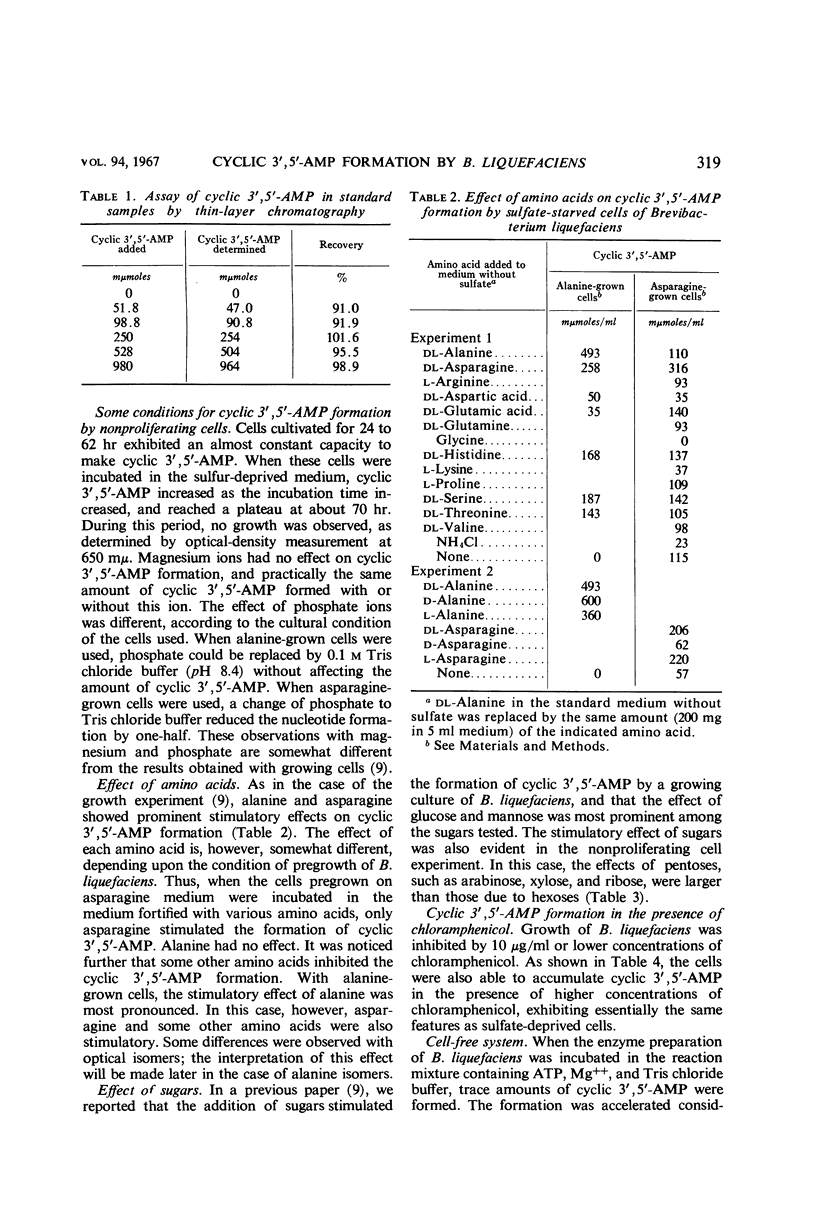
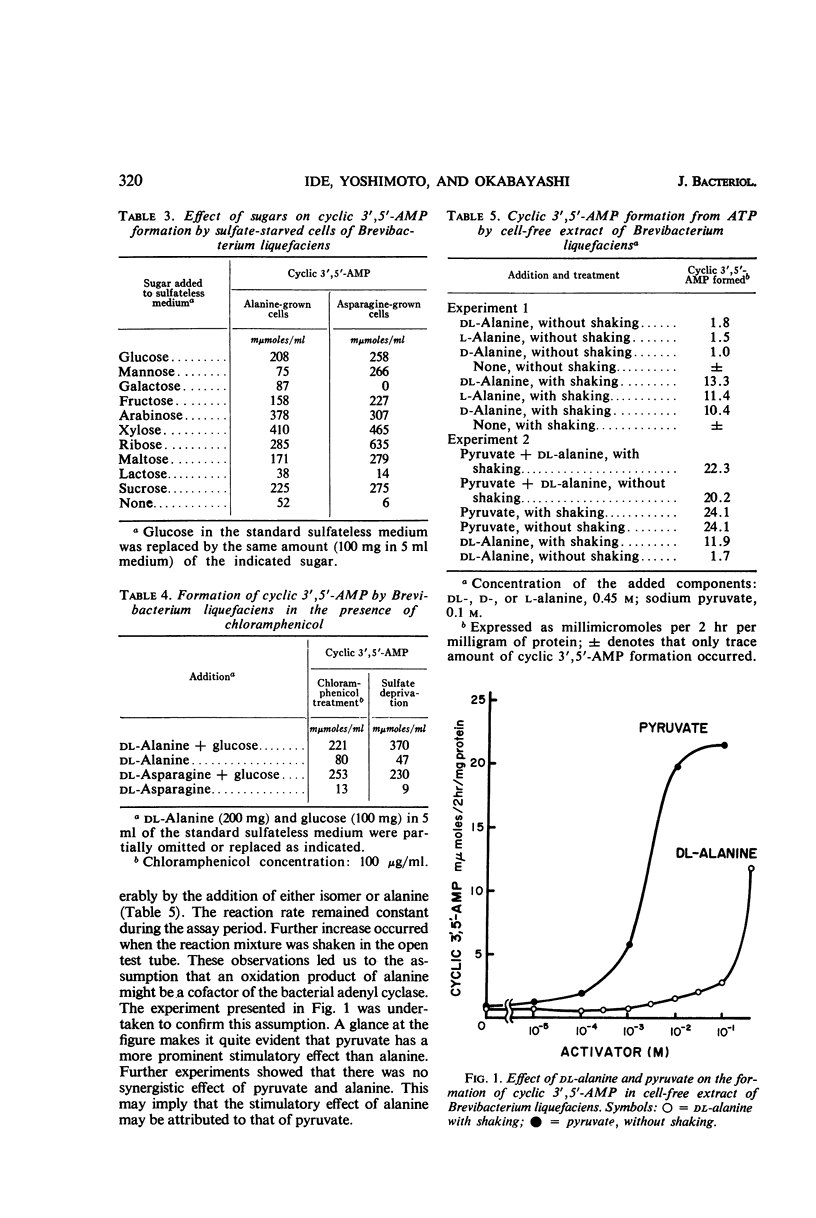
Abstract
The formation of adenosine cyclic 3′,5′-phosphate by Brevibacterium liquefaciens ATCC 14929 was studied with the use of nonproliferating cells and cell-free extract. With nonproliferating cells provided by deprivation of sulfate, the formation of this nucleotide was accelerated by adding some amino acids and sugars. Among amino acids tested, alanine and asparagine were most effective. Pentoses were more favorable than hexoses and other sugars. Formation of adenosine cyclic 3′,5′-phosphate was observed also with chloramphenicol-treated cells. Experiments on cell-free extract showed that addition of alanine or pyruvate stimulated the formation of adenosine cyclic 3′,5′-phosphate from adenosine-5′-triphosphate. When alanine was added to the cell-free system, shaking of the reaction mixture further increased the amount of the nucleotide, but pyruvate was far more effective than alanine. No synergistic effect of alanine and pyruvate was observed. Some enzyme activity was observed which decomposed adenosine cyclic 3′,5′-phosphate, but it was weak as compared with adenyl cyclase activity in the presence of pyruvate. From the results obtained, it appears that pyruvate may act as an activating factor of adenyl cyclase in Brevibacterium liquefaciens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., WALTON G. M. KINETICS OF REGULATORY ENZYMES. ESCHERICHIA COLI PHOSPHOFRUCTOKINASE. J Biol Chem. 1965 Feb;240:757–763. [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Hirata M., Hayaishi O. Pyruvate dependent adenyl cyclase activity of Brevibacterium liquefaciens. Biochem Biophys Res Commun. 1965 Nov 22;21(4):361–365. doi: 10.1016/0006-291x(65)90202-0. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- OKABAYASHI T., IDE M., YOSHIMOTO A. Excretion of adenosine-3',5'-phosphate in the culture broth of Brevibacterium liquefaciens. Arch Biochem Biophys. 1963 Jan;100:158–159. doi: 10.1016/0003-9861(63)90046-8. [DOI] [PubMed] [Google Scholar]
- OKABAYASHI T., YOSHIMOTO A., IDE M. OCCURRENCE OF NUCLEOTIDES IN CULTURE FLUIDS OF MICROORGANISMS. V. EXCRETION OF ADENOSINE CYCLIC 3',5'-PHOSPHATE BY BREVIBACTERIUM LIQUEFACIENS SP. N. J Bacteriol. 1963 Nov;86:930–936. doi: 10.1128/jb.86.5.930-936.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAIAH A., HATHAWAY J. A., ATKINSON D. E. ADENYLATE AS A METABOLIC REGULATOR. EFFECT ON YEAST PHOSPHOFRUCTOKINASE KINETICS. J Biol Chem. 1964 Nov;239:3619–3622. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- THRELFALL C. J. An analytical procedure for the acid-soluble phosphorus compounds in rat-skeletal muscle. Biochem J. 1957 Apr;65(4):694–699. doi: 10.1042/bj0650694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñuela E., Salas M. L., Salas M., Sols A. Two interconvertible forms of yeast phosphofructokinase with different sensitivity to endproduct inhibition. Biochem Biophys Res Commun. 1964 Mar 26;15(3):243–249. doi: 10.1016/0006-291x(64)90154-8. [DOI] [PubMed] [Google Scholar]


