Abstract
Electron cryo-microscopy (cryo-EM) images are commonly collected using either charge-coupled devices (CCD) or photographic film. Both film and the current generation of 16 megapixel (4k × 4k) CCD cameras have yielded high-resolution structures. Yet, despite the many advantages of CCD cameras, more than two times as many structures of biological macromolecules have been published in recent years using photographic film. The continued preference to film, especially for subnanometer-resolution structures, may be partially influenced by the finer sampling and larger effective specimen imaging area offered by film. Large format digital cameras may finally allow them to overtake film as the preferred detector for cryo-EM. We have evaluated a 111-megapixel (10k × 10k) CCD camera with a 9 μm pixel size. The spectral signal-to-noise ratios of low dose images of carbon film indicate that this detector is capable of providing signal up to at least 2/5 Nyquist frequency potentially retrievable for 3-D reconstructions of biological specimens, resulting in more than double the effective specimen imaging area of existing 4k × 4k CCD cameras. We verified our estimates using frozen-hydrated ε15 bacteriophage as a biological test specimen with previously determined structure, yielding a ~7 Å resolution single particle reconstruction from only 80 CCD frames. Finally, we explored the limits of current CCD technology by comparing the performance of this detector to various CCD cameras used for recording data yielding subnanometer resolution cryo-EM structures submitted to the Electron Microscopy Data Bank (http://www.emdatabank.org/).
Keywords: Cryo-EM, Electron cryo-microscopy, 10k × 10k CCD, Charge-coupled devices, Electron detection
1. Introduction
Charge-coupled device (CCD) cameras offer numerous advantages compared to photographic film for transmission electron microscopy (TEM) data collection (see reviews: Mooney, 2007; Faruqi and Subramaniam, 2000). Principally, digital CCD images are immediately available for data processing and analysis without requiring chemical development and scanning. This is not only convenient but also improves the quality and efficiency of data collection by facilitating on-demand image analysis for the purpose of evaluation of instrument stability, defocus setting, astigmatism correction, and coma-free alignment for high-resolution imaging (Ishizuka, 1994). The ability to directly digitize images also enables automated data collection (Zhang et al., 2009; Zhang et al., 2003; Carragher et al., 2000). Furthermore, CCD cameras have improved linearity and dynamic range (Brink and Chiu, 1994), as well as increased low-frequency contrast in comparison to photographic film (Booth et al., 2006; Sander et al., 2005). Therefore, although photographic film may provide slightly higher inherent resolution (McMullan et al., 2009), the numerous other advantages of CCD cameras have made them an attractive choice for practical cryo-EM imaging.
Using both 200 and 300 kV microscopes, our laboratory has previously evaluated the performance of the Gatan (Pleasanton, CA) US4000 CCD camera (model 895), which is 16 megapixels (4096 × 4096) with 15 μm pixel size (Booth et al., 2006; Chen et al., 2008). We demonstrated that the latest version of this camera is capable of recording usable signal up to at least 2/3 Nyquist frequency (Chen et al., 2008), a result that has been subsequently validated by many biological structural studies, including the recent 4.3 Å (~62% Nyquist) resolution structure of the archaeal chaperonin Mm-cpn (Zhang et al., 2010).
Despite the advantages and demonstrated performance of CCD cameras for electron cryo-microscopy (cryo-EM), CCD cameras remain much less popular than photographic film for both medium and high-resolution imaging (Fig. 1). One reason for the continued use of film may be the finer sampling offered by film, which allows for imaging at lower magnifications to provide much larger effective specimen imaging area. For example, the 4.3 Å structure of Mm-cpn was generated from CCD images acquired at 112,000× detector magnification (Zhang et al., 2010). In contrast, a similar resolution reconstruction of the chaperonin GroEL was generated from photographic film acquired at only 60,000× detector magnification (Ludtke et al., 2008). Although both reconstructions reached similarly high resolutions, a much lower magnification was used when recording on photographic film, yielding a much larger total specimen area—and thus a much larger number of particles—captured in each image.
Figure 1.
The Electron Microscopy Data Bank (http://www.emdatabank.org) was mined to determine the usage of film and CCD detectors in biological TEM. Each EMDB entry was categorized according to the reported detection device (film or CCD) and the reported reconstruction resolution—either 1–10 nm (A) or subnanometer (B) resolution. The dashed lines (right axis) show the fraction of the EMDB entries that used a CCD detector. The results indicate that CCDs have been slowly gaining popularity for both low- and high-resolution studies, yet film has remained ~3× more popular overall.
New large-format CCD cameras may offer all the advantages of a CCD camera while providing a large imaging area similar to photographic film. Gatan (Pleasanton, CA) has recently developed the US10000XP (model 990) TEM CCD camera, which is a 111 megapixel (10560 × 10560) detector with 9 μm pixel size (Lee et al., 2010). While this 10k × 10k detector has ~6.6× as many pixels as a 4k × 4k (16 megapixel) camera, the pixels are 40% smaller for finer sampling. As such, the physical size of the US10000XP is ~2.4× the physical size of the 16-megapixel US4000 camera.
In this study, we have evaluated the performance of the US10000XP CCD camera on a 300 kV electron microscope and compared its performance to the previously characterized US4000 CCD camera. In the process, we have also explored the limits of current CCD technology by comparing the performance of both of these cameras to results published using other CCD detectors.
2. Materials and methods
2.1. Electron cryo-microscopy
In order to ensure the relevance of our tests, microscope conditions were set for routine high-resolution data collection. Images were collected at liquid nitrogen temperature (~100 K) on a JEM-3200FSC transmission electron cryo-microscope (JEOL Inc, Tokyo, Japan) operating at 300 kV, with an in-column energy filter slit width of 25 eV. Prior to imaging, astigmatism correction and evaluation of the stage stability were completed using a 10×-binned live view from the CCD camera of amorphous carbon film image at 400,000× nominal microscope magnification. For data collection, lower magnification images were captured on a US10000XP CCD camera (Gatan, Pleasanton, CA) using Digital Micrograph software (Gatan, Pleasanton, CA). The total specimen exposure for each image was ~20 e−/Å2 over 1 second. Specimen exposure rates were estimated using the microscope screen current, which was calibrated previously using a Faraday cage. Exposure rates were also verified for images of carbon film and biological specimens by examining the mean detector counts per pixel. Based on the mean pixel values from flood beam images at varying total exposures, we estimated the conversion rate of the US10000XP camera to be ~84 counts per primary electron.
2.2. MTF and DQE calculations
The modulation transfer function (MTF) and detective quantum efficiency (DQE) were measured using images of the microscope’s beam stop. Just before installation of the US10000XP camera, we collected beam stop and uniform flood-beam images on the previously installed 4k × 4k US4000 camera so that we could assess the performance of the two cameras with nearly identical microscope conditions. We examined the difference between consecutive images of the beam stop to ensure its position remained stable. After installing the new CCD camera, we collected the same set of images using the 10k × 10k US10000XP camera.
Initially, each beam stop image was gain-normalized by dividing the dark-corrected beam stop image by a dark-corrected uniform flood-beam image. In our case, the images of the beam stop were not gain corrected automatically as is normally done. Instead, we performed gain correction manually and immediately after the beam stop image to ensure that the uniform (gain reference) image had exactly the same electron exposure and microscope conditions as the beam stop image. All gain-corrected beam stop images were then rotated by exactly 90° using pixel-by-pixel transposition, so that the edges of the beam stop were ~30° from vertical, providing an optimal orientation for the MTF and DQE calculation algorithms that we used (Mooney, 2007). The rotated beam stop images were then clipped to include only the central 70% of the area from each image. Uniform flood beam images from the US10000XP CCD were also clipped to 4096 × 4096 to facilitate Fast Fourier Transform (FFT) calculations to determine the noise power spectrum (NPS).
Calculation of the MTF and DQE were carried out using Digital Micrograph scripts. We calculated the MTF for both the left and right beam stop edge in each of the 4 images collected with each camera, yielding a total of 8 MTF curves for each camera. Subsequently, each MTF curve was used to calculate the DQE, using the average noise power spectrum from two uniform flood-beam images without any specimen in the field-of-view.
2.3. High magnification calibration
Magnification calibration was completed using commercially available graphitized carbon grids and negatively-stained catalase crystal grids (Electron Microscopy Services, Fort Washington, PA). Graphitized carbon was used to calibrate nominal microscope magnifications from 50,000× to 150,000×. Images were collected at 0–1 μm under-focus, with the focus set to ensure that the CTF zeros did not mask the characteristic graphite peak at 1/3.35 Å−1. For each nominal microscope magnification, images of at least 8 different areas of graphite crystal were collected. The Fourier transforms of boxes with at least 1024×1024 pixels of graphite crystal revealed a bright ring at 1/3.4 Å−1, which we fit with a circle centered at the origin. The radius of the circle in pixels was then used to calculate the Ångströms-per-pixel sampling and the effective detector magnification.
Since the graphite peak was not detectable at 40,000× microscope magnification and below, we also used commercially available negatively-stained catalase crystals for magnification calibration. Unbinned images of catalase crystals were collected at nominal microscope magnifications from 40,000× to 100,000×. Regions (2048 × 2048) containing catalase crystal were processed using 2dx software (Gipson et al., 2007) to determine the lattice spacing, overall crystal tilt, and corresponding detector magnification from the Fourier transformed image regions based on the published commercial specification of 8.75 × 6.85 nm unit cell spacing.
2.4. Carbon film imaging and analysis
The carbon support film on the graphitized carbon grids was used for analysis of the spectral signal-to-noise ratios generated by the CCD camera. At each nominal microscope magnification from 40,000× to 100,000×, images of the carbon film were collected across a range of 0.3 – 3.5 μm under-focus. The Fourier transform of the image was examined to check for drift and astigmatism before saving it for subsequent analysis. The noise profile was estimated by log-linear interpolation of the CTF zeros from the circularly averaged power spectrum. The measured power spectrum and the estimated noise profile were used to calculate the spectral signal-to-noise ratios (SNR) for each image. The maximum spatial frequency containing usable signal was determined as the point where the peaks of the SNR curve fell near zero and were no longer unambiguously distinguishable (~0.03 SNR).
2.5. ε15 bacteriophage imaging and analysis
The structure of ε15 bacteriophage is well-known and has a published cryo-EM density map at 4.5 Å resolution (Jiang et al., 2008). As such, it is an ideal specimen for determining the practical performance limits of new technology, and we have used it previously to assess the performance of the US4000 camera (Booth et al., 2006; Chen et al., 2008). Quantifoil 400 mesh R2/2 holey carbon grids (Quantifoil Micro Tools GmbH, Jena, Germany) were cleaned with acetone and glow discharged. A thin (~100 Å) amorphous carbon film was floated on a water bath and then applied to one side of the grid. Purified ε15 bacteriophage (Jiang et al., 2006) was added to the grid and subsequently vitrified by plunging into liquid ethane using a Vitrobot Mark IV (FEI Inc, Hillsboro, OR). Grids were stored in liquid nitrogen until being transferred into the microscope column for imaging.
Images of ε15 bacteriophage were collected at a nominal microscope magnification of 40,000×, corresponding to a detector magnification of ~70,000× and ~1.3 Å/pixel sampling. Particle boxing and automated CTF fitting were completed using EMAN2 (Tang et al., 2007). CTF parameters were manually determined using the EMAN program ctfit (Ludtke et al., 1999). Alignment parameters for each particle were determined by the Multi-Path Simulated Annealing algorithm (Liu et al., 2007), and were used to generate an icosahedral three-dimensional reconstruction of ε15 bacteriophage using the weighted back-projection scheme of EMAN’s make3d program. Resolution was estimated by Fourier shell correlation using two half data sets (Harauz and van Heel, 1986). Surface representations of density maps were generated using Chimera (Goddard et al., 2005).
3. Results
3.1. Detector characterization
Initially, we characterized the detector independent of any specimen. In this case, detector performance is often characterized by two related measures: the modulation transfer function (MTF) and the detective quantum efficiency (DQE) (Mooney, 2007; Meyer and Kirkland, 2000). The MTF quantifies the frequency response of the detector by measuring the ratio of output to input contrast of a hard edge (in our case, the beam stop edge) at various frequencies. The DQE is defined as the output intensity-derived signal-to-noise ratio (SNR) divided by the input intensity-derived SNR, and thus is analogous to the envelope function of an electron microscope describing the detector’s degradation of the overall input while accounting for the background noise.
Figure 2 shows the MTF and DQE measurements for the US4000 and the US10000XP cameras. On the left (A,C), the MTF and DQE are shown in terms of the fraction of Nyquist frequency, which is independent of pixel size and describes the detector’s sampling efficiency. However, since the pixel sizes of the two detectors is different, we also show the same curves on the right (B,D) in terms of absolute spatial frequency (lp/mm), which accounts for differences in pixel size and thus describes the detector’s effective resolution for practical imaging. Nyquist frequency is the reciprocal of two-times the pixel size (i.e., 1 / (2 × 15 μm/pixel) = 1 / (30 lp/μm) = 33 lp/mm for the US4000, and 1 / (2 × 9 μm/pixel) = 1 / (18 lp/μm) = 55 lp/mm for the US10000XP).
Figure 2.
Comparison of the US4000 and US10000XP cameras. The modulation transfer function (MTF) is shown in terms of the Nyquist frequency (A), as well as the spatial frequency (B). The curves represent averages calculated from four images of the beam-stop edges. These MTFs were subsequently used to calculate the detective quantum efficiency (DQE) in terms of the Nyquist frequency (C) and the spatial frequency (D). The dashed lines indicate the 0.07 threshold value, corresponding to ~2/3 (0.66) Nyquist and ~2/5 (0.44) Nyquist for the US4000 and the US10000XP cameras, respectively. Error bars in both the MTF and DQE plots represent the standard deviation of multiple measurements at each spatial frequency.
In terms of Nyquist frequency, both the MTF and DQE of the US10000XP fall below the US4000 (Fig. 2A,C), implying that the US10000XP does not perform as well per-pixel as the US4000. We previously showed that the US4000 is capable of providing usable signal up to at least 2/3 Nyquist frequency for single particle reconstructions. This empirical result corresponds to a DQE threshold value of ~0.07 (Fig. 2C). The DQE of the US10000XP camera crosses this threshold value at ~0.44 Nyquist, suggesting that the US10000XP may produce images capable of generating three-dimensional reconstructions with resolution up to at least 2/5 Nyquist.
So while the US10000XP does not perform as well as the US4000 per pixel (i.e., in terms of fraction of Nyquist frequency), the US10000XP slightly outperforms the US4000 in terms of absolute spatial resolution (Fig. 2B,D). The MTF of the US10000XP shows a modest improvement of ~30% for spatial frequencies above 10 lp/mm, implying that the US10000XP may provide improved absolute spatial resolution for any given detector magnification. However, despite the improvement in the MTF, the DQEs of the two cameras are statistically equivalent up to the DQE value of ~0.07, the empirical threshold for usable signal in a cryo-EM single-particle reconstruction. Thus, the DQE curves indicate that the two detectors should perform similarly in terms of the achievable resolution at any given detector magnification.
3.2. Carbon film image performance evaluation
The spectral signal-to-noise ratio (SNR) from images of amorphous carbon film has been previously shown to provide a reasonable measure for evaluating detector performance (Booth et al., 2006). Amorphous carbon is appropriate for evaluating the performance of a CCD since its scattering power is similar to biological samples without exhibiting significant radiation damage. Since the SNR of each image depends on the exposure used, we chose an exposure of 20 e−/Å2 for each image, which is similar to the exposure we use for routine high-resolution cryo-EM.
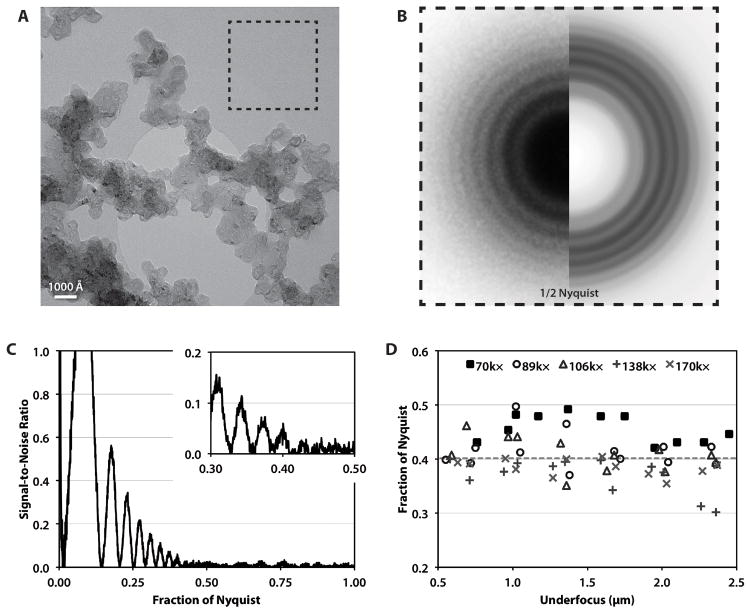
Figure 3A shows an image from the US10000XP CCD camera of a graphitized carbon grid. The box indicates an area of amorphous carbon film used for analysis of the SNR. In contrast to the image shown, the images used for our analysis contained only thin amorphous carbon film (without the graphite crystal) throughout the entire field of view. The Fourier transform of such an image shows the characteristic contrast transfer function (CTF) rings. At all magnifications and defocus values examined, we were not able to visually distinguish CTF rings past 1/2 Nyquist with the US10000XP (Fig. 3B).
Figure 3.
(A) A full CCD frame of graphitized carbon at ~70,000× detector magnification. The black box marks an example area of carbon film used to measure the decay of the signal-to-noise ratio (SNR). (B) The central part of the Fourier transform of an area of carbon film. The boundaries of the box correspond to 1/2 Nyquist. The right half has been processed to better visualize the Thon rings. (C) The spectral SNR based on the rotationally averaged Fourier transform (power spectrum) in B. (D) The spatial frequency (as a fraction of the detector Nyquist frequency) where SNR peaks were no longer distinguishable from noise. The results indicate that the CCD is capable of recording usable signal up to at least ~2/5 Nyquist (dashed line).
We used the rotational average of each image’s Fourier transform to determine the noise profile and the SNR. Figure 3C shows a SNR curve characteristic of an image acquired at ~70,000× detector magnification. The overall sinusoidal behavior of the SNR is due to the contrast transfer function at the chosen defocus setting, so we are primarily interested in the degradation of the SNR peaks. At increasing spatial frequency, the SNR of each peak decreases toward zero, until the regular peaks are no longer distinguishable from the SNR noise. In the example shown (Fig. 3C), this occurs just slightly beyond 2/5 Nyquist.
To ensure that the SNR was limited by the detector and not the microscope, we examined the SNR from additional images of carbon film across a range of detector magnifications, from 70,000× to 170,000×, with constant specimen exposure. If the detector is the primary limit of image resolution, the SNR peaks should show similar degradation in terms of the detector’s Nyquist frequency across a broad range of magnifications. At all magnifications examined, the SNR peaks decayed to near zero at approximately 2/5 Nyquist (Fig. 3D), confirming that the US10000XP limits the detected signal to approximately 2/5 Nyquist at electron doses typical of biological cryo-EM. However, while all magnifications decay near 2/5 Nyquist, there is a slight trend for lower magnification images (e.g. 70,000×), to reach slightly higher resolutions (in terms of the fraction of Nyquist frequency). This may be attributable by the higher electron exposure per pixel on the detector at lower magnifications (see §3.3 and 4.3).
3.3 Exposure series of carbon film
We further investigated the practical SNR performance of the US10000XP by collecting a series of carbon film images with varying exposures. For precise control over the relative exposures of each image, we kept the electron exposure constant at 10 e− /Ų on the specimen and varied the exposure time from 0.1–4.0 seconds. The calibrated detector magnification was used to convert the specimen exposure to the per-pixel exposure on the detector. Images were collected at low magnification (35,000×), and only SNR peaks at 15 Å or lower resolution were examined to exclude the impact of any small specimen drift over long exposures.
Figure 4A shows the SNR curves from carbon film images at two different exposure levels. The “1× exposure” corresponds to an equivalent exposure on the specimen of 20 e− /Ų when imaging at 170,000× detector magnification, (which is at the upper end of magnifications typically used for high-resolution imaging). With this exposure, the SNR decays to near zero at ~2/5 Nyquist, as we previously observed for carbon film (Fig. 3). However, if the exposure is increased by an order of magnitude (10×), the SNR peaks not only increase in amplitude but also are visible at much higher spatial frequencies, with peaks clearly distinguishable up to at least 3/5 Nyquist. This represents the maximum performance predicted by the MTF and DQE (Fig. 2), which fall to zero just past 3/5 Nyquist.
Figure 4.
(A) The SNR curves of images of carbon film for a broad range of total electron exposures. 1× exposure is typical for high-resolution cryo-EM imaging, and corresponds to 20 e− /Ų specimen exposure at 170,000× detector magnification. Increasing the exposure by an order of magnitude improves the resolution performance of the detector to ~3/5 Nyquist. (B) The relative change in SNR peak amplitude at varying detector exposures up to the detector saturation point (~400 e− /pixel). The arrow indicates the data point corresponding to the 1× exposure curve in (A).
Figure 4B shows the relative change in the SNR peak amplitudes over a broad range of detector exposures, from a minimum of 6 e− /pixel up to 400 e− /pixel, which is near the saturation point (maximum acceptable exposure) of the detector. The plot reveals that the SNR is directly proportional to the total exposure, as is expected from the Poisson statistics of photon/electron counting, called “shot noise” (see review: Janesick, 2001). As the total exposure increases, shot noise causes the SNR to scale linearly (since the SNR is calculated from Fourier intensities, which is amplitude squared). For subnanometer target resolutions, radiation damage considerations limit cryo-EM exposures of biological specimens to ~20–30 e− /Ų (Bammes et al., 2010). We have conservatively used 20 e− /Ų for our performance tests. At this level of exposure (indicated by the arrow), the SNR is only ~20% of the maximum performance of the detector.
3.4. ε15 bacteriophage image performance evaluation
To validate our carbon film performance test, we used frozen, hydrated ε15 bacteriophage as a biological test specimen, with a previously published structure and backbone trace to 4.5 Å resolution (Jiang et al., 2008). We acquired 80 CCD frames of ε15 bacteriophage with the US10000XP camera at 40,000× microscope (~70,000× detector) magnification. Figure 5A shows a typical 10k × 10k image of ε15 bacteriophage, containing ~100 particles. Thon rings from the Fourier transforms of the images disappeared at lower resolutions than 1/2 Nyquist (Fig. 5B). The spectral SNR curves showed detectable CTF oscillations to approximately 2/5 Nyquist (Fig. 5C), in agreement with our carbon film experiments (Fig. 3). From the 80 CCD frames we acquired, we boxed ~7,800 particles, far more than necessary to reach a subnanometer resolution (Liu et al., 2007). This data was used to generate an icosahedral single-particle reconstruction (Fig. 5D) using 10 iterations of the cross-common-lines-based MPSA algorithm (Liu et al., 2007). The Fourier shell correlation (FSC) of reconstructions from two half-data sets crosses 0.5 at 7.2 Å (0.35 Nyquist) and 0.143 at 5.7 Å (0.45 Nyquist) (Rosenthal and Henderson, 2003) (Fig. 5E). At this resolution, we could unambiguously visualize α-helices (Fig. 5F). Thus, in agreement with our carbon film results, the US10000XP can be used to generate three-dimensional reconstructions of biological macromolecules with resolution of at least 2/5 Nyquist.
Figure 5.
(A) A full CCD frame of ε15 bacteriophage at ~70,000× detector magnification at ~0.6 μm under-focus. (B) The central portion of the Fourier transform of the image in A. The boundaries of the box correspond to 1/2 Nyquist. The right half has been processed to better visualize the Thon rings. (C) The rotationally-averaged power spectrum (left axis) and spectral SNR (right axis) from the Fourier transform in B. (D) The icosahedral 3D reconstruction, with the 3-fold symmetry axis in the middle of the image shown. (E) The Fourier shell correlation (FSC) between reconstructions of two half data sets reveals the final resolution is 7.2 Å (0.35 Nyquist) by 0.5 FSC or 5.7 Å (0.45 Nyquist) by 0.143 FSC. (F) A view of the 3-fold symmetry axis from the inside of the capsid. Secondary structure is clearly distinguishable, including the long α-helix in the zoomed view.
3.5. Binning performance test
Since we could not distinguish usable signal above 1/2 Nyquist at the electron exposure allowable for cryo-EM specimens, we also investigated the performance of the camera in 2×-binned mode. Binning integrates multiple pixels on the detector to increase the apparent pixel size and the per-pixel contrast. Initially, we collected consecutive pairs of images of amorphous carbon film at 106,000× detector magnification in unbinned and 2×-binned modes. The SNR from unbinned and 2×-binned images showed identical SNR degradation and maximum resolution (Fig. 6A). We could not detect any negative effects or loss of resolution from 2×-binning with the US10000XP. The effect of 2×-binning appeared to be truncating the negligible noise in the upper half of the spatial frequency spectrum, with no loss of usable information overall. Thus, the coarser sampling of 2×-binned images results in detectable carbon film signal up to at least 4/5 Nyquist. However, it is not clear whether three-dimensional single particle reconstructions could fully realize this resolution. In our experience, reconstruction resolutions have been limited to ~2/3 Nyquist, possibly due to the influence of interpolation artifacts at high frequencies (Orlova et al., 1997).
Figure 6.
(A) Regions of carbon film were successively imaged using unbinned and 2×-binned acquisition at 70,600× detector magnification and the same defocus. The spectral SNRs for the unbinned and 2×-binned images were indistinguishable (as shown in the example SNRs at 1.3 μm under-focus). (B) Images of frozen-hydrated ε15 bacteriophage were also collected using unbinned and 2×-binned data acquisition. Experimental B-factors (spectral signal decay) are statistically equivalent for unbinned and 2×-binned images.
Images of ε15 bacteriophage were also collected at ~70,000× detector magnification in unbinned and 2×-binned modes. The envelope functions (as measured by the experimental B-factors (Saad et al., 2001)) from the CTF fits were statistically equivalent between unbinned and 2×-binned images (Fig. 6B), again implying that 2×-binning causes no detectable information loss for individual cryo-EM images.
4. Discussion
4.1 Significance
Numerous factors affect the ultimate achievable resolution in electron cryo-microscopy, including the detector, radiation damage, imaging exposure, defocus, spatial and temporal coherence, beam alignment, etc. (Glaeser et al., 2007; Zhou and Chiu, 1993). Of all these factors, one of the most significant is the detector, which imposes discrete sampling, a limited field of view, and imperfect SNR transfer of the microscopy image. Therefore, choosing an electron detector is critical to achieving optimal cryo-EM images.
Over the past decade, CCD cameras have slowly gained popularity for cryo-EM data collection (Fig. 1). Although camera manufacturers typically provide specifications detailing the performance of their cameras, these are often insufficient to accurately predict the achievable resolution under low-dose, cryo-EM conditions. Thus, as CCD technology for TEM imaging has developed, testing under practical experimental conditions has remained vital to understanding and characterizing the capabilities of these detectors (Downing and Hendrickson, 1999; Zhang et al., 2003; Booth et al., 2004; Sander et al., 2005; Booth et al., 2006; Chen et al., 2008). Up to now, most of the detectors that have been characterized have been 4k × 4k or smaller, with 15 μm or larger pixel size, and it has remained unclear whether smaller pixel sizes could improve the resolution of CCD detectors.
In this study, we have thoroughly tested the newest generation of large-format CCD detectors, represented by the Gatan 10k × 10k US10000XP camera, with 9 μm pixels. We began with an industry standard for evaluating CCD performance using the MTF and DQE (Fig. 2), which suggests the maximum performance of the detector without respect for specific experimental conditions. Subsequently, we used carbon film to evaluate the SNR output under low-dose imaging conditions typical of cryo-EM (Figs. 3), and at a variety of exposures (Fig. 4). Finally, we completed a practical reconstruction of frozen-hydrated ε15 bacteriophage (Fig. 5) to closely replicate conditions used for real cryo-EM experiments of radiation-sensitive specimens.
4.2. Comparison with the 4k × 4k CCD
We previously showed that the 4k × 4k US4000 camera (15 μm pixels) could acquire usable signal and yield three-dimensional reconstructions with resolution up to at least 2/3 Nyquist (Chen et al., 2008). Similarly, we have shown here that the 10k × 10k US10000XP camera (9 μm pixels) is capable of detecting usable signal up to at least 2/5 Nyquist at a typical electron exposure used for biological specimens (Figs. 3,5). The ratio of the performance in terms of Nyquist frequency (2/5 to 2/3) is precisely equal to the ratio of the pixel sizes of the two cameras (9 to 15 μm), indicating that the finer per-pixel sampling does not render improved effective detector resolution.
To explicitly compare the two cameras, we compared the SNRs of images of amorphous carbon film collected on the US4000 and the US10000XP at nearly equivalent microscope conditions and detector magnifications (106,000×). Though we observed some differences in the absolute SNR peak heights, the overall SNR degradation was comparable between the two detectors (Fig. 7A). Based on 35 carbon film images from the US4000 and 18 carbon film images from the US10000XP, across a defocus range of 0.4 – 3.4 μm, the maximum frequency with detectable SNR peaks was statistically equivalent for the two cameras (p = 0.55) at the practical electron exposure allowable for frozen, hydrated specimens. Thus, we found that at the same detector magnification, the US4000 and US10000XP cameras detect signal up to the same spatial frequency limit. Note that the detector magnification is generally much higher than the nominal microscope magnification due to the physical distance between the film and CCD image planes.
Figure 7.
Comparison of the US4000 and US10000XP CCD cameras. (A) Images of carbon film were taken with each camera at 106,000× detector magnification. Similar to Fig. 3, the spectral SNR was calculated for each image. Comparison of two carbon film images with similar defocuses shows similar SNR degradation. (B) Maximum resolution and the total imaging area for each microscope magnification for each camera. The result shows that, for any given resolution target, the US10000XP provides approximately 2.4× the total imaging area.
We calculated the effective achievable resolution at each detector magnification for the US4000 and US10000XP CCD cameras, corresponding to 2/3 and 2/5 of Nyquist frequency, respectively (Fig. 8A). The maximum achievable resolution at each detector magnification follows exactly the same curve for the two cameras, defined by 22.5 μm effective physical spacing on the detector. The US4000 with 15 μm pixel size can acquire usable signal up to 2/3 Nyquist, implying that the effective resolution at the detector plane is 15 μm ÷ 2/3 = 22.5 μm. Similarly, the US10000XP with 9 μm pixel size can acquire usable signal up to 2/5 Nyquist, implying that the effective resolution at the detector plane is 9 μm ÷ 2/5 = 22.5 μm. We conclude that the practically achievable spatial resolution is identical for the two detectors, regardless of differences in pixel size.
Figure 8.
(A) Data points show 2/3 and 2/5 Nyquist at each detector magnification for the US4000 and US10000XP, respectively. The points for each detector lie on the same curve (green), modeled by the equation shown, representing an information limit at the detector plane of ~22.5 μm pixel size. (B) All subnanometer resolution entries in the EMDB in terms of detector type and magnification, including entries from our laboratory (yellow center). None of the CCD data (blue) reaches substantially higher resolution than our model (green curve), implying that pixel size may not be the overall limiting factor for CCD resolution. Note the reported resolution criteria vary.
Finally, while the US10000XP contains nearly 6.6 times as many pixels as the US4000 camera, the finer sampling of the US10000XP results in a CCD whose physical size is only 2.4 times that of the US4000. For a smaller pixel camera, one would hope to be able to image at a lower magnification relative to that of a larger pixel camera to achieve the same resolution while providing a larger field of view. However, we found that the achievable resolution at any given detector magnification is identical for both CCD detectors (Fig. 8A). Therefore, the finer sampling of the US10000XP does not allow for imaging at lower magnifications. Based on the Ångströms per pixel sampling and the maximum achievable resolution at each microscope magnification, we calculated the total specimen imaging area per frame for each camera (Fig. 7B). For any given target resolution, the US10000XP provides more than double (~2.4×) the total imaging area as the smaller US4000 camera. This is highly desirable for cryo-EM of biological specimens, as it maximizes the number of particles per CCD frame, which not only enhances the data collection efficiency but also facilitates more accurate CTF parameter determination for each frame. Additionally, the larger effective image area of large-format CCD detectors is particularly advantageous for whole-cell tomography, where the trade-off between sampling and imaging area is an important consideration.
Due to the large size of the US10000XP camera, we collected more than enough particles for a 7 Å reconstruction of ε15 bacteriophage from only 80 CCD frames (Fig. 5). Although 7800 particles is more than enough to attain our target resolution (Liu et al., 2007), we chose to use this many particles to ensure sufficient sampling of defocus, and to ensure that the resulting resolution was limited by the CCD detector and not the number of particles. In this example, the particles were well populated in each CCD frame. However, many other biological specimens are difficult to prepare similarly well, resulting in very few particles per frame and preventing high resolution imaging due to the inefficient imaging and the inability to accurately determine CTF parameters from so few particles per frame.
4.3. Generalized performance of CCD detectors
The two CCD detectors that we have examined here appear to be limited to a maximum resolution of 22.5 μm on the detector plane. The question remains of whether this limit is coincidental to the two specific detectors we have tested, or if this limit can be generalized. We further explored the resolution limits across a broad range of detectors in cryo-EM by mining the metadata for the published structures in the publicly accessible Electron Microscopy Data Bank (http://www.emdatabank.org) (Lawson et al., 2011). Figure 8B shows the self-reported resolution and detector magnification of all subnanometer resolution structures in the EMDB as of 3 January 2011. Multiple structures with resolution better than 5 Å have been generated using photographic film at relatively low magnifications (<60,000×), confirming that electron microscopes are capable for providing high-resolution information at these low magnifications. In contrast, the three high-resolution (<5 Å) structures based on images from CCD cameras used much higher detector magnifications (>100,000×). With one minor exception (Zhang et al., 2009), none of the structures from CCD cameras were better than the apparent resolution limit of 22.5 μm physical spacing on the detector (Fig. 8A), indicating that this limit may be consistent for all currently available CCD cameras, regardless of their pixel size for images recorded at typical electron exposure for cryo-EM experiments.
It is important to note that the 22.5 μm resolution limit is an empirical limit for electron cryo-microscopy of radiation-sensitive samples, and we have not found any definitive theoretical explanations for this empirical limit. Furthermore, at unlimited exposures, the US10000XP is capable of providing signal up to 0.6 Nyquist (Fig. 4), corresponding to 15 μm effective resolution on the detector. We therefore conclude that the performance of the US10000XP—and presumably other modern CCD detectors (Fig. 8B)—is limited by the low electron exposures imposed by cryo-EM imaging. It may be possible to improve upon this 22.5 μm limit with advances in CCD technology. Alternatively, digital imaging using completely different technologies, such as CMOS-based direct electron detectors, have shown promise in providing improved DQE/MTF compared to the current generation CCDs (Milazzo et al., 2010; McMullan et al., 2009), which may result in improved spatial resolution.
Since exposure is the principal limit of CCD performance, the magnification should be carefully considered for cryo-EM imaging. For any given specimen, the detector shot noise is related to the per-pixel exposure. If the total exposure is restricted by radiation damage considerations to ~20–30 e− /Ų (Bammes et al., 2010), the per-pixel exposure can only be increased by lowering the magnification. This explains why we observe marginal magnification dependence in our carbon film performance tests (Fig. 3), with the lowest detector magnification (70,000×) providing slightly higher resolution. Therefore, in order to optimize the per-pixel exposure‚ and thus the image SNR‚ we recommend using the lowest magnification necessary to provide the targeted imaging resolution.
Finally, the 22.5 μm empirical sampling limit at the detector plane implies that a CCD detector with a smaller pixel size would oversample the signal. For example, a CCD detector with pixel size of 22.5 μm can provide detectable signal up to its Nyquist frequency at the restricted electron exposure. A different CCD detector with pixel size of 15 μm instead of the current 9 μm based on the same technology (with a 22.5 μm limit), would provide detectable signal only up to 15 μm ÷ 22.5 μm = 2/3 Nyquist, which is considered to be oversampling. However, if we consider the benefits of oversampling for overcoming negative effects such as aliasing, it appears the choice of a pixel size slightly smaller than 22.5 μm would be an optimal design in the current CCD technology.
Our data shows that the 9 μm pixel size of the US10000XP camera allows for 2×-binning without any information loss in individual images (Fig. 6). In this case, the effective pixel size of the binned images is 18 μm, providing highly efficient imaging with detectable signal up to at least 18 μm ÷ 22.5 μm = 4/5 Nyquist.
5. Conclusion
CCD cameras provide multiple advantages compared to film, and their capabilities in producing high-resolution reconstructions of biological macromolecules continue to be demonstrated by published structures. For cryo-EM, a primary disadvantage of CCD cameras, as compared to photographic film, appears to be the limited imaging area due to the high magnifications required by CCD cameras. Large format CCD cameras, such as the 10k × 10k Gatan US10000XP, can provide ~2.4× larger total imaging area with the same achievable resolution, and thus may finally supplant photographic film for high-resolution cryo-EM imaging. As a practical demonstration of this performance, we generated a ~7 Å resolution structure of ε15 bacteriophage with visible α-helices from only 80 CCD frames.
We found that the effective resolution of CCD detectors for biological cryo-EM is currently limited to approximately 22.5 μm physical spacing on the detector. This limit is primarily imposed by the low exposures required for radiation-sensitive specimens. The US10000XP camera records usable signal up to this limit, with individual 2×-binned images providing spectral information up to at least 4/5 Nyquist frequency. Compared to the 4k × 4k Gatan US4000 CCD camera, this represents a 20% improvement in imaging efficiency while providing more than 2.4× the total specimen imaging area.
Acknowledgments
This work is supported by the National Institutes of Health through the National Center for Macromolecular Imaging (P41RR002250 and PN2EY016525), NIH Training Programs from the Keck Center for Interdisciplinary Bioscience Training of the Gulf Coast Consortia (T32GM008280 and T15LM007093), and the Robert Welch Foundation (Q1242).
We also thank Jonathan King and Peter Weigele at the Massachusetts Institute of Technology (MIT) for the ε15 bacteriophage sample, and Paul Mooney and Brian Lee at Gatan (Pleasanton, CA) for the Digital Micrograph script for calculating the MTF and DQE from uniform and beam stop images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bammes BE, Jakana J, Schmid MF, Chiu W. Radiation damage effects at four specimen temperatures from 4 to 100 K. J Struc Biol. 2010;169:331–41. doi: 10.1016/j.jsb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CR, Jiang W, Baker ML, Zhou ZH, Ludtke SJ, et al. A 9 Å single particle reconstruction from CCD captured images on a 200 kV electron cryo-microscope. J Struct Biol. 2004;147:116–127. doi: 10.1016/j.jsb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Booth CR, Jakana J, Chiu W. Assessing the capabilities of a 4k×4k CCD camera for electron cryo-microscopy. J Struct Biol. 2006;156:556–63. doi: 10.1016/j.jsb.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Brink J, Chiu W. Applications of a slow-scan CCD camera in protein electron crystallography. J Struct Biol. 1994;113:23–34. doi: 10.1006/jsbi.1994.1029. [DOI] [PubMed] [Google Scholar]
- Carragher B, Kisseberth N, Kriegman D, Milligan RA, Potter CS, et al. Leginon: an automated system for acquisition of images from vitreous ice specimens. J Struct Biol. 2000;132:33–45. doi: 10.1006/jsbi.2000.4314. [DOI] [PubMed] [Google Scholar]
- Chen DH, Jakana J, Liu X, Schmid MF, Chiu W. Achievable resolution from images of biological specimens acquired from a 4k×4k CCD camera in a 300-kV electron cryomicroscope. J Struct Biol. 2008;163:45–52. doi: 10.1016/j.jsb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing KH, Hendrickson FM. Performance of a 2k CCD camera designed for electron crystallography at 400 kV. Ultramiscroscopy. 1999;75:215–233. doi: 10.1016/s0304-3991(98)00065-5. [DOI] [PubMed] [Google Scholar]
- Faruqi AR, Subramaniam S. CCD detectors in high-resolution biological electron microscopy. Quart Rev Biophys. 2000;33:1–27. doi: 10.1017/s0033583500003577. [DOI] [PubMed] [Google Scholar]
- Gipson B, Zeng X, Zhang ZY, Stahlberg H. 2dx - user-friendly image processing for 2D crystals. J Struct Biol. 2007;157:64–72. doi: 10.1016/j.jsb.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Glaeser RM, Downing K, DeRosier D, Chiu W, Frank J. Electron Crystallography of Biological Macromolecules. Oxford University Press; New York: 2007. [Google Scholar]
- Goddard TD, Huang CC, Ferrin TE. Software extensions to UCSF chimera for interactive visualization of large molecular assemblies. Structure. 2005;13:473–482. doi: 10.1016/j.str.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Harauz G, van Heel M. Exact filters for general geometry three dimensional reconstruction. Optik. 1986;73:146–156. [Google Scholar]
- Ishizuka K. Coma-free alignment of a high-resolution electron microscope with three-fold astigmatism. Ultramicroscopy. 1994;55:407–418. doi: 10.1016/0304-3991(95)00155-7. [DOI] [PubMed] [Google Scholar]
- Janesick JR. Scientific charge-coupled devices. SPIE Press; Bellingham, WA: 2001. [Google Scholar]
- Jiang W, Chang J, Jakana J, Weigele P, King J, et al. Structure of epsilon15 bacteriophage reveals genome organization and DNA packaging/injection apparatus. Nature. 2006;439:612–616. doi: 10.1038/nature04487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Baker ML, Jakana J, Weigele PR, King J, et al. Backbone structure of the infectious epsilon15 virus capsid revealed by electron cryomicroscopy. Nature. 2008;451:1130–34. doi: 10.1038/nature06665. [DOI] [PubMed] [Google Scholar]
- Lee B, Mollon B, Mooney P. 300kV performance of a 10k × 10k scintillator- and fiber-coupled CCD camera for transmission electron microscopes. Microsc Microanal. 2010;16(S2):64–65. [Google Scholar]
- Liu X, Jiang W, Jakana J, Chiu W. Averaging tens to hundreds of icosahedral particle images to resolve protein secondary structure elements using a Multi-path Simulated Annealing optimization algorithm. J Struct Biol. 2007;160:11–27. doi: 10.1016/j.jsb.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke SJ, Baldwin PR, Chiu W. EMAN: semi-automated software for high resolution single particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- Ludtke SJ, Baker ML, Chen DH, Song JL, Chuang DT, et al. De novo backbone trace of GroEL from single particle electron cryomicroscopy. Structure. 2008;16:441–8. doi: 10.1016/j.str.2008.02.007. [DOI] [PubMed] [Google Scholar]
- McMullan G, Chen S, Henderson R, Faruqi AR. Detective quantum efficiency of electron area detectors in electron microscopy. Ultramicroscopy. 2009;109:1126–43. doi: 10.1016/j.ultramic.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RR, Kirkland AI. Characterisation of the signal and noise transfer of CCD cameras for electron detection. Microsc Res Tech. 2000;49:269–280. doi: 10.1002/(SICI)1097-0029(20000501)49:3<269::AID-JEMT5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Milazzo AC, Moldovan G, Lanman J, Jin L, Bouwer JC, et al. Characterization of a direct detection device imaging camera for transmission electron microscopy. Ultramicroscopy. 2010;110:744–7. doi: 10.1016/j.ultramic.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney PE. Optimization of image collection for cellular electron microscopy. Methods Cell Biol. 2007;79:661–719. doi: 10.1016/S0091-679X(06)79027-6. [DOI] [PubMed] [Google Scholar]
- Orlova EV, Dube P, Harris JR, Beckmann E, Zemlin F, Markl J, van Heel M. Structure of Keyhole Limpet Hemocyanin Type 1 (KLH1) at 15 Å resolution by electron cryomicroscopy and angular reconstitution. J Mol Biol. 1997;271:417–437. doi: 10.1006/jmbi.1997.1182. [DOI] [PubMed] [Google Scholar]
- Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J Mol Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Saad A, Ludtke SJ, Jakana J, Rixon FJ, Tsuruta H, et al. Fourier amplitude decay of electron cryomicroscopic images of single particles and effects on structure determination. J Struct Biol. 2001;133:32–42. doi: 10.1006/jsbi.2001.4330. [DOI] [PubMed] [Google Scholar]
- Sander B, Golas MM, Stark H. Advantages of CCD detectors for de novo three-dimensional structure determination in single-particle electron microscopy. J Struct Biol. 2005;151:92–105. doi: 10.1016/j.jsb.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, et al. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang P, Borgnia MJ, Mooney P, Shi D, Pan M, et al. Automated image acquisition and processing using a new generation of 4K × 4K CCD cameras for cryo electron microscopic studies of macromolecular assemblies. J Struct Biol. 2003;143:135–144. doi: 10.1016/s1047-8477(03)00124-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Nakamura N, Shimizu Y, Liang N, Liu X, et al. JADAS: a customizable automated data acquisition system and its application to ice-embedded single particles. J Struct Biol. 2009;165:1–9. doi: 10.1016/j.jsb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Baker ML, Schröder GF, Douglas NR, Reissmann S, et al. Mechanism of folding chamber closure in a group II chaperonin. Nature. 2010;463:379–83. doi: 10.1038/nature08701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Chiu W. Prospects for using an IVEM with a FEG for imaging macromolecules towards atomic resolution. Ultramicroscopy. 1993;49:407–16. doi: 10.1016/0304-3991(93)90246-t. [DOI] [PubMed] [Google Scholar]