mg protein\10 min\ mM) |
1.8 ± 0.5 | ||||
| Average % gly-sar transported by PepT1 (0.02-1 mM) |
69 | 85 | 87 | 83 |
Kinetic characteristics of PepT1-mediated gly-sar uptake using different methods to correct total uptake for passive uptake (n=9). * when appropriate
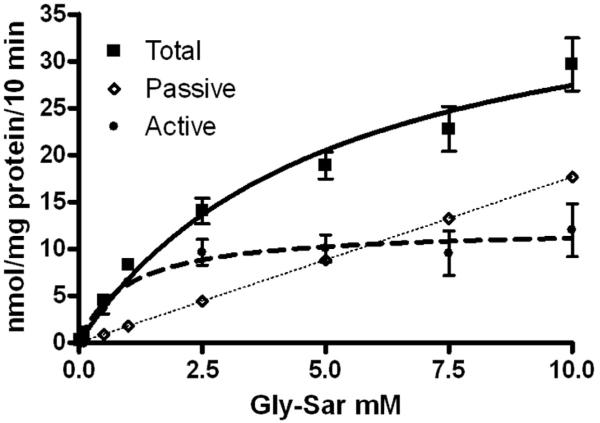
Analyzing total uptake by the modified Michaelis-Menten equation resulted in values for Km, Vmax, and Kd of 1.0±0.8 mM, 12.2±5.3 nmol\mg protein\10 min, and 1.8±0.5 nmol\mg protein\10 min\mM, respectively (table 3). In order to separate graphically the transporter-mediated and passive components of uptake, Kd was multiplied by the tested concentrations of gly-sar to estimate passive uptake. Estimated values of passive uptake were subtracted from total uptake to determine transporter-mediated uptake which was graphed using the Michaelis-Menton equation (fig 3). This approach resulted in a curve of transporter-mediated uptake that lies between but was not significantly different than transporter-mediated curves corrected for passive uptake using pH 8.0, cold temperature, and 50 mM gly-leu (p>0.05 respectively) (fig 2 and table 2).
Figure 3.
Graphic representation of the passive and active components of absorption using the modified Michaelis-Menten equation (eq 2). Uptake values are presented as mean ± the standard error of the mean (n=9).
When the amount of gly-sar estimated to be transported by PepT1 was analyzed for each method over the concentration range of 0.02-1 mM, the method using pH 8.0 to determine passive uptake estimated 69% of the gly-sar taken up was transported by PepT1, while the methods of 50 mM gly-leu, cold temperature, and the modified Michaelis-Menten equation resulted in estimates that were remarkably consistent − 85%, 87%, and 83%, respectively (table 3).
Discussion
In our previous in vitro study of dipeptide uptake by PepT1 in whole tissue, we used the method of Matthews et al. to correct total uptake for passive uptake in order to study transporter-mediated uptake. With this method, passive uptake at lesser concentrations is estimated from total uptake at much greater (10-100x) concentrations of gly-sar. As substrate concentration increases, so does passive uptake, and once the transporter is saturated, the linear increase in total uptake is attributed only to passive uptake independent of a membrane transport protein, i.e. passive diffusion and mucosal adherence (33-35). When we applied this method to our cell culture work, however, we suspected this method to be less reliable, yielding poor results and prompting us to seek a better technique. A thorough search of the literature yielded the methods that we tested against one another in this experiment. To our knowledge, this is the first study comparing the three methods (cold temperature, markedly greater concentration of a different dipeptide, and modified Michaelis-Menten kinetics) used most commonly to correct total uptake of peptides by passive uptake to estimate the specific PepT1-mediated uptake.
All methods for estimating passive uptake were reproducible. Cold temperature, a markedly greater concentration of a different dipeptide, the modified Michaelis-Menten equation appear to be relatively equivalent and differ significantly from using an increased pH to quench the proton gradient-dependent, transporter-mediated uptake of PepT1. A pH of 8.0 was chosen based on previous studies demonstrating that PepT1 functions most efficiently at pH 6.0 – a pH at which a proton gradient from the extracellular environment into the cell is present. Our assumption was that at pH 8.0 (1/100 of the proton gradient at pH 6.0), the proton gradient to transport dipeptides would be abolished. We were concerned that at more alkaline pHs, issues of cell membrane stability would be present, altering more variables in our experimental setup than transport of dipeptides by PepT1. Additionally, it is possible that at pH 8.0, passive diffusion of dipeptides is increased due to alteration of several character istics of the cell membrane which might account for the increase in passive absorption of dipeptides that we observed in our experiments (figure 1). Also possible is that at pH 8.0, sufficient protons were present to create a small gradient into the cell allowing for PepT1 to continue transporting gly-sar into the cell. This possibility could explain the increased absorption at pH 8.0 compared to the other methods tested.
A relative advantage of using modified Michaelis-Menten kinetics when compared to the other methods is that it is quicker to compute and does not require “extra” experiments to be conducted or additional solutions to be prepared. Because of the consistency of our results with three of the four tested methods, we maintain that cold temperature, a markedly greater concentration of a different dipeptide, and the modified Michaelis-Menten equation are more accurate than correction using a pH 8.0 based on this inter-method variation. Although it is possible that the Km and Vmax estimated for PepT1 using the three equivalent methods are incorrect and those estimated using the increased pH method are more accurate, such a scenario seems unlikely, because the other three methods gave very similar and congruent values of passive uptake of gly-sar. Our data could be criticized, because we do not present evidence confirming or disproving that other transporters are or are not involved. We and others have confirmed the presence of PepT1 in the Caco-2 cell line (3, 22, 24-26, 28). We believe, however, that our estimation of Km and Vmax of PepT1 for gly-sar is consistent with previously reported values under different conditions. While Km is relatively easy to compare between studies, Vmax is more difficult because of the variety of durations of incubation that have been evaluated and the manner in which it is reported (i.e. per min, per 10 min, per min after 10 min incubation, etc.). Km may also vary depending on a wide variety of experimental factors, including modifications to PepT1 with tags (myc, hemagglutinin, green fluorescent protein etc.), transfection into a non-PepT1 expressing cell, cloning, or other alterations. The most convincing evidence from the results of our study using a cold temperature, a markedly greater concentration of a second dipeptide, and/or the modified Michaelis-Menten equation to correct total uptake for passive uptake is the estimate that ~80% of gly-sar is taken up by a transporter-mediated process at concentrations of gly-sar ≤ 1 mM (table 3). This value is similar to and consistent with PepT1 knockout and transfection studies comparing dipeptide uptake in PepT1 and non-PepT1 containing cells/tissues. (1, 4, 5, 29).
This information should allow investigators studying PepT1 to make an informed, evidence-based decision about methods required to correct for passive uptake. This topic is of considerable importance in the study of epithelial transport when trying to determine potential methods to augment peptide absorption under various disease states, such as short bowel syndrome, mucosal disease, or other disorders of absorption. As for other membrane transport proteins, PepT1 is believed to be regulated in part by translocation to the apical membrane from preformed intracellular stores of cytoplasmic PepT1. To study this important mechanism, accurate measurements of transporter-mediated uptake are imperative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To be presented in part at the 6th Annual Academic Surgical Congress in Huntington Beach, CA on February 5, 2011.
References
- 1.Bhardwaj RK, Herrera-Ruiz D, Sinko PJ, Gudmundsson OS, Knipp G. Delineation of human peptide transporter 1 (hPepT1)-mediated uptake and transport of substrates with varying transporter affinities utilizing stably transfected hPepT1/Madin-Darby canine kidney clones and Caco-2 cells. J Pharmacol Exp Ther. 2005;314:1093–1100. doi: 10.1124/jpet.105.087148. [DOI] [PubMed] [Google Scholar]
- 2.Brandsch M, Miyamoto Y, Ganapathy V, Leibach FH. Expression and protein kinase C-dependent regulation of peptide/H+ co-transport system in the Caco-2 human colon carcinoma cell line. Biochem J. 1994;299(Pt 1):253–260. doi: 10.1042/bj2990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza VM, Buckley DJ, Buckley AR, Pauletti GM. Extracellular glucose concentration alters functional activity of the intestinal oligopeptide transporter (PepT-1) in Caco-2 cells. J Pharm Sci. 2003;92:594–603. doi: 10.1002/jps.10325. [DOI] [PubMed] [Google Scholar]
- 4.Herrera-Ruiz D, Faria TN, Bhardwaj RK, Timoszyk J, Gudmundsson OS, Moench P, Wall DA, Smith RL, Knipp GT. A novel hPepT1 stably transfected cell line: establishing a correlation between expression and function. Mol Pharm. 2004;1:136–144. doi: 10.1021/mp034011l. [DOI] [PubMed] [Google Scholar]
- 5.Chu C, Okamoto CT, Hamm-Alvarez SF, Lee VH. Stable transfection of MDCK cells with epitope-tagged human PepT1. Pharm Res. 2004;21:1970–1973. doi: 10.1023/b:pham.0000048186.86886.e6. [DOI] [PubMed] [Google Scholar]
- 6.Okamura M, Terada T, Katsura T, Saito H, Inui K. Inhibitory effect of zinc on PEPT1-mediated transport of glycylsarcosine and beta-lactam antibiotics in human intestinal cell line Caco-2. Pharm Res. 2003;20:1389–1393. doi: 10.1023/a:1025797808703. [DOI] [PubMed] [Google Scholar]
- 7.Fatima J, Iqbal CW, Houghton SG, Kasparek MS, Duenes JA, Zheng Y, Sarr MG. Hexose transporter expression and function in mouse small intestine: role of diurnal rhythm. J Gastrointest Surg. 2009;13:634–641. doi: 10.1007/s11605-008-0776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal CW, Fatima J, Duenes J, Houghton SG, Kasparek MS, Sarr MG. Expression and function of intestinal hexose transporters after small intestinal denervation. Surgery. 2009;146:100–112. doi: 10.1016/j.surg.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal CW, Qandeel HG, Zheng Y, Duenes JA, Sarr MG. Mechanisms of ileal adaptation for glucose absorption after proximal-based small bowel resection. J Gastrointest Surg. 2008;12:1854–1864. doi: 10.1007/s11605-008-0666-9. discussion 1864-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton SG, Zarroug AE, Duenes JA, Fernandez-Zapico ME, Sarr MG. The diurnal periodicity of hexose transporter mRNA and protein levels in the rat jejunum: role of vagal innervation. Surgery. 2006;139:542–549. doi: 10.1016/j.surg.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Houghton SG, Iqbal CW, Duenes JA, Fatima J, Kasparek MS, Sarr MG. Coordinated, diurnal hexose transporter expression in rat small bowel: implications for small bowel resection. Surgery. 2008;143:79–93. doi: 10.1016/j.surg.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447:610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- 13.Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol. 2003;285:G779–788. doi: 10.1152/ajpgi.00056.2003. [DOI] [PubMed] [Google Scholar]
- 14.Knutter I, Kottra G, Fischer W, Daniel H, Brandsch M. High-affinity interaction of sartans with H+/peptide transporters. Drug Metab Dispos. 2009;37:143–149. doi: 10.1124/dmd.108.022418. [DOI] [PubMed] [Google Scholar]
- 15.Knutter I, Wollesky C, Kottra G, Hahn MG, Fischer W, Zebisch K, Neubert RH, Daniel H, Brandsch M. Transport of angiotensin-converting enzyme inhibitors by H+/peptide transporters revisited. J Pharmacol Exp Ther. 2008;327:432–441. doi: 10.1124/jpet.108.143339. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Okuda M, Terada T, Sasaki S, Inui K. Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of beta-lactam antibiotics in the intestine and kidney. J Pharmacol Exp Ther. 1995;275:1631–1637. [PubMed] [Google Scholar]
- 17.Ogihara T, Kano T, Wagatsuma T, Wada S, Yabuuchi H, Enomoto S, Morimoto K, Shirasaka Y, Kobayashi S, Tamai I. Oseltamivir (tamiflu) is a substrate of peptide transporter 1. Drug Metab Dispos. 2009;37:1676–1681. doi: 10.1124/dmd.109.026922. [DOI] [PubMed] [Google Scholar]
- 18.Sugawara M, Huang W, Fei YJ, Leibach FH, Ganapathy V, Ganapathy ME. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J Pharm Sci. 2000;89:781–789. doi: 10.1002/(SICI)1520-6017(200006)89:6<781::AID-JPS10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, Lee CP, Oh DM, Sadee W, Amidon GL. 5′-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998;15:1154–1159. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- 20.Foster DR, Landowski CP, Zheng X, Amidon GL, Welage LS. Interferon-gamma increases expression of the di/tri-peptide transporter, h-PEPT1, and dipeptide transport in cultured human intestinal monolayers. Pharmacol Res. 2009;59:215–220. doi: 10.1016/j.phrs.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Thamotharan M, Bawani SZ, Zhou X, Adibi SA. Hormonal regulation of oligopeptide transporter pept-1 in a human intestinal cell line. Am J Physiol. 1999;276:C821–826. doi: 10.1152/ajpcell.1999.276.4.C821. [DOI] [PubMed] [Google Scholar]
- 22.Ashida K, Katsura T, Motohashi H, Saito H, Inui K. Thyroid hormone regulates the activity and expression of the peptide transporter PEPT1 in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G617–623. doi: 10.1152/ajpgi.00344.2001. [DOI] [PubMed] [Google Scholar]
- 23.Terada T, Saito H, Mukai M, Inui K. Recognition of beta-lactam antibiotics by rat peptide transporters, PEPT1 and PEPT2, in LLC-PK1 cells. Am J Physiol. 1997;273:F706–711. doi: 10.1152/ajprenal.1997.273.5.F706. [DOI] [PubMed] [Google Scholar]
- 24.Terada T, Sawada K, Saito H, Hashimoto Y, Inui K. Functional characteristics of basolateral peptide transporter in the human intestinal cell line Caco-2. Am J Physiol. 1999;276:G1435–1441. doi: 10.1152/ajpgi.1999.276.6.G1435. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto S, Saito H, Inui K. Transport characteristics of ceftibuten, a new cephaloporin antibiotic, via the apical H+/dipeptide cotransport system in human intestinal cell line Caco-2: regulation by cell growth. Pharm Res. 1995;12:1483–1487. doi: 10.1023/a:1016235404598. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen CU, Amstrup J, Nielsen R, Steffansen B, Frokjaer S, Brodin B. Epidermal growth factor and insulin short-term increase hPepT1-mediated glycylsarcosine uptake in Caco-2 cells. Acta Physiol Scand. 2003;178:139–148. doi: 10.1046/j.1365-201X.2003.01113.x. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen CU, Amstrup J, Steffansen B, Frokjaer S, Brodin B. Epidermal growth factor inhibits glycylsarcosine transport and hPepT1 expression in a human intestinal cell line. Am J Physiol Gastrointest Liver Physiol. 2001;281:G191–199. doi: 10.1152/ajpgi.2001.281.1.G191. [DOI] [PubMed] [Google Scholar]
- 28.Saito H, Inui K. Dipeptide transporters in apical and basolateral membranes of the human intestinal cell line Caco-2. Am J Physiol. 1993;265:G289–294. doi: 10.1152/ajpgi.1993.265.2.G289. [DOI] [PubMed] [Google Scholar]
- 29.Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- 30.Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Peranzi G, Merlin D, Laburthe M, Lewin MJ, Roze C, Bado A. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001;108:1483–1494. doi: 10.1172/JCI13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalmasso G, Nguyen HT, Yan Y, Charrier-Hisamuddin L, Sitaraman SV, Merlin D. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS One. 2008;3:e2476. doi: 10.1371/journal.pone.0002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Souza VM, Shertzer HG, Menon AG, Pauletti GM. High glucose concentration in isotonic media alters caco-2 cell permeability. AAPS PharmSci. 2003;5:E24. doi: 10.1208/ps050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qandeel HG, Alonso F, Hernandez DJ, Duenes JA, Zheng Y, Scow JS, Sarr MG. Role of vagal innervation in diurnal rhythm of intestinal peptide transporter 1 (PEPT1) J Gastrointest Surg. 2009;13:1976–1985. doi: 10.1007/s11605-009-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qandeel HG, Duenes JA, Zheng Y, Sarr MG. Diurnal expression and function of peptide transporter 1 (PEPT1) J Surg Res. 2009;156:123–128. doi: 10.1016/j.jss.2009.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews DM, Gandy RH, Taylor E, Burston D. Influx of two dipeptides, glycylsarcosine and L-glutamyl-L-glutamic acid, into hamster jejunum in vitro. Clin Sci (Lond) 1979;56:15–23. doi: 10.1042/cs0560015. [DOI] [PubMed] [Google Scholar]