Abstract
The trigonal bipyramidal high-spin (S = 2) oxoiron(IV) complex [FeIV(O)(TMG2dien)(CH3CN)]2+ (7) was synthesized and spectroscopically characterized. Substitution of the CH3CN ligand by anions, demonstrated here for X = N3− and Cl−, yielded further S = 2 oxoiron(IV) complexes of general formulation [FeIV(O)(TMG2dien)(X)]+ (7-X). The reduced steric bulk of 7 relative to the published S = 2 complex [FeIV(O)(TMG3tren)]2+ (2) was reflected by enhanced rates of intermolecular substrate oxidation.
Non-heme monoiron oxygen activating enzymes perform a remarkably diverse array of highly selective oxidative transformations, 1 Most have iron centers with a 2-His-1-carboxylate facial triad structural motif, and their catalytic cycles often involve oxoiron(IV) intermediates as oxidants. Within the past several years, such oxoiron(IV) species have been trapped and spectroscopically characterized in several enzymes and found in all cases to be high spin (S = 2).2 In contrast, the overwhelming majority of existing synthetic oxoiron(IV) complexes have S = 1 ground states.3 To date the only published examples of S = 2 oxoiron(IV) complexes are [FeIV(O)(H2O)5]2+ (1),4 [FeIV(O)(TMG3tren)]2+ (2, TMG3tren = 1,1,1-tris{2-[N2-(1,1,3,3-tetramethylguanidino)]-ethyl}amine))5 and [FeIV(O)(H3buea)]− (3, H3buea = tris[(N′-tert-butylureaylato)-N-ethylene]amine trianion).6 The crystallographically characterized trigonal bipyramidal (TBP) complex 2 was found to react rapidly via intramolecular ligand hydroxylation (t1/2 at 25 °C = 30sec), but reacted with external hydrocarbon substrates at rates comparable to those of existing S = 1 complexes. Given that many DFT studies predict more facile H-atom abstraction by S = 2 oxoiron(IV) centers than their S = 1 counterparts,7 the intermolecular reactivity observed for 2 was disappointingly sluggish, a fact attributed to a steric retardation of reaction due to the bulk of the tetramethylguanidine donors.5a,8
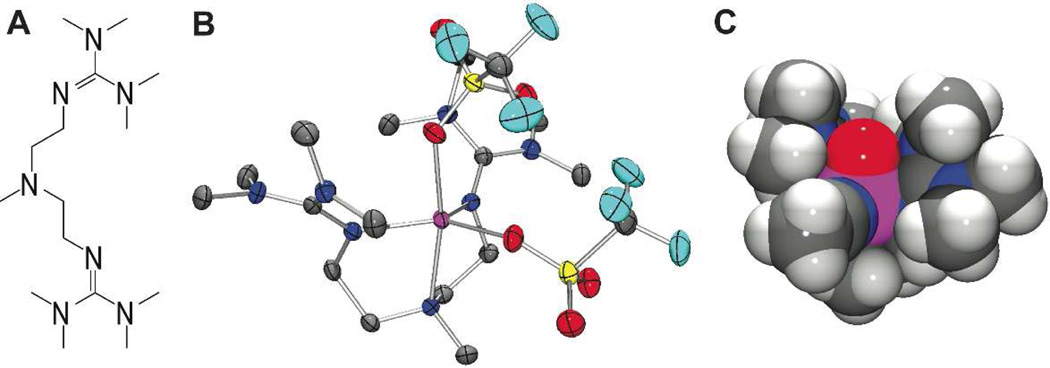
In an effort to assess and rationalize the inherent reactivity of the S = 2 oxoiron(IV) center in 2, we sought to (i) reduce the steric bulk of the supporting ligand and (ii) expand the palette of existing high-spin oxoiron(IV) complexes. Both aims are easily accommodated by replacement of one arm of the tripodal TMG3tren ligand with a methyl group to yield the tridentate TMG2dien ligand (Figure 1A).
Figure 1.
A: Structure of TMG2dien ligand. B: Thermal ellipsoid plot of [FeII(TMG2dien)(OTf)2] (4), showing 50% probability ellipsoids. Hydrogen atoms have been omitted for clarity. Selected bond distances (Å): Fe-Oaxial, 2.2012(15); Fe-Oequatorial, 2.0816(15); Fe-Naxial, 2.2835(17); Fe-Nguanidine(ave), 2.0597(17). C: Space-filling model of DFT-generated structure of 7. Atom color scheme: C, gray; F, light blue; Fe, magenta; H, white; N, blue; O, red; S, yellow.
The iron(II) starting material used in this study, [FeII(TMG2dien)(OTf)2] (4), was prepared by thallium(I) salt metathesis of the chloride ligands in [FeII(TMG2dien)(Cl)2] (5), which was itself generated by combination of equimolar quantities of TMG2dien and FeCl2. The high-resolution X-ray structures of 4 and 5 (Figures 1B and S1, Tables S1 and S2) reveal 5-coordinate complexes with a geometry that is intermediate between square pyramidal (SP) and TBP (τ = 0.64 and 0.56, respectively). 9 This contrasts with the strictly TBP geometry of [FeII(TMG3tren)(OTf)](OTf) (6, τ = 0.96), the iron(II) starting material used in the generation of 2.5a
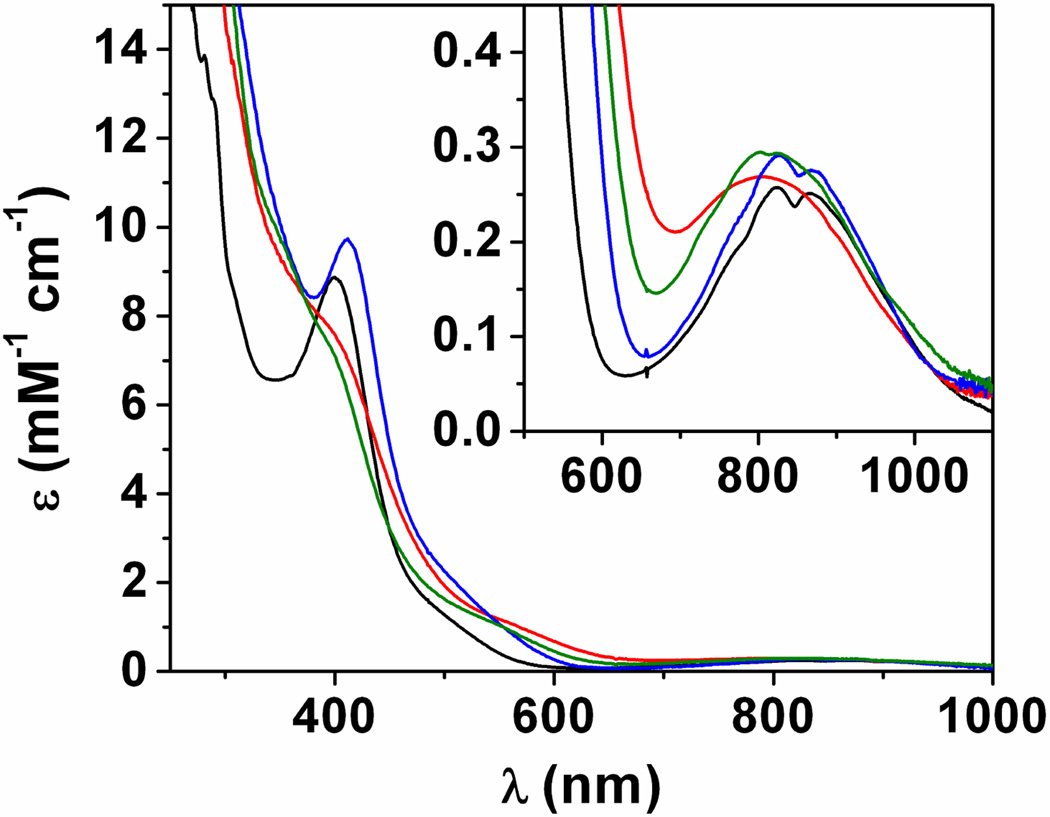
Treatment of a CH3CN solution of 4 with 2-(tert-butylsulfonyl) iodosylbenzene (2-(tBuSO2)C6H4IO)10 yields an orange-brown species 7 (t1/2 at −30 °C ≈ 0.5 h vs. 4.5 h for 2) whose electronic spectrum is reminiscent of 2 (Figure 2, Table 1), with a weak near IR (NIR) band centered at 805 nm (270 M−1 cm−1) and an intense UV absorption having a shoulder at ca. 380 nm (8200 M−1 cm−1). Notably, maximization of the intensity of the aforementioned NIR band requires addition of 2.5 – 3 equiv of oxidant. Mössbauer spectroscopy revealed that reaction with a single equivalent of oxidant leads to substoichiometric yields of 7 (45 %), with the remainder of the iron content being associated primarily with unreacted 4 (Figure S2). The absence of other iron products is consistent with non-productive reaction of 2-(tBuSO2)C6H4IO due to metal-catalyzed disproportionation, a process that has been documented for this oxidant.10 Additionally, the 19F NMR spectrum of 7 in CD3CN displayed a single peak at −80 ppm, which is indicative of free triflate and suggests that the potentially cis-labile site is filled by a solvent ligand. This observation, combined with the other data detailed herein, leads to formulation of 7 as [FeIV(O)(TMG2dien)(CH3CN)]2+.
Figure 2.
Main: electronic spectra of 2 (black line), 7 (red line), 7-N3 (blue line) and 7-Cl (green line) in CH3CN solution. Inset: expansion of the NIR region features.
Table 1.
Spectroscopic parameters of selected S = 2 oxoiron(IV) complexes.
| Complex | λmax [nm] (εmax [M cm−1]) |
νFe=O [cm−1] |
D [cm−1] |
E/D |
Ax,y,z/gnβn [T] |
ΔEQ [mm s−1] |
η | δ [mm s−1] |
E0 [eV] |
EPE [eV] (area) |
|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 380 (8200),a 805 (270) |
808 | 4.5b 4.2c |
0.09b 0.10c |
−13.9, −15.8, −26.0 | 0.58d | 0.5 | 0.08 | 7123.6 | 7113.3 (19.9), 7115.0 (3.1) |
| 7-N3 | 412 (9700), 827 (290), 867 (275) |
833 | 4.6b 5.0c |
0.04b 0.05c |
−15.5, −14.5, −27.0 | −0.30e | 0.35 | 0.12 | 7124.2 | 7113.8 (24.7), 7115.7 (9.2), |
| 7-Cl | 385 (7800),a 803 (295), 825 (293) |
810 | 4.1b 4.2c |
0.13b 0.14c |
−15.1, −15.4, −26.6 | 0.41 | 0.53 | 0.08 | 7123.9 | 7113.9 (21.9), 7115.7 (2.1) |
| 2f | 400 (8900), 825 (260), 865 (250) |
843 | 5.0 | 0.02 | −15.5, −14.8, −28.0 | −0.29 | 0 | 0.09 | 7123.2 | 7113.8 (23.9), 7115.6 (3.1) |
| 3g | 350 (4200), 440 (3100), 550 (1900), 808 (280) |
798 | 4.0 | 0.03 | - | 0.43 | - | 0.02 | - | - |
| 1h | 320 | - | 9.7 | 0 | −20.3, −20.3, nd | −0.33 | 0 | 0.38 | 7126 | 60–70 |
| TauD-Ji | 318 | 821 | 10.5 | 0.01 | −18.4, −17.6, −31.0 | −0.9 | 0 | 0.30 | 7123.8j |
Shoulder.
Determined by Mössbauer spectroscopy.
Determined by EPR.
The electric field gradient (EFG) tensor and the A-tensor are rotated relative to the ZFS tensor by αEFG = 50°, βEFG = 45° and αA = 55° (WMOSS convention); see SI for comments.
The EFG tensor and the A-tensor are rotated by αEFG = 30°, βEFG = 60° and αA = 20°.
Reference 5a.
Reference 6.
Reference 4.
Assuming an Fe foil reference E of 7112.0 eV.
One of the primary motivations for the development of the chemistry of the TMG2dien ligand was to generate a TBP high-spin oxoiron(IV) complex incorporating a labile site. To test the viability of our approach, tetraalkylammonium azide and chloride salts were added to pre-formed solutions of iron(IV) complex 7. This led to near instantaneous UV-Vis spectral changes (Figure 2, Table 1), consistent with substitution of the CH3CN ligand and formation of new complexes formulated as [FeIV(O)(TMG2dien)(X)]+ (7-X, X = N3, Cl), with the resultant spectra retaining the same general features as the parent complex 7 (i.e. a weak NIR band and an intense UV feature that trails into the visible region). Notably, 7-N3 is of comparable stability to 7, but 7-Cl undergoes self-decay at a significantly accelerated rate (t1/2 at −30 °C ≈ 34 and 2 min. for 7-N3 and 7-Cl, respectively).
The presence of a terminal Fe=O unit in complexes 7, 7-N3, and 7-Cl was confirmed by resonance Raman spectroscopy (Figure S3), which yielded ν(Fe=O) vibrational modes at 807, 833 and 810 cm−1, respectively. In close agreement with our expectations based upon Hooke’s Law (∆ν theoretical ≈ 36 – 37 cm−1), these bands shifted upon 18O-labelling to 773, 795 and 775 cm−1, respectively.
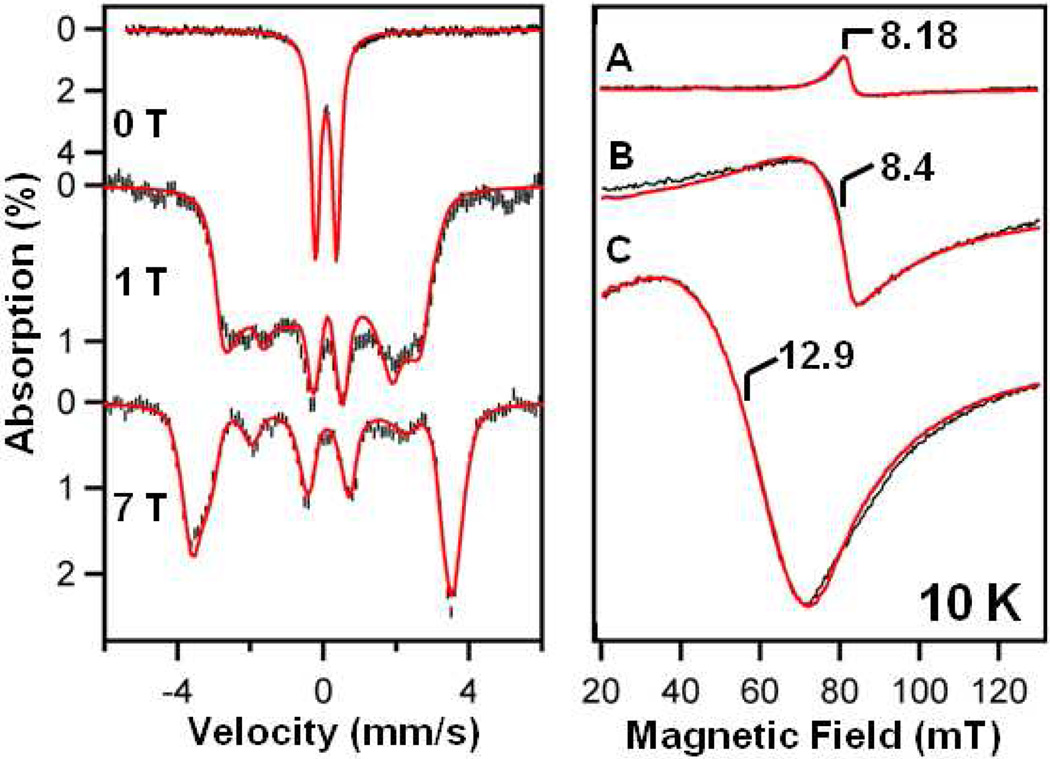
Mössbauer spectroscopy confirms that complexes 7, 7-N3, and 7-Cl all contain S = 2 oxoiron(IV) centers (Figures 3 (left panel) and S4–S7), with zero-field spectra each exhibiting a doublet with isomer shift δ ≈ 0.1 mm s−1 (Table 1). Their distinct quadrupole splittings, ∆ EQ, confirm formation of new anion complexes. The three species were obtained in high yield, with 7, 7-N3, and 7-Cl accounting for 88, 80 and 87 % (all ca. ±4%) of the Fe present, respectively. Minor iron(III) impurities account for the remainder. Fitting Mössbauer spectra observed at variable applied fields, B, to an S = 2 spin Hamiltonian (see SI) yielded zero-field splitting (ZFS) parameters (D, E/D) and hyperfine parameters that compare well with other S = 2 oxo-iron(IV) species.
Figure 3.
Left panel: Selected 4.2 K Mössbauer spectra of 7 in CH3CN (black) recorded in parallel applied magnetic fields, as indicated. For all spectra, a high-spin FeIII impurity, representing ~ 12% of the iron, has been subtracted from the raw data. Solid red lines are spectral simulations using the parameters in Table 1. Additional spectra are shown in Figures S4 – S7. Right panel: X-band EPR spectra (black) of (A) 7-N3, (B) 7, and (C) 7-Cl. Red lines are spectral simulations for (A) D = 5.0 cm−1, E/D = 0.05, σE/D = 0.02; (B) D = 4.2 cm−1, E/D = 0.1, σE/D = 0.03; (C) D = 4.2 cm−1, E/D = 0.14, σE/D = 0.016. In all simulations, intrinsic g values were kept isotopic (gx = gy = gz = 2). σE/D is the width of an assumed Gaussian distribution of E/D. Additional spectra are shown in Figures S8 – S10. Experimental conditions: B1‖ B; temperature, 10 K; microwave power, 2 mW (for B and C) and 20 mW (for A) at 9.28 GHz.
The high-spin ground state of 7, 7-N3, and 7-Cl and the D and E/D values obtained by Mössbauer spectroscopy were confirmed by parallel mode EPR (Figure 3 (right panel) and S8–S10). The X-band spectra of 7, 7-N3 and 7-Cl all displayed broad resonances at g ~ 8 – 13 that originate from the excited state MS = ± 2 quasi-doublet of an S = 2 multiplet. As the intensity of such signals is proportional to (E/D)4, quantitative simulations allowed quite accurate determinations of E/D (Table 1).11
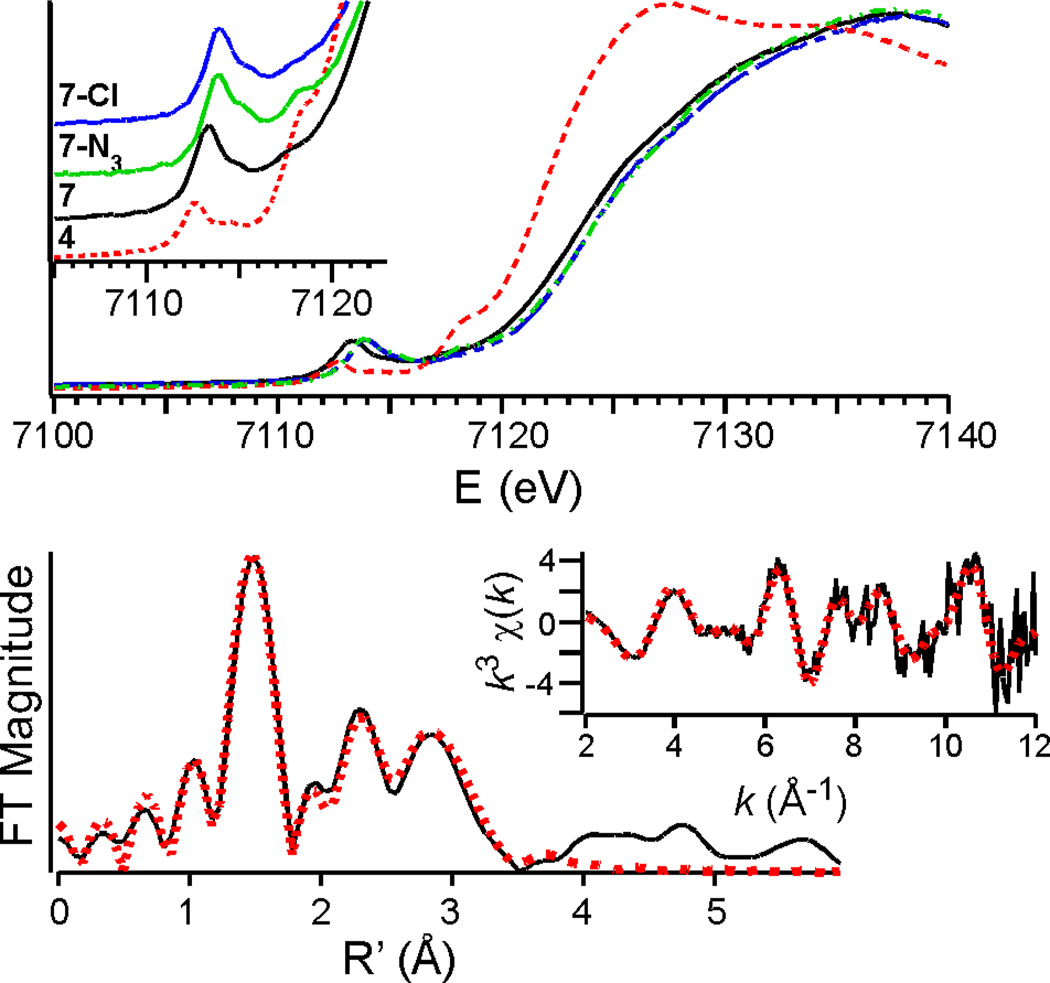
Consistent with their assignment as oxoiron(IV) complexes, 7, 7-N3 and 7-Cl exhibit XAS edge energies (E0) of ~7124 eV (Fig. 4, Table 1) that are in the range observed for other oxoiron(IV) complexes and blue-shifted by ≥ 1.7 eV relative to the iron(II) starting material 4 (E0 = 7121.9 eV). Unlike S = 1 oxoiron(IV) complexes, which exhibit a single symmetrical pre-edge feature,12 7, 7-N3 and 7-Cl all exhibit pre-edge features composed of two discernible peaks (Figure 4 top and Table S3), originating from 1s→3d electronic transitions. Consequently, two Gaussians are required to model them successfully, as predicted by DFT for high-spin iron(IV) complexes.13
Figure 4.
Top: X-ray absorption edge spectra of 4 (red), 7 (black), 7-N3 (green), and 7-Cl (blue). Inset: Expansion of the pre-edge region. Bottom: Fe K-edge unfiltered EXAFS data (k3χ (k), inset) and the corresponding Fourier transform for 7-Cl. The red dots and solid black lines correspond to the experimental data and fits, respectively. Fit of 7 and further details of the EXAFS analysis are provided in the Supporting Information.
Analysis of the EXAFS data for both 7 and 7-Cl furnished best fits (Tables S4 and S5, Figures 4 and S11) with an O/N scatterer at ~1.65 Å that corresponds to the Fe=O unit. (Thus far, we have been unable to collect high-quality EXAFS data for 7-N3 due to rapid photo-decomposition.) These distances are comparable to the Fe=O lengths observed in the X-ray structures of 2 and 3 (1.661(2) and 1.680(1) Å, respectively),5a,6 the EXAFS of enzymatic oxoiron(IV) intermediates,2e,14b,15 and the plethora of existing S = 1 oxoiron(IV) complexes.3,16 Both 7 and 7-Cl also have a shell of O/N scatterers at ca. 1.94 Å, four in the former case and three in the latter, assigned to the N-donors of the supporting ligands. This Fe-N distance is shorter than that found for 2 by EXAFS (1.99 Å),5a reflecting the lower steric constraints of the TMG2dien ligand. Lastly, 7-Cl has a Cl scatterer at 2.27 Å, a distance that is very similar to the 2.31 Å Fe–Cl distance obtained by EXAFS for the chloroferryl intermediate of the α-ketoglutarate-dependent aliphatic halogenase SyrB2.2e
DFT calculations for 7, 7-N3 and 7-Cl further support our S = 2 spin state assignment for these three complexes (Tables S6– S13). Complex 7, 7-N3, and 7-Cl all have a 5A ground state with four d electrons located in two half-filled E levels (Table S7). Spin populations calculated for the iron and the oxo atoms, respectively, are +3.0(1) and +0.6(1) (Tables S9 and S10), similar to the values obtained for 1, 2, and TauD-J.4a,5a,14c The DFT geometry-optimized structures of 7, 7-N3 and 7-Cl (Figures 1C and S12, Table S8) exhibit geometries that are best described as TBP (τ = 0.79, 0.83 and 0.72, respectively)9 and have Fe=O bond lengths of ~1.65 Å, in close agreement with values obtained from EXAFS. In contrast, the Fe-Cl distance of 2.35 Å calculated for 7-Cl is somewhat longer than the value of 2.27 Å determined by EXAFS. Lastly, the DFT-calculated spin-dipolar contribution to the 57Fe A-tensor is in good agreement with the experimental data (Table S6), indicating that the z axis of the spin Hamiltonian (determined by the ZFS tensor) is oriented along the Fe-O bond (within about 5°).
In addition to creating a S = 2 oxoiron(IV) complex with a cis-labile site, it was anticipated that removing one of the bulky tetramethylguanidinyl donors of the TMG3tren ligand to give TMG2dien would provide substrates greater access to the FeIV=O unit, thereby allowing the inherent reactivity properties of the S = 2 oxoiron(IV) center to be manifested. Consistent with these expectations, the oxo-transfer reaction of 7 to PPh3 proceeded so rapidly that we were unable to accurately measure the associated rate constants at −30 °C for comparison with published data for other oxoiron(IV) complexes listed in Table 2. Additionally, H-atom abstraction from 1,4-cyclohexadiene (CHD) and 9,10-dihydroanthracene (DHA), substrates with similarly weak C–H bonds but differing steric profiles, proceeded at comparable rates, with respective second-order rate constants 15 and 630 times larger than for the more sterically hindered 2 (Table 2, Figures S13–S15).
Table 2.
Second-order rate constants (k2) observed in reactions of FeIV=O complexes with substrates.
| Complex a | k2 [M−1 s−1] in CH3CN solution at −30°C b | ||
|---|---|---|---|
| CHD | DHA | PPh3 | |
| 7 | 18 | 57 | c |
| 2 d | 1.2 | 0.090 | 1.1 |
| 8 d | 1.3 | 2.0 | 1.5 |
| 9 d | 0.018 | 0.016 | 0.22 |
| 10 e | 94 | 310 | c |
[FeIV(O)(N4Py)]2+ (8, N4Py = bis(2-pyridylmethyl)-bis(2-pyridyl)-methylamine), [FeIV(O)(TMC)(CH3CN)]2+ (9, TMC = 1,4,8,11-tetramethylcyclam), [FeIV(O)(Me3NTB)]2+ (10, Me3NTB = tris((N-methyl-benzimidazol-2-yl)methyl)amine)).
Reaction kinetics for 2, 8 and 9 were measured using 1.0 mM complex. For 7 the larger rates of reaction required the use of 0.1–0.2 mM complex and similarly reduced concentrations of substrate.
This reaction was too fast for measurement of kobs.
Kinetic data from reference 5a.
Kinetic data at −40 °C taken from reference 17.
Notably, 7 exhibits reactivity more than one and three orders of magnitude greater than the S = 1 complexes [FeIV(O)(N4Py)]2+ (8) and [FeIV(O)(TMC)(CH3CN)]2+ (9) (Table 2), respectively, which would appear to support DFT-based predictions of a more reactive S = 2 FeIV=O center relative to a S = 1 FeIV=O center.7 However, 7 is an order of magnitude less reactive than the recently reported S = 1 complex [FeIV(O)(Me3NTB)]2+ (10).17 This fact serves to highlight the difficulty of making such comparisons without consideration of the thermodynamic and steric consequences of the differing ligand environments of the various complexes. Thus far, there is only one pair of closely related complexes that have identical ligand environments, namely [(HO)(L)FeIII/IV–O–FeIV(L)(O)] where L = tris(3,5-dimethyl-4-methoxypyridyl–2-methyl)amine, but differ in having a S = 1 or S = 2 oxoiron(IV) unit.18 Remarkably, the high-spin FeIIIFeIV complex was found to be a thousandfold more reactive than the low-spin FeIVFeIV complex, thereby providing support for the DFT-based predictions.
In summary, we have described the synthesis of the high-spin oxoiron(IV) complex [FeIV(O)(TMG2dien)(CH3CN)]2+ (7), which is related to the S = 2 complex [FeIV(O)(TMG3dien)(CH3CN)]2+ (2) by replacement of one of the tetramethylguanidinyl arms of the TMG3tren ligands by a methyl group and inclusion of a solvent ligand in its place. This modification provides greater access to the FeIV=O subunit, eliminating the selectivity for smaller substrates exhibited by 2 and resulting in a significant increase in the rates of intermolecular reactions. Furthermore, the introduction of CH3CN as an equatorial ligand in 7 provides a means to access a series of closely related anion substituted S = 2 oxoiron(IV) complexes that are highly amenable to characterization, as illustrated here for [FeIV(O)(TMG2dien)(X)]+ (7-X, X = N3, Cl). This offers the promise of elucidating spectroscopic and reactivity trends as a function of the electronic properties of an S = 2 oxoiron(IV) center and may provide answers to specific bio-relevant questions, such as the reason for the omnipresence of carboxylato ligands in non-heme enzymes1 and for the inherent preference for halogen versus oxygen atom rebound in non-heme iron halogenase enzymes. 19
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH (grants GM33162 to LQ and EB001475 to EM and postdoctoral fellowships ES017390 to MAC and GM093479 to KMVH) and the NSF (grants CHE1058248 to LQ and CHE070073 to ELB through Teragrid resources provided by NCSA). XAS data were collected on beamline X3B at the National Synchrotron Light Source (NSLS), Brookhaven National Laboratory. NSLS is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. We thank Michael Sullivan for technical assistance with our XAS experiments. Data collection and structure solution were conducted by Victor G. Young, Jr. at the X-Ray Crystallographic Laboratory, Department of Chemistry, University of Minnesota.
Footnotes
SUPPORTING INFORMATION PARAGRAPH: Experimental and synthetic details, resonance Raman spectra and additional Xray crystallographic, Mössbauer, EPR, XAS, DFT and kinetics information. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Krebs C, Galonić Fujimori D, Walsh CT, Bollinger JM., Jr Acc. Chem. Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kovaleva EG, Lipscomb JD. Nat. Chem. Biol. 2008;4:186–193. doi: 10.1038/nchembio.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Eser BE, Barr EW, Frantom PA, Saleh L, Bollinger JM, Jr, Krebs C, Fitzpatrick PF. J. Am. Chem. Soc. 2007;129:11334–11335. doi: 10.1021/ja074446s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Price JC, Barr EW, Tirupati B, Bollinger JM, Jr, Krebs C. Biochemistry. 2003;42:7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]; (c) Hoffart LM, Barr EW, Guyer RB, Bollinger JM, Jr, Krebs C. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14738–14743. doi: 10.1073/pnas.0604005103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Galonić DP, Barr EW, Walsh CT, Bollinger JM, Jr, Krebs C. Nat. Chem. Biol. 2007;3:113–116. doi: 10.1038/nchembio856. [DOI] [PubMed] [Google Scholar]; (e) Matthews ML, Krest CM, Krest CM, Barr EW, Vaillancourt FH, Walsh CT, Green MT, Krebs C, Bollinger JM., Jr Biochemistry. 2009;48:4331–4343. doi: 10.1021/bi900109z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Que L., Jr Acc. Chem. Res. 2007;40:493–500. doi: 10.1021/ar700024g. [DOI] [PubMed] [Google Scholar]
- 4.Pestovsky O, Stoian S, Bominaar EL, Shan X, Münck E, Que L, Jr, Bakac A. Angew. Chem., Int. Ed. 2005;44:6871–6874. doi: 10.1002/anie.200502686. [DOI] [PubMed] [Google Scholar]
- 5.(a) England J, Martinho M, Farquhar ER, Frisch JR, Bominaar EL, Münck E, Que L., Jr Angew. Chem., Int. Ed. 2009;48:3622–3626. doi: 10.1002/anie.200900863. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) England J, Guo Y, Farquhar ER, Young VG, Jr, Münck E, Que L., Jr J. Am. Chem. Soc. 2010;132:8635–8644. doi: 10.1021/ja100366c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacy DC, Gupta R, Stone KL, Greaves J, Ziller JW, Hendrich MP, Borovik AS. J. Am. Chem. Soc. 2010;132:12188–12190. doi: 10.1021/ja1047818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Ye S, Neese F. Curr. Opin. Chem. Biol. 2009;13:89–98. doi: 10.1016/j.cbpa.2009.02.007. [DOI] [PubMed] [Google Scholar]; (b) Decker A, Rohde J-U, Klinker EJ, Wong SD, Que L, Jr, Solomon EI. J. Am. Chem. Soc. 2007;129:15983–15996. doi: 10.1021/ja074900s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bernasconi L, Louwerse MJ, Baerends EJ. Eur. J. Inorg. Chem. 2007:3023–3033. [Google Scholar]; (d) Hirao H, Kumar D, Que L, Jr, Shaik S. J. Am. Chem. Soc. 2006;128:8590–8606. doi: 10.1021/ja061609o. [DOI] [PubMed] [Google Scholar]
- 8.(a) Janardanan D, Wang Y, Schyman P, Que L, Jr, Shaik S. Angew. Chem., Int. Ed. 2010;49:3342–3345. doi: 10.1002/anie.201000004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wong SD, Bell CB, III, Liu LV, Kwak Y, England J, Zhao J, Que L, Jr, Solomon EI. Angew. Chem. Int. Ed. 2011;50:3215–3218. doi: 10.1002/anie.201007692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addison AW, Rao TN, Reedijk J, Van Rijn J, Verschoor GC. J. Chem. Soc., Dalton Trans. 1984:1349–1356. [Google Scholar]
- 10.Macikenas D, Skrzypczak-Jankun E, Protasiewicz JD. J. Am. Chem. Soc. 1999;121:7164–7165. [Google Scholar]
- 11.Surerus KK, Hendrich MP, Christie PD, Rottgardt D, Orme-Johnson WH, Münck E. J. Am. Chem. Soc. 1992;114:8579–8590. [Google Scholar]
- 12.(a) Jackson TA, Rohde JU, Seo MS, Sastri CV, DeHont R, Stubna A, Ohta T, Kitagawa T, MAünck E, Nam W, Que L., Jr J. Am. Chem. Soc. 2008;130:12394–12407. doi: 10.1021/ja8022576. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rohde J-U, Torelli S, Shan X, Lim MH, Klinker EJ, Kaizer J, Chen K, Nam W, Que L., Jr J. Am. Chem. Soc. 2004;126:16750–16761. doi: 10.1021/ja047667w. [DOI] [PubMed] [Google Scholar]
- 13.Berry JF, DeBeer George S, Neese F. Phys. Chem. Chem. Phys. 2008;10:4361–4374. doi: 10.1039/b801803k. [DOI] [PubMed] [Google Scholar]
- 14.(a) Proshlyakov DA, Henshaw TF, Monterosso GR, Ryle MJ, Hausinger RP. J. Am. Chem. Soc. 2004;126:1022–1023. doi: 10.1021/ja039113j. [DOI] [PubMed] [Google Scholar]; (b) Riggs-Gelasco PJ, Price JC, Guyer RB, Brehm JH, Barr EW, Bollinger JM, Jr, Krebs C. J. Am. Chem. Soc. 2004;126:8108–8109. doi: 10.1021/ja048255q. [DOI] [PubMed] [Google Scholar]; (c) Sinnecker S, Svensen N, Barr EW, Ye S, Bollinger JM, Jr, Neese F, Krebs C. J. Am. Chem. Soc. 2007;129:6168–6179. doi: 10.1021/ja067899q. [DOI] [PubMed] [Google Scholar]
- 15.Fujimori DG, Barr EW, Matthews ML, Koch GM, Yonce JR, Walsh CT, Bollinger JM, Jr, Krebs C, Riggs-Gelasco PJ. J. Am. Chem. Soc. 2007;129:13408–13409. doi: 10.1021/ja076454e. [DOI] [PubMed] [Google Scholar]
- 16.(a) Rohde J-U, In J-H, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L., Jr Science. 2003;299:1037–1039. doi: 10.1126/science.299.5609.1037. [DOI] [PubMed] [Google Scholar]; (b) Klinker EJ, Kaizer J, Brennessel WW, Woodrum NL, Cramer CJ, Que L., Jr Angew. Chem., Int. Ed. 2005;44:3690–3694. doi: 10.1002/anie.200500485. [DOI] [PubMed] [Google Scholar]; (c) Thibon A, England J, Martinho M, Young VG, Jr, Frisch JR, Guillot R, Girerd J-J, Münck E, Que L, Jr, Banse F. Angew. Chem., Int. Ed. 2008;47:7064–7067. doi: 10.1002/anie.200801832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo MS, Kim NH, Cho K-B, So JE, Park SK, ClAémancey M, Garcia-Serres R, Latour J-M, Shaik S, Nam W. Chem. Sci. 2011;2:1039–1045. [Google Scholar]
- 18.Xue G, De Hont R, Münck E, Que L., Jr Nat. Chem. 2010;2:400–405. doi: 10.1038/nchem.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Matthews ML, Neumann CS, Miles LA, Grove TL, Booker SJ, Krebs C, Walsh CT, Bollinger JM., Jr Proc. Natl. Acad. Sci. U.S.A. 2009;106:17723–17728. doi: 10.1073/pnas.0909649106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) de Visser SP, Latifi R. J. Phys. Chem. B. 2008;113:12–14. doi: 10.1021/jp8097632. [DOI] [PubMed] [Google Scholar]; (c) Kulik HJ, Blasiak LC, Marzari N, Drennan CL. J. Am. Chem. Soc. 2009;131:14426–14433. doi: 10.1021/ja905206k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Borowski T, Noack H, RadonÌ M, Zych K, Siegbahn PEM. J. Am. Chem. Soc. 2010;132:12887–12898. doi: 10.1021/ja101877a. [DOI] [PubMed] [Google Scholar]; (e) Pandian S, Vincent MA, Hillier IH, Burton NA. Dalton Trans. 2009:6201–6207. doi: 10.1039/b906866j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.