Abstract
Epidemiologic and scientific research indicates that diet and other lifestyle factors have a significant influence on the risk of developing colorectal cancer. Obesity, consumption of red meat, a Western pattern diet, alcohol, and smoking influence one’s risk of developing colorectal cancer while physical activity, vitamin D, post-menopausal estrogen use, aspirin and NSAIDs decrease one’s risk. Until recently, it was largely unknown if any of these modifiable factors influence the outcomes of patients already diagnosed with colorectal cancer. However, data are emerging of factors that may influence disease recurrences and mortality for colorectal cancer survivors. Prospective observational studies have shown that increased exercise after diagnosis and avoidance of a Western pattern diet are associated with reduced risk of cancer recurrence and improved overall survival in early- stage colorectal cancer after standard therapy. Patients with class II and III obesity (BMI ≥ 35 kg/m2) have a modestly increased risk of recurrence. Regular use of aspirin or cyclooxygenase-2 inhibitors decrease recurrence rates as well as increased serum vitamin D levels. In contrast, change of weight after diagnosis or smoking status (never, past or current) are not associated with outcomes after diagnosis. The data supporting these observations will be reviewed, potential mechanisms of actions will be discussed and next steps forward will be proposed.
In 2010, an estimated 142,570 individuals will be diagnosed with colorectal cancer and 51,370 will die from the disease in the United States.1 Eighty percent will be diagnosed without clinical or radiographic evidence of metastatic disease. Depending on the stage of disease and pathological features of the tumor, recurrence rates between 10–60% have been demonstrated. Greater than 50% of patients with 4 or more lymph nodes will recur or die at 5 years after diagnosis despite standard treatment with surgery and adjuvant chemotherapy. There is a clear need to further optimize the outcomes for patients with colorectal cancer.
Epidemiologic and scientific research indicates that diet and other lifestyle factors have a significant influence on the risk of developing colorectal cancer.2 Obesity, consumption of red meat or a Western pattern diet, alcohol, and smoking increase one’s risk of developing colorectal cancer while physical activity, calcium, vitamin D, post-menopausal estrogen use, aspirin or non steroidal anti-inflammatory usage decrease one’s risk. Until recently, it was largely unknown if any of these modifiable factors influence the outcomes of patients already diagnosed with colorectal cancer. However, data are emerging of host factors that may impact disease recurrences and mortality for colorectal cancer survivors. The sources of these data remain limited to observational studies, though interventional trials are now underway.
Physical activity in colorectal cancer survivors
Lack of physical activity has been the most consistently demonstrated lifestyle factor increasing the risk of developing colorectal cancer, particularly colon cancer.3 The International Agency for Research on Cancer concluded that the evidence supports a causal relation between inactivity and colorectal cancer risk.4 In a recent meta-analysis of 52 studies, Wolin and colleagues reported an inverse association between physical activity and primary colon cancer with an overall relative risk (RR) of 0.76 (95% confidence interval [CI]: 0.72–0.81), comparing the most to least active individuals across studies.5 No association has been observed between physical activity and the primary risk of rectal cancer.3
In the past five years, data have emerged for the potential influence of exercise in colorectal cancer survivors with stage I to III disease. In a study of physical activity and disease outcomes, 526 colorectal cancer survivors were identified from a cohort of 41,528 Australians that had a pre-diagnosis assessment of physical activity.6 Increased exercise was associated with improved disease-specific survival (adjusted hazard ratio [HR] = 0.73; 95% CI: 0.54 –1.00). In subgroup analyses, the association seemed restricted to stage II and III tumors (HR=0.49; 95% CI: 0.30–0.79). In efforts to correlate these findings with molecular markers, the investigators reported that physically active colorectal cancer survivors had higher insulin-like growth factor binding protein-3 (IGFBP-3), which was associated with a significant reduction in disease-specific death (HR=0.52; 95% CI: 0.33–0.83).7 These data suggest that the association between physical activity and disease-specific survival in colorectal cancer survivors may be through the insulin-like growth factor (IGF) axis, particularly IGFBP-3.
Three studies have examined the association between post-diagnosis physical activity and disease outcomes in colorectal cancer survivors (Table 1).8–10 A prospective, observational study of 832 patients who participated in an adjuvant study comparing 5-fluorouracil (5-FU) and leucovorin to irinotecan, 5-FU and leucovorin (IFL) for stage III colon cancer (CALGB 89803) found that higher levels of self-reported physical activity approximately 6 months after completion of chemotherapy (at least 18 metabolic equivalent task [MET]-hours/week) were associated with superior disease-free, recurrence-free, and overall survival, adjusted for known prognostic factors, including body mass index (BMI). 10 For reference, 3 MET-hours is equivalent to one hour average pace walking.
Table 1.
Association between physical activity and outcome in colorectal cancer survivors
| CALGB 89803 | Nurse’s Health Study | Health Professionals Follow-up Study | ||||
|---|---|---|---|---|---|---|
| MET-hours/week | Adjusted HR Disease-Free Survival (95% CI) | Adjusted HR Overall Survival (95% CI) | Adjusted HR for CRC-specific mortality (95% CI) | Adjusted HR for overall mortality (95% CI) | Adjusted HR for CRC- specific mortality (95% CI) | Adjusted HR for overall mortality (95% CI) |
| < 3 | Referent | Referent | Referent | Referent | Referent | Referent |
| 3–8.9 | 0.87 (0.58–1.29) | 0.85(0.49–1.49) | 0.92 (0.50–1.69) | 0.77 (0.48–1.23) | 1.06 (0.55–2.08) | 1.00 (0.68–1.48) |
| 9–17.9 | 0.90 (0.57–1.40) | 0.71 (0.36–1.41) | 0.57 (0.27–1.20) | 0.50 (0.28–0.90) | 1.30 (0.65–2.59) | 1.12 (0.74–1.70) |
| 18–26.9 | 0.51 (0.26–0.97) | 0.71 (0.32–1.59) | 0.39 (0.18–0.82) for >18 MET-hrs/week | 0.43 (0.25–0.74) for >18 MET-hrs/week | 0.76 (0.33–1.77) | 0.74 (0.46–1.20) |
| ≥ 27 | 0.55 (0.33–0.91) | 0.37 (0.16–0.82) | 0.47 (0.24–0.92) | 0.59 (0.41–0.86) | ||
| P trend | 0.01 | 0.01 | 0.008 | 0.003 | 0.002 | 0.0003 |
CALGB = Cancer and Leukemia Group B; HR = hazard ratio; CRC = colorectal cancer
In a second study, a cohort of 573 women diagnosed with stages I – III colorectal cancer who participate in the Nurses’ Health Study and self-reported leisure-time physical activity before diagnosis and 1–4 years after diagnosis was utilized to test the association between exercise before diagnosis and after diagnosis and survival outcomes.8 Women who were most physically active experienced 61% reduced colorectal cancer–specific mortality and 57% reduced overall mortality compared to inactive women, adjusted for other prognostic factors. Level of physical activity prior to diagnosis was not associated with mortality.
A similar study to that of the Nurse’s Health Study was recently reported utilizing male colorectal cancer survivors from the Health Professionals Follow-up Study.9 Amongst 668 men with stage I–III colorectal cancer, more than 27 MET-hours per week of exercise had an adjusted HR for colorectal cancer-specific mortality of 0.47 (95% CI: 0.24–0.92) compared with men who engaged in 3 or less MET hours per week.
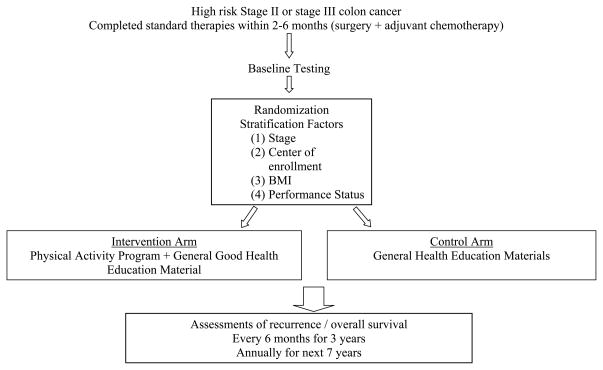
Based on these data, a multinational trial in Canada and Australia, the Colon Health and Life-Long Exercise Change (CHALLENGE) trial, was developed to determine the effects of a 3 year structured and supervised physical activity intervention on disease outcomes in 962 high-risk stage II and III colon cancer survivors who have completed adjuvant chemotherapy within the previous 2–6 months (Figure 1).11 The primary end point is disease-free survival and secondary endpoints include patient-reported outcomes, health-related fitness, biologic correlative markers, and an economic analysis. The trial is currently open to accrual.
Figure 1.
Colon Health and Life-Long Exercise Change Trial (CHALLENGE) Schema
Several observational studies have shown that higher physical activity levels or meeting physical activity guidelines is associated with better patient-reported quality of life, physical functioning, and fatigue.12–17 To date, only one randomized trial of an exercise intervention in colorectal cancer survivors has been published.18 There was appreciable contamination in the comparison group, impacting the intention-to-treat analyses. In a secondary analysis, participants whose fitness increased over the course of the intervention, regardless of group assignment, reported significantly improved quality of life, physical functioning, and psychosocial distress compared to participants whose fitness decreased.
Adiposity in colorectal cancer survivors
The impact of adiposity on outcomes in non-metastatic colorectal cancer has been less certain.19–24 There are relatively consistent data that obese subjects have an increased risk of developing colorectal cancer.25 However, prospective observational cohort studies in colon or rectal cancer survivors have only shown a modest association with outcomes.20,22 When detected, the association has primarily been restricted to those with BMI ≥ 35 mg/m2 (class II and III obesity), with approximately 25% worsening of disease-free survival.
Only one study has observed the influence of change in weight after diagnosis on cancer recurrences and survival.20 In breast cancer survivors, gain in weight has been associated with increased risk of cancer in some,26,27 but not all studies.28,29 Increasing weight after diagnosis (between time on adjuvant therapy and 6 months after completion of adjuvant therapy) was not associated with disease-free survival or overall survival in the CALGB 89803 cohort.
Diet and Colon Cancer Outcomes
Diet has been extensively studied as a risk factor for the development of colorectal cancer.25,30–33 In contrast, few studies have examined the association between diet and outcomes in colorectal cancer survivors.34–36 Slattery and colleagues studied 411 colon cancer patients from two population-based case-control studies and observed an improved survival with increasing consumption of calories, fat, and protein.34 Among 148 patients with colorectal cancer, Dray et al reported improved survival with increasing consumption of calories based on dietary information prior to diagnosis.36 These two studies were limited by their heterogeneous patient population that included all stages of disease, small sample size, lack of data on cancer treatment, and limited capacity to adjust for other prognostic factors.
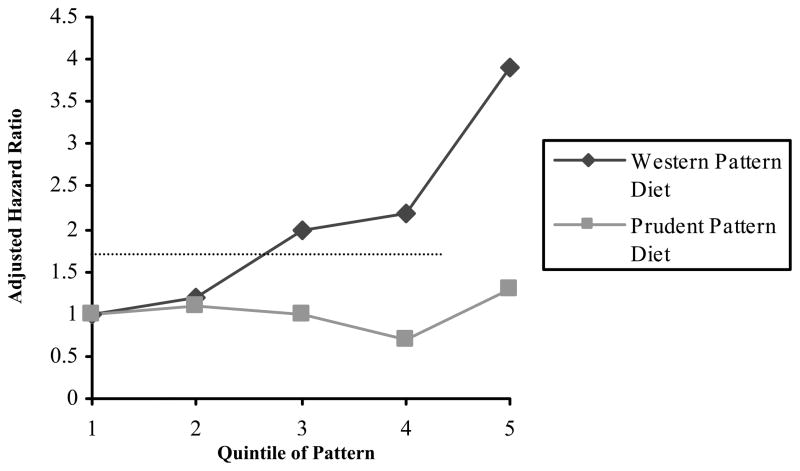
To address these concerns, a prospective observational study on diet in 1,009 colon cancer survivors from CALGB 89803 was reported in which participants completed a food frequency questionnaire during adjuvant therapy and 6 months after the completion of adjuvant therapy.35 Using factor analysis, two major dietary patterns were identified, prudent and Western pattern. The prudent pattern was characterized by high fruit and vegetable, poultry and fish intakes; the Western pattern was characterized by high meat, fat, refined grains and dessert intakes. All patients were assigned a relative value along the spectrum of both dietary patterns. The two patterns were not correlated with each other (Spearman correlation coefficient = 0.02). Western pattern diet was associated with disease-free and overall survival (Figure 2). Compared with patients in the lowest quintile of Western pattern diet, those in the highest quintile experienced worse disease-free survival, with an adjusted HR of 3.25 (95% CI: 2.04–5.19; P for trend <0.0001). In that patient cohort, patients in the highest quintile of Western pattern diet consumed a mean intake of 6 servings of red meat per week, 5.6 servings of processed meat per week, 5.8 servings of refined grains daily and 2.5 servings of sugary desserts daily.37 In comparison, those in the lowest quintile of Western pattern diet reported a mean intake of 2.3 servings of red meat and 1.8 servings of processed meats per week and 2.0 servings of refined grains and less than 1 serving of sugary desserts daily. In contrast, the prudent pattern diet was not significantly associated to cancer recurrence or mortality. Since these patterns are mutually exclusive, based on the data, the data would suggest an avoidance or reduction of foods associated with a Western pattern diet but not necessarily an increase in foods associated with the prudent pattern diet.
Figure 2.
Diet Patterns and Disease-Free Survival in Stage III Colon Cancer35
Potential Mechanisms of Energy Balance Factors and Outcomes in Colorectal Cancer Survivors
Physical activity, adiposity and diet influence energy balance within individuals. Molecular pathways associated with alterations in energy balance are a leading potential mechanism for the association between these host factors and outcomes in colorectal cancer survivors. Shifts in energy balance may lead to changes in insulin and insulin-like growth factors (IGF) levels as well as sensitivity to insulin.38–40 In observational studies, colon cancer risk is elevated in individuals with higher circulating levels of insulin or C-peptide (a marker of insulin secretion)41–43 and IGF-1 or IGF-1/IGF binding protein (IGFBP)-3 ratio.44–48 Preclinically, insulin stimulates pathways that increase levels of free IGF-1, and both insulin and IGF-1 promote cell proliferation and inhibit apoptosis in colon cancer cells.49–53 In one study, obesity and physical inactivity influenced colon cancer risk primarily through the insulin axis, but non-hyperinsulinemic (ie. non-overweight, physically active) men were still at elevated risk if they had high IGF-1 levels, suggesting that high circulating IGF-1 (or IGF-1/IGFBP-3 ratio) and hyperinsulinemia may represent two different axes that influence colorectal neoplasia risk.54
The presumed cause of disease recurrence and colorectal cancer-specific mortality in patients initially diagnosed with non-metastatic colorectal cancer is the growth of micrometastatic cells in distant organs. Physical activity, obesity and a Western pattern diet may influence insulin and insulin-like growth factors, which subsequently stimulate growth and inhibit apotosis of micrometastases. Among 373 patients diagnosed with nonmetastatic colorectal cancer in two prospective observational cohort studies, those in the top quartile of plasma C-peptide had an age-adjusted HR for death of 1.87 (95% CI: 1.04 to 3.36; P = 0.03 for trend), whereas those in the top quartile of circulating IGFBP-1 had a significant reduction in mortality (HR = 0.48; 95% CI: 0.28 to 0.84; P = 0.02 for trend), both compared to patients in respective bottom quartiles.
Other potential mechanisms for the influence of physical activity, adiposity and diet are alterations in vitamin D (as these host factors influence vitamin D levels), changes in hormones (particularly estrogen), inflammation and immune modulation.
Aspirin and Nonsteroidal Anti-inflammatory Agents (NSAIDs)
Aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) have long been studied as agents that may influence cancer development and progression.55 Data from observational studies and intervention trials consistently demonstrate that usage of these agents reduces the risk of colorectal adenomas and/or cancer.55–60 Hypotheses for the mechanism of action of these agents include inhibition of the cyclooxygenase (COX) family of enzymes (increasing arachidonic acid which stimulates the conversion of sphingomyelin to ceramide that mediates apoptosis as well as altering prostaglandin production which will decrease angiogenic factors), inhibition of the activation of nuclear factor- -B, interference of the binding of peroxisome-proliferator–activated receptor (PPAR ) to DNA and other potential non-COX-mediated pathways.61 Regardless of the precise mechanism, the data is so consistent that causality with colorectal cancer development is generally accepted.
In a randomized trial of 517 patients with prior stage I–III colorectal cancer, patients randomized to 325 mg daily aspirin experienced a reduction in number of subsequent polyps (adjusted relative risk 0.65 [95% CI: 0.46–0.91]) as well as time to detection of a first adenoma (HR = 0.64 [95% CI: 0.43 – 0.94]).62 Recently, two prospective cohorts of colorectal cancer survivors have suggested an association between use of aspirin or cyclooxygenase 2 (COX-2) inhibitors and cancer recurrence and survival in non-metastatic colorectal cancer patients. Chan and colleagues reported that among 1,279 stage I – III colorectal cancer survivors, those who regularly used aspirin after diagnosis experienced an adjusted HR for colorectal cancer specific mortality of 0.71 (95% CI: 0.53–0.95) and for overall mortality of 0.79 (95% CI: 0.65–0.97), compared to nonusers.63 When restricting the analyses to the 719 participants who did not use aspirin before diagnosis, aspirin use initiated after diagnosis was associated with an adjusted HR for colorectal cancer specific mortality of 0.53 (95% CI: 0.33–0.86).
In the CALGB 89803 cohort of stage III colon cancer patients, regular aspirin use (defined as consistent use while on adjuvant therapy and 6 months after the completion of adjuvant therapy) had a statistically significant 54% improvement in disease-free survival compared to non-regular users.64 A similar impact was seen in patients who were on COX-2 inhibitors (celecoxib or rofecoxib).
The Vioxx in Colorectal Cancer Therapy: Definition of Optimal Regime (VICTOR) trial was a randomized controlled trial in the United Kingdom evaluating the role of rofecoxib for stage II and III colon cancer who completed adjuvant therapy.65 The trial activated in April 2002 but was terminated in September 2004 after withdrawal of rofecoxib from the market. Prior to study closure, 2,434 patients were randomized to placebo, 2 years of rofecoxib or 5 years of rofecoxib, with a median duration of time on trial of 7.4 months. The hazard ratio for disease-free survival with median follow-up of 3 years was 0.90 (95% CI, 0.77 – 1.06), favoring rofecoxib amongst stage II and III patients.
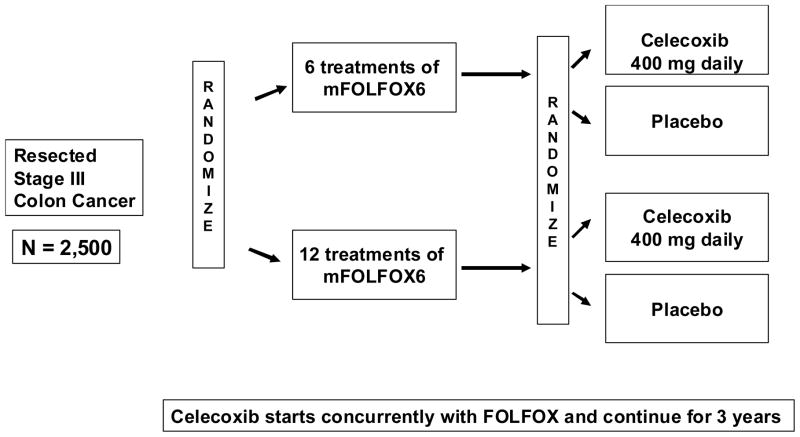
Given these data, the Cancer and Leukemia Group B and Southwestern Oncology Group recently activated a randomized trial to test celecoxib as an adjunctive to standard therapy in stage III colon cancer patients (Figure 3). The trial has a 2 × 2 randomization design, with the first randomization of 6 versus 12 treatments of 5-FU, leucovorin and oxaliplatin (FOLFOX) and the second randomization of celecoxib versus placebo initiated with FOLFOX and continuing for three years. Data testing duration of FOLFOX therapy will be pooled with at least 3 other trials in Europe to test for noninferiority. The trial will test for superiority in disease-free survival of celecoxib compared to placebo.
Figure 3.
Schema of CALGB/SWOG 80702 Phase III adjuvant therapy trial for stage III colon cancer patients
Vitamin D
Increasing evidence suggests an association between vitamin D and development of colorectal cancer.66 A meta-analysis of five epidemiologic studies found a 51% decrease in the risk of colorectal cancer associated with plasma 25(OH)D levels in the highest quintile compared to those in the lowest quintile (P<0.0001).67 The cellular effects of 1–25-dihydroxycholecalciferol [1,25(OH)2D] are principally mediated through the vitamin D receptor (VDR), which regulates the transcription of genes involved in cellular differentiation and inhibition of proliferation.68,69 Well differentiated human colon cancer cell lines have higher VDR expression 70 and the antiproliferative effects of vitamin D only occur in cell lines expressing high levels of VDR.71
The role of vitamin D in cancer survivors has recently generated much interest.72 Ng and colleagues reported results of a cohort of 304 participants of the Nurse’s Health Study and Health Professional Follow-up Study who were diagnosed with colorectal cancer and had a serum vitamin D level at least 2 years prior to diagnosis and found that those with the highest level of pre-diagnosis vitamin D had a statistically significant 55% improvement in overall mortality compared to those with the lowest level.73 In an expanded effort using those cohorts, Ng and colleagues predicted serum 25 (OH) D levels in 1,107 subjects using race, geographic region, dietary vitamin D intake, BMI, and physical activity.74 Compared with levels in the lowest quintile, participants with predicted 25 (OH) D levels in the highest quintile had an adjusted HR of 0.50 (95% CI, 0.26–0.95) for cancer-specific mortality and 0.62 (95% CI, 0.42–0.93) for overall mortality.
Cigarette smoking
Smoking has been associated with the development of colorectal cancer and may contribute up to 22% of cases.75,76 Using data from CALGB 89803, Jackson and colleagues recently reported no association between smoking status (current, past and never smoker) or total tobacco usage and disease-free survival amongst patients with stage III colon cancer.77 However, when limiting analyses to early years smoking intensity, the adjusted HR for disease-free survival for subjects with at least 12 pack-years of smoking prior to age 30 was 1.37 (95% CI 1.02–1.84) compared to never smoking (p trend 0.04). These results imply that smoking early in life may eventually lead to more aggressive colon cancers with higher risk of recurrence.
Summary
Cancer survivors often seek answers as to what additional steps they can take to increase the likelihood of “beating” their cancer. Patients frequently ask their oncologist what they should eat, does exercise matter, what supplements to take, and what else they can do. As with chemotherapy, no such modifiable diet or lifestyle intervention is likely to benefit all patients. However, it is becoming increasingly evident that certain factors may influence some patients’ outcome. There are clear limitations to these data at this point. The data are observational at this point, albeit prospective with efforts to minimize bias and confounding. A randomized clinical trial is currently underway to test exercise and another to test celecoxib as adjunct to standard adjuvant therapy. The data is also principally limited to non-metastatic colorectal cancer. There are current efforts to collect similar prospective data of diet, lifestyle and supplements in patients undergoing chemotherapy with metastatic colorectal cancer. Further, there are studies underwent to test potential tumor and host biomarkers that may predict more or less likelihood of benefit or detriment from certain diet and lifestyle factors. These data are needed to better define a set of guidelines for colorectal cancer survivors.
Table 2.
Summary of studies of body mass index in colorectal cancer survivors
| Author | Year of Diagnosis | Sample size | Stages of Disease | Outcome measure | Hazard Ratio (95% CI) or P value (compared to normal weight) |
|---|---|---|---|---|---|
| Tartter78 | 1976–1979 | 279 | Duke B2, C1, C2 colon cancer | Recurrence rate | P = 0.003 for above median weight |
| Meyerhardt19 | 1988–1992 | 3759 | Duke B2, B3, C colon cancer | Disease-free survival | 1.11 (0.94–1.30) BMI ≥ 30 kg/m2 |
| Overall survival | 1.11 (0.96–1.29) BMI ≥ 30 kg/m | ||||
| Meyerhardt 21 | 1990–1992 | 1792 | TNM Stage II and III rectal cancer | Disease-free survival | 1.10 (0.91–1.32) BMI ≥ 30 kg/m2 |
| Overall survival | 1.09 (0.90–1.33) BMI ≥ 30 kg/m2 | ||||
| Local recurrences | 1.31 (0.91–1.88) BMI ≥ 30 kg/m2 | ||||
| Dignam 22 | 1989–1994 | 4288 | Duke B and C colon cancer | Disease-free survival | 1.06 (0.93–1.21) BMI 30–34.9 kg/m2 1.27 (1.05–1.53) BMI ≥ 35 kg/m2 |
| Colon cancer events | 1.04 (0.88–1.24) BMI 30–34.9 kg/m2 1.38 (1.10–1.73) BMI ≥ 35 kg/m2 |
||||
| Meyerhardt 20 | 1999–2001 | 1053 | TNM Stage III colon cancer | Disease-free survival | 1.00 (0.72–1.40) BMI 30–34.9 kg/m2 1.24 (0.84–1.83) BMI ≥ 35 kg/m2 |
| Recurrence-free survival | 0.97 (0.69–1.37) BMI 30–34.9 kg/m2 1.27 (0.85–1.89) BMI ≥ 35 kg/m2 |
||||
| Overall survival | 0.90 (0.61–1.34) BMI 30–34.9 kg/m2 0.87 (0.54–1.42) BMI ≥ 35 kg/m2 |
||||
| Hines 24 | 1981–2001 | 496 | TNM Stage I-IV colon cancer | Overall survival | 0.77 (0.61–0.97) BMI ≥ 25 all stages 0.92 (0.65–1.30) stage I–II 0.92 (0.59–1.45) stage III 0.58 (0.37–0.90) stage IV |
TNM = tumor, node, metastases staging; CI = confidence interval
Acknowledgments
Acknowledgement of research support: Jeffrey Meyerhardt receives research support from NCI grants R01 CA149222, R01 CA118553, P50 CA127003, P01CA87969 and P01 CA55075.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, Colditz GA. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108(3):433–42. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7(3):204–13. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 4.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11 (Suppl 2):S94–100. [PubMed] [Google Scholar]
- 5.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100(4):611–6. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55(1):62–7. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haydon AM, Macinnis RJ, English DR, Morris H, Giles GG. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55(5):689–94. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, Fuchs CS. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169(22):2102–8. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 11.Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, Friedenreich CM, Au HJ, Brundage MD, Tu D, Dhillon H, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15(6):271–8. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 13.Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215–26. doi: 10.1089/acm.1997.3.215. [DOI] [PubMed] [Google Scholar]
- 14.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21(2):171–9. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BL, Trentham-Dietz A, Koltyn KF, Colbert LH. Physical activity and function in older, long-term colorectal cancer survivors. Cancer Causes Control. 2009;20(5):775–84. doi: 10.1007/s10552-008-9292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch BM, Cerin E, Owen N, Aitken JF. Associations of leisure-time physical activity with quality of life in a large, population-based sample of colorectal cancer survivors. Cancer Causes Control. 2007;18(7):735–42. doi: 10.1007/s10552-007-9016-6. [DOI] [PubMed] [Google Scholar]
- 17.Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum. 2008;51(8):1242–8. doi: 10.1007/s10350-008-9324-2. [DOI] [PubMed] [Google Scholar]
- 18.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12(4):347–57. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB, 3rd, Macdonald JS, Fuchs CS. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98(3):484–95. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26(25):4109–15. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerhardt JA, Tepper JE, Niedzwiecki D, Hollis DR, McCollum AD, Brady D, O’Connell MJ, Mayer RJ, Cummings B, Willett C, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22(4):648–57. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 22.Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O’Connell MJ, Wolmark N. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98(22):1647–54. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 23.Shibakita M, Yoshimura H, Tachibana M, Ueda S, Nagasue N. Body mass index influences long-term outcome in patients with colorectal cancer. Hepatogastroenterology. 57(97):62–9. [PubMed] [Google Scholar]
- 24.Hines R, Shanmugam C, Waterbor J, McGwin G, Funkhouser E, Coffey C, Posey J, Manne U. Effect of Comorbidity and Body Mass Index on the Survival of African-American and Caucasian Patients With Colon Cancer. Cancer. 2009;115:5798–806. doi: 10.1002/cncr.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannucci E. Diet, body weight, and colorectal cancer: a summary of the epidemiologic evidence. J Womens Health (Larchmt) 2003;12(2):173–82. doi: 10.1089/154099903321576574. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–8. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 27.Camoriano JK, Loprinzi CL, Ingle JN, Therneau TM, Krook JE, Veeder MH. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol. 1990;8(8):1327–34. doi: 10.1200/JCO.1990.8.8.1327. [DOI] [PubMed] [Google Scholar]
- 28.Caan BJ, Emond JA, Natarajan L, Castillo A, Gunderson EP, Habel L, Jones L, Newman VA, Rock CL, Slattery ML, et al. Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat. 2006;99(1):47–57. doi: 10.1007/s10549-006-9179-y. [DOI] [PubMed] [Google Scholar]
- 29.Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, Weltzien E. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19(10):1319–28. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31(4):925–43. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 31.Thun MJ, Calle EE, Namboodiri MM, Flanders WD, Coates RJ, Byers T, Boffetta P, Garfinkel L, Heath CW., Jr Risk factors for fatal colon cancer in a large prospective study. J Natl Cancer Inst. 1992;84(19):1491–500. doi: 10.1093/jnci/84.19.1491. [DOI] [PubMed] [Google Scholar]
- 32.Martinez ME. Primary prevention of colorectal cancer: lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 33.Bostick RM, Potter JD, Kushi LH, Sellers TA, Steinmetz KA, McKenzie DR, Gapstur SM, Folsom AR. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States) Cancer Causes Control. 1994;5:38–52. doi: 10.1007/BF01830725. [DOI] [PubMed] [Google Scholar]
- 34.Slattery ML, French TK, Egger MJ, Lyon JL. Diet and survival of patients with colon cancer in Utah: is there an association? Int J Epidemiol. 1989;18(4):792–7. doi: 10.1093/ije/18.4.792. [DOI] [PubMed] [Google Scholar]
- 35.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. Jama. 2007;298(7):754–64. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 36.Dray X, Boutron-Ruault MC, Bertrais S, Sapinho D, Benhamiche-Bouvier AM, Faivre J. Influence of dietary factors on colorectal cancer survival. Gut. 2003;52(6):868–73. doi: 10.1136/gut.52.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyerhardt JA, Fuchs C. Cancer Recurrence and Survival Associated With Dietary Patterns in Stage III Colon Cancer-Reply. Jama. 2007;298(19):2263. doi: 10.1001/jama.298.19.2263-a. [DOI] [PubMed] [Google Scholar]
- 38.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6(2):164–79. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 39.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11 Suppl):3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 40.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60(1):91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 41.Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91(13):1147–54. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 42.Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92(19):1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 43.Palmqvist R, Stattin P, Rinaldi S, Biessy C, Stenling R, Riboli E, Hallmans G, Kaaks R. Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int J Cancer. 2003;107(1):89–93. doi: 10.1002/ijc.11362. [DOI] [PubMed] [Google Scholar]
- 44.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91(7):620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 45.Manousos O, Souglakos J, Bosetti C, Tzonou A, Chatzidakis V, Trichopoulos D, Adami HO, Mantzoros C. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer. 1999;83(1):15–7. doi: 10.1002/(sici)1097-0215(19990924)83:1<15::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 46.Renehan AG, Painter JE, Atkin WS, Potten CS, Shalet SM, O’Dwyer ST. High-risk colorectal adenomas and serum insulin-like growth factors. Br J Surg. 2001;88(1):107–13. doi: 10.1046/j.1365-2168.2001.01645.x. [DOI] [PubMed] [Google Scholar]
- 47.Palmqvist R, Hallmans G, Rinaldi S, Biessy C, Stenling R, Riboli E, Kaaks R. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50(5):642–6. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Probst-Hensch NM, Yuan JM, Stanczyk FZ, Gao YT, Ross RK, Yu MC. IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. Br J Cancer. 2001;85(11):1695–9. doi: 10.1054/bjoc.2001.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollak MN, Perdue JF, Margolese RG, Baer K, Richard M. Presence of somatomedin receptors on primary human breast and colon carcinomas. Cancer Lett. 1987;38(1–2):223–30. doi: 10.1016/0304-3835(87)90218-7. [DOI] [PubMed] [Google Scholar]
- 50.Guo YS, Narayan S, Yallampalli C, Singh P. Characterization of insulinlike growth factor I receptors in human colon cancer. Gastroenterology. 1992;102(4 Pt 1):1101–8. [PubMed] [Google Scholar]
- 51.Watkins L, Lewis L, Levine A. Characterization of the synergistic effect of insulin and transferrin and the regulation of their receptors on a human colon carcinoma cell line. Int J Cancer. 1990;45:372–375. doi: 10.1002/ijc.2910450227. [DOI] [PubMed] [Google Scholar]
- 52.Koenuma M, Yamori T, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res. 1989;80(1):51–8. doi: 10.1111/j.1349-7006.1989.tb02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjork J, Nilsson J, Hultcrantz R, Johansson C. Growth-regulatory effects of sensory neuropeptides, epidermal growth factor, insulin, and somatostatin on the non-transformed intestinal epithelial cell line IEC-6 and the colon cancer cell line HT 29. Scand J Gastroenterol. 1993;28(10):879–84. doi: 10.3109/00365529309103129. [DOI] [PubMed] [Google Scholar]
- 54.Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, Gaziano JM, Stampfer MJ. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004;96(7):546–53. doi: 10.1093/jnci/djh082. [DOI] [PubMed] [Google Scholar]
- 55.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 56.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 57.Cross JT, Poole EM, Ulrich CM. A review of gene-drug interactions for nonsteroidal anti-inflammatory drug use in preventing colorectal neoplasia. Pharmacogenomics J. 2008;8(4):237–47. doi: 10.1038/sj.tpj.6500487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan AT. Aspirin, non-steroidal anti-inflammatory drugs and colorectal neoplasia: future challenges in chemoprevention. Cancer Causes Control. 2003;14(5):413–8. doi: 10.1023/a:1024986220526. [DOI] [PubMed] [Google Scholar]
- 59.Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, Bolognese JA, Oxenius B, Horgan K, Loftus S, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–82. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 60.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. Jama. 2005;294(8):914–23. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 62.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 63.Chan AT, Ogino S, Fuchs CS. Aspirin Use and Survival After Diagnosis of Colorectal Cancer. Jama. 2009;302(6):649–659. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuchs C, Meyerhardt JA, Heseltine DL, Niedzwiecki D, Hollis D, Chan AT, Saltz LB, Schilsky RL, Mayer RJ. Influence of regular aspirin use on survival for patients with stage III colon cancer: Findings from Intergroup trial CALGB 89803. J Clin Oncol. 2005;23(16S) Abstract 3530. [Google Scholar]
- 65.Kerr DJ, Dunn JA, Langman MJ, Smith JL, Midgley RS, Iveson CL, McConkey CC. VICTOR: A phase III placebo-controlled trial of rofecoxib in colorectal cancer patients following surgical resection. J Clin Oncol. 2008 May 20;26(suppl) abstr 4038. [Google Scholar]
- 66.Giovannucci E. Epidemiological evidence for vitamin D and colorectal cancer. J Bone Miner Res. 2007;22 (Suppl 2):V81–5. doi: 10.1359/jbmr.07s206. [DOI] [PubMed] [Google Scholar]
- 67.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32(3):210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984;72(6):1323–5. [PubMed] [Google Scholar]
- 69.Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13(4):719–64. doi: 10.1210/edrv-13-4-719. [DOI] [PubMed] [Google Scholar]
- 70.Shabahang M, Buras RR, Davoodi F, Schumaker LM, Nauta RJ, Evans SR. 1,25-Dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res. 1993;53(16):3712–8. [PubMed] [Google Scholar]
- 71.Evans SR, Schwartz AM, Shchepotin EI, Uskokovic M, Shchepotin IB. Growth inhibitory effects of 1,25-dihydroxyvitamin D3 and its synthetic analogue, 1alpha,25-dihydroxy-16-ene-23yne-26,27-hexafluoro-19-nor-cholecalcifero l (Ro 25–6760), on a human colon cancer xenograft. Clin Cancer Res. 1998;4(11):2869–76. [PubMed] [Google Scholar]
- 72.Goodwin PJ. Vitamin D in cancer patients: above all, do no harm. J Clin Oncol. 2009;27(13):2117–9. doi: 10.1200/JCO.2008.20.8629. [DOI] [PubMed] [Google Scholar]
- 73.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, Fuchs CS. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–91. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 74.Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, Hollis BW, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101(6):916–23. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giovannucci E, Martinez ME. Tobacco, colorectal cancer, and adenomas: a review of the evidence. J Natl Cancer Inst. 1996;88(23):1717–30. doi: 10.1093/jnci/88.23.1717. [DOI] [PubMed] [Google Scholar]
- 76.Heineman EF, Zahm SH, McLaughlin JK, Vaught JB. Increased risk of colorectal cancer among smokers: results of a 26-year follow-up of US veterans and a review. Int J Cancer. 1994;59(6):728–38. doi: 10.1002/ijc.2910590603. [DOI] [PubMed] [Google Scholar]
- 77.Rocha RL, Hilsenbeck SG, Jackson JG, VanDenBerg CL, Weng C, Lee AV, Yee D. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin Cancer Res. 1997;3(1):103–9. [PubMed] [Google Scholar]
- 78.Tartter PI, Slater G, Papatestas AE, Aufses AH., Jr Cholesterol, weight, height, Quetelet’s index, and colon cancer recurrence. J Surg Oncol. 1984;27(4):232–5. doi: 10.1002/jso.2930270407. [DOI] [PubMed] [Google Scholar]