Abstract
Viscosity is proposed to modulate diastolic function, but only limited understanding of the source(s) of viscosity exists. In-vitro experiments have shown that the proline-glutamic acid-valine-lysine (PEVK) rich element of titin interacts with actin, causing a viscous force in the sarcomere. It is unknown whether this mechanism contributes to viscosity in-vivo. We tested the hypothesis that PEVK-actin interaction causes cardiac viscosity and is important in-vivo via an integrative physiological study on a unique PEVK-knockout (KO) model. Both skinned cardiomyocytes and papillary muscle fibers were isolated from wildtype (WT) and PEVK KO mice and passive viscosity was examined using stretch-hold-release and sinusoidal analysis. Viscosity was reduced by ~60% in KO myocytes and ~50% in muscle fibers at room temperature. The PEVK-actin interaction was not modulated by temperature or diastolic calcium, but was increased by lattice compression. Stretch-hold and sinusoidal frequency protocols on intact isolated mouse hearts showed a smaller, 30–40% reduction in viscosity, possibly due to actomyosin interactions, and showed that microtubules did not contribute to viscosity. Transmitral Doppler echocardiography similarly revealed a 40% decrease in LV chamber viscosity in the PEVK KO in-vivo. This integrative study is the first to quantify the influence of a specific molecular (PEVK-actin) viscosity in-vivo and shows that PEVK-actin interactions are an important physiological source of viscosity.
Keywords: Titin/connectin, actin, diastole, in-vivo, passive force
INTRODUCTION
Similar to all biological materials the myocardium is viscoelastic, with viscosity revealed as a force that resists deformation and that increases with strain rate. Viscosity is likely to be a key aspect of cardiac function affecting ventricular relaxation and the resistance to filling during diastole and, importantly, may play a role in diastolic dysfunction[1–2]. While the underlying mechanism(s) of cardiac viscosity has not been firmly established, it has been hypothesized, based on in vitro studies, that titin is a major source of viscosity[3]. At more than 3MDa, titin is the largest protein known and contains an elastic region composed of serially-linked immunoglobulin-like (Ig) domains, a unique N2B sequence and a proline-glutamic acid-valine-lysine (PEVK) rich element. Although it was originally thought that reversible unfolding of titin’s Ig domains might be a source of viscosity[4–5], subsequent single molecule and simulation studies have shown that the forces required for unfolding are likely to exceed physiological levels[6–10]. More recent protein studies have shown that the PEVK spring element, but not the Ig or N2B domains, produces a viscous interaction by interacting with actin[11–13]. The functional effect of this PEVK-actin interaction has been studied in, for example, in vitro motility assays[12] and in single myofibrils extracted with gelsolin (to remove the actin filament) or degraded with trypsin (to degrade titin)[12, 14], but conclusive evidence for a functional role of PEVK-actin interactions in-vivo remains to be established. In this study, we examined the physiological relevance of PEVK-actin interaction in a set of integrative experiments from skinned cells to in-vivo physiology, utilizing a unique PEVK knockout (KO) mouse model[15]. The PEVK KO mouse has a deletion of PEVK exons expressed in all muscle tissues and that are the only ones expressed in the dominant mouse cardiac N2B isoform[15–16], making it well suited for studying the effect of PEVK-actin interactions in-vivo.
MATERIALS AND METHODS (For details, see Supplement)
ANIMALS
The PEVK KO mouse is a conventional KO in which exons 219-225 have been deleted from the titin gene[15]. We used male, age-matched (mean age 5 months) PEVK KO and WT mice for all studies. A N2B KO mouse model, a conventional KO of exon 49, encoding the N2B region of titin[17] provided additional fibers for comparison. All experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and followed the U.S. National Institutes of Health “Using Animals in Intramural Research” guidelines for animal use.
SKINNED CELL AND FIBER PREPARATION AND MECHANICS
Skinned WT and PEVK KO cardiomyocytes were stretched from their slack sarcomere length (SL) to SL 2.15µm, with ramp speeds from 0.01 to 10 lengths/s, held for 20 seconds, a sinusoidal length change was imposed using frequencies from 0.1Hz to 100Hz, and were then released (Fig.1A). Skinned muscle fibers were obtained from WT, PEVK KO and N2B KO mice. Fibers were stretched from slack SL to 2.15µm, at speeds from 0.1 to 50.0 lengths/s, held for 90 seconds, a sinusoidal frequency sweep was applied with frequencies from 0.1 to 400Hz, and were then released. Myofilaments were extracted and protocols repeated to reveal the ECM vs. titin-based passive properties (see supplement for details). WT and PEVK KO fibers were studied at 22 and 37°C in the presence and absence of blebbistatin[18]; WT and PEVK KO tissues were also studied in relaxing solution that contained 3% Dextran, to examine more physiological lattice spacing, and a calcium level of ~150 nmol to examine the effect of diastolic calcium. Stretch-hold release experiments were used to calculate the viscous stress and viscosity[3] and frequency sweeps analyzed to obtain the viscous moduli[19–20].
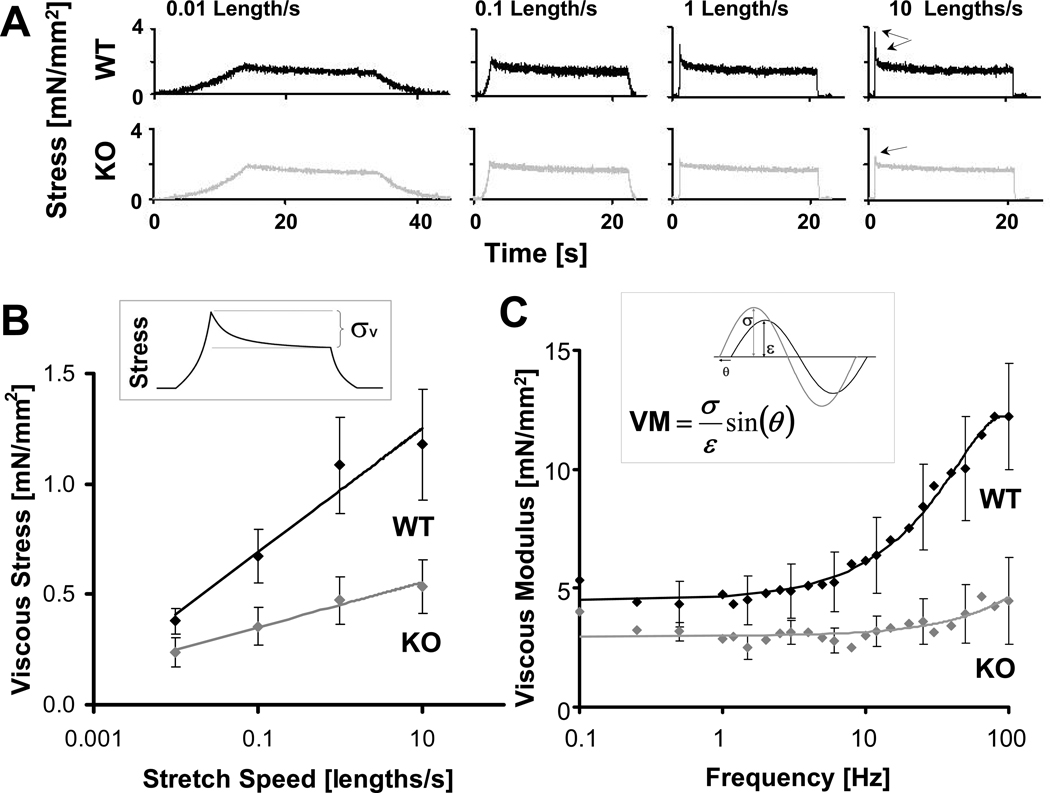
Figure 1.
Viscosity in skinned cardiomyocytes. A) Example of stretch-hold-release experiments for WT (black) and KO (gray) cardiomyocytes stretched at different speeds. Stress relaxation during the hold phase is less pronounced in the KO cells (arrows). B) KO (gray) cells have a significantly reduced viscous stress at all speeds and a 67% reduction in the coefficient of viscosity (slope) compared to WT (black). Inset: Schematic of how viscous stress (σv) is defined. C) KO (gray) cells have smaller viscous moduli than WT (black) cells. (Every third error bar (SE) plotted for clarity.) Inset: Schematic showing how the viscous modulus (VM) is calculated from the magnitude of the stress (σ, gray), the length change (ε, black) and the phase delay (θ) between steady state stress and length traces. (Length change was normalized to prep length and viscous modulus is in stress per fractional length change).
ISOLATED HEART MECHANICS
Isolated perfused mouse hearts were prepared as previously described[15, 17]. Ramp-hold volume perturbations (corresponding to a midwall strain rate of ~1 length/s) were used to obtain viscous stress in hearts; sinusoidal oscillations were imposed at frequencies from 10 to 60Hz. Isolated hearts were also treated with colchicine to evaluate the contribution of microtubules to viscosity; qRT-PCR of tubulin expression levels and immunofluorescence staining of microtubules in WT and PEVK KO hearts was also performed (see supplemental data for details). Data was converted to wall stress[15] and viscous stress and viscous moduli were calculated.
IN-VIVO ECHOCARDIOGRAPHIC EXPERIMENTS
Transmitral Doppler echocardiography provided the E-wave acceleration time (AT) and deceleration time (DT) and the difference (DT-AT) used as a marker for viscosity[21]. Additional quantitative, independent viscosity measurements were obtained according to a kinematic model defined by the Parameterized Diastolic Filling Formalism[21] providing a viscosity (c) parameter.
STATISTICS
T-Test or ANOVA was used to calculate significance as appropriate; p-values <0.05 were considered significant. See Supplement for details.
RESULTS
VISCOSITY IN CARDIAC TISSUES
To study the extent to which the interaction between titin’s PEVK element and actin contribute to passive viscosity, we characterized the viscosity of WT and PEVK KO mouse cardiac myocytes and myocardial tissues using two experimental approaches. First, we imposed stretch-hold-release protocols using multiple stretch speeds from slack SL to SL 2.15 µm (see Fig.1A and Fig.2A for examples) to probe viscosity in the physiologic sarcomere length range of the mouse[22–23]. The peak minus steady-state stress during the hold phase was characterized as a viscous stress (Fig.1B, inset)[3]. Viscosity was calculated as the slope of the viscous stress versus (log) stretch speed. Second, a sinusoidal analysis at a wide range of frequencies was used to determine the complex stiffness and phase shift between imposed length and measured force change (Fig.1C, inset), and from this we calculated the viscous modulus at each frequency. These two methodologies provide a broad base for evaluating the viscosity of cardiac myocytes and tissues.
Figure 2.
Viscosity in skinned cardiac tissue. Skinned WT and KO papillary muscle fibers were stretched at 4 speeds. In (A) the slowest (0.1 length/s; black) and fastest (50 lengths/s; gray) speeds are shown superimposed for WT (left) and KO (right) tissues with WT showing a higher speed dependence of viscous stress-relaxation (Arrows). B) KO tissues (gray) have a reduced viscous stress and a 47% reduction in the coefficient of viscosity compared to WT (black) tissues. Myofilament extraction reveals almost no viscous phenotype for the ECM. C) Viscous moduli are significantly reduced in the KO vs. WT tissues, but no ECM effect. (Viscous modulus was normalized to slack fiber length).
The peak minus steady state viscous stress in cardiomyocytes was different at all speeds and, importantly, a 67% reduction in viscosity was found in PEVK KO cells (Fig.1B). This suggests that, while viscosity is not completely removed, a majority of the viscosity in myocytes is abolished in the PEVK KO. For myocardial tissues with an extracellular matrix (ECM), we performed experiments on skinned cardiac papillary muscle fibers and determined the titin- and ECM-based viscosity (see Methods). Again, WT and KO fibers showed differences in viscous stresses, and importantly, a 46% reduction in titin-based viscosity in the PEVK KO was found (Fig.2B), which supports the conclusions from the myocyte data that in the KO viscosity is much less than in the WT. The viscosity of the ECM is much smaller than that of the myofilaments and is not different between WT and KO (Fig.2B). Thus, the PEVK KO has significantly less titin-based viscosity than WT tissue.
The viscous moduli of the cells was significantly reduced in the KO compared to the WT, at all frequencies tested (Fig.1C). Importantly, at physiological frequencies in the mouse (10–12 Hz) and man (1–2Hz), we found on average a 50% decrease in viscous moduli of cardiomyocytes. Oscillations imposed on muscle fibers support the findings in cells (Fig.2C). At physiological frequencies, we also found an average 37% decrease in viscous moduli from WT to KO tissues. Again, myofilament extracted sinusoidal responses indicated that there is no difference between WT and KO in viscosity due to the ECM. Overall these data support the finding obtained with the stretch-hold-release experiments that compared to WT, PEVK KO tissues have a much reduced viscosity.
The deletion in the PEVK KO eliminates the PEVK spring element from titin’s extensible region and, as a result, increases the strain of the remaining spring elements more than in WT muscle for a given sarcomere stretch[15]. This increased strain, one could argue, might pull titin away from the thin filament and lower thereby viscosity. We tested whether increased strain itself lowers viscosity by studying a mouse model in which the N2B element has been deleted (N2B KO) and where a given sarcomere stretch also results in a larger strain than in WT[17]. We have previously shown that the extension of the N2B and PEVK elements are similar at the maximal sarcomere length used in this study (at 2.15 µm, both provide ~50 nm of extensibility)[24]. Thus, if increased titin strain lowers viscosity in the PEVK KO, we expect viscosity to be similarly reduced in the N2B KO. However, viscosity was not different in N2B KO mice compared to WT (Supplemental Fig.S1A), suggesting that the PEVK deletion, not increased strain, reduces viscosity in the PEVK KO. The increased titin strain in the PEVK KO also appears to not cause functional or structural changes elsewhere in the sarcomere, as suggested by an absence of differences in maximal active tension in WT and PEVK KO tissues, an absence of differences in expression and phosphorylation of thin and thick filament proteins and an absence of differences in sarcomeric ultrastructure (Supplemental Fig.S1 and Fig.S2, see also[15]). In summary, reduced viscosity is unique to the PEVK KO model which supports that the effect is caused by abolishing PEVK-actin interaction.
The skinned fiber mechanics experiments were performed at room temperature (RT, 24°C), and we also evaluated the effect of physiological temperature (37°C) on viscosity. Because we recently showed that physiological temperature results in a low level of actomyosin interaction[25], we performed these experiments both in the absence and presence of the actomyosin inhibitor blebbistatin[18]. At physiological temperature, viscosity was increased in both WT and PEVK KO tissues; this effect was abolished by blebbistatin (Fig.3). At room temperature, blebbistatin did not change viscosity suggesting an absence of actomyosin interactions at this temperature (Fig.3). Thus, increasing from room temperature to physiological temperature increases viscosity, an increase that can be accounted for by actomyosin interaction.
Figure 3.
A) Effect of actomyosin inhibitor blebbistatin on passive viscosity of skinned muscle at room (RT, 24°C) and physiological temperature (37°C). At RT blebbistatin has no effect on viscosity. Physiological temperature increases viscosity but this increase is abolished by blebbistatin. Finding are the same in WT (left) and PEVK KO (right) tissues. * p<0.05 vs RT -.
Because skinning results in myofilament lattice expansion[26], we also evaluated the effect of lattice compression with dextran T-500 on viscosity in the presence of blebbistatin to eliminate the influence of actomyosin interactions. At room temperature, lattice compression by dextran led to a 32±4% viscosity increase in WT tissues; there was no significant change in PEVK KO tissues (−6±2%) (Supplemental Fig.S3). The increase by lattice compression in WT tissues at physiological temperature was 50±7% (p<0.05) and again there was no effect in the PEVK KO tissues (14±12%)) (Supplemental Fig.S3). These findings suggest that physiological lattice compression increases PEVK-actin interaction in WT tissues.
Finally, we tested the effect of diastolic levels of calcium on viscosity, and used for this a calcium concentration of 150 nmol, or pCa 6.8[27] at room temperature. No change in the viscosity was measured in WT or PEVK KO fibers compared to standard pCa 9.0 relaxing solution (−3±4% in WT and 4±3% in PEVK KO, Supplemental Fig.S4). Thus, diastolic calcium does not appear to directly influence PEVK-actin interactions.
VISCOSITY IN MOUSE HEARTS
In order to quantify the difference in viscosity between WT and PEVK KO mice at the level of the whole heart, we performed ex-vivo isolated heart experiments in which we imposed stretch-hold-release protocols and sinusoidal oscillations during the diastolic period (see Methods). Due to limitations in pacing and the frequency response of the isolated heart system, we only used one stretch speed (corresponding to a LV midwall strain rate of approximately 1 length/s; Fig.4A), and calculated viscous (peak minus steady state) wall stress. The viscous stress was different between WT and PEVK KO (p=0.02) demonstrating that KO hearts have a 32% reduction in viscosity compared to WT (Fig.4B). The viscous moduli of the KO hearts during sinusoidal oscillation (10Hz example shown in Fig.4C) showed an average 43±2% decrease from WT hearts (Fig.4D). Because microtubules have been hypothesized as a source of viscosity[28–29], we also examined their effect in WT and PEVK KO hearts. Colchicine has been shown to rapidly depolymerize microtubules within minutes of perfusion at low concentrations[30]; thus, stretch-hold experiments were repeated before and repeatedly during 30 minutes of colchicine perfusion. Our data shows that there was no change in viscous index (Supplemental Fig.S5A), and we confirmed that tubulin expression was not different by qRT-PCR and immunofluorescence staining, indicating no difference between mRNA expression or polymerized tubulin (Supplemental Fig.S5B-C). These results indicate that microtubule depolymerization did not affect viscosity in either WT or PEVK KO LVs.
Figure 4.
Diastolic viscosity in isolated hearts. A) Typical stretch-hold-release experiment in heart; viscous stress was calculated as the peak minus stress at end of hold. B) KO (gray) hearts have a 32% reduction in viscous stress compare to WT (black,*p=0.01). C) Example sinusoidal oscillation (10Hz) induced on the beating heart; viscous modulus was calculated from the oscillation during the diastolic plateau (box). D) Viscous moduli calculated at 6 frequencies during a diastolic plateau. KO hearts show an average 43% reduction in viscous moduli compared to WT.
We also performed cardiac Doppler ultrasound on WT and KO mice to determine the in-vivo contribution of PEVK-actin interaction to viscosity. The deceleration time (DT) during early diastolic filling (E-wave) was shorter in the KO animals (Fig.5; Table.1) consistent with increased stiffness shortening the early diastolic filling period as published previously[15]. However, only for a perfectly symmetric E-wave is DT inversely related to stiffness alone; with the contribution of viscosity, the symmetry is broken and acceleration time (AT) shortens and DT is prolonged[21]. The analysis of AT revealed a prolongation in the KO (p<0.008, Table 1). We examined the symmetry (difference between DT and AT) as a quantifiable measurement of viscosity and found that the KO diastolic inflow patterns are more symmetric (Table 1, Fig.5), suggesting significant reduction in viscosity. These measurements required the use of anesthetized mice to obtain clear E-waves and prevent the atrial contraction from starting before the E-wave decelerates (heart rate WT=338±22 bpm, KO=361±16 bpm; ns). However, we show that AT and DT measurements are not dependent on heart rate (Supplemental Fig.S6), consistent with findings in humans[31]. To corroborate these findings with an independent model of viscosity, we obtained stiffness and viscosity indexes by fitting the data to a kinematic model that derives an index of viscosity (c) (Fig.5C–D)[21]. This model suggests that the viscosity had a significant 42% decrease from WT to KO values (p<0.005, Table 1). Thus, using various methods the KO hearts consistently show a viscosity decrease.
Figure 5.
Viscosity in-vivo. Transmitral Doppler from WT (A) and KO (B) hearts show an asymmetric E-wave for the WT and a more symmetric E-wave in the KO. AT (gray line) is shorter in WT (12ms) than KO (19ms) while DT (black line) is longer in WT (29ms) than KO (25ms). Characterizing the same WT (C) and KO (D) hearts using a viscoelastic model indicates that the WT has a prolonged deceleration phenotype (tail) while the KO has a more symmetric shape that corresponds to a reduced viscosity. (Vertical scale lines denote 50cm/s velocity. Images contrast-adjusted for clarity.)
Table 1.
In-vivo Echocardiographic Viscosity Parameters.
| Standard Parameters | Kinematic Parameter | |||
|---|---|---|---|---|
| AT [ms] | DT [ms] | DT-AT [ms] | c [1/s] | |
| WT | 12.4±0.4 | 28.8±1.0 | 16.4±0.7 | 163±13 |
| KO | 15.4±0.9 | 25.1±0.7 | 9.7±1.1 | 94±13 |
| p-value | 0.008 | 0.009 | <0.001 | 0.005 |
|
Parameter Interpretation |
↑=↓viscosity or ↓stiffness |
↑=↑viscosity or ↓stiffness |
↑=↑viscosity | ↑=↑viscosity |
AT: acceleration time; DT: Deceleration time; c: independent kinematic viscosity parameter.
DISCUSSION
Viscosity is a resistive force that opposes the expansion of the left ventricle during the filling phase of the heart, affecting diastolic relaxation[1] and early filling[2, 16, 21]. Titin has been suggested to be an important source of viscosity[3, 27], and we focused on the role of titin’s PEVK region, which has been suggested to generate viscosity by interacting transiently with the actin filament[12, 14, 16, 32–33]. The PEVK KO provides a unique opportunity to probe the effects of PEVK-actin interactions from single cardiac cells to in-vivo levels. Our results reveal at all levels of investigation that viscosity is reduced in the PEVK KO, supporting an important role for PEVK-actin interaction in diastole.
Results from skinned tissues show that myocardial viscosity is significantly reduced in the PEVK KO. However, viscosity is not completely abolished in skinned myocytes and skinned muscle and the source of the remaining viscosity warrants discussion. In the PEVK KO, exons 219-225 have been deleted from the titin gene[15], exons that are expressed in all known titin isoforms[34] and that comprise the full PEVK region of the dominant N2B cardiac titin isoform. A second cardiac isoform, the N2BA cardiac titin isoform, is larger than N2B titin (~3.3 vs. ~3.0 MDa) and contains PEVK exons 219-225 but also additional PEVK sequences that are found in the N-terminal and mid region of the PEVK gene sequence[35], which also interact with F-actin[33]. However, recent work based on thin filament extraction[16] indicates that due to N2BA titin’s longer extensible region, PEVK-actin interaction in the N2BA cardiac isoform contributes ~50% less to viscosity than the N2B isoform (this is likely due to the longer extensible region of the N2BA isoform). Combined with the knowledge that in the mouse LV ~20% of total titin is N2BA titin[15], this suggests that remaining PEVK elements in the KO tissues can at most account for ~10% of the total viscosity, considerably less than the remaining viscosity that was found in the skinned myocytes and myocardium of the PEVK KO. It is also possible that other elements of titin’s spring region explain the remaining viscosity. Potential sources include unfolding of titin’s Ig domains and unfolding of secondary and tertiary structures contained in the large unique sequence in the N2B element of titin[36]. Although at physiological stretch rates unfolding of Ig domains is thought to require forces that exceed physiological levels[6–7, 9–10], the unfolding probability will be higher in the PEVK KO (due to its increased strain[15]). Thus, it is possible that Ig domain unfolding takes place in the KO and explains part of the remaining viscosity. Alternatively, the deletion of the PEVK element increases the strain on the remaining spring elements but it seems unlikely that this affects viscosity because no viscosity change was found in the N2B KO where strain is also increased but the PEVK element is still present (Supplemental Fig.S1A and C).
Although the PEVK KO does not affect the sarcomere in ways that alters the sarcomeric structure or sarcomeric protein expression in detectable ways (Supplemental Fig.S1B and S2), viscous sources in the sarcomere in addition to titin might exist. At physiological temperatures, actomyosin interaction may be a source of viscosity (see also below) because when the actomyosin inhibitor blebbistatin is added a reduction in viscosity is detected in both WT and PEVK KO skinned tissue (Fig.3). However, at room temperature no such effect is seen and remaining viscosity in the PEVK KO requires additional explanations. Friction between thin and thick filaments has also been suggested as a source of viscosity[37]. Our experiments in which myofilament spacing was reduced with dextran, however, did not reveal a lattice spacing effect on viscosity in the PEVK KO skinned tissue (Supplemental Fig.S3). This lack of change in viscosity in the PEVK KO suggests that under our experimental conditions thin-thick filament friction does not contribute to our measured viscosity. Our experiments also suggest that direct calcium binding to PEVK[38] does not influence viscosity (Supplemental Fig.S4), consistent with findings of Kulke et al[14]. Finally, microtubules have been proposed to be a source of viscosity[29] but because they depolymerize in skinned preparations, especially during their cold storage phase[28–30] they are unlikely to explain the remaining viscosity of skinned cells and tissues of the PEVK KO mice. In summary, our findings provide evidence that PEVK-actin interaction contributes at least 50% to passive viscosity of skinned myocytes and muscle and suggests that the remaining viscosity might be due, at least in part, to the remaining PEVK sequence in the N2BA isoform and/or unfolding of titin’s Ig-like domains that is enhanced in the PEVK KO.
PHYSIOLOGICAL IMPORTANCE OF PEVK-BASED VISCOSITY
To address whether the findings in skinned cardiomyocytes and cardiac tissues are relevant in the intact heart we performed ex-vivo isolated heart and in-vivo echocardiography experiments. In the ex-vivo experiments on isolated hearts we used stretch-hold and sinusoidal analysis experiments, both of which revealed that PEVK KO hearts had a ~30% reduction in viscosity. Similar findings were made in the in-vivo analysis using Doppler echocardiography. We evaluated both acceleration and deceleration times of the E-wave diastolic filling pattern (Table.1) and used the kinematic model known as the Parameterized Diastolic Filling Formalism[21, 39]. The model has previously revealed that the consequence of a high viscous index is an asymmetric E-wave[21]; simply it suggests that the acceleration time will shorten and deceleration time will lengthen as any viscous source provides more viscosity. The model predicts a 42% reduction in viscosity in the KO, which is slightly larger than the ~30% obtained in the ex-vivo isolated heart studies. Thus, the quantification via DT-AT (symmetry) and kinematic modeling (the viscous parameter c) both indicate a significant reduction of viscosity in the PEVK KO and is the first report of the in-vivo effects of a molecular viscous source.
The viscosity reduction at the ex-vivo and in-vivo heart levels is less than that measured in skinned cells and tissues. Because our in vitro experiments found that the ECM contributes little to viscosity, it seems unlikely that this difference in viscosity originates from the ECM. It is also unlikely that the difference is caused by microtubules because colchicine (which depolymerizes microtubules) had no effect on viscosity in the isolated heart experiments (Supplemental Fig.S5). However, it is possible that titin-based viscosity is less in-vivo than in vitro, for example the titin-actin interactions might be reduced by the S100A1/Ca2+ complex[12, 16] that is present in-vivo but absent in the in vitro experiments with skinned preparations. Alternatively, actomyosin interaction during diastole might also play a role. Our findings in skinned muscle showed actomyosin interactions at physiological temperatures that can be inhibited by blebbistatin (Fig.3). This inhibition suggests that actomyosin interactions provides an active source of viscosity in both WT and KO at the ex-vivo and in-vivo levels, and this might explain, at least in part, the reduced fractional amount of total viscosity that is caused by the PEVK-actin interactions. Finally, PEVK-actin based viscosity is likely to be larger during diastole than systole, because the strain rate during diastole is higher than in systole[40–41] and in addition PEVK-actin based viscosity is likely to be reduced by S100A1/Ca2+[12]. Thus, PEVK-actin interactions are a source of viscosity that is most prominent during diastole.
Sources of physiological modulation of passive viscosity may be seen in recent findings that protein kinase-C modulates the conformation of the PEVK element and causes increases in the viscous and elastic moduli[20, 42]. Because kinases have been found to be differentially expressed in diastolic dysfunction and heart failure[43], modulation of viscosity by post-translational modification of the PEVK merits future examination. Because the PEVK-actin interactions are not only present in stiff mouse tissues but also in compliant tissues[16], understanding the molecular basis and modulation of titin-based viscosity is relevant in future diagnosis and treatment of disease states such as diastolic heart failure[2].
In conclusion, deletion of the PEVK exons 219-225 in the mouse greatly lowers viscosity in skinned and intact conditions and significantly affects the passive diastolic inflow in-vivo. As the ventricle fills in early diastole via release of stored elastic energy, an un-damped system may over shoot the desired equilibrium position and result in instabilities[16, 44]. Such over shoot might increase the possibility of Ig domain unfolding and reduce the optimal overlap between the thick and thin filaments. Doppler ultrasound data supports the hypothesis that viscosity guides the ventricle to its diastasis (equilibrium) condition before atrial contraction, suggesting the importance of viscous modulation of diastolic function. Thus, utilizing a unique mouse model deficient in PEVK exons 219-225 allowed us to quantify a molecular source of diastolic viscosity and show that PEVK-actin interaction is an important physiological source of viscosity.
RESEARCH HIGHLIGHTS.
Our goal was to study the role of titin-based viscosity in diastolic function.
The titin PEVK element interacts in vitro with actin to create viscosity; we used a mouse deficient in PEVK.
PEVK KO reduces viscosity >50% in skinned tissue in-vitro and >30% in hearts in-vivo.
The PEVK-actin interaction is an important physiologic source of viscosity.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance of Luann Wyly for expert animal care and Nathan E Cromer for echocardiography.
SOURCES OF FUNDING
This work was supported by grants from the American Heart Association (0825748G) and the NIH (T32 HL07249-31) to CSC; by the Deutsche Forschungsgemeinschaft to MG; and the NIH (R01 HL062881) and a generous gift from Alan and Alfie Norville to HLG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Chung CS, Kovács SJ. Physical determinants of left ventricular isovolumic pressure decline: model prediction with in vivo validation. Am J Physiol Heart Circ Physiol. 2008 Apr 1;294(4):H1589–H1596. doi: 10.1152/ajpheart.00990.2007. [DOI] [PubMed] [Google Scholar]
- 2.Kass DA, Bronzwaer JGF, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004 Jun 25;94(12):1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 3.De Tombe PP, ter Keurs HE. An internal viscous element limits unloaded velocity of sarcomere shortening in rat myocardium. J Physiol (Lond) 1992 Aug 1;454:619–642. doi: 10.1113/jphysiol.1992.sp019283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson HP. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci USA. 1994 Oct 11;91(21):10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tskhovrebova L, Trinick J, Sleep JA, Simmons RM. Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature. 1997 May 15;387(6630):308–312. doi: 10.1038/387308a0. [DOI] [PubMed] [Google Scholar]
- 6.Rief M, Gautel M, Schemmel A, Gaub HE. The mechanical stability of immunoglobulin and fibronectin III domains in the muscle protein titin measured by atomic force microscopy. Biophys J. 1998 Dec;75(6):3008–3014. doi: 10.1016/S0006-3495(98)77741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautel M, Lehtonen E, Pietruschka F. Assembly of the cardiac I-band region of titin/connectin: expression of the cardiac-specific regions and their structural relation to the elastic segments. J Muscle Res Cell Motil. 1996 Aug;17(4):449–461. doi: 10.1007/BF00123361. [DOI] [PubMed] [Google Scholar]
- 8.Trombitás K, Greaser M, Labeit S, Jin JP, Kellermayer M, Helmes M, et al. Titin extensibility in situ: entropic elasticity of permanently folded and permanently unfolded molecular segments. J Cell Biol. 1998 Feb 23;140(4):853–859. doi: 10.1083/jcb.140.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trombitás K, Wu Y, McNabb M, Greaser M, Kellermayer MSZ, Labeit S, et al. Molecular basis of passive stress relaxation in human soleus fibers: assessment of the role of immunoglobulin-like domain unfolding. Biophys J. 2003 Nov 1;85(5):3142–3153. doi: 10.1016/S0006-3495(03)74732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmes M, Trombitás K, Centner T, Kellermayer M, Labeit S, Linke WA, et al. Mechanically driven contour-length adjustment in rat cardiac titin's unique N2B sequence: titin is an adjustable spring. Circ Res. 1999 Jun 11;84(11):1339–1352. doi: 10.1161/01.res.84.11.1339. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez-Cruz G, Van Heerden AH, Wang K. Modular motif, structural folds and affinity profiles of the PEVK segment of human fetal skeletal muscle titin. J Biol Chem. 2001 Mar 9;276(10):7442–7449. doi: 10.1074/jbc.M008851200. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki R, Berri M, Wu Y, Trombitas K, McNabb M, Kellermayer MS, et al. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J. 2001 Oct;81(4):2297–2313. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linke WA, Ivemeyer M, Labeit S, Hinssen H, Ruegg JC, Gautel M. Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophys J. 1997 Aug;73(2):905–919. doi: 10.1016/S0006-3495(97)78123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, et al. Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res. 2001 Nov 9;89(10):874–881. doi: 10.1161/hh2201.099453. [DOI] [PubMed] [Google Scholar]
- 15.Granzier HL, Radke MH, Peng J, Westermann D, Nelson OL, Rost K, et al. Truncation of titin's elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009 Sep 11;105(6):557–564. doi: 10.1161/CIRCRESAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukushima H, Chung CS, Granzier HL. Titin-isoform dependence of titin-actin interaction and its regulation by S100A1/Ca2+ in skinned myocardium. J Biomed Biotechnol. 2010 Jan 1;2010:727239. doi: 10.1155/2010/727239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, et al. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farman GP, Tachampa K, Mateja R, Cazorla O, Lacampagne A, de Tombe PP. Blebbistatin: use as inhibitor of muscle contraction. Pflugers Arch. 2008 Mar 1;455(6):995–1005. doi: 10.1007/s00424-007-0375-3. [DOI] [PubMed] [Google Scholar]
- 19.Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, et al. PKC phosphorylation of titin's PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009 Sep 25;105(7):631–638. doi: 10.1161/CIRCRESAHA.109.198465. 17 p following 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson BD, Hidalgo CG, Gotthardt M, Granzier HL. Excision of titin's cardiac PEVK spring element abolishes PKCalpha-induced increases in myocardial stiffness. J Mol Cell Cardiol. 2010 May 1;48(5):972–978. doi: 10.1016/j.yjmcc.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shmuylovich L, Kovács SJ. E-wave deceleration time may not provide an accurate determination of LV chamber stiffness if LV relaxation/viscoelasticity is unknown. Am J Physiol Heart Circ Physiol. 2007 Jun 1;292(6):H2712–H2720. doi: 10.1152/ajpheart.01068.2006. [DOI] [PubMed] [Google Scholar]
- 22.Chung CS, Granzier HL. Contribution of titin and extracellular matrix to passive pressure and measurement of sarcomere length in the mouse left ventricle. J Mol Cell Cardiol. 2011 Apr;50(4):731–739. doi: 10.1016/j.yjmcc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toh R, Shinohara M, Takaya T, Yamashita T, Masuda S, Kawashima S, et al. An X-Ray diffraction study on mouse cardiac cross-bridge function in vivo: effects of adrenergic {beta}-stimulation. Biophys J. 2006 Mar 1;90(5):1723–1728. doi: 10.1529/biophysj.105.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trombitas K, Freiburg A, Centner T, Labeit S, Granzier H. Molecular dissection of N2B cardiac titin's extensibility. Biophys J. 1999 Dec;77(6):3189–3196. doi: 10.1016/S0006-3495(99)77149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King NM, Methawasin M, Nedrud J, Harrell N, Chung CS, Helmes M, et al. Mouse intact cardiac myocyte mechanics: cross-bridge and titin-based stress in unactivated cells. J Gen Physiol. 2011 Jan;137(1):81–91. doi: 10.1085/jgp.201010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millman BM. The filament lattice of striated muscle. Physiol Rev. 1998 Apr 1;78(2):359–391. doi: 10.1152/physrev.1998.78.2.359. [DOI] [PubMed] [Google Scholar]
- 27.Stuyvers BD, Miura M, ter Keurs HE. Dynamics of viscoelastic properties of rat cardiac sarcomeres during the diastolic interval: involvement of Ca2+ J Physiol (Lond) 1997 Aug 1;502( Pt 3):661–677. doi: 10.1111/j.1469-7793.1997.661bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris TS, Baicu CF, Conrad CH, Koide M, Buckley JM, Barnes M, et al. Constitutive properties of hypertrophied myocardium: cellular contribution to changes in myocardial stiffness. Am J Physiol Heart Circ Physiol. 2002 Jun 1;282(6):H2173–H2182. doi: 10.1152/ajpheart.00480.2001. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura S, Nagai S, Katoh M, Yamashita H, Saeki Y, Okada J, et al. Microtubules modulate the stiffness of cardiomyocytes against shear stress. Circ Res. 2006 Jan 6;98(1):81–87. doi: 10.1161/01.RES.0000197785.51819.e8. [DOI] [PubMed] [Google Scholar]
- 30.Tagawa H, Rozich JD, Tsutsui H, Narishige T, Kuppuswamy D, Sato H, et al. Basis for increased microtubules in pressure-hypertrophied cardiocytes. Circulation. 1996 Mar 15;93(6):1230–1243. doi: 10.1161/01.cir.93.6.1230. [DOI] [PubMed] [Google Scholar]
- 31.Chung CS, Karamanoglu M, Kovacs SJ. Duration of diastole and its phases as a function of heart rate during supine bicycle exercise. Am J Physiol Heart Circ Physiol. 2004 Nov;287(5):H2003–H2008. doi: 10.1152/ajpheart.00404.2004. [DOI] [PubMed] [Google Scholar]
- 32.Bianco P, Nagy A, Kengyel A, Szatmári D, Mártonfalvi Z, Huber T, et al. Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys J. 2007 Sep 14;93(6):2102–2109. doi: 10.1529/biophysj.107.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagy A, Cacciafesta P, Grama L, Kengyel A, Malnasi-Csizmadia A, Kellermayer MS. Differential actin binding along the PEVK domain of skeletal muscle titin. J Cell Sci. 2004 Nov 15;117(Pt 24):5781–5789. doi: 10.1242/jcs.01501. [DOI] [PubMed] [Google Scholar]
- 34.Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, et al. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001 Nov 23;89(11):1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 35.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004 Mar 5;94(4):505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe K, Nair P, Labeit D, Kellermayer MSZ, Greaser M, Labeit S, et al. Molecular mechanics of cardiac titin's PEVK and N2B spring elements. J Biol Chem. 2002 Mar 29;277(13):11549–11558. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 37.Mutungi G, Ranatunga KW. Tension relaxation after stretch in resting mammalian muscle fibers: stretch activation at physiological temperatures. Biophys J. 1996 Mar 1;70(3):1432–1438. doi: 10.1016/S0006-3495(96)79702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, et al. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13716–13721. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovács SJ, Barzilai B, Pérez JE. Evaluation of diastolic function with Doppler echocardiography: the PDF formalism. Am J Physiol. 1987 Jan 1;252(1 Pt 2):H178–H187. doi: 10.1152/ajpheart.1987.252.1.H178. [DOI] [PubMed] [Google Scholar]
- 40.Nottin S, Doucende G, Schuster-Beck I, Dauzat M, Obert P. Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete's heart. J Physiol. 2008 Oct 1;586(Pt 19):4721–4733. doi: 10.1113/jphysiol.2008.156323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoylen A, Slordahl S, Skjelvan GK, Heimdal A, Skjaerpe T. Strain rate imaging in normal and reduced diastolic function: comparison with pulsed Doppler tissue imaging of the mitral annulus. J Am Soc Echocardiogr. 2001 Apr;14(4):264–274. doi: 10.1067/mje.2001.110375. [DOI] [PubMed] [Google Scholar]
- 42.Anderson BR, Bogomolovas J, Labeit S, Granzier H. The effects of PKCalpha phosphorylation on the extensibility of titin's PEVK element. J Struct Biol. 2010 Feb 10; doi: 10.1016/j.jsb.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borbély A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009 Mar 27;104(6):780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 44.Shmuylovich L, Chung CS, Kovács SJ, Yellin EL, Nikolic SD. Left ventricular volume during diastasis IS/IS NOT the physiologic in-vivo equilibrium volume and IS/IS NOT related to diastolic suction. J Appl Physiol. 2009 Dec 24; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.