Abstract
We studied stress hormones and foraging of nocturnal Acomys cahirinus and diurnal A. russatus in field populations as well as in two field enclosures populated by both species and two field enclosures with individuals of A. russatus alone. When alone, A. russatus individuals become also nocturnally active. We asked whether nocturnally active A. russatus will respond to moon phase and whether this response will be obtained also in diurnally active individuals. We studied giving-up densities (GUDs) in artificial foraging patches and fecal cortisol metabolite levels. Both species exhibited elevated fecal cortisol metabolite levels and foraged to higher GUDs in full moon nights; thus A. russatus retains physiological response and behavioral patterns that correlate with full moon conditions, as can be expected in nocturnal rodents, in spite of its diurnal activity. The endocrinological and behavioral response of this diurnal species to moon phase reflects its evolutionary heritage.
Introduction
Rodents are vulnerable to predation [1]–[3]. As most rodent species are nocturnal [4], owls, many of which are small mammal specialists, pose a serious threat. While earlier ecological studies have focused on the population dynamics of predator-prey relationships, the past two decades have seen a growing realization that behavioral responses of prey to predation risk have further profound ecological implications (e.g., [5]–[7]). Therefore, considerable research has been devoted to recording rodent behavioral responses to direct and indirect cues of owl predation risk and to understanding their ecological implications (e.g., [8]–[14]). Among indirect cues, particular attention has been aimed at the response of rodents to moon phase as a surrogate for predation pressure (e.g., [9], [11], [15]–[20]).
Owl strike success rate is high [21], particularly under elevated illumination [14], [22], and successful strikes are invariably lethal. As learning from experience is simply too costly, accurate perception of predation risk from owls and relevant behavioral responses may be predominantly genetically determined. Indeed, captive-bred individuals that have never encountered owls in nature were repeatedly found to respond behaviorally to changes in predation risk and illumination levels (e.g., [23]–[26]). The mechanism underlying these responses may include the elevation of glucocorticoids (GC). In this case, nocturnal rodents will respond adaptively to elevated illumination during full moon nights, by increasing GC levels that cause a reduction in foraging and therefore in predation risk. It has been suggested that apart from being a response to the stressor itself, GCs have a role in preparing the individual to an expected stressor [27]. In these cases, GC concentrations increase in anticipation of a challenge, rather than in response to the challenge itself. Such preparative action requires that the stressor (predation in the current work) will be statistically predictable [27]–[29]; because moonlight increases predation risk, and because elevated moonlight occurs with regularity in the lunar cycle, risk of predation by nocturnal raptors is entirely predictable, and it would be attractive to hypothesize that a lunar rhythm in stress hormones has evolved in response (but see Discussion).
In laboratory biomedical experiments using rodents, light is routinely used as a cue for stress. Light pulses during the night were shown to cause an increase in GC levels in rats [30], GC treatment was shown to increase anxiety and conditioned fear [31]–[34], and acute corticosterone elevation enhanced antipredator behaviors in tree lizard species [35]. At least some of these responses may be genetically determined; experimental studies in laboratory rodents as well as in captive-bred wild species that have never encountered predators have shown that exposure to light, a predator, or even its voice or odors, can cause an increase in stress hormone levels (e.g., [30], [35]–[38]). In some cases increased GC levels led to increases in food intake, in particular of highly palatable foods [39], [40], but in most cases increased GC levels result in a decrease in food intake (reviewed by [41]). The effect of lunar phase on rodent activity under natural conditions at night has been studied extensively by ecologists, the role of light as a stressor is clear on experimental grounds, and the connection between the behavioral response to risk and stress hormones is well established; however the effect of lunar phase on stress hormones in rodents in the field was never studied.
Although most current mammals retain the mammalian ancestral state of nocturnality, species within both closely and distantly related taxa have evolved to become diurnal (reviewed by [42]). This shift released many species from the threat of owl predation, exposing them to diurnal predators, whose predation efficiency is not influenced by lunar cycle. However, since predation by owls is a strong selective force which has produced ‘hard-wired’ endocrinological and behavioral responses, diurnally active rodents may retain these responses. Alternatively, these adaptations may be lost with release from natural selection (e.g., [43], [44]).
We studied lunar cycle patterns of stress hormone levels and foraging behavior of nocturnal and diurnally active rodents asking whether stress hormone levels correlate with moon phase, whether foraging behavior correlates with moon phase, and whether they do so both during the day and during the night. Acomys russatus is a diurnally active rocky desert rodent but in absence of its nocturnal congener (A. cahirinus) it is also active at night [45]–[47]. Molecular phylogenetic research suggested that the A. russatus lineage diverged ca. 6–8 million years ago, but it has been suggested that the shift to diurnality occurred at the evolutionary scale ca. 0.3–0.5 million years ago when it encountered the younger lineage of A. cahirinus [48, and Volobouev pers. com.].
In the field, spiny mice are preyed upon at night by Blanford's foxes (Vulpes cana, although they form only a small part of the fox's diet, [49]), owls (Hume's Tawny Owl, Strix butleri [50]), and snakes (saw-scaled viper, Echis coloratus); during the day they are probably preyed upon by diurnal raptors (Common Kestrel, Falco tinnunculus) [51]. General indirect evidence for the evolutionary significance of predation are spines on spiny mouse rumps, in particular those of A. russatus, a histological mechanism for tail loss [52], and relative immunity to viper venoms [53].
A. cahirinus reduce their foraging activity as a result of predation risk by owls in open habitats during moonlit nights, as has been demonstrated for rodents of sandy deserts [19]. In response to owl calls, the level of stress hormones of A. cahirinus increases [23], and their motor behavior changes with rising illumination levels.
Nocturnal Blanford's foxes pose a risk of predation in open areas, regardless of moon phase, probably reinforcing preference of sheltered microhabitats driven primarily by owl predation [51], [54].
The saw-scaled viper is active at Ein Gedi during the warm summer months. Predation by vipers is a threat primarily under boulders during the day (where these nocturnal sit-and-wait predators rest curled up); during night, it is a threats both under and between boulders and in open areas, habitats where snakes either lie still or move actively at night [55]. Consequently both spiny mouse species reduce their foraging in sheltered microhabitats, and shift their foraging activity to more open microhabitats in summer [51]. Thus, in summer the selective pressure posed by vipers counters that of owl predation risk during night (see also [56] and that of physiological stress during the day, regardless of moon phase.
In sum, being active during the day poses different challenges to A. russatus than being active at night, among them differences in predation regimes [19], [51], [54]. Specifically, risk of predation by owls should be a threat only during the night. Indeed, analysis of pellets of the resident owl at Ein Gedi, the tawny owl, confirms that in nature owls take only nocturnal A. cahirinus [19]. In response, A. cahirinus prefer to forage in sheltered microhabitats where they are safe from avian predators [51] and reduce their foraging in the open during moonlit nights [19].
Light increases activity in diurnal mammals (positive masking) and suppresses it in nocturnal ones (negative masking), while darkness acts in the opposite way [57]–[60]. In order for a nocturnal species to evolve into a diurnal one or for an individual to move from a nocturnal to a diurnal activity niche, the negative masking effects of light on activity must be overcome. In accord, masking effect of light in rodent species which show individual differences in activity patterns, was associated in predictable ways with the overall activity pattern that the individuals exhibited at the time of testing [57]–[60]. In previous experiments we found that light suppresses activity and body temperature levels of A. cahirinus in the laboratory as well as in the field (negative masking), but not in A. russatus [61], [62].
It appears that A. russatus is a nocturnal species forced into diurnality; it retains several nocturnal physiological and morphological traits, including nocturnal circadian rhythms, but it has evolved also some morphological and physiological adaptations for diurnal activity [42], [46], [61], [63]–[69]. Is its response to lunar phase also evolutionarily constrained? Will A. russatus, active in nature only during the day [46], [67], respond to the lunar cycle when a manipulative field experiment enables it to extend activity into the night? If so, will stress hormone levels and diurnal activity patterns respond to moon phase as well?
In order to address these questions, we carried out a field study, including a controlled and replicated removal experiment in field enclosures, enabling us to gain insight into the role of the lunar cycle on stress hormones levels and on foraging behavior (see also [45]) during day and night. Specifically, we compared fecal stress hormone levels and nocturnal foraging behavior of A. cahirinus and A. russatus during new moon and full moon nights and diurnal foraging behavior of A. russatus in the days that follow them.
We framed our working hypotheses as follows:
A. cahirinus stress hormone levels will increase, and it will reduce foraging behavior on full moon nights.
If A. russatus retains its response to moon phase, then when a manipulative field experiment enables it to extend its activity into the night, it will respond to the lunar cycle in a similar manner to A. cahirinus; its stress hormone levels will increase, and it will reduce foraging behavior on full moon nights.
If that is the case, A. russatus stress hormone levels and diurnal foraging behavior may be affected by moon phase as well, in either an adaptive way (decreased stress hormone levels and increased diurnal foraging to compensate for reduced nocturnal foraging) or a maladaptive way that reflects an evolutionary constraint (increased stress hormone levels and decreased foraging during the day in response to moon phase).
Our alternative hypothesis was that the endocrinological and behavioral response to moon phase has been lost in this diurnally active species and that A. russatus behavior and stress hormones levels will not be affected by moon phase.
Materials and Methods
The study was conducted in four research enclosures, 2 populated with both A. cahirinus and A. russatus, and two populated with A. russatus only (for details see below), as well as in free ranging populations of both species (for hormone levels only).
Populating experimental enclosures
We conducted the study at the Ein Gedi Nature Reserve, in the Judean Desert near the Dead Sea (31°28′N, 35°23′E, 300 m below sea level). We erected four mouse-proof enclosures (20×50 m) over linear rock terraces (see details and feeding regime in [45]). As we could not be certain that the food and water naturally available in the enclosures were sufficient to sustain the spiny mice, a limited amount of food (commercial rodent pellets and sunflower seeds) was added every two weeks at the end of each trapping session, and between foraging experiments (see protocol bellow). Food was not supplemented at the time of the experiments. Rodents in the enclosures are exposed to the same suite of predators they face in nature in this area (foxes, snakes, owls, and probably raptors) [52]–[54].
We captured spiny mice at the Ein Gedi area in fall (1999), marked them individually, and introduced them into the enclosures (from which resident individuals were trapped and removed), allowing them several months to acclimate. The first experiment took place during June and July, 2000. We placed Sherman live traps in the enclosures for at least two consecutive days and nights every other week; individuals of both species can be easily and repeatedly trapped and both show similar trappability [70]. Traps were closed before sunrise and sunset, checked for trapped rodents, and then reopened only after sunrise or complete darkness. By doing this, we could monitor population size and clearly define the time of activity (nocturnal or diurnal).
We removed or released surplus mice that were born in the enclosures to regulate population size, held constant as from ca. 6 months prior to the experiment. We monitored the sex ratios, kept at ca. 1∶1 for each species. Foraging experiments began in June (2000) after trapping results indicated a shift in activity patterns of A. russatus held alone (see [45]); there were 8 individuals of each species in the two species enclosures and 16 individuals of A. russatus in the A. russatus enclosures.
Fecal stress hormones
Fecal cortisol metabolite levels of free living spiny mice
We trapped free-living spiny mice using 100–200 Sherman live traps during full and new moon nights during December–June 2003–2004 (A. russatus: new moon n = 19, full moon n = 12; A. cahirinus: new moon n = 18, full moon n = 22). Traps were set at different areas in each sampling session, and mice were marked with paint before they were released in order to avoid re-sampling. Only one fecal sample was collected from each individual. Sampling sessions lasted 24–72 h, dependent on trapping success. A. russatus were always trapped during the day, while A. cahirinus were always trapped during the night. Trapping is a stressor; in spiny mice, trapping stress is evident in the fecal stress hormone metabolite levels after 8–12 h (see Text S1). Traps that were opened at sunset and sunrise were checked 6–8 or 4–6 h later, respectively, to avoid measuring trapping stress. Traps opened at sunrise were checked 4–6 hours later to avoid heat stress to the animals. Hence, fecal stress hormone levels reflect the time interval before the animals entered the traps. Feces were collected from the traps and stored in 95% ethanol in −20°C until extraction.
Stress hormone levels in the enclosures
During June and July 2003, we trapped individuals in the A. russatus enclosures during days following full moon (n = 12) and new moon (n = 12) nights, using 10 Sherman live traps in each enclosure. Each individual was sampled once during each moon phase. Traps were opened at sunrise and checked 4–6 h later. Food was not supplemented at the time of the feces collection.
Hormone extraction
Hormones were extracted based on Harper and Austad [71]. Before extraction, feces were dried in open air to constant weight. 10 ml of ethanol were added to each sample, and the samples were boiled for 20 minutes at 90°C. The ethanol was transferred to a new tube, another 10 ml of ethanol were added to the original tube, and boiled again. The ethanol from the two boiling sessions was combined and dried using a sample concentrator (Techne) using nitrogen at 60°C (heat block, Ori-block 08-3), rinsed with ethyl acetate: hexane (3∶2, v/v) solution, and dried again. Samples were stored in −20°C until analyzed.
In spiny mice, unlike most rodents, the major glucocorticoid is cortisol [23], [72]. Fecal cortisol metabolite was measured using a tritiated RIA kit for unextracted serum (ICN biomedical, inc., catalog number 07-221105) in duplicates. The assay was validated for both species: Biological validity, which included two experiments; 1. Serial sampling before and after a stressful event (trapping, including a control group without stress), which showed that trapping stress causes a significant effect on fecal cortisol metabolite levels after 8–12 h, demonstrating that the technique can detect biologically meaningful changes in GC levels (paired t-tests: A. russatus: 4 hr: t = −0.33, df = 14, p = 0.37; 8 hr: t = −1.15, df = 14, p = 0.13; 12 hr: t = −2.61, df = 11, p<0.05, A. cahirinus: 4 hr: t = −0.81, df = 14, p = 0.21; 8 hr: t = −0.33, df = 13, p = 0.51; 12 hr: t = −1.49, df = 13, p = 0.07; Figure S1). 2. Describing the naturally occurring diurnal variation in the fecal cortisol metabolite levels, which also indicate biological relevance. Protocol validity: cortisol (from standard solutions) and cortisol-like immuno-reactivity in spiny mice feces extract diluted in parallel in the ICN cortisol RIA (p = 0.0052, R2 = 0.817 for A. russatus and p = 0.0083, R2 = 0.828 for A. cahirinus; Figure S2). We also extracted an increasing amount of homogenized pool of fecal matter and found a correlation between fecal mass extracted and fecal cortisol metabolite levels in the extract (simple regression analysis: A. russatus mass effect −1430.9±291.1, R2 = 0.83, t = 4.9, df = 5, p<0.01; A. cahirinus mass effect −698.3±127.1, R2 = 0.86, t = 5.5, df = 5, p<0.01; [µg/dL±SD]; Figure S3. For all validation methods and results Text S1). Our within-assay coefficients of variation were 0%–5%, and the between assay coefficient of variation was 6.5%. Before analysis, samples were re-constituted using 100–500 µl ethanol, according to their fecal mass. The dilution factor was taken into account in the concentration calculation. We used a control solution (BIO-RAD liphocheck immunoassay plus control) as a quality control.
Studying foraging behavior with artificial food patches
We studied foraging behavior of spiny mice using the giving-up density method (GUD, [73]), which assumes that a forager is behaving optimally and that the density of food remaining in the patch when it gives up foraging corresponds to a harvest rate at which the energetic gain from foraging just balances the metabolic cost of foraging, the cost of the perceived risk of predation in foraging in that patch, cost of interference, and the missed opportunity cost of not foraging elsewhere or indulging in other fitness enhancing activities [9], [17]. During both day and night, standard artificial food patches maintained similar metabolic costs of foraging associated with digging for food in the trays and similar missed opportunity costs from not foraging in other artificial food patches; therefore, differences between different days during the lunar cycle can be ascribed to differences in the perceived risk of predation. Predation risks during day and during night appeared constant across enclosures. Rarely, a viper settled for a day or two in one of the enclosures and dramatically affected foraging activity in the entire enclosure but not other enclosures. Such a case happened in the second new moon experiment. Therefore, we omitted the number of trays foraged in these days from our analysis.
Our artificial food patches comprised aluminum trays (30×20×4 cm) containing two liters of finely sifted local soil and two grams of crushed and sieved sunflower seed (1–2 mm length), mixed thoroughly and protected from foraging birds by heavy wire frames and fine filament fish netting (see [51] for details). Each experiment was preceded by three days and nights of pre-baiting to ensure that the trays had been discovered by rodents.
We placed nine trays in each enclosure, divided into 3 stations, 3 trays in each. The tray stations were placed ca. 12 m apart and trays in each station were placed in a triangle ca. 3 m apart from one another. We collected two types of data: a) number of trays foraged, as a measure of total activity level (since spiny mouse numbers were held constant across enclosures); b) giving-up densities, which should be independent of the number of mice that have visited the tray [73].
Experimental protocol
We studied nocturnal foraging microhabitat use and efficiencies of A. russatus in absence of A. cahirinus, and of A. cahirinus on full moon and new moon nights (June and July). We also studied diurnal foraging of A. russatus during days following full and new moon nights. Four runs were conducted: two on full moon days and nights and two on new moon days and nights. Each experiment lasted a week: three days of pre-baiting and population monitoring using traps followed by four days of actual foraging experiments, when no trapping took place. We examined trays for footprints and GUDs measured at sunrise and at sunset, as in [51]. We determined identities of foragers in the two species enclosures (A. russatus or A. cahirinus) based on trapping results (discussed also in [45]). In brief, trapping results revealed that presence of A. cahirinus had a significant effect on activity times of A. russatus [45]. When kept together, A. cahirinus and A. russatus were temporally partitioned: A. cahirinus was nocturnal while A. russatus was diurnal [45]. In the A. russatus enclosures, A. russatus were trapped also during the night but still significantly more frequently during the day [45].
A.russatus night trappings showed that 10 different individuals were trapped during the night (of 13 night trappings), suggesting that nighttime activity was widespread among individuals of A. russatus in the A. russatus enclosures [45].
Statistical analyses
We collected data from several trays at the same enclosures multiple times. As a result, if the same individual foraged in several trays on multiple occasions, these differences in GUD would not represent independent data points. We dealt with this issue by analyzing the trays data with the enclosures as the experimental units. We used mixed-effects modeling to analyze data because of the repeated measurements and the hierarchical nature of the sampling (trays sub-samples within each enclosure). Such models allow for the use of all data while correcting for pseudoreplication below the enclosure level (e.g. [74]). We used Bayesian inference because of the observational nature of the study [75] and ran the statistical models using a Markov Chain Monte Carlo (MCMC) simulation implemented in the JAGS computer program [76]. We used non-informative priors for all model parameters and used the R CODA software package [77] to calculate parameters' estimation (with standard deviations and 95%, 99%, and 99.5% confidence intervals [CI]), and to test their convergence (by convergence criteria described in detail in [78] and in [79]).
We analyzed (1) presence and absence of foraging on trays using generalized linear mixed-effects models (GLMM) (binomial data), (2) the GUD data using linear mixed-effects models (LMM), and (3) the amount of seeds eaten at each enclosure using LMM. In each model, we separated the statistical inference for each species: for A. cahirinus, we examined the effect lunar phase, and for A. russatus we examined the effect of competition (presence or absence of A. cahirinus), lunar phase, and activity phase. We then statistically compared the effect of moon phase between the species. At models 1 and 2, we added the enclosure type, enclosure index, line of trays, microhabitat, the day of measurement and the Julian month as random factors. At model 3, we added the enclosure type, enclosure index, the day of measurement and the Julian month as random factors.
We used deviance information criterion (DIC; [80]), which can be seen as the AIC Bayesian counterpart for model selection, to find whether each of the random factors contributed to the fit of the models. We found that all of the above random factors contributed to the models adequacy. We report estimates ± SD and the most significant level of CI. Two-way ANOVA on log transformed data was used for testing the effect of moon phase on fecal cortisol metabolite levels. The residuals of the LMMs and Two-way Anova models were normally distributed, based on graphical examination of their histogram (see Zuur et al. 2009).
Results
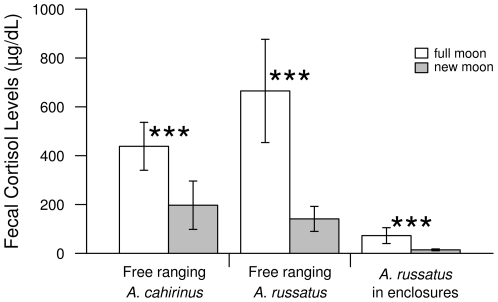
We found that moon phase influenced fecal cortisol metabolite levels in both free ranging diurnal A. russatus and nocturnal A. cahirinus. In both species, fecal cortisol metabolite levels were significantly higher in full moon nights and the days following them than during new moon nights and the days following them (df = 1, F = 16.7, p<0.001, Figure 1). A similar pattern was found in A. russatus in the two species enclosures, where they are active both during the day and during the night (no moon*enclosure/free interaction, df = 2, F = 2.4, p = 0.1, Figure 1). Fecal cortisol metabolite levels were significantly higher in free ranging spiny mice then in spiny mice in the enclosures (df = 2, F = 16.3, p<0.001).
Figure 1. Fecal cortisol metabolite levels of free ranging diurnal A. russatus and nocturnal A. cahirinus, and of diurnal A. russatus in A. russatus enclosures during full moon and new moon nights and the following days (free ranging diurnal A. russatus new moon n = 19, full moon n = 12; free ranging nocturnal A. cahirinus new moon n = 18, full moon n = 22; A. russatus enclosures diurnal A. russatus new moon n = 9, full moon n = 8. *** p<0.001).
Moon phase influenced fecal cortisol metabolite levels in both free ranging diurnal A. russatus and nocturnal A. cahirinus. In both, fecal cortisol metabolite levels were significantly higher in full moon nights than during new moon nights. A similar pattern was found in A. russatus in the A. russatus enclosures, where they are active both during the day and during the nights.
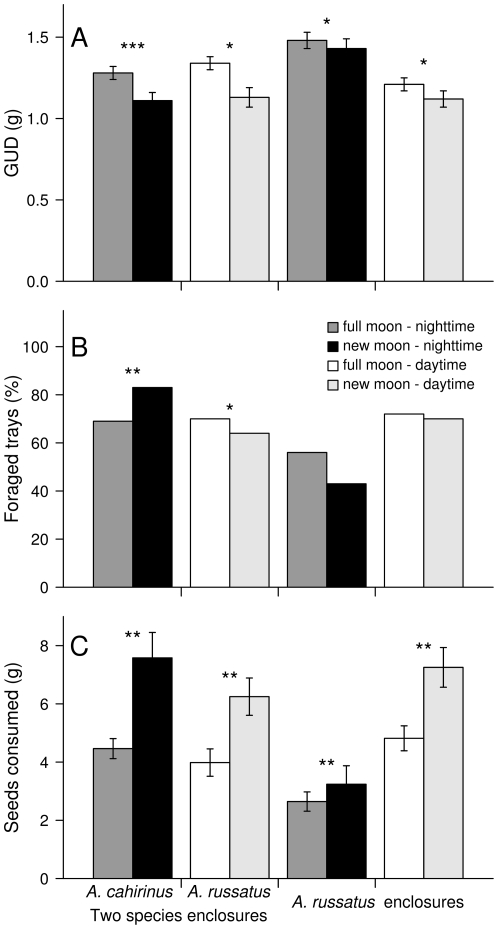
Moon phase influenced patch foraging probabilities at the two species enclosures and influenced foraging efficiency in both treatments and in both diel parts (Figure 2, Table 1). Based on the results of Gutman et al [42], nocturnal foraging in the two species enclosures was attributed to A. cahirinus while diurnal foraging in the two species enclosures and all foraging in the A. russatus enclosures is attributed to A. russatus. Therefore, it can be concluded that moon phase influenced patch foraging probabilities and foraging efficiency of both Acomys species (Figure 2). Specifically, moon phase had a significant effect on patch foraging probabilities only in the two species enclosures, where during daytime A. russatus patch foraging probabilities were lower during new moon than during full moon (Moon (new) main effect: 0.59±0.62; Moon (new) * Competition (yes): −2.13±0.90; Moon (new) effect in the presence of interspecific competition: −1.56±0.64), while during nighttime A. cahirinus patch foraging probabilities were higher during new moon than full moon nights (Moon (new) effect: 0.87±0.29). Hence, in the two species enclosures, moon phase effect was significantly different between the species (Δnew moon effect [A. cahirinus – A. russatus] = −2.43±0.71). Patch foraging probabilities of A. russatus were lower during nighttime compared to daytime (day part [night] effect: −3.13±0.65) in which A. russatus foraged in fewer patches during nighttime regardless of moon phase (Figure 2, Table 1).
Figure 2. Mean GUDs (g ± SE, A) Percent of trays foraged (B), and total seeds consumption (g ± SE, C) of A. russatus and A. cahirinus in the two species enclosures and A. russatus enclosures during full moon nights and the following days.
In the two species enclosures nocturnal foraging is ascribed to A. cahirinus and diurnal foraging to A. russatus. In the A. russatus enclosures both diurnal and nocturnal foraging were carried out by A. russatus * p<0.05, ** p<0.01, ***p<0.001. A: Moon phase influenced GUDs, even during daytime: patches were foraged by A. russatus to significantly lower GUDs under new moon vs. full moon during both parts of the diel cycle. However, patches were foraged to a significantly lower GUDs during daytime than during nighttime. A. cahirinus also foraged to significantly lower GUDs under new moon. The effect of moon was significantly higher on A. cahirinus compared to A. russatus. B: Moon phase had a significant effect on patch foraging probabilities only in the two species enclosures, where during daytime A. russatus patch foraging probabilities were lower during new moon than during full moon. During nighttime A. cahirinus patch foraging probabilities were higher during new moon than full moon nights. In the A. russatus enclosures patch foraging probabilities of A. russatus were lower during nighttime compared to daytime, in which A. russatus foraged in fewer patches during nighttime regardless of moon phase. C: Moon phase influenced the amount of seeds eaten at each enclosure: A. russatus consumed a higher amount of seeds under new moon vs. full moon during both parts of the dial cycle. However, significantly less amount of seeds were eaten during A. russatus nocturnal foraging than during diurnal foraging. A. cahirinus also consumed a higher amount of seeds under new moon. Moreover, the effect of moon was not significantly different between the species.
Table 1. Results of the mixed-effects generalized linear mixed-effects models (GLMM) (binomial data) for presence and absence of foraging on trays, the linear mixed-effects models (LMM) for the GUD, and LMM for the amount of seeds eaten of A. russatus and A. cahirinus in the two species enclosures and A. russatus enclosures during full moon nights and the following days.
| Test | Estimate | SD | 95% CI | 99% CI | 99.5% CI | Significance | |
| Foraged | A. cahirinus | ||||||
| trays (log odds) | Intercept | 0.30 | 0.60 | −0.44, 2.05 | −1.42, 3.90 | −2.97, 4.69 | NS |
| New moon effect | 0.87 | 0.29 | 0.31, 1.46 | 0.12, 1.64 | −0.09, 1.84 | ** | |
| A. russatus | |||||||
| Intercept | 0.21 | 1.86 | −4.96, 3.88 | −7.16, 6.32 | −9.31, 8.65 | NS | |
| New moon | 0.59 | 0.62 | −0.64, 1.79 | −1.00, 2.20 | −1.39, 2.60 | NS | |
| Night | −3.13 | 0.65 | −4.45, −1.89 | −4.91, −1.58 | −5.39, −1.07 | *** | |
| Competition | −0.01 | 3.20 | −6.66, 5.55 | −16.69, 11.02 | −25.27, 18.05 | NS | |
| moon(new) and daypart(night) | −1.05 | 0.76 | −2.54, 0.45 | −3.05, 0.89 | −3.53, 1.36 | NS | |
| moon(new) and competition(yes) | −2.13 | 0.90 | −3.97, −0.45 | −4.57, 0.07 | −5.51, 0.73 | * | |
| Δnew moon effect [A. cahirinus – A. russatus] | −2.43 | 0.71 | −4.96, 3.88 | −7.16, 6.32 | −9.31, 8.65 | NS | |
| Giving Up | A. cahirinus | ||||||
| Densities | Intercept | 0.29 | 0.18 | −0.11, 0.62 | −0.40, 1.00 | −0.71, 1.27 | NS |
| (GUD, g) | New moon effect | −0.30 | 0.05 | −0.4, −0.21 | −0.43, −0.18 | −0.46, −0.13 | *** |
| A. russatus | |||||||
| Intercept | 0.29 | 0.19 | −0.12, 0.70 | −0.49, 1.02 | −0.80, 1.40 | NS | |
| New moon | −0.13 | 0.05 | −0.23, −0.03 | −0.26, 0.0004 | −0.33, 0.03 | * | |
| Night | 0.39 | 0.05 | 0.29, 0.49 | 0.26, 0.52 | 0.23, 0.56 | *** | |
| Competition | 0.12 | 0.30 | −0.44, 0.69 | −1.04, 1.30 | −2.34, 1.90 | NS | |
| moon(new) and daypart(night) | 0.05 | 0.08 | −0.10, 0.21 | −0.15, 0.27 | −0.22, 0.33 | NS | |
| moon(new) and competition(yes) | −0.06 | 0.07 | −0.20, 0.08 | −0.25, 0.12 | −0.30, 0.18 | NS | |
| Δnew moon effect [A. cahirinus – A. russatus] | −0.17 | 0.07 | −0.30, −0.03 | −0.35, 0.01 | −0.41, 0.08 | * | |
| Consumed | A. cahirinus | ||||||
| Seeds (g) | Intercept | 1.49 | 1.18 | −0.35, 3.56 | −3.97, 6.53 | −12.05, 10.82 | NS |
| New moon effect | 3.00 | 1.00 | 1.07, 4.92 | 0.30, 5.65 | −0.81, 7.20 | ** | |
| A. russatus | |||||||
| Intercept | 1.56 | 1.14 | −0.23, 3.21 | −4.21, 5.47 | −12.56, 11.94 | NS | |
| New moon | 2.37 | 0.72 | 0.95, 3.77 | 0.45, 4.22 | −0.65, 5.22 | ** | |
| Night | −2.55 | 0.46 | −3.44, −1.64 | −3.76, −1.31 | −4.12, −0.83 | *** | |
| Competition | −0.83 | 2.06 | −4.76, 3.02 | −8.57, 7.77 | −16.59, 16.51 | NS | |
| moon(new) and daypart(night) | −1.73 | 1.05 | −3.77, 0.35 | −4.44, 1.10 | −5.50, 2.60 | NS | |
| moon(new) and competition(yes) | −0.18 | 1.02 | −2.15, 1.75 | −2.83, 2.47 | −3.82, 3.94 | NS | |
| Δnew moon effect [A. cahirinus – A. russatus] | 0.63 | 1.23 | −1.74, 3.02 | −2.58, 3.79 | −3.82, 5.61 | NS |
In the two species enclosures nocturnal foraging is ascribed to A. cahirinus and diurnal foraging to A. russatus. In the A. russatus enclosures both diurnal and nocturnal foraging were carried out by A. russatus. NS: 95% CI span zero.
*95% CI don't span zero,
**99% CI don't span zero,
***99.5% CI don't span zero.
Moon phase also influenced GUDs, even during daytime (Figure 2, Table 1): patches were foraged by A. russatus to significantly lower GUDs under new moon vs. full moon (Moon (new) effect:−0.13±0.05) during both parts of the dial cycle (no significant interaction with day part term, 0.05±0.08). No significant main effect of competition was found (Competition (yes) effect: 0.12±0.30). However, patches were foraged to a significantly lower GUDs during daytime than during nighttime (day part (night) effect: 0.39±0.05). No significant day-part * moon phase interaction (0.05±0.08) was found. A. cahirinus also foraged to significantly lower GUDs under new moon (Moon (new) effect: −0.30±0.05). Moreover, the effect of moon was significantly higher on A. cahirinus compared to A. russatus (Δnew moon effect [A. cahirinus – A. russatus] = −0.17±0.07).
Moon phase also influenced the total amount of seeds eaten at each enclosure at both daytime and nighttime (Figure 2, Table 1): significantly more seeds were consumed by A. russatus under new moon vs. full moon (Moon (new) effect: 2.37±0.72) during both parts of the dial cycle (no significant interaction with day part term, −1.73±1.05). Competition had no significant main effect (Competition (yes) effect: −0.83±2.06). However, A. russatus consumed significantly more seeds during daytime than during nighttime (day part (night) effect: −2.55±0.46). There was no significant day-part * moon phase interaction (−1.73±1.05). A. cahirinus also consumed significantly more seeds under new moon (Moon (new) effect: 3.00±1.00). The effect of moon was not significantly different between A. cahirinus and A. russatus (Δnew moon effect [A. cahirinus – A. russatus] = 0.63±1.23).
Discussion
Moon phase affected fecal cortisol metabolite levels and foraging behavior of populations of both species in all experimental conditions; fecal cortisol metabolite levels were elevated as were GUDs.
During full moon nights, A. cahirinus visited fewer trays than during new moon nights; the opposite pattern occurred in A. russatus, but it was significant only in the two species enclosures (where the species is diurnal). Nevertheless, the total amount of seeds consumed was lower in full moon nights and the days following them for all populations, indicating a significant effect of moon phase on foraging behavior. The fact that in spite of their foraging in more patches, total seeds consumed was lower during full moon nights suggests two possible scenarios: a) that A. russatus had shorter visits to each tray, resulting in shorter foraging time per patch and therefore higher GUD values; b) similar time was spent on each patch, but more time was devoted to vigilance (in response to the elevated perceived predation risk [81]), resulting in less efficient foraging in each patch. Be that as it may, the increase in the number of trays foraged during full moon night or days did not compensate for the increased GUDs.
The behavioral response may be mediated, at least in part, by the elevated cortisol levels, reflected in our study by change in fecal cortisol metabolite levels, as has previously been demonstrated under laboratory settings. GCs were shown to have an effect on different behaviors that may result in reduced foraging. For example, in a light dark box test, GC treatment resulted in increased latency in the dark compartment, and in the elevated plus maze (elevated maze with two exposed and two sheltered arms) it increased time spent in the sheltered arms [31]–[34]. Tree lizards treated with GC responded more quickly to the predator and hid longer than control lizards [35], and in the Adelie penguin (Pygoscelis adeliae) individuals with high pre-foraging corticosterone levels spent less time foraging, and stayed closed to the colony than penguins with low pre-foraging corticosterone levels [82], so baseline corticosterone levels were correlated with foraging behavior. We suggest that elevated GC levels in spiny mice resulted in increased anxiety and fear, which influenced their foraging behavior. As mentioned earlier, GC may also affect foraging behavior via their effect on food consumption, so one could speculate that it is GC levels directly reducing consumption. However, since the relationships between GC levels and food consumption are complex, this cannot be determined from this study.
A. russatus are active in nature only during the day and have probably been so for millennia [42], [66], so individuals in the wild and in our two species enclosures do not routinely experience predation risk by owls, nor have their ancestors. The fact that when A. russatus revert to nocturnal activity, they respond to moon phase both in hormone levels and foraging behavior, suggests that this ‘hard-wired’ response is evolutionarily constrained; A. russatus individuals respond to elevated illumination although in all likelihood they have never faced risk of owl predation, suggesting that this response is genetically determined and was not lost in spite of the shift in activity patterns [46], [63], [67], [68]. Previous research shows that although A. russatus are diurnally active and have even evolved some adaptations for this activity pattern (reviewed by [42], [66]), they retain various physiological and morphological traits of nocturnal mammals [46], [61], [63], [64], [68], [69].
Behavior is considered to be of great evolutionary plasticity – changing prior to genetic changes; “in circumstances where rapid reaction to environmental events is required, behavior must invariably be more flexible than genetic change” [83]. A. russatus's behavioral rigidity indicates that avoidance behavior, reduced foraging during moonlit nights, is extremely stable to the point that it is retained in a diurnally active rodent.
More surprising is the fact the diurnal populations of A. russatus also respond to moon phase. Since predation efficiency of diurnal predators is not expected to be influenced by moon phase the previous night, moon phase is not expected to affect predation risk during the subsequent day, so diurnal moon-phase related behavior is probably not adaptive. In fact, it is the opposite of that expected if diurnal foraging should compensate for reduced nocturnal foraging, as has been described in several studies of other species: increased activity at dusk or dawn during full moon nights (Dipodomys merriami, [16]), more evenly spread activity in response to a predator in enclosed bank voles (Clethrionomys glareolus, [84]), increased diurnal activity bouts after exposure to light intensities similar to full moon light (Phyllotis xanthopygus, [85]), and inversion in activity patterns of Norwegian rats at high red fox densities [86]. How can we explain the response of diurnal A. russatus to moon phase?
Root voles (Microtus oeconomus) are active during both day and night; Halle [87] found that they reduced their activity in response to full moon nights and also during the days following them, “suggesting possible lunar periodicity in behavior of root voles” [87]. However, experiments carried out in controlled conditions demonstrate that rodents, including A. cahirinus, respond behaviorally to elevated illumination regardless of natural moon-phase [8], [13], [26], [88], [89]. If this response is mediated by GC, then GC should have lunar periodicity, but such endogenous periodicity was never described in mammals; lunar periodicity is currently known only in marine species, apparently a response to physical changes in tides that result from moon phase shifts (reviewed by [90]).
An alternative hypothesis can be based on the concept of a “memory window” [91] in which an optimal forager's knowledge of the environment and thereby its behavior are affected by the recent past; i.e., by a running average of the prior×patches (or t-times) [92]. Thereafter patch profitability is compared against this updated parameter rather than the unknown global value [92]. Perhaps A. russatus exhibited reduced foraging efficiency during days that followed moonlit nights because of a “memory” of increased risk of predation during the full moon night. This hypothesis is in accord with the preparative action of GCs [27]. Working with laboratory rats and mouse strains, which never encountered a predator, investigators use stimuli such as predator odor and smell, and even light to induce stress response. These studies rely on existence of an association between the stimulus, and the actual stressor. Such association is widely used in the study of stress: exposure to pairs of emotionally neutral stimuli such as sound, coupled with aversive stimuli, triggers a learning process, resulting in an acquired stress response to the neutral stimuli [93]. Such associations exist in animals that were never exposed to the actual stressor (e.g., individuals that were never exposed to a predator respond to its odor), suggesting that they are genetically determined, and can explain the mechanism for the “memory window” resulting in increased GC levels and reduced foraging in diurnally active A. russatus.
Indeed experiments of predator-prey interactions have shown the significance of previous experience [28]. For example, mice exposed to a live cat remained in their burrows for over 14 h [94], and an impaired long-term memory of 16–22 days followed the predatory stress [95]. Bank voles reduced activity when enclosed with a weasel, and recovered only days after its removal [84]. Kotler [56] found that gerbils had a slow rate of recovery (1–5 days) in foraging activity after being held with a barn owl. Sih et al. [29] point that prey may have difficulty in detecting and responding to a decrease in risk.
In sum, we found that both species exhibit high fecal cortisol metabolite levels and reduced foraging when the moon is full. We suggest that reduced foraging may be mediated by increased GC levels. More surprising are increased GC levels and reduced foraging in diurnally active A. russatus during days that follow moonlit nights. Not only does this behavior have no straightforward adaptive value, it can reduce fitness of these individuals (resulting from decreased food consumption and higher GC levels). We suggest that GC levels increase in response to previous experience – elevated risk of predation during moonlit nights, and that these elevated hormone levels induce predator-avoidance behavior in the field. Hormonal and behavioral responses to predation risk appear ‘hard wired’ in A. russatus, remaining evolutionarily constrained in spite of the shift in activity patterns.
Supporting Information
The effect of trapping on mean fecal cortisol metabolite levels (µg/dL ± SE) in (A) A. russatus and (B) A. cahirinus (experimental group – filled bars, n = 15, control group – empty bars, n = 15). * - P<0.05.
(TIFF)
Daily rhythms in mean fecal cortisol metabolites level (○, % ± SE), relative activity levels (×, % ± SE) and body temperature (•, °C ± SE) of A. russatus (A, n = 10) and A. cahirinus (B, n = 9). Data for fecal cortisol metabolite levels and activity levels are presented as % of the highest value obtained for each individual. Dark background represents the dark hours.
(TIFF)
The relationship between increasing pooled fecal mass (g) of A. russatus (A) and A. cahirinus (B) extracted and fecal cortisol metabolite levels (µg/dL). Dashed line represents the regression line: A. russatus– R2 = 0.83, p<0.01; A. cahirinus – R2 = 0.86, p<0.01).
(TIFF)
Validation of fecal cortisol measurements.
(DOC)
Acknowledgments
We thank Arieh Landsman for his tireless and cheerful assistance in the field, D. Ucitel, A. Barash, S. Bar-David, and U. Roll for their assistance in conducting the field experiments, A. Shkolnik for his constant advice and support, the Ein Gedi Field School of the Society for the Protection of Nature in Israel for their hospitality and warmth, and the Israel Nature and Parks Authority for their help.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by a National Geographic Society grant (6293-98) to T. Dayan (http://www.nationalgeographic.com/) and an ISF grant (04320021) to T. Dayan and N. Kronfeld-Schor (http://www.isf.org.il/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ebensperger LA. A review of the evolutionary causes of rodent group-living. Acta Theriologica. 2001;46:115–144. [Google Scholar]
- 2.Hanski I, Henttonen H, Korpimaki E, Oksanen L, Turchin P. Small-rodent dynamics and predation. Ecology. 2001;82:1505–1520. [Google Scholar]
- 3.Sundell J. Experimental tests of the role of predation in the population dynamics of voles and lemmings. Mammal Rev. 2006;36:107–141. [Google Scholar]
- 4.Roll U, Dayan T, Kronfeld-Schor N. On the role of phylogeny in determining activity patterns of rodents. Evol Ecol. 2006;20:479–490. [Google Scholar]
- 5.Lima SL. Nonlethal effects in the ecology of predator-prey interactions. Bio Science. 1998;48:25–34. [Google Scholar]
- 6.Nelson EH, Matthews CE, Rosenheim JA. Predators reduce prey population growth by inducing changes in prey behavior. Washington, DC: Ecological Society of America; 2004. 6 [Google Scholar]
- 7.Schmitz OJ, Suttle KB. Effects of top predator species on direct and indirect interactions in a food web. Ecology. 2001;82:2072–2081. [Google Scholar]
- 8.Abramsky Z, Rosenzweig ML, Subach A. The costs of apprehensive foraging. Ecology. 2002;83:1330–1340. [Google Scholar]
- 9.Bouskila A. Interactions between predation risk and competition - a field-study of kangaroo rats and snakes. Ecology. 1995;76:165–178. [Google Scholar]
- 10.Brown JS. Desert rodent community structure – A test of 4 mechanisms of coexistence. Ecol Monogr. 1989;59:1–20. [Google Scholar]
- 11.Brown JS, Kotler BP, Smith RJ, Wirtz WO. The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia. 1988;76:408–415. doi: 10.1007/BF00377036. [DOI] [PubMed] [Google Scholar]
- 12.Kotler BP, Brown JS. Mechanisms of coexistence of optimal foragers as determinants of local abundances and distributions of desert granivores. J Mammal. 1999;80:361–374. [Google Scholar]
- 13.Kotler BP, Brown JS, Hasson O. Factors affecting gerbil foraging behavior and rates of owl predation. Ecology. 1991;72:2249–2260. [Google Scholar]
- 14.Longland WS, Price MV. Direct observations of owls and heteromyid rodents: can predation risk explain microhabitat use? Ecology. 1991;72:2261–2273. [Google Scholar]
- 15.Bowers MA. Seed removal experiments on desert rodents - the microhabitat by moonlight effect. J Mammal. 1988;69:201–204. [Google Scholar]
- 16.Daly M, Behrends PR, Wilson MI, Jacobs LF. Behavioral modulation of predation risk – moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys-merriami. Anim Behav. 1992;44:1–9. [Google Scholar]
- 17.Kotler BP, Brown JS. Environmental heterogeneity and the coexistence of desert rodents. Annu Rev Ecol Syst. 1988;19:281–307. [Google Scholar]
- 18.Kotler BP, Brown JS, Dall SRX, Gresser S, Ganey D, et al. Foraging games between gerbils and their predators: temporal dynamics of resource depletion and apprehension in gerbils. Evol Ecol Res. 2002;4:495–518. [Google Scholar]
- 19.Mandelik Y, Jones M, Dayan T. Structurally complex habitat and sensory adaptations mediate the behavioural responses of a desert rodent to an indirect cue for increased predation risk. Evol Ecol Res. 2003;5:501–515. [Google Scholar]
- 20.Price MV, Waser NM, Bass TA. Effects of moonlight on microhabitat use by desert rodents. J Mammal. 1984;65:353–356. [Google Scholar]
- 21.Curio E. The ethology of predation. Berlin: Springer-Verlag; 1976. [Google Scholar]
- 22.Clarke JA. Moonlights influence on predatory prey interactions between short-eared owls (Asio-Flammeus) and deermice (Peromyscus-maniculatus). Behav Ecol Sociobiol. 1983;13:205–209. [Google Scholar]
- 23.Eilam D, Dayan T, Ben-Eliyahu S, Schulman I, Shefer G, et al. Differential behavioural and hormonal responses of voles and spiny mice to owl calls. Anim Behav. 1999;58:1085–1093. doi: 10.1006/anbe.1999.1224. [DOI] [PubMed] [Google Scholar]
- 24.Hendrie CA, Weiss SM, Eilam D. Exploration and predation models of anxiety: Evidence from laboratory and wild species. Pharmacol Biochem Behav. 1996;54:13–20. doi: 10.1016/0091-3057(95)02176-0. [DOI] [PubMed] [Google Scholar]
- 25.Hendrie CA, Weiss SM, Eilam D. Behavioral response of wild rodents to the calls of an owl: a comparative study. J Zool. 1998;254:439–446. [Google Scholar]
- 26.Mandelik Y, Dayan T, Eilam D. Foraging activity of Acomys cahirinus under different illumination levels: Comparing giving-up densities to direct behavioral observations. Isr J Zool. 2000;46:167–168. [Google Scholar]
- 27.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari MCO, Sih A, Chivers DP. The paradox of risk allocation: a review and prospectus. Anim Behav. 2009;78:579–585. [Google Scholar]
- 29.Sih A, Ziemba R, Harding KC. New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol Evol. 2000;15:3–4. doi: 10.1016/s0169-5347(99)01766-8. [DOI] [PubMed] [Google Scholar]
- 30.Mohawk JA, Pargament JM, Lee TM. Circadian dependence of corticosterone release to light exposure in the rat. Physiol Behav. 2007;92:800–806. doi: 10.1016/j.physbeh.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ardayfio P, Kim KS. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav Neurosci. 2006;120:1267–1267. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
- 32.Conrad CD, MacMillan DD, Tsekhanov S, Wright RL, Baran SE, et al. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiol Learn Mem. 2004;81:185–199. doi: 10.1016/j.nlm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. P Natl Acad Sci USA. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skorzewska A, Bidzinski A, Lehner M, Turzynska D, Sobolewska A, et al. The effects of acute corticosterone administration on anxiety, endogenous corticosterone, and c-Fos expression in the rat brain. Horm Behav. 2007;52:317–325. doi: 10.1016/j.yhbeh.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Thaker M, Lima SL, Hews DK. Acute corticosterone elevation enhances antipredator behaviors in male tree lizard morphs. Horm Behav. 2009;56:51–57. doi: 10.1016/j.yhbeh.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Cockrem JF, Silverin B. Sight of a predator can stimulate a corticosterone response in the great tit (Parus major) San Diego, CA: Elsevier; 2002. 8. [DOI] [PubMed] [Google Scholar]
- 37.Monclús R, Rödel H, Palme R, Holst D, Miguel J. Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology. 2006;16:25–29. [Google Scholar]
- 38.Thomas RM, Urban JH, Peterson DA. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp Neurol. 2006;201:308–315. doi: 10.1016/j.expneurol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, et al. Glucocorticoids, chronic stress, and obesity. Prog Brain Res. 2006;153:75–105. doi: 10.1016/S0079-6123(06)53004-3. [DOI] [PubMed] [Google Scholar]
- 41.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Kronfeld-Schor N, Dayan T. Activity patterns of rodents: the physiological ecology of biological rhythms. Biol Rhythm Res. 2008;39:193–211. [Google Scholar]
- 43.O'Steen S, Cullum AJ, Bennett AF. Rapid evolution of escape ability in Trinidadian guppies (Poecilia reticulata). Evolution. 2002;56:776–784. doi: 10.1111/j.0014-3820.2002.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 44.Stoks R, McPeek MA, Mitchell JL. Evolution of prey behavior in response to changes in predation regime: Damselflies in fish and dragonfly lakes. Evolution. 2003;57:574–585. doi: 10.1554/0014-3820(2003)057[0574:EOPBIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 45.Gutman R, Dayan T. Temporal partitioning: An experiment with two species of spiny mice. Ecology. 2005;86:164–173. [Google Scholar]
- 46.Levy O, Dayan T, Kronfeld-Schor N. The relationship between the golden spiny mouse circadian system and its diurnal activity: An experimental field enclosures and laboratory study. Chronobiol Int. 2007;24:599–613. doi: 10.1080/07420520701534640. [DOI] [PubMed] [Google Scholar]
- 47.Shkolnik A. Diurnal activity in a small desert rodent. Int J Biometeorol. 1971;15:115–120. doi: 10.1007/BF01803884. [DOI] [PubMed] [Google Scholar]
- 48.Volobouev V, Auffray JC, Debat V, Denys C, Gautun JC, et al. Species delimitation in the Acomys cahirinus-dimidiatus complex (Rodentia, Muridae) inferred from chromosomal and morphological analyses. Biol J Linn Soc. 2007;91:203–214. [Google Scholar]
- 49.Geffen E, Hefner R, Macdonald DW, Ucko M. Diet and foraging behavior of blanford foxes, Vulpes cana, in Israel. J Mammal. 1992;73:395–402. [Google Scholar]
- 50.Mandelik Y. Foraging microhabitat use and foraging efficiencies of the common spiny mouse, Acomys cahirinus (in Hebrew, English summary). 1999. Master's dissertation. Tel Aviv: Tel Aviv University.
- 51.Jones M, Mandelik Y, Dayan T. Coexistence of temporally partitioned spiny mice: Roles of habitat structure and foraging behavior. Ecology. 2001;82:2164–2176. [Google Scholar]
- 52.Shargal E, Rath-Wolfson L, Kronfeld N, Dayan T. Ecological and histological aspects of tail loss in spiny mice (Rodentia: Muridae, Acomys) with a review of its occurrence in rodents. J Zool. 1999;249:187–193. [Google Scholar]
- 53.Weissenberg S, Bouskila A, Dayan T. Resistance of the common spiny mouse (Acomys cahirinus) to the strikes of the Palestine saw-scaled viper (Echis coloratus). Isr J Zool. 1997;43:119. [Google Scholar]
- 54.Jones M, Dayan T. Foraging behavior and microhabitat use by spiny mice, Acomys cahirinus and A. russatus, in the presence of Blanford's fox (Vulpes cana) odor. J Chem Ecol. 2000;26:455–469. [Google Scholar]
- 55.Mendelssohn H. On the biology of the venomous snakes of Israel (II). Isr J Zool. 1965;14:185–212. [PubMed] [Google Scholar]
- 56.Kotler BP. Behavioral resource depression and decaying perceived risk of predation in two species of coexisting gerbils. Behav Ecol Sociobiol. 1992;30:239–244. [Google Scholar]
- 57.Hagenauer MH, Lee TM. Circadian organization of the diurnal Caviomorph rodent, Octodon degus. Biol Rhythm Res. 2008;39:269–289. [Google Scholar]
- 58.Redlin U, Mrosovsky N. Nocturnal activity in a diurnal rodent (Arvicanthis niloticus): The importance of masking. J of Biol Rhythm. 2004;19:58–67. doi: 10.1177/0748730403260371. [DOI] [PubMed] [Google Scholar]
- 59.Vivanco P, Rol MA, Madrid JA. Two steady-entrainment phases and graded masking effects by light generate different circadian chronotypes in octodon degus. Chronobiol Int. 2009;26:219–241. doi: 10.1080/07420520902768203. [DOI] [PubMed] [Google Scholar]
- 60.Weinert D, Weinandy R, Gattermann R. Photic and non-photic effects on the daily activity pattern of Mongolian gerbils. Physiol Behav. 2007;90:325–333. doi: 10.1016/j.physbeh.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 61.Cohen R, Smale L, Kronfeld-Schor N. Masking and temporal niche switches in spiny mice. J Biol Rhythm. 2010;25:47–52. doi: 10.1177/0748730409351672. [DOI] [PubMed] [Google Scholar]
- 62.Rotics S, Dayan T, Levy O, Kronfeld-Schor N. Light masking in the field: an experiment with nocturnal and diurnal spiny mice under semi-natural field conditions. Chronobiol Int. 2011;28:70–75. doi: 10.3109/07420528.2010.525674. [DOI] [PubMed] [Google Scholar]
- 63.Cohen R, Kronfeld-Schor N. Individual variability and photic entrainment of circadian rhythms in golden spiny mice. Physiol Behav. 2006;87:563–574. doi: 10.1016/j.physbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Cohen R, Kronfeld-Schor N, Ramanathan C, Baumgras A, Smale L. The substructure of the suprachiasmatic nucleus: similarities between nocturnal and diurnal spiny mice. Brain Behav Evol. 2010;75:9–22. doi: 10.1159/000282172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gutman R, Choshniak I, Kronfeld-Schor N. Defending body mass during food restriction in Acomys russatus: a desert rodent that does not store food. Am J Physiol. 2006;290:R881–R891. doi: 10.1152/ajpregu.00156.2005. [DOI] [PubMed] [Google Scholar]
- 66.Kronfeld-Schor N, Dayan T. Partitioning of time as an ecological resource. Annu Rev Ecol Evol S. 2003;34:153–181. [Google Scholar]
- 67.Kronfeld-Schor N, Dayan T, Elvert R, Haim A, Zisapel N, et al. On the use of the time axis for ecological separation: Diel rhythms as an evolutionary constraint. Am Nat. 2001;158:451–457. doi: 10.1086/321991. [DOI] [PubMed] [Google Scholar]
- 68.Kronfeld-Schor N, Dayan T, Jones ME, Kremer I, Mandelik Y, et al. Retinal structure and foraging microhabitat use of the golden spiny mouse (Acomys russatus). J Mammal. 2001;82:1016–1025. [Google Scholar]
- 69.Kronfeld-Schor N, Shargal E, Haim A, Dayan T, Zisapel N, et al. Temporal partitioning among diurnally and nocturnally active desert spiny mice: energy and water turnover costs. J Therm Biol. 2001;26:139–142. doi: 10.1016/s0306-4565(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 70.Shargal E, Kronfeld-Schor N, Dayan T. Population biology and spatial relationships of coexisting spiny mice (Acomys) in Israel. J Mammal. 2000;81:1046–1052. [Google Scholar]
- 71.Harper JM, Austad SN. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool. 2000;73:12–22. doi: 10.1086/316721. [DOI] [PubMed] [Google Scholar]
- 72.Lamers WH, Mooren PG, Griep H, Endert E, Degenhart HJ, et al. Hormones in perinatal rat and spiny mouse: relation to altricial and precocial timing of birth. Am J Physiol. 1986;251:E78–85. doi: 10.1152/ajpendo.1986.251.1.E78. [DOI] [PubMed] [Google Scholar]
- 73.Brown JS. Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol. 1988;22:37–47. [Google Scholar]
- 74.Crawley MJ. Statistical Computing: An Introduction to Data Analysis using S-plus. New York: John Wiley and Sons; 2002. [Google Scholar]
- 75.Anderson DR, Burnham KP, Thompson WL. Null hypothesis testing: Problems, prevalence, and an alternative. J Wildlife Manage. 2000;64:912–923. [Google Scholar]
- 76.Plummer M. JAGS: Just Another Gibbs Sampler. 2008. Version 1.0.3, available for download in http://www-fis.iarc.fr/~martyn/software/jags/
- 77.Plummer M, Best N, Cowles K, Vines K. coda: Output analysis and diagnostics for MCMC. 2009. R package version 0.13–4.
- 78.Cowles MK, Carlin BP. Markov chain Monte Carlo convergence diagnostics: A comparative review. J Am Stat Assoc. 1996;91:883–904. [Google Scholar]
- 79.Mengersen K, Robert C, Guihenneuc-Jouyaux C. MCMC convergence diagnostics: a “reviewww”. In: Elliott P, Wakefield JC, Best NG, Briggs D, editors. Spatial epidemiology: methods and applications. Oxford: Oxford University; 2000. pp. 393–414. [Google Scholar]
- 80.Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J Roy Stat Soc B. 2002;64:583–616. [Google Scholar]
- 81.Brown JS. Vigilance, patch use and habitat selection: Foraging under predation risk. Evol Ecol Res. 1999;1:49–71. [Google Scholar]
- 82.Angelier F, Bost CA, Giraudeau M, Bouteloup G, Dano S, et al. Corticosterone and foraging behavior in a diving seabird: The Adelie penguin, Pygoscelis adeliae. Gen Comp Endocr. 2008;156:134–144. doi: 10.1016/j.ygcen.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Hendrie CA, Weiss SM. The development of an animal model of panic with predictive and face validity. In: Cooper SJ, Hendrie CA, editors. Ethology and psychopharmacology. West Sussex, England: John Woley and Sons Ltd; 1994. [Google Scholar]
- 84.Jedrzejewska B, Jedrzejewski W. Antipredatory behaviour of bank voles and prey choice of weasels – enclosure experiments. Ann Zool Fenn. 1990;27:321–328. [Google Scholar]
- 85.Kramer KM, Birney EC. Effect of light intensity on activity patterns of Patagonian leaf-eared mice, Phyllotis xanthopygus. J Mammal. 2001;82:535–544. [Google Scholar]
- 86.Fenn MGP, Macdonald DW. Use of middens by red foxes - risk reverses rhythms of rats. J Mammal. 1995;76:130–136. [Google Scholar]
- 87.Halle S. Effects of extrinsic factors on activity of root voles, Microtus oeconous. J Mammal. 1995;76:88–99. [Google Scholar]
- 88.Abramsky Z, Rosenzweig ML, Belmaker J, Bar A. The impact of long-term continuous risk of predation on two species of gerbils. Can J Zool. 2004;82:464–474. [Google Scholar]
- 89.Kotler BP, Ayal Y, Subach A. Effects of predatory risk and resource renewal on the timing of foraging activity in a gerbil community. Oecologia. 1994;100:391–396. doi: 10.1007/BF00317860. [DOI] [PubMed] [Google Scholar]
- 90.Foster RG, Roenneberg T. Human responses to the geophysical daily, annual and lunar cycles. Curr Biol. 2008;18:R784–R794. doi: 10.1016/j.cub.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 91.Cowie RJ, Krebs JR. Optimal foraging in patchy environments. In: Anderson RM, Turner BD, Taylor LR, editors. Population dynamics. Oxford, England: Blackwell; 1979. pp. 183–205. [Google Scholar]
- 92.Crawford LL. Local contrast and memory windows as proximate foraging mechanisms. Z Tierpsychol. 1983;63:283–293. [Google Scholar]
- 93.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 94.Blanchard RJ, Parmigiani S, Bjornson, et al. Antipredator behavior of Swiss-Webster mice in a visible burrow system. New York, NY, USA: Wiley-Liss; 1995. [Google Scholar]
- 95.El Hage W, Griebel G, Belzung C. Long-term impaired memory following predatory stress in mice. Physiol Behav. 2006;87:45–50. doi: 10.1016/j.physbeh.2005.08.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effect of trapping on mean fecal cortisol metabolite levels (µg/dL ± SE) in (A) A. russatus and (B) A. cahirinus (experimental group – filled bars, n = 15, control group – empty bars, n = 15). * - P<0.05.
(TIFF)
Daily rhythms in mean fecal cortisol metabolites level (○, % ± SE), relative activity levels (×, % ± SE) and body temperature (•, °C ± SE) of A. russatus (A, n = 10) and A. cahirinus (B, n = 9). Data for fecal cortisol metabolite levels and activity levels are presented as % of the highest value obtained for each individual. Dark background represents the dark hours.
(TIFF)
The relationship between increasing pooled fecal mass (g) of A. russatus (A) and A. cahirinus (B) extracted and fecal cortisol metabolite levels (µg/dL). Dashed line represents the regression line: A. russatus– R2 = 0.83, p<0.01; A. cahirinus – R2 = 0.86, p<0.01).
(TIFF)
Validation of fecal cortisol measurements.
(DOC)