Abstract
The cultivated Pacific oyster Crassostrea gigas has suffered for decades large scale summer mortality phenomenon resulting from the interaction between the environment parameters, the oyster physiological and/or genetic status and the presence of pathogenic microorganisms including Vibrio species. To obtain a general picture of the molecular mechanisms implicated in C. gigas immune responsiveness to circumvent Vibrio infections, we have developed the first deep sequencing study of the transcriptome of hemocytes, the immunocompetent cells. Using Digital Gene Expression (DGE), we generated a transcript catalog of up-regulated genes from oysters surviving infection with virulent Vibrio strains (Vibrio splendidus LGP32 and V. aestuarianus LPi 02/41) compared to an avirulent one, V. tasmaniensis LMG 20012T. For that an original experimental infection protocol was developed in which only animals that were able to survive infections were considered for the DGE approach. We report the identification of cellular and immune functions that characterize the oyster capability to survive pathogenic Vibrio infections. Functional annotations highlight genes related to signal transduction of immune response, cell adhesion and communication as well as cellular processes and defence mechanisms of phagocytosis, actin cytosqueleton reorganization, cell trafficking and autophagy, but also antioxidant and anti-apoptotic reactions. In addition, quantitative PCR analysis reveals the first identification of pathogen-specific signatures in oyster gene regulation, which opens the way for in depth molecular studies of oyster-pathogen interaction and pathogenesis. This work is a prerequisite for the identification of those physiological traits controlling oyster capacity to survive a Vibrio infection and, subsequently, for a better understanding of the phenomenon of summer mortality.
Introduction
Aquatic organisms and particularly marine invertebrates, such as the oyster Crassostrea gigas, harbour an abundant and diverse microflora on their surface (epibiosis) or inside their tissues (endobiosis) where Vibrio splendidus is found as a dominant culturable vibrio. With Evolution, the oysters have developed effective systems for maintaining their homeostasis and for controlling potentially harmful and pathogenic bacteria. However, for decades, the cultivated Pacific oyster C. gigas is suffering large scale summer mortalities that are reported in all areas of the world where this species is cultivated [1]. Mortalities result from the interaction between the environment, the oyster physiological and/or genetic status and the presence of pathogenic microorganisms [1]. Beside the recent identification of a virulent microvariant of an Herpes virus, the OsHV-1 [2], Vibrio strains of V. splendidus and V. aestuarianus groups have been repeatedly associated to summer mortality episodes [3] and the virulence of some strains has been demonstrated through C. gigas experimental infections [4], [5].
Considerable effort has been invested in advanced genomic technologies to understand and characterize the major traits that govern the tolerance of oysters to stressful culture conditions or to pathogenic bacteria [6], [7], [8], [9], [10], [11]. In particular, immune-related genes have been characterized from C. gigas. Briefly, a variety of antimicrobials have been fully characterized, namely a Bactericidal/Permeability-Increasing protein, Cg-BPI [12] and antimicrobial peptide families, Cg-Defensins (Cg-Defs) [13], Cg-Proline rich peptides (Cg-Prps) [14], and a great diversity has been demonstrated in terms of sequences and potential antimicrobial activities [15]. Tissue Inhibitor Metalloprotease (TIMP)-encoding gene has been shown to be induced upon microbial challenge, the expression of which would be controlled by a Rel/NF-κB pathway [16], [17]. The oyster major plasma protein, Cg-EcSOD (extracellular Superoxide Dismutase) which appears to display anti-oxidant and LPS-binding properties [18] has recently been shown to be used as an opsonin for V. splendidus LGP32 phagocytosis through its RGD sequence [19]. Otherwise, oyster infection with V. aestuarianus results in a decrease in Cg-EcSOD transcripts in circulating hemocytes [20]. However, whereas most of these immune genes were shown to be modulated during infections, the molecular mechanisms by which the oyster can survive virulent Vibrio infections remained totally unknown.
Here, our objective was to develop a better understanding of the genetic-level responses of oysters to pathogenic vibrios and to identify genes that are involved in immune responsiveness to circumvent the infections. In this attempt, we have performed a comprehensive analysis of the transcriptome of oyster immunity (hemocytes), using Digital Gene Expression (DGE) [21], an improved version of the Serial Analysis of Gene Expression (SAGE) technique [22]. These methods generate genome-wide and high-throughput transcription profiles and provide qualitative and quantitative gene expression data that do not depend on prior identification of transcript information (sequence homologies or gene function). For the DGE approach, we have developed an original experimental infection protocol, considering individual monitoring of the oyster successful response in terms of survival to pathogenic Vibrio infections versus non pathogenic ones. Thus, two DGE libraries were constructed from hemocytes of oysters surviving infections by virulent V. splendidus LGP32 and V. aestuarianus LPi 02/41 on the one hand, and by V. tasmaniensis LMG 20012T, an avirulent strain related to the V. splendidus group, on the other hand. The study aimed to compare the expression data of the two libraries and, beyond gene identification and functional annotation, to explore the putative functions related to the capability of oysters to circumvent and to survive vibrioses. This is the first report on genome-wide transcriptional analysis of oyster survival-responsiveness to virulent vibrios.
Results
Oyster survival to virulent Vibrio species
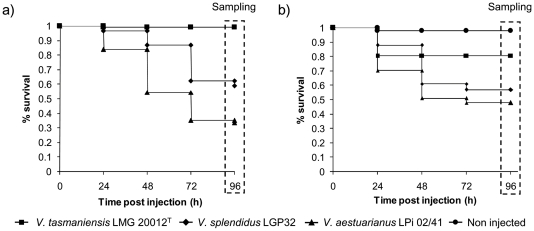
To strengthen the accuracy of our transcriptomic approach, two independent experimental infections have been performed for (i) the construction of hemocyte DGE libraries and for (ii) the exploration of the DGE data by qPCR, using respectively oysters from the Atlantic coast and Mediterranean Lagoon that differ in abiotic and biotic environmental conditions. After infections, the mortalities were individually monitored and Kaplan–Meier survival curves were generated for the different groups of oysters ( Figure 1 ).
Figure 1. Kaplan-Meier survival curves of C. gigas oysters during infections with virulent Vibrio strains, V. aestuarianus LPi 02/41 or V. splendidus LGP32, and with avirulent V. tasmaniensis LMG 20012T.
Infections for (a) construction of the hemocyte DGE libraries and (b) for gene expression exploration by qPCR analysis, with non injected oysters as control. For the two experiments, hemolymph was individually sampled from oysters that survived the infection at 96 h post-infection corresponding to the end of mortalities.
For the Atlantic oysters, the survival rates were 58.9% for the animals infected by virulent V. splendidus LGP32 (8.107 CFU/oyster), 33.3% for the oysters infected with the highly virulent V. aestuarianus LPi 02/41 (2.107 CFU/oyster) and 96.9% for those injected with the avirulent V. tasmaniensis LMG 20012T. The peaks of mortalities were reached at 72 h and 48 h post-injection respectively for the V. splendidus and V. aestuarianus strains ( Figure 1a ). Kaplan–Meier survival curves revealed statistical differences between the oysters injected either with V. splendidus LGP32, V. aestuarianus LPi 02/41 or the avirulent strain (p<0.0001, Log-Rank and Wilcoxon tests). For the Mediterranean Lagoon oysters, the two groups of individuals infected with V. splendidus LGP32 (4.108 CFU/animal) or V. aestuarianus LPi 02/41 (8.107 CFU/animal) displayed respectively 57% and 48% survival, with peaks of mortalities reached at 48 h post-infection for both bacteria strains. For the third group injected with V. tasmaniensis LMG 20012T (2.108 CFU/animal), 80% survival was observed at 24 h post-injection whereas 98% survival were recorded for the fourth non-injected oyster group used as control ( Figure 1b ). Generated Kaplan–Meier survival curves revealed significant differences (p<0.0001, Log-Rank and Wilcoxon tests, DDL = 2) between the four experimental infections. The survival curves differed statistically between the two independent infections (p<0.0001, Log-Rank and Wilcoxon tests, DDL = 5), but no differences were observed between Atlantic oysters injected with V. tasmaniensis and non injected Mediterranean oysters (p>0.05, Log-Rank and Wilcoxon tests, DDL = 5). We showed that higher dose of V. splendidus LGP32 (4.108 CFU/oyster) was requested for Mediterranean oysters than for the Atlantic ones (8.107 CFU/oyster) to reach similar mortality rates. This would confirm variabilities previously observed in oyster susceptibility to infections according to their geographic origins (Duperthuy, pers. comm.).
To consider only animals that have been able to survive infections, hemolymph for hemocyte RNA extraction was individually collected at 96 h post-infection when mortalities were no more recorded.
General characteristics of hemocyte DGE libraries
To have access to the hemocyte transcriptome and to establish a complete quantitative and qualitative gene expression database for the oyster immune functions and survival to virulent vibrios, two DGE libraries were generated from pooled hemocyte RNAs of the Atlantic oysters (i) surviving virulent V. splendidus LGP32 and V. aestuarianus LPi 02/41 infections (SVir library) and (ii) those challenged with the avirulent strain, V. tasmaniensis LMG 20012T (SaVir library). Sequencing of these two libraries resulted in a total of 6,983,680 DGE tags. Characteristics of the libraries are summarized in Table 1 . According to P-value (0.001) and occurrence of tags (incidence of each tag in libraries) in the DGE libraries, 56,871 unique tags have been identified from both libraries (GEO accession numbers GSM667899 and GSM667900). Comparison of the tag occurrences between libraries revealed that 22,187 unique tags are differentially represented between libraries (fold change >2), composed of 9,815 tags more represented in SVir library and 12,372 tags more represented in SaVir library. By using Blast search on the C. gigas 29,745 unique ESTs (http://www.sigenae.org/aquafirst/), these DGE tags were found to be associated to 4,374 ESTs corresponding to 3,931 unique ESTs (1,610 for SVir and 2,321 for SaVir), which have been further considered for functional annotation of genes related to survival response to virulent versus non virulent vibrios. The relatively low percentage of homology found (19.7%) is mainly due to the fact that the ESTs assembled in the GigasDatabase were obtained from different developmental stages and oyster tissues that include only 13,898 ESTs from hemocytes [10].
Table 1. General characteristics of DGE libraries generated from oysters surviving virulent (SVir) and avirulent (SaVir) Vibrio infections.
| DGE library | SVir | SaVir |
| Sequenced tags | 3,349,884 | 3,633,796 |
| Unique tags | 52,224 | 52,528 |
| Specific tags | 3,647 | 4,343 |
| Tags differentially represented (≥2 fold change) | 9,812 | 12,372 |
| Number of tags which match with EST | 1,782 | 2,592 |
| Unique EST | 1,610 | 2,321 |
| EST with B2GO annotation | 393 | 766 |
| EST with KEGG annotation | 271 | 564 |
Exploration of differential gene expressions according to Vibrio strains
The accuracy of our DGE approach have been validated by qPCR analysis. To reinforce this exploration, we used an independent experimental infection with oysters from Mediterranean Lagoon instead of Atlantic coast. For that, 18 genes more represented in SVir library and 17 genes more represented in SaVir library (2-fold change) were randomly chosen. Transcript levels were measured from hemocytes of oysters that survived infections with the virulent strains and the avirulent control. In addition, non-injected oysters were used as a control group ( Figure 1b ). Hierarchical clustering of the 18 SVir gene expression profiles distinguished, through clusters of conditions (CC), surviving oysters to virulent Vibrio strain infections (CC 3 and 4) to injected oysters with avirulent Vibrio strain (CC 2) and non injected oysters (CC 1) with a range of differential expression of 6.9-fold ( Figure 2a ). We can notice than up-regulated genes were found in all clusters of expression (CE) independently of the virulent Vibrio strains injected. Comparatively expression profiles of the 17 SaVir genes did not discriminate oyster response according to the Vibrio strains or control in the clustering (Figure S1). Furthermore, for these genes, a 2.6-fold change of differential expression was observed, weaker than obtained for SVir genes.
Figure 2. Gene expression analysis by qPCR.
(a) Hierarchical clustering analysis and differential expression of 18 genes from SVir library. Hemocyte gene expression profiles were analysed in biological replicates from oysters: non injected as control (C), surviving infections with avirulent V. tasmaniensis LMG 20012T (T), with virulent V. splendidus LGP32 (S) and virulent V. aestuarianus LPi 02/41 (A). Each biological replicata was constituted by a pool of hemocyte RNA from ten oysters, and between 2 and 5 replicates were analysed for each experimental condition. Each cell in the matrix corresponds to the expression level of one gene in a sample. The intensity of the color from green to red indicates the magnitude of differential expression (see color scale at the bottom of the image). Relative expressions were calculated according the 2−(ΔΔCt) method normalized with elongation factor-1α (EF-1α). Each value was calculated in reference to the mean of ΔCt of all conditions (relative expression = 1). The dendrograms at the top of the figures indicate relationship among experimental conditions which define clusters of conditions (CC). The dendrograms at the left of the figures indicate relationship among the profiles of the selected genes which define clusters of expression (CE), after clustering analysis using Multiple Array Viewer software. Gene #1: Inhibitor of apoptosis; #2: Baculoviral IAP repeat-containing protein 4; #3: Enhancer of kinase suppressor of Ras2; #4: Rac GTPase-activating protein1; #5: Proteasome 216S subunit, non-ATPase 11a; #6: Glyceraldhyde 3-phosphate dehydrogenase; #7: Thioredoxin; #8: C-type lectin 2 like protein; #9: Metallothionein IV; #10: F-box only protein37; #11: Cystatin B-like protein; #12: Heat shock protein 22 isoform 1; #13: L-rhamnose-binding lectin; #14: Microsomal glutathione S-tranferase; #15: Cystatin A; #16: Interferon-induced protein 44; #17: Cullin-associated and neddylation-dissociated 1. Hierarchical clustering was contructed with Multiple Array Viewer software using average linkage clustering with Pearson correlation as the default distance metric. (b) Examples of gene expression profiles defining pathogen- or challenge-specific signatures. SVir genes; line 1: similar response to both virulent strains; line 2: response induced by V. splendidus LGP32; line 3: response induced by V. aestuarianus LPi 02/41; line 4: SaVir genes. Different letters indicate significant variation between conditions (p<0.05) determined using the non-parametric multiple comparison test ANOVA of Kruskal-Wallis.
Pathogen-specific signatures are evidenced among DGE genes
It is noteworthy that hierarchical clustering evidenced pathogen-specific and shared signatures upon bacterial challenges. Three major clusters of expressions (CE) were evidenced for SVir genes. Indeed, we observed genes up-regulated only in response to both virulent Vibrio compared to control or avirulent Vibrio injection (as examples: baculoviral IAP repeat containing protein 4, fascin or C-type lectin). Besides, genes were seen to be up-regulated in response to V. splendidus associated to cluster of expression 1 (as examples: Enhancer of kinase suppressor of ras 2, Metallothionein IV and Thioredoxin), while up-regulated genes in response to V. aestuarianus infection were found in clusters of expression 2 and 3 (as examples: Heat shock protein 22, Cystatin A2 and L-rhamnose-binding lectin) ( Figure 2b ). Various expression profiles were found for the SaVir genes tested, such as a down-regulation upon virulent Vibrio infections (as an example, the putative 26S proteasome non-ATPase regulatory subunit 7), up-regulation by the avirulent strain compared to virulent strains (Metaxin 3) or up-regulated indistinctly by the avirulent and virulent Vibrio compared to unchallenged oysters (no Blast hit sequence wy0aba12yi21fm1.1.a.cg.2).
Functional annotation
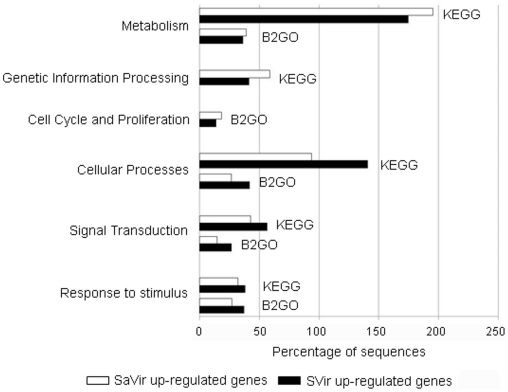
Altogether, the qPCR analyses which were performed on independent experimental infections and distinct biological material corroborated the DGE quantitative data. This supported further bioinformatic and Blast2GO and KEGG annotations [23] aiming to gain a general picture of the functional profiles of oyster survival- and infection-responsive genes identified by DGE approach. The 3,931 unique ESTs differentially represented between libraries (2-fold change) were annotated into 7 and 5 functional groups based on GO terms and KO terms respectively (Figure S2). SaVir up-regulated genes appeared to be assigned to “cell cycle and proliferation”, “metabolism” and “genetic information processing” ( Figure 3 and Figure S2). SVir up-regulated genes were assigned into groups related to “cellular processes” that included “cell motility and communication”, “cell growth and death” and “cytosqueleton”, to “signal transduction”, and to “response to stimulus” ( Figure 3 and Figure S2). Genes without GO terms were annotated manually (using Gene Cards informations) to enrich functional annotations in both DGE libraries.
Figure 3. Functional annotation of unique ESTs differentially represented between SVir and SaVir libraries, respectively.
Categorization was based on KO terms of the Kyoto Encyclopedia of Genes and Genomes (KEGG) using KEGG Automatic Annotation Server (KAAS), and on GO terms of Biological Process using Blast2GO software.
Biological functions related to oyster successful response to virulent Vibrio infections
We focused on further identification of biological functions that may influence the successful immune response to virulent vibrios. Considering their occurrence in SVir versus SaVir DGE library, a list of genes has been established that highlights different pathogen-responsive immune functions (Table S1). This list has been generated from the analysis of full data provided in Supplementary file giving information on DGE tag sequences, C. gigas EST names, and on Blast2GO and KEGG functionnal annotation (Table S2). Several immune-related genes already characterized in oyster have been evidenced with no differential representation between the SVir and SaVir libraries (Table S2).
Immune response is characterized by genes related to signaling pathways notably involved in innate immunity (Toll-like/NF-κB and MAPK), from membrane receptors (Toll-like receptor) to regulating intermediaries (MAP kinases, G-protein, and NF-κB inhibitor) and transcription factors (LITAF-like protein). In addition, signaling and interaction molecules such as cytokine receptors were evidenced as well as recognition molecules such as lectins. Besides, proteases, protease inhibitors and stress proteins (such as heat shock proteins and metallothionein) were found with various immune effectors such as antimicrobial (lysozyme). Cell adhesion and communication category is dominated in SVir by regulating elements associated to cellular processes represented by genes related to cell membrane molecules like integrins, collagen or tetraspanins. Cytosqueleton reorganisation appears as a dominant group in terms of number of sequences associated to cellular and response processes related to actin (calcium dependent) and tubulin reorganization. Particularly, actin related genes associated to endocytosis were evidenced such as genes coding for membrane receptors associated to vesicule formation or to endosome and lysosome. In addition, trafficking and autophagy are highlighted by genes encoding several trafficking proteins and regulators of transport associated to cytosqueleton network. Respiratory chain, dominated by regulating and response elements, is represented by genes associated to oxidative stress, several potent antioxidants and potential DNA repair elements associated to respiratory burst. Apoptosis is noticed with both regulating pro-apoptotic related genes such as caspases but also anti-apoptotic ones, such as Baculoviral IAP repeat-containing proteins, involved in inhibition of tumor necrosis factor receptor-associated factors. Finally, Cellular differentiation and proliferation category is represented by regulating and response elements like genes involved in TGF-β and Wnt signaling pathways and positive or negative regulators of these pathways (such as small GTPase).
Discussion
Here, we report on the first deep sequencing study of the transcriptome of hemocytes, immunocompetent cells, of the oyster Crassostrea gigas. We have identified by DGE approach those defence mechanisms related to the oyster successful response and survival to virulent Vibrios comparatively to non virulent Vibrio infection. Only the animals that were able to survive infections were considered for the DGE library construction, i.e. hemocytes were collected after the peak of mortalities, 96 h post-infections. To fulfill this requirement, oysters have been experimentally infected by injection of the bacteria into the adductor muscle, since this method is the only reproducible and standardized procedure to induce mortality of oysters with controlled doses of virulent Vibrio [24]. Comparatively, the immersion in virulent Vibrio-containing sea water does not consistently induce disease with well defined peaks of mortalities. It is noteworthy that, in our experimental conditions, bacteria virulence mechanisms that can breach the first line of oyster defences have been bypassed. Besides, potential recognition processes have not been highlighted in the analyses. Thus, the present study is not exhaustive. By considering surviving oysters after the peak of mortalities, we have focused on late processes of the immune responses relevant to bacteria elimination and infection resolution, instead of defence mechanisms occurring early post-infection.
Functional annotation of genes differentially represented between the SVir and SaVir DGE libraries has revealed biological processes that may characterize the successful immune response of oysters to pathogenic vibrios versus a non pathogenic one. However, because C. gigas is a non-model organism, it is noteworthy that such annotations may only suggest putative functions. The response to avirulent Vibrio was enriched in genes related to cell metabolism and cell cycle which may reflect hemocyte homeostasis recovery upon non-pathogenic challenge. Comparatively, in respect to oyster survival to infections, we found enrichment for genes related to signal transduction, signaling molecules and interactions, implicated in the regulation of immune response, as well as to cell adhesion and communication. In addition, the hemocyte transcript catalog we generated highlights cellular processes and defence mechanisms related to phagocytic events, actin cytosqueleton reorganization, cell trafficking and autophagy, but also to oxidative antioxidant and anti-apoptotic reactions.
The hemocyte gene repertoire of oysters able to circumvent pathogenic infections revealed the presence of pathogen recognition molecules and elements of signaling pathways involved in immune responses and inflammatory processes. Two lectins were seen up-regulated in response to virulent vibrios, a homologue to L-rhamnose-binding lectin (RBL) identified as constitutively expressed in Hydractinia [25] and the oyster C-type lectin. RBLs are pattern recognition receptors in vertebrates; they have opsonising properties and are activators of pro-inflammatory cytokines [26]. Interestingly, here, the putative RBL was seen to be exclusively expressed in response to the virulent vibrios according to a pathogen-specific expresssion signature for V. aestuarianus. C-type lectins are Ca+-dependent carbohydrate binding proteins. Upon pathogen recognition, they are known as mediating various immune responses in invertebrates and vertebrates such as autophagy, phagosome maturation or apoptosis [27], [28]. One can notice that in the DGE libraries, oyster galectin, a galactosidase-binding lectin known to be involved in infections [29], was not seen differentially expressed according to the bacterial virulence (Table S2). However, in vertebrates, galectins play dual functions as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) [30]. Among pattern recognition receptors (PRRs), a Toll-like receptor homologous to Toll-9 from Anopheles was evidenced as differentially expressed, whereas the Toll-like receptor 1, recently described in oyster [31], did not (Table S2). Our results enriched the Toll-like repertoire in oyster, but further investigations will determine the respective involvement of these molecules in innate immune pathways, particularly the Toll/NF-κB pathway. Among SVir up-regulated genes, we identified several components of C. gigas NF-κB pathway [17] as well as LITAF (LPS-Induced TNF-α Factor) transcription factor already identified in C. gigas [32]. LITAF signaling pathway plays a major role in regulating various mouse inflammatory cytokines in response to LPS stimulation [33]. Here, cytokine receptor and cytokine-induced protein categories related to inflammation processes were also evidenced with up-regulated putative tumor necrosis factor ligand superfamily member 10, and Interferon-induced protein 44. Proinflammatory effects of virulent Vibrio infections were also revealed by over representation, in oyster SVir library, of components of MAPK signaling pathway. In abalones, pathogenic V. harveyi avoids both phagocytosis and ROS production and reduces p38 MAPK [34].
Besides cytokines and associated proteins, protease inhibitors were also found. Among them, Cg-TIMP, an inhibitor of metalloproteinases, which has NF-κB binding sites in promoting region, was shown to be implicated in oyster wound healing and defence mechanisms [16]. Several putative cystatins A and B and serpins were also identified whose putative role in invertebrate immune defence was suggested by previous genomic studies [35]. Vibrios secrete extracellular metalloproteases whose toxicity has been demonstrated both for V. splendidus LGP32 [36] and V. aestuarianus strain [37]. Thus, we can hypothesize that such protease inhibitors neutralize the Vibrio toxins. Moreover, members of cystatin superfamily have immunomodulatory properties and cytokine regulating properties thus preventing excessive inflammation (for review [38]). Cystatins may also interact with TIMPs and other proteases in patho-physiological processes that require tissue remodeling and that range from cell survival and proliferation, to differentiation and cell signaling [39]. High representation of sequences for stress proteins was shown, including various heat shock proteins (HSPs) and oyster metallothioneins (MTs). MTs are ubiquitous metal binding proteins though to be involved in detoxifying of heavy metals, in free radical scavenging but also in inflammatory responses [40].
Surprisingly, whereas we evidenced the potential involvement of immune response regulating pathways, few antimicrobials were seen to be significantly involved in the response to infections. Thus, no differential representation of antimicrobial transcripts was observed in the DGE data, namely for Cg-BPI, Bactericidal/Permeability-Increasing protein [12], Cg-Prps, proline-rich peptides [14], or for the new oyster big defensin family (Rosa et al. in prep). However, lysozyme transcripts were significantly increased in hemocytes from oyster surviving virulent Vibrio infection that motivates further characterization of this antimicrobial. Indeed, until now, three lysozymes have been characterized in oyster, mainly from mantle, gills or digestive gland with potential digestive functions [41]. Finally, one can mention the high representation of transcripts with homologies with Complement component 1q (C1q) in the SVir library. C1q domain containing (C1q-DC) proteins are ubiquitous in animal kingdom and many of them remain to be characterized. Nevertheless, C1q-DC proteins display many functions in immunity including clearance of pathogens in vertebrates [42] and invertebrates as well [43].
The DGE data presented here revealed the importance of genes involved in cell adhesion and communication that may contribute to oyster post-infection recovery. Among them, we showed several integrins including an oyster β-integrin that plays an important role in phagocytosis [44]. Integrins are also cell adhesion receptors for proteins of the extracellular proteins such as collagens evidenced here. Collagens maintain tissue integrity but also play significant role in regulating cell functions. Therefore, collagen production may indicate potential repairement of the lesions caused by virulent Vibrio infection. Besides, interestingly, members of tetraspanin superfamily were also significantly represented. Tetraspanins are small transmembrane proteins that, once interacting with immune receptors such as integrins, play important role in cellular processes such as migration, proliferation. They are immune modulators of signaling pathways (NF-κB and kinases) that induce cytokine production following pathogenic recognition [45]. Whereas tetraspanin associated to phagocytic receptors contribute to facilitate phagocytosis processes, microbial pathogens can also exploit tetraspanin to enter in host cell for further colonization and invasion [46]. Interestingly, V. splendidus LGP32 uses β-integrin through its outer membrane protein OmpU to invade oyster hemocyte and further impair defence fonctions [19]. Whether tetraspanins intervene also in V. splendidus LGP32 pathogenesis remains to be established. However, because tetraspanin transcript enrichment was shown here to be associated to the oyster successful response to circumvent Vibrio infection, it is likely that they display a role in modulating inflammatory responses. In several mollusk species, pathogenic vibrios (i.e. V. aestuarianus) use cytotoxins (metalloproteases and extracellular products - ECPs) as part of their virulence mechanisms by impairing cellular processes including adhesiveness, migration, morphogenesis and phagocytosis [47], [48]. Thus, in our study, the oyster capability to have circumvented the infection is reflected by the enrichment of gene transcripts related to cell adhesion and motility, and consequently to cytosqueleton reorganization.
We evidenced numerous molecules involved in cytoskeleton rearrangements which are essential for various cellular processes dealing with morphogenesis, chemotaxis, migration but also phagocytosis and intracellular transports [49]. Thus, actin cytoskeleton changes are also involved in host-pathogen interactions. However, many pathogens subvert these cellular machineries for invading and surviving into host cells [50] as also reported for the oyster pathogen V. splendidus LGP32 [19]. Here, the DGE approach greatly contributed to characterize in C. gigas numerous genes, actors of cellular defence mechanisms, but consequently also potential targets for Vibrio virulence. Among those, one can cite overexpression of genes related to endocytosis, lysosome and active state of actin as well as to tubulin reorganization. All those cellular motor genes (myosin light chain, dynein arm light chain, kinesin-like protein) involved in intracellular trafficking or exocytosis of immune effectors may contribute to removal of invading pathogen and homeostasis recovery. This is particularly highlighted by the modulation of genes we classified in autophagy and microtubule transport. Authophagy is part of the microbicidal defence system. This conserved mechanism plays roles in degrading intracellular pathogens but also in regulator of innate immunity particularly the inflammatory or systemic immune response [51].
Apoptosis, another specialized form of programmed cell death, was also evidenced in our data with different pro- or anti-death effectors such as caspases, cytochrome c or IAPs. Baculoviral IAP repeat-containing 2/3/4 is described to inhibit apoptosis by binding to tumor necrosis factor receptor-associated factors [52]. Thus, hemocytes of surviving oysters up-regulate a variety of anti-apoptotic genes which could modulate inflammatory cytokine pathways and oxidative stress potentially toxic for the cell. Besides activation by inflammatory pathways, apoptosis as autophagy can be triggered by reactive oxygen species (ROS) [53]. Here, the successful response of oyster to virulent Vibrio infection revealed up-regulated genes related to respiratory chain and particularly in ROS production, a microbicidal defence reaction known in oyster [54], as well as putative genes coding for anti-oxidants. In C. gigas, hemocyte oxidative metabolism has been shown to be enhanced following virulent V. aestuarianus infection when superoxide dismutase Cg-EcSOD gene was down-regulated, likely being a pathogen adaption for impairing hemocyte functions and survival [20]. With respect to V. splendidus LGP32, we have shown that the Vibrio uses Cg-EcSOD as opsonin for hemocyte invasion and evades defence reaction by limiting ROS production [19]. Our DGE data provided several components of the oxidative stress that may contribute to pathogen elimination and to anti-inflammatory reactions, in the context of oyster survival to infections.
Our study highlighted signaling pathways and regulators we classified in cell differentiation, closely related to the different functions described above. Those are homologous to activins and TGF-β inducible early growth response proteins, members of the TGF-β signaling pathway which regulates a wide spectrum of cellular functions such as proliferation, apoptosis, differentiation and migration [55]. Additionally, the involvement of Wnt-signaling pathway and numerous members of the Rho protein family such as Cdc42 and small GTPases were evidenced. They are important actors for cellular processes such as migration, chemiotaxis or phagocytosis and they are involved in cellular functions related to actin cytoskeleton regulation. Thus, Rho GTPases are also known to be specific targets for bacterial cytotoxins [56].
Here, qPCR analyses aimed at validating the differential gene expression evidenced by DGE. So, independent experimental infections were performed separately with the different virulent and avirulent vibrios. Using oysters from the Mediterranean sea instead of the Atlantic ocean, we showed that the differential gene expression was independent of the oyster origin. Interestingly, pathogen-specific signatures were evidenced upon bacterial challenge. To our knowledge, this is shown for the first time in this invertebrate. The different oyster infections resulted in both responses shared by the three Vibrio strains whatever their virulence or avirulence, and specific responses to virulent vibrios. This is consistent with recent data on virulence mechanisms and pathogenesis that greatly differ between V. aestuarianus strain and V. splendidus LGP32. V. aestuarianus was shown to affect hemocyte phagocytosis and adhesiveness properties by the secretion of extracellular products and to enhance the production of ROS [48]. Regarding V. splendidus LGP32, a metalloprotease from ECPs (extracellular products) has been associated to toxicity and its outer membrane protein OmpU has been evidenced as a virulent factor [24], [36]. Recently, V. splendidus LGP32 has been shown to be a facultative intracellular pathogen which invades the oyster hemocytes through OmpU adhesin/β-integrin recognition and survives by impairing phagosome acidification and ROS production [19]. In this work, we demonstrated that bacterial invasion induces hemocyte cytoskeleton reorganizations by analysing expression of genes evidenced in the present DGE study.
Concluding remarks
This genome-wide expression profiling aimed at a better understanding of the molecular mechanisms that control or contribute to the anti-infectious response of this marine bivalve mollusc with economical importance. The data we generated for characterizing the transcriptome of the oyster immune functions could be enriched by further progress on genomic resources of this non-model organism. Nevertheless, we provided here a catalog of genes and cellular or immune functions that are potential targets for mechanisms of pathogen resistance and escape to the immune response, that may concern not only Vibrio species but also viruses. Further exploitation of our DGE libraries opens the way to (i) the in-depth characterization of pathogen-specific gene expression signatures and (ii) the description of the effects of vibrio/virus co-infections that may impair oyster immune defences. Indeed, it is now considered that Herpes virus OsHV-1 together with Vibrio species, such as V. splendidus LGP32 present in the oyster microbiota, may contribute to C. gigas mortality outbreaks. Finally, this work is a prerequisite for the identification of those physiological traits controlling oyster survival capacity and subsequently for a better understanding of the phenomenon of summer mortality.
Materials and Methods
Bacterial strains
Two strains belonging to the Vibrio splendidus polyphyletic group were considered, namely the oyster pathogen V. splendidus LGP32 [4] and V. tasmaniensis LMG 20012T used as an avirulent strain [57]. Additionally, virulent V. aestuarianus LPi 02/41 isolated during oyster mortality events was chosen as representative of this dominant Vibrio species [3]. The strains were grown under agitation at 20°C in artificial sea water (ASW) [58] supplemented with 4 g/l bactopeptone and 1 g/l yeast extract (referred to as Zobell medium) for 18 h. Bacterial concentrations were evaluated by optical density (OD) at 600 nm (UltraspecIII, Pharmacia Biotech), an OD value value of 1 corresponding to 109 colonies forming units (CFU)/ml for V. aestuarianus LPi 02/41 and to 2.109 for V. splendidus LGP32 and V. tasmaniensis LMG 20012T. Bacteria were centrifuged (15 min, 3,000×g, 20°C) and suspended in autoclaved ASW at the concentration calculated for the experimental injections.
Oysters and experimental infections
For DGE library construction, adult (2 year-old) oysters, Crassostrea gigas, were purchased from an Atlantic oyster farm (La Tremblade, France) and acclimatized in the Ifremer laboratory (LGP, La Tremblade, France) over a 1-week period in aerated 0.45 µm-filtered seawater. The temperature was maintained at 20°C during the trial. A total of 390 oysters was individually tagged and distributed in three groups in separate tanks. For infection, oysters were first anesthetized for 3 h in aerated 50 g/l MgCl2 bath (2/3 v/v sea water/freshwater) containing phytoplankton (Chaetoceros gracilis and Isochrysis galbana). Experimental infections were performed as previously described by injecting bacteria into the posterior adductor muscle [24]. One group of 180 oysters was injected with 8.107 CFU of V. splendidus LGP32 per animal under 100 µl, and a second group (180 osyters) with 2.107 CFU/animal of V. aestuarianus LPi 01/42. The third group (30 oysters) was injected with 8.107 CFU/animal of the avirulent Vibrio tasmaniensis LMG 20012T.
A second experimental infection was performed for qPCR analyses with adult oysters (2 year-old) obtained from a Mediterranean commercial hatchery (Sodimer, Montpellier, France). Oysters were acclimatized at 20°C and maintained in tanks with UV-treated and biologically filtered sea water in the experimental aquaculture platform of Ifremer Palavas (France). To allow the intramuscular injection of bacteria suspensions, a small cut was made in the side of oyster shells, adjacent to the adductor muscle. A total of 300 oysters were divided into four groups. Two groups of 100 oysters were respectively infected by virulent Vibrio strains, 4.108 CFU/animal of V. splendidus LGP32 and 2.107 CFU/animal of V. aestuarianus LPi 02/41. The third group of 50 oysters was injected with 2.108 CFU/animal of the avirulent strain V. tasmaniensis LMG 20012T. Finally, the fourth group of 50 non infected oysters was used as control for the experiment to assess mortality due to the handling of the animals.
For both experiments, mortalities were monitored daily and, at the end of mortalities, hemolymph was individually collected from surviving oysters by withdrawing 0.5 to 1 ml from the posterior muscle adductor using a precooled 2 ml syringe for further RNA extraction. The non-parametric method of Kaplan-Meier (XLSTAT 2008.7.02) test was used to estimate survival rates and the Log-Rank and Wilcoxon values for comparing differences between the groups. All experimental infections were performed according to the Ifremer animal care guideline and policy.
RNA extraction
Hemocyte samples from individual oysters were obtained by hemolymph centrifugation at 1,500×g for 15 min at 4°C. Each pellet was lysed in 1 ml of TRIzol® reagent (Invitrogen®) for total RNA extraction according to the manufacturer's instructions. Total RNA amount and purity were checked by using spectrophotometrer NanoDrop ND-1000 (Thermo Scientific, Les Ulis, France) and the integrity of total RNA was analyzed by agarose-electrophoresis.
DGE library construction and sequencing
Two DGE libraries were constructed from hemocyte total RNA of oysters surviving Vibrio infections: SVir from pooled RNA samples from surviving individuals of virulent infections with V. splendidus LGP32 and V. aestuarianus LPi 02/41; SaVir from individuals injected with the avirulent V. tasmaniensis LMG 20012T.
Sequence tag preparation was done with Illumina's Digital Gene Expression Tag Profiling Kit according to the manufacturer's protocol (version 2.1B). For both libraries, 7 µg of total RNA (from 10 oysters, 0.7 µg per oyster) was incubated with oligo-dT beads. First- and second-strand cDNA syntheses were performed using superscript II reverse transcription kit according to the manufacturer's instructions (Invitrogen). The cDNAs were cleaved using the NlaIII anchoring enzyme. Subsequently, digested cDNAs were ligated with the GEX adapter 1 containing a restriction site of MmEI. The second digestion with MmeI was performed, which cuts 17 bp downstream of the CATG site. At this point, the fragments detach from the beads. The GEX adapter 2 was ligated to the 3' end of the tag. A PCR amplification with 15 cycles using Phusion polymerase (Finnzymes) was performed with primers complementary to the adapter sequences to enrich the samples for the desired fragments. The resulting fragments of 85 bp were purified by excision from a 6% polyacrylamide TBE gel. The DNA was eluted from the gel debris with 1× NEBuffer 2 by gentle rotation for 2 h at room temperature. Gel debris were removed using Spin-X Cellulose Acetate Filter (2 ml, 0.45 µm) and the DNA was precipitated by adding 10 µl of 3 M sodium acetate (pH 5.2) and 325 µl of cold ethanol, followed by centrifugation at 13,000×g for 20 min. After washing the pellet with 70% ethanol, the DNA was resuspended in 10 µl of 10 mM Tris-HCl (pH 8.5) and quantified by using Nanodrop 1000 spectrophotometer.
Cluster generation was performed after applying 4 pM of each sample to the individual lanes of the Illumina 1G flowcell. After hybridization of the sequencing primer to the single-stranded products, 18 cycles of base incorporation were carried out on the 1G analyzer according to the manufacturer's instructions. Image analysis and base calling were performed using the Illumina Pipeline, where sequence tags were obtained after purity filtering. This was followed by sorting and counting the unique tags.
DGE library characteristics and functional annotation of tags differentially represented between libraries
The sequence files of each DGE library were analyzed with BIOTAG software (Skuldtech, Montpellier, France). The statistical value of DGE data comparisons, as a function of tag counts, was calculated by assuming that each tag has an equal chance of being detected. For several highly expressed transcripts, we checked that tag frequencies in successive sequence batches were distributed in agreement with a binomial law [59]. Selected genes were chosen based on a comparison between the two libraries, combined with the significance threshold of the observed variations (p-value<0.01). Tag to gene mapping was performed using EST collection from C. gigas which contains 29,745 unique sequences (7,940 contigs and 21,805 singletons), generated from different oyster tissues including hemocytes, and stored on the platform of Sigenae-INRA Toulouse (http://www.sigenae.org/aquafirst/). For tag to gene mapping, the virtual tags were extracted from all contigs and singletons.
Sequence functional annotation analyses were performed using two ways of classification. First we used Blast2GO software v1.3.3. (http://www.blast2go.org/start_blast2go). Briefly, Blast2GO uses BlastX available through the National Center for Biotechnology Information (NCBI) with a user-defined threshold to find similar sequences from the NCBI (nr database). Sequences which found homology with annotated sequences were annotated according to the gene ontology (GO) terms. The hierarchical representation of the gene ontology is structured according to different levels, from the top (level 1) parents corresponding to the three main GO categories (cellular component, biological process and molecular function) to the lowest more specialized child terms level 2, 3, 4 etc. In the present research, GO annotations were represented at level 3 of biological process. Due to the redundancy of term attribution, we have manually condensed group functionally related gene and term into seven functional groups to clarify annotation. Second, we have used the KEGG Automatic Annotation Server (Kyoto encyclopedia of genes and genomes, http://www.genome.jp/tools/kaas/) with SBH method for EST annotation. This server provides functional annotation of genes by BlastX comparisons against the manually curated KEGG GENES database, for ortholog assignment and pathway mapping. In addition, we have used GenesCards version 3 to obtain functionnal description of indentified genes (http://www.genecards.org/).
Real-time quantitative PCR analysis
To validate the quantitative data of DGE libraries, we have quantified the expression levels of 35 selected genes from SVir or SaVir DGE libraries by quantitative PCR (qPCR) from pooled hemocyte total RNAs in biological replicates (10 oysters per replicata) from four experimental conditions: (i) uninfected oysters, (ii) oysters injected with avirulent V. tasmaniensis LMG 20012T, (iii) oysters that survived infection with virulent V. splendidus LGP32 or (iv) V. aestuarianus LPi 02/41. These experimental conditions were analysed in biological replicates of ten oysters per replicata. The first cDNA strand was synthesized from 700 ng of purified total RNA from hemocytes, using MMLV Reverse Transcriptase kit, according to the manufacturer's instructions (Invitrogen®), in 20 µl of reaction volume. qPCR amplifications were performed in the LightCycler 480 (Roche) in a final volume of 5 µl containing 5 mM MgCl2, 0.33 µM of each primer, 2.5 µl of reaction mix (LightCycler 480 SYBR Green I Master 2X) and 1 µl of each reverse transcribed RNA (diluted 1∶9). The list of oligonucleotide primers used to amplify target genes is shown in (Table S3). Each qPCR reaction was performed with an initial denaturation step of 10 min at 95°C followed by an amplification of the target cDNA for 40 cycles, each cycle consisting of a denaturation at 95°C for 10 s, annealing at 57°C for 20 s and elongation at 72°C for 25 s. Specificity of the qPCR product was analyzed by melting curve analysis. To determine the qPCR efficiency of each primer pair used, standard curves were generated using six serial dilutions (1∶1, 1∶3, 1∶7, 1∶15, 1∶31, 1∶63) of a unique cDNA sample constituted from a pool of all cDNAs obtained from each condition; qPCR efficiencies of tested genes varied between 1.87 and 1.99. Results are shown as changes in relative expression normalized with the elongation factor 1-alpha reference gene (EF-1α, GenBank accession number AB122066) using the 2−(ΔΔCt) method described by Pfaffl [60]. Global hierarchical clustering of qPCR data was performed with Multiple Array Viewer software (version 4.6.2, http://www.tm4.org/mev/) using avarage linkage clustering with Pearson correlation as the default distance metric. In addition, statitical analyses gene by gene were conducted using Statistica Statsoft software version 6 with the non-parametric multiple comparison test ANOVA of Kruskal-Wallis were considered significantly different at p<0.05.
Supporting Information
List of functional groups and related hemocyte up-regulated genes from oysters surviving virulent versus avirulent Vibrio infections.
(PDF)
List of annotated genes from hemocyte DGE libraries used for functional annotations.
(XLSX)
List of primers.
(XLSX)
Hierarchical clustering analysis and differential expression of 17 genes from SaVir library. Hemocyte gene expression profiles were analysed in biological replicates from oysters: non injected as control (C), surviving infections with avirulent V. tasmaniensis LMG 20012T (T), with virulent V. splendidus LGP32 (S) and virulent V. aestuarianus LPi 02/41 (A). Each cell in the matrix corresponds to the expression level of one gene in a sample. The intensity of the color from green to red indicates the magnitude of differential expression (see color scale at the bottom of the image). Relative expressions were calculated according the 2−(ΔΔCt) method normalized with elongation factor-1α (EF-1α). Each value was calculated in reference to the mean of ΔCt of all conditions (relative expression = 1). The dendrograms at the top of the figures indicate relationship among experimental conditions which define clusters of conditions (CC). The dendrograms at the left of the figures indicate relationship among the profiles of the selected genes which define clusters of expression (CE), after clustering analysis using Multiple Array Viewer software.
(TIF)
Gene ontology assignment of 1,610 and 2,321 unique ESTs differentially represented between SVir and SaVir libraries, respectively. (a) Categorization based on GO terms assignment from 3nd level of Biological Process using Blast2GO software. (b) Categorization based on KO terms (level A and B) of the Kyoto Encyclopedia of Genes and Genomes (KEGG) using KEGG Automatic Annotation Server (KAAS).
(TIF)
Acknowledgments
We thank Denis Saulnier for support for experimental infections done at the Ifremer laboratory of La Tremblade, Julie Fievet and Marc Leroy for experiments at the Aquaculture Platform of Ifremer Palavas. RZ and RDR were supported by doctoral fellowships from Ifremer and Languedoc-Roussillon region and CNPq – Brazil, respectively. Data used in this work were partly produced through molecular genetic analysis technical facilities of the SFR “Montpellier Environnement Biodiversité” and the “Plateforme qPHD UM2/Montpellier GenomiX”.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding from the Institut Français de Recherche et d'Exploitation de la Mer (Ifremer) and the Centre National de la Recherche Scientifique (Cnrs) grant from ANR (Agence Nationale de la Recherche) Genanimal project Cg-Physiogene (N°07/5210875/F). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Samain JF, McCombie H. Quae, editor. Summer mortality of Pacific oyster Crassostrea gigas. 2008. 379
- 2.Segarra A, Pepin JF, Arzul I, Morga B, Faury N, et al. Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Res. 2010;153:92–99. doi: 10.1016/j.virusres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Garnier M, Labreuche Y, Garcia C, Robert M, Nicolas JL. Evidence for the involvement of pathogenic bacteria in summer mortalities of the Pacific oyster Crassostrea gigas. Microb Ecol. 2007;53:187–196. doi: 10.1007/s00248-006-9061-9. [DOI] [PubMed] [Google Scholar]
- 4.Gay M, Renault T, Pons AM, Le Roux F. Two Vibrio splendidus related strains collaborate to kill Crassostrea gigas: taxonomy and host alterations. Dis Aquat Organ. 2004;62:65–74. doi: 10.3354/dao062065. [DOI] [PubMed] [Google Scholar]
- 5.Garnier M, Labreuche Y, Nicolas JL. Molecular and phenotypic characterization of Vibrio aestuarianus subsp. francensis subsp. nov., a pathogen of the oyster Crassostrea gigas. Syst Appl Microbiol. 2008;31:358–365. doi: 10.1016/j.syapm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Gueguen Y, Cadoret JP, Flament D, Barreau-Roumiguiere C, Girardot AL, et al. Immune gene discovery by expressed sequence tags generated from hemocytes of the bacteria-challenged oyster, Crassostrea gigas. Gene. 2003;303:139–145. doi: 10.1016/s0378-1119(02)01149-6. [DOI] [PubMed] [Google Scholar]
- 7.Tanguy A, Bierne N, Saavedra C, Pina B, Bachère E, et al. Increasing genomic information in bivalves through new EST collections in four species: development of new genetic markers for environmental studies and genome evolution. Gene. 2008;408:27–36. doi: 10.1016/j.gene.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Lang RP, Bayne CJ, Camara MD, Cunningham C, Jenny MJ, et al. Transcriptome profiling of selectively bred Pacific oyster Crassostrea gigas families that differ in tolerance of heat shock. Mar Biotechnol. 2009;11:650–668. doi: 10.1007/s10126-009-9181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taris N, Lang RP, Reno PW, Camara MD. Transcriptome response of the Pacific oyster (Crassostrea gigas) to infection with Vibrio tubiashii using cDNA AFLP differential display. Anim Genet. 2009;40:663–677. doi: 10.1111/j.1365-2052.2009.01894.x. [DOI] [PubMed] [Google Scholar]
- 10.Fleury E, Huvet A, Lelong C, De Lorgeril J, Boulo V, et al. Generation and analysis of a 29,745 unique Expressed Sequence Tags from the Pacific oyster (Crassostrea gigas) assembled into a publicly accessible database: the GigasDatabase. BMC Genomics. 2009;10:341–356. doi: 10.1186/1471-2164-10-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleury E, Moal J, Boulo V, Daniel JY, Mazurais D, et al. Microarray-based identification of gonad transcripts differentially expressed between lines of Pacific oyster selected to be resistant or susceptible to summer mortality. Mar Biotechnol (NY) 2010;12:326–339. doi: 10.1007/s10126-009-9227-9. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez M, Gueguen Y, Destoumieux-Garzón D, Romestand B, Fievet J, et al. Evidence of a bactericidal permeability increasing protein in an invertebrate, the Crassostrea gigas Cg-BPI. Proc Natl Acad Sci U S A. 2007;104:17759–17764. doi: 10.1073/pnas.0702281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gueguen Y, Herpin A, Aumelas A, Garnier J, Fievet J, et al. Characterization of a defensin from the oyster Crassostrea gigas. Recombinant production, folding, solution structure, antimicrobial activities, and gene expression. J Biol Chem. 2006;281:313–323. doi: 10.1074/jbc.M510850200. [DOI] [PubMed] [Google Scholar]
- 14.Gueguen Y, Romestand B, Fievet J, Schmitt P, Destoumieux-Garzón D, et al. Oyster hemocytes express a proline-rich peptide displaying synergistic antimicrobial activity with a defensin. Mol Immunol. 2009;46:516–522. doi: 10.1016/j.molimm.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt P, Gueguen Y, Desmarais E, Bachère E, de Lorgeril J. Molecular diversity of antimicrobial effectors in the oyster Crassostrea gigas. BMC Evol Biol. 2010;10:23. doi: 10.1186/1471-2148-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montagnani C, Avarre JC, de Lorgeril J, Quiquand M, Boulo V, et al. First evidence of the activation of Cg-timp, an immune response component of Pacific oysters, through a damage-associated molecular pattern pathway. Dev Comp Immunol. 2007;31:1–11. doi: 10.1016/j.dci.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Montagnani C, Labreuche Y, Escoubas JM. Cg-IkappaB, a new member of the IkappaB protein family characterized in the pacific oyster Crassostrea gigas. Dev Comp Immunol. 2008;32:182–190. doi: 10.1016/j.dci.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez M, Romestand B, Fievet J, Huvet A, Lebart MC, et al. Evidence in oyster of a plasma extracellular superoxide dismutase which binds LPS. Biochem Biophys Res Commun. 2005;338:1089–1097. doi: 10.1016/j.bbrc.2005.10.075. [DOI] [PubMed] [Google Scholar]
- 19.Duperthuy M, Schmitt P, Garzón E, Caro A, Rosa RD, et al. Use of OmpU porins for attachment and invasion of Crassostrea gigas immune cells by the oyster pathogen Vibrio splendidus. Proc Natl Acad Sci U S A. 2011;108:2993–2998. doi: 10.1073/pnas.1015326108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labreuche Y, Lambert C, Soudant P, Boulo V, Huvet A, et al. Cellular and molecular hemocyte responses of the Pacific oyster, Crassostrea gigas, following bacterial infection with Vibrio aestuarianus strain 01/32. Microbes Infect. 2006;8:2715–2724. doi: 10.1016/j.micinf.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Morin R, Bainbridge M, Fejes A, Hirst M, Krzywinski M, et al. Profiling the HeLa S3 transcriptome using randomly primed cDNA and massively parallel short-read sequencing. Biotechniques. 2008;45:81–94. doi: 10.2144/000112900. [DOI] [PubMed] [Google Scholar]
- 22.Velculescu VE, Zhang L, Vogelstein B, Kinzler KW. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- 23.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 24.Duperthuy M, Binesse J, Le Roux F, Romestand B, Caro A, et al. The major outer membrane protein OmpU of Vibrio splendidus contributes to host antimicrobial peptide resistance and is required for virulence in the oyster Crassostrea gigas. Environ Microbiol. 2010;12:951–963. doi: 10.1111/j.1462-2920.2009.02138.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz RS, Hodes-Villamar L, Fitzpatrick KA, Fain MG, Hughes AL, et al. A gene family of putative immune recognition molecules in the hydroid Hydractinia. Immunogenetics. 2007;59:233–246. doi: 10.1007/s00251-006-0179-1. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe Y, Tateno H, Nakamura-Tsuruta S, Kominami J, Hirabayashi J, et al. The function of rhamnose-binding lectin in innate immunity by restricted binding to Gb3. Dev Comp Immunol. 2009;33:187–197. doi: 10.1016/j.dci.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanne A, Ma B, Boudou F, Tailleux L, Botella H, et al. A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med. 2009;206:2205–2220. doi: 10.1084/jem.20090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7:424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Li L, Zhang G. A Crassostrea gigas Toll-like receptor and comparative analysis of TLR pathway in invertebrates. Fish Shellfish Immunol. 2011;30:653–660. doi: 10.1016/j.fsi.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Park EM, Kim YO, Nam BH, Kong HJ, Kim WJ, et al. Cloning, characterization and expression analysis of the gene for a putative lipopolysaccharide-induced TNF-alpha factor of the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2008;24:11–17. doi: 10.1016/j.fsi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: Evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci U S A. 2006;103:13777–13782. doi: 10.1073/pnas.0605988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travers MA, Le Bouffant R, Friedman CS, Buzin F, Cougard B, et al. Pathogenic Vibrio harveyi, in contrast to non-pathogenic strains, intervenes with the p38 MAPK pathway to avoid an abalone haemocyte immune response. J Cell Biochem. 2009;106:152–160. doi: 10.1002/jcb.21990. [DOI] [PubMed] [Google Scholar]
- 35.Guillou F, Mitta G, Galinier R, Coustau C. Identification and expression of gene transcripts generated during an anti-parasitic response in Biomphalaria glabrata. Dev Comp Immunol. 2007;31:657–671. doi: 10.1016/j.dci.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Binesse J, Delsert C, Saulnier D, Champomier-Verges MC, Zagorec M, et al. Metalloprotease vsm is the major determinant of toxicity for extracellular products of Vibrio splendidus. Appl Environ Microbiol. 2008;74:7108–7117. doi: 10.1128/AEM.01261-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labreuche Y, Le Roux F, Henry J, Zatylny C, Huvet A, et al. Vibrio aestuarianus zinc metalloprotease causes lethality in the Pacific oyster Crassostrea gigas and impairs the host cellular immune defenses. Fish Shellfish Immunol. 2010;29:753–758. doi: 10.1016/j.fsi.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Ochieng J, Chaudhuri G. Cystatin superfamily. J Health Care Poor Underserved. 2010;21:51–70. doi: 10.1353/hpu.0.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopitar-Jerala N. The role of cystatins in cells of the immune system. FEBS Lett. 2006;580:6295–6301. doi: 10.1016/j.febslet.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 40.Kanekiyo M, Itoh N, Kawasaki A, Matsuyama A, Matsuda K, et al. Metallothionein modulates lipopolysaccharide-stimulated tumour necrosis factor expression in mouse peritoneal macrophages. Biochem J. 2002;361:363–369. doi: 10.1042/0264-6021:3610363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue Q, Hellberg ME, Schey KL, Itoh N, Eytan RI, et al. A new lysozyme from the eastern oyster, Crassostrea virginica, and a possible evolutionary pathway for i-type lysozymes in bivalves from host defense to digestion. BMC Evol Biol. 2010;10:213. doi: 10.1186/1471-2148-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kishore U, Reid KB. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Song L, Li C, Zhao J, Wang H, et al. A novel C1q-domain-containing protein from Zhikong scallop Chlamys farreri with lipopolysaccharide binding activity. Fish Shellfish Immunol. 2008;25:281–289. doi: 10.1016/j.fsi.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Terahara K, Takahashi KG, Nakamura A, Osada M, Yoda M, et al. Differences in integrin-dependent phagocytosis among three hemocyte subpopulations of the Pacific oyster “Crassostrea gigas”. Dev Comp Immunol. 2006;30:667–683. doi: 10.1016/j.dci.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 46.van Spriel AB, Figdor CG. The role of tetraspanins in the pathogenesis of infectious diseases. Microbes Infect. 2010;12:106–112. doi: 10.1016/j.micinf.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Allam B, Ford SE. Effects of the pathogenic Vibrio tapetis on defence factors of susceptible and non-susceptible bivalve species: I. Haemocyte changes following in vitro challenge. Fish Shellfish Immunol. 2006;20:374–383. doi: 10.1016/j.fsi.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Labreuche Y, Soudant P, Gonçalves M, Lambert C, Nicolas JL. Effects of extracellular products from the pathogenic Vibrio aestuarianus strain 01/32 on lethality and cellular immune responses of the oyster Crassostrea gigas. Dev Comp Immunol. 2006;30:367–379. doi: 10.1016/j.dci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 50.Rottner K, Lommel S, Wehland J, Stradal TEB. Pathogen-induced actin filament rearrangement in infectious diseases. J Pathol. 2004;204:396–406. doi: 10.1002/path.1638. [DOI] [PubMed] [Google Scholar]
- 51.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng C, Kabaleeswaran V, Wang Y, Cheng G, Wu H. Crystal structures of the TRAF2: cIAP2 and the TRAF1: TRAF2: cIAP2 complexes: affinity, specificity, and regulation. Mol Cell. 2010;38:101–113. doi: 10.1016/j.molcel.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bachère E, Boulo V, Godin P, Goggin L, Hervio D, et al. In vitro chemiluminescence studies of marine bivalve defence mechanisms and responses against specific pathogens. Dev Comp Immunol. 1991;15:S102. [Google Scholar]
- 55.Subramaniam M, Hawse JR, Johnsen SA, Spelsberg TC. Role of TIEG1 in biological processes and disease states. J Cell Biochem. 2007;102:539–548. doi: 10.1002/jcb.21492. [DOI] [PubMed] [Google Scholar]
- 56.Aktories K, Barbieri JT. Bacterial cytotoxins: targeting eukaryotic switches. Nat Rev Microbiol. 2005;3:397–410. doi: 10.1038/nrmicro1150. [DOI] [PubMed] [Google Scholar]
- 57.Thompson FL, Thompson CC, Swings J. Vibrio tasmaniensis sp. nov., isolated from Atlantic salmon (Salmo salar L.). Syst Appl Microbiol. 2003;26:65–69. doi: 10.1078/072320203322337326. [DOI] [PubMed] [Google Scholar]
- 58.Saulnier D, Avarre JC, Le Moullac G, Ansquer D, Levy P, et al. Evidence that Vibrio penaeicida is the putative etiological agent of syndrome 93 in New Caledonia and development of a rapid and sensitive PCR assay for its detection in shrimp and sea water. Dis Aquatic Org. 2000;40:109–115. doi: 10.3354/dao040109. [DOI] [PubMed] [Google Scholar]
- 59.Piquemal D, Commes T, Manchon L, Lejeune M, Ferraz C, et al. Transcriptome analysis of monocytic leukemia cell differentiation. Genomics. 2002;80:361–371. doi: 10.1006/geno.2002.6836. [DOI] [PubMed] [Google Scholar]
- 60.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of functional groups and related hemocyte up-regulated genes from oysters surviving virulent versus avirulent Vibrio infections.
(PDF)
List of annotated genes from hemocyte DGE libraries used for functional annotations.
(XLSX)
List of primers.
(XLSX)
Hierarchical clustering analysis and differential expression of 17 genes from SaVir library. Hemocyte gene expression profiles were analysed in biological replicates from oysters: non injected as control (C), surviving infections with avirulent V. tasmaniensis LMG 20012T (T), with virulent V. splendidus LGP32 (S) and virulent V. aestuarianus LPi 02/41 (A). Each cell in the matrix corresponds to the expression level of one gene in a sample. The intensity of the color from green to red indicates the magnitude of differential expression (see color scale at the bottom of the image). Relative expressions were calculated according the 2−(ΔΔCt) method normalized with elongation factor-1α (EF-1α). Each value was calculated in reference to the mean of ΔCt of all conditions (relative expression = 1). The dendrograms at the top of the figures indicate relationship among experimental conditions which define clusters of conditions (CC). The dendrograms at the left of the figures indicate relationship among the profiles of the selected genes which define clusters of expression (CE), after clustering analysis using Multiple Array Viewer software.
(TIF)
Gene ontology assignment of 1,610 and 2,321 unique ESTs differentially represented between SVir and SaVir libraries, respectively. (a) Categorization based on GO terms assignment from 3nd level of Biological Process using Blast2GO software. (b) Categorization based on KO terms (level A and B) of the Kyoto Encyclopedia of Genes and Genomes (KEGG) using KEGG Automatic Annotation Server (KAAS).
(TIF)