Abstract
Study Objective
To determine the electrocardiographic (ECG) effects of co-administration of lofexidine and methadone.
Design
Prospective, double-blind study.
Subjects
Fourteen participants with physical dependence on opioids at an outpatient drug treatment research clinic.
Methods
Participants were stabilized on methadone maintenance therapy (80 mg/day), then received escalating doses of lofexidine for eight weeks. ECGs were performed during peak plasma lofexidine levels. Pre-specified outcome measures were mean and maximal changes in heart rate, PR, QRS, and QTc intervals (1) when stabilized on methadone and (2) after lofexidine (0.4 mg) co-administration.
Main Results
Repeated-measures regression showed no changes in HR, PR, QRS, or QTc after methadone stabilization, but a significant decrease in mean HR (mean change −8.0 ± 7.3 bpm, p=0.0006) after initiating lofexidine. When data were analyzed using maximal ECG response, again, there were no significant changes during methadone induction compared to pretreatment, but there were significant changes in all four ECG parameters when lofexidine was coadministered: decreased HR (−9.6 ± 5.8 bpm, p<0.0001) and increased PR interval (+11.1 ± 19.8 ms, p=0.026), QRS interval (+3.7 ± 4.3 ms, p=0.002), and QTc interval (+21.9 ± 40.8 ms, p=0.018). In three participants, the QTc prolongation was clinically significant (> 40 ms).
Conclusion
Pending larger studies, our data suggest that coadministration of lofexidine and methadone should be prescribed cautiously, preferably with ECG monitoring.
Keywords: Methadone, Lofexidine, ECG, QTc interval
Introduction
Currently, over 200,000 patients in the US are enrolled in methadone maintenance for opioid dependence1. The cardiac arrhythmia torsade de pointes has been reported in patients maintained on methadone and its effects appear to be dose-dependent2,3. Although arrhythmia development may be a result of multiple factors, methadone may be an independent causal agent, interfering with the important repolarizing current IKR, and prolonging QTc4. QTc prolongation serves as a surrogate for risk of torsade5. Therefore, methadone should be used cautiously with other medications that have QTc-prolonging properties. In addition, QTc prolongation may be augmented in the setting of decreased autonomic tone6; therefore, the delayed repolarization associated with methadone could be magnified by medications that predispose to bradycardia.
Lofexidine, 2[1-(2,6-dichlorophenoxy)-ethyl]-2-imidazoline hydrochloride, is an alpha-2 agonist that is used for opioid detoxification7,8 and to assist in transferring patients from methadone to buprenorphine maintenance9. It has also been shown in both animal and human models to prevent relapse to alcohol, cocaine, and heroin use outside the context of acute withdrawal10–13; thus, independent of its role in detoxification, lofexidine may also help prevent relapse during methadone maintenance.
Both methadone and lofexidine exhibit cardiac properties: both drugs may cause bradycardia and hypotension. Methadone has been shown to prolong the QTc interval14, but also has the potential to augment bradycardia due to calcium-channel blockade15 and through acetyl-cholinesterase inhibition16. Lofexidine possesses both alpha-2 adrenergic agonist activity and peripheral agonist activity at postganglionic muscarinic receptors17. Although lofexidine has not definitively been shown to cause QTc prolongation, we have reported a single case of marked QTc prolongation during methadone and lofexidine co-administration18 and recently reported hypotension caused by this combination19. Given the two drugs’ overlapping cardiovascular properties, we prospectively examined the cardiac conduction and repolarization effects of the coadministration of methadone and lofexidine.
Methods
Study Design and Subjects
This report contains secondary findings from a pilot dose-escalation study of the safety of lofexidine and methadone co-administration. The study methods and primary findings have been described in detail previously19. The study protocol was approved by the Institutional Review Board of the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, conducted under an investigational new drug application approved by the United States Food and Drug Administration; the study was conducted in accord with the Helsinki Declaration of 1975. Between January 2001 and February 2002, we recruited opioid-dependent participants from Baltimore, Maryland, and the surrounding area. After giving written informed consent, participants underwent a history and physical with screening tests, including a 12-lead electrocardiogram (ECG) and three-minute rhythm strip.
All participants were between 18 to 45 years old and physically dependent on opioids, as determined by DSM-IV criteria, self-reported 30-day use, and urine toxicology screens. Participants were excluded for unstable Axis I psychiatric disorders, cognitive impairment, pregnancy, relative hypotension (blood pressure consistently below 110/70 mm Hg), relative bradycardia (heart rate consistently below 50 beats per minute [bpm]), chronic hypertension, myocardial infarction, stroke, coronary artery disease, creatinine > 1.7, or use of medications with lofexidine interactions (antihypertensives, antiepileptics, psychoactives, hypoglycemics, anticholinergics, antiparkinsonians).
Study Drugs
Oral methadone was administered daily throughout the study in a constant 50 ml volume (Mallinckrodt Pharmaceuticals, St Louis, MO), starting at 30 mg on Day 1 and ending at the maintenance dose of 80 mg. This dose is within the range used in community treatment programs20. Participants came to the clinic seven days a week for medication. After three weeks of stabilization on methadone, participants began receiving daily lofexidine or lofexidine placebo administered at the same time as methadone. Lofexidine (0.2 mg tablets) and matching placebo (Forum Products Inc., Redhill Surry, United Kingdom) were scheduled to be administered once per day for one-week intervals according to the following schedule: Weeks 1 and 2 lofexidine 0 or 0.4 mg double blind in random order; Week 3 lofexidine 0.6 mg; Week 4 lofexidine 0.8 mg; Week 5 lofexidine 1.0 mg; Week 6 lofexidine 1.2 mg; Week 7 lofexidine 1.4 mg; Week 8 lofexidine 1.6 mg. Additional study details were reported previously19.
Outcome Measures
A twelve-lead electrocardiogram was performed before the first administration of each lofexidine dose and at 5.0 hours post-dose between 10:00 AM and 4:00 PM, using a GE MAC 5000 (Milwaukee, WI, USA). For a subset of participants, additional ECG monitoring was performed: at 3.0, 3.5, 4.0, and 4.5 hours post-dose in addition to 5.0 hours post-dose. Tracings were obtained supine, and were recorded at 25 mm/second speed. Automated PR interval, QRS duration, QT interval, and QTC interval were tabulated. To compare the accuracy of automated interpretation, each ECG was over read by a single cardiologist (MJK), who was blinded to time interval, methadone dose, lofexidine dose, gender, and age. QT intervals were measured manually with calipers. The QTc interval was then calculated using Bazett’s formula (QTc=QT/√RR, where RR denotes the interval from the onset of one QRS complex to the onset of the next QRS complex.) The QT interval was measured from the first downward deflection from the isoelectric PR interval to the visual return of the T wave to the TP segment. QT intervals were measured preferentially in lead II. U waves were not incorporated into the QT measurement. Each automated measurement (rate, rhythm, PR interval, QRS, QT, QTc) was compared to the manual reading; manual readings were subsequently used for data analysis.
Statistical Analysis
For the primary analysis, we examined QTc, PR, QRS, and HR at three time points: (1) prior to study enrollment, when the participant was taking neither methadone nor lofexidine (referred to as T0); (2) after stabilization on methadone but before the first lofexidine dose (T1); and (3) during peak plasma levels, 5 hours after receiving the first active lofexidine dose (0.4 mg) (T2). All measurements were available for all participants (n=14) at all three time points. A repeated-measures linear regression analysis (SAS Proc Mixed) with two single-df planned contrasts was performed for each outcome measure: T0 vs. T1, and T1 vs. T2. Since multiple measures were taken during peak plasma levels following drug administration (3–5 hours post-dose) for a subset of participants (n=6), a secondary analysis was performed to assess maximal drug effects. For this analysis, the measures selected for the outcome were the maximum QTc, maximum PR, maximum QRS, and minimum HR values post-dose (for the 8 participants without multiple measures this was the 5-hour post-dose value, as in the primary analysis); the same regression technique was employed as for the primary analysis.
A secondary analysis examined dose effects of lofexidine on the largest subset of participants for whom multiple-dose data were available (n=7). This analysis was analogous to the primary analysis, substituting doses of lofexidine (0.4 mg, 0.6 mg, 0.8 mg) for T0, T1, and T2. As in the primary analysis, the methadone maintenance dose remained constant (80 mg/day), and the same outcome measures were used (QTc, PR, QRS, and HR) at the same time points (5 hours post-dose initially, then the analysis was repeated using the maximum post-dose reading). A repeated-measures linear regression analysis with two single-df planned contrasts was performed for each outcome measure: comparing 0.4 mg to 0.6 mg, and 0.4 mg to 0.8 mg.
All repeated-measures regression models used an unstructured covariance structure. Spearman correlation coefficients were calculated to determine the level of agreement between automated ECG measurements and manual readings. SAS version 9.0 was used for all analyses (SAS Institute, Cary NC).
Results
Subject Characteristics
Of the 26 participants initially enrolled in the study, 12 left or were disqualified for administrative/non-medical reasons prior to receiving lofexidine. Analysis was limited to the 14 participants who received at least one dose of lofexidine. They were aged 34.9 ± 5.3 years; 6 were women (42.9%) and 8 were African-American (57.1%). All smoked tobacco, and nine abused cocaine. Otherwise, the participants were generally healthy and did not have hepatitis C, HIV, potassium below 3.5 mmol/L, or structural heart disease. None of the participants were on any prescription medicines. Those who remained in the study long enough to receive 0.6 and 0.8 mg lofexidine did not differ from the others in terms of race/ethnicity or sex.
A total of 289 ECGs were performed and interpreted by automated and manual readings. The dataset used for this analysis consisted of 63 complete sets of ECG measurements: 7 participants had data for the T0, T1, and T2 time points and for the lofexidine dose analyses (0.4 mg, 0.6 mg, 0.8 mg) and 7 participants had data for only the T0, T1, T2 time points. Automated QTc readings were correlated strongly with manual readings (r= +0.59) over all participants and doses (n=60 pairs, because one participant was missing cardiologist-read values for 3 time points); manual readings were utilized for all analyses.
Baseline ECG findings (Table 1) were consistent with expectations for a young population free of structural heart disease. Among the 14 participants, the highest dose of lofexidine reached in the dose escalation was 0.4 mg in 7 participants, 0.8 mg in 2, 1.4 mg in 3, and 1.6 mg in 2 participants. Dose escalation was limited by side effects such as hypotension or sedation, as we previously reported19.
Table 1.
Baseline Electrocardiographic Findings (n=14)
| Electrocardiographic findingsa | Number |
|---|---|
| Normal sinus rhythm | 7 |
| Sinus bradycardia (HR<60 bpm) | 7 |
| U waves | 2 |
| Nonspecific ST changes | 1 |
| Left ventricular hypertrophy | 1 |
Other ECG findings evaluated at baseline included: sinus tachycardia, prolonged QTc (QTc > 450 ms in men, >470 ms in women), right ventricular hypertrophy, right and left bundle branch block, previous myocardial infarction, and premature ventricular contractions; no abnormalities were found.
The Effect of Lofexidine on ECG Measures
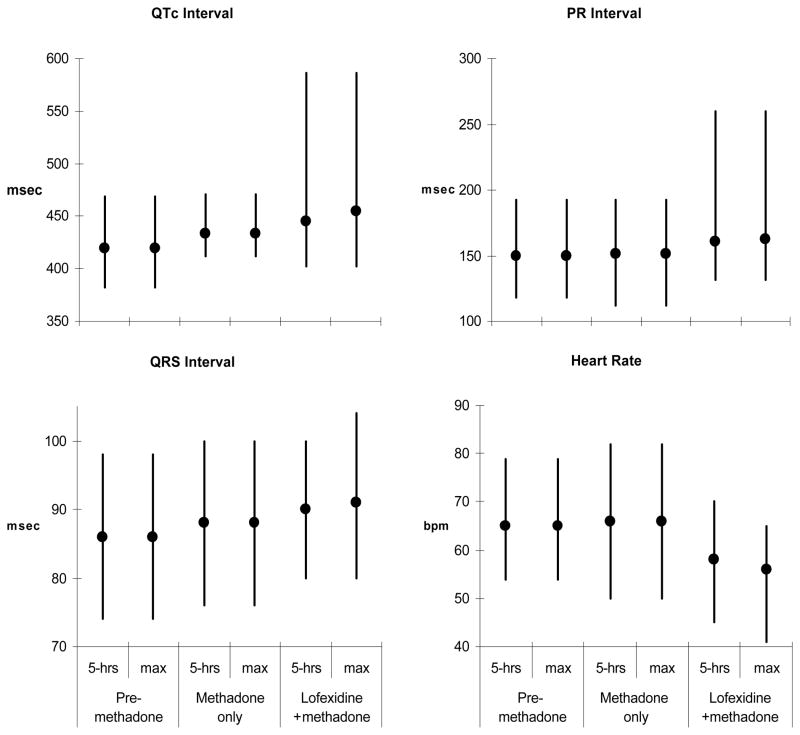
Repeated-measures regression showed no statistically significant differences in mean HR, PR, QRS, or QTc in participants on methadone compared to their unmedicated baselines. When 0.4 mg of lofexidine was added to methadone, there was a statistically significant decrease in HR (mean decrease=8.0 ± 7.3 bpm, F1,26=15.01, p=0.0006) but no significant change in the other three parameters. However when the analysis was performed using the maximum observed values for the six participants with multiple post-dose measures (and the 5-hour post-dose value for the other 8 participants), again there were no significant changes in any parameter comparing methadone to baseline, but significant changes in all four parameters comparing lofexidine plus methadone to methadone alone: HR (mean maximal decrease=9.6 ± 5.8 bpm, F1,26=22.95, p<0.0001), PR interval (mean maximal increase=11.1 ± 19.8, F1,26=5.58, p=0.026), QRS interval (mean maximal increase=3.7 ± 4.3, F1,26=11.8, p=0.002), and QTc interval (mean maximal increase=21.9 ± 40.8, F1,26=6.34, p=0.018). Figure 1 shows minimum, maximum, and mean values for all four parameters for each condition; the response at 5 hours post-dose and the maximum drug response are both shown.
Figure 1.
Electrocardiographic parameters (HR, PR, QRS, QTc) while participants (n=14) were: illicit drug-free, on methadone, and immediately following the first dose of lofexidine 0.4 mg. The vertical line indicates the range (minimum – maximum), the circle indicates the mean value. Responses at five hours post-dose and maximum drug responses are shown.
The mean (maximal) increase in QTc was 21.9 ms. In addition, three participants, all female, had clinically important increases (>40 ms) in QTc interval after administration of lofexidine 0.4 mg. The first participant increased from baseline 462 ms to 586 ms on both drugs; the second participant increased from 419 ms to 464 ms; the third participant increased from 428 ms to 493 ms. In these three participants, QTc prolongation resolved after lofexidine was discontinued. One other female participant developed a substantial increase in PR interval with lofexidine 0.4 mg. Baseline PR was 200 ms, reaching a maximum duration of 500 ms by lofexidine 1.0 mg. She never developed high-grade atrioventricular block and was otherwise asymptomatic during the study.
Lofexidine Dose Effects on ECG Measures
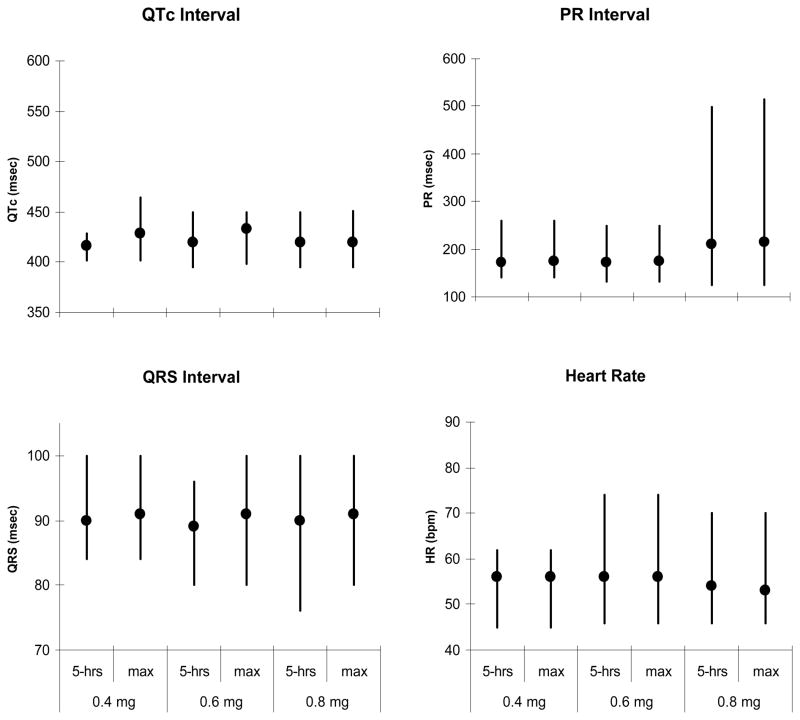
A secondary analysis of the dose-dependent effect of lofexidine escalation comprised a subset of participants (n=7) who received doses of 0.6 mg and 0.8 mg as well as 0.4 mg. There were no statistically significant dose effects for any ECG parameters, comparing 0.4 mg to 0.6 mg, and 0.6 mg to 0.8 mg. This lack of significant dose effects was observed when measures taken at 5 hours post-dose were used in analysis, as well as when the maximum post-dose measures were used. The minimum, maximum, and mean values for all four parameters by dose are shown in Figure 2.
Figure 2.
Electrocardiographic parameters (HR, PR, QRS, QTc) while participants (n=7) were on methadone and lofexidine 0.4, 0.6, and 0.8 mg. The vertical line indicates the range (minimum – maximum), the circle indicates the mean value. Responses at five hours post-dose and maximum drug responses are shown.
Discussion
Lofexidine decreases opioid withdrawal symptoms and has been used extensively for this purpose in the United Kingdom7. Recent and ongoing investigations support its utility for transitioning patients from methadone to buprenorphine maintenance and for relapse prevention beyond the period of acute withdrawal9,13. Given its potential for expanded coadministration, understanding the cardiovascular effects of combining lofexidine with methadone is important. We have reported previously that the combination of lofexidine and methadone may result in significant hypotension, sedation, and cognitive deficits compared to placebo19. Furthermore, methadone is known to cause dose-dependent increases in QTc prolongation and has been associated with the polymorphic ventricular arrhythmia torsade de pointes2, which often results from the confluence of several factors, such as the combination of medications. Therefore, before augmenting methadone maintenance with another maintenance medication, it is important to identify combinations that could increase arrhythmic potential. It should be noted that a great number of methadone-maintained patients also abuse cocaine; cocaine, like methadone, can block the delayed cardiac rectifier potassium channel, which is the primary mechanism for drug-induced QTc prolongation21.
This is the first study to examine potential effects of lofexidine and methadone co-administration on cardiac conduction and repolarization. It was designed solely as a safety study; consequently, generalizability is limited by small sample size (n=14). Due to side effects such as hypotension, most patients were not escalated to the target dose of 1.6 mg lofexidine, so a full range of lofexidine dose effects could not be examined. We found no significant changes in mean heart rate, PR interval, QRS duration, or QTc interval after methadone administration, though a larger prospective study has shown that methadone monotherapy increases QTc interval modestly despite having no effect on QRS duration14. When the starting dose of lofexidine was added (0.4 mg), we found a statistically significant decrease in heart rate. When maximal ECG responses were examined, we found statistically significant changes in all four parameters, most importantly a mean maximum increase in QTc interval of 21.9 ms. In addition, three female subjects developed marked (> 40 ms increase from baseline) QTc prolongation. No additional changes were seen with lofexidine dose escalation, but only a small number of patients were tested at the higher doses, precluding assessment of a dose-dependent impact on cardiac conduction and repolarization. Another limitation of our study is the lesser precision of hand calipers for measuring QTc interval (approximately 10 ms) compared with electronic measurement22.
The known electrocardiographic properties of methadone and lofexidine provide possible biological mechanisms for the effects seen in our study. Methadone may induce bradycardia through its anticholinergic and calcium-channel-blocking properties15,16,23,24, while lofexidine induces bradycardia through CNS alpha agonism. Methadone can prolong the QTc interval4, and perhaps this effect is exacerbated by a lofexidine-induced decrease in sympathetic tone and increase in parasympathetic tone. Although a recent study demonstrated that sympathetic surge may lead to a net prolongation in ventricular repolarization25, autonomic blockade has previously been shown to exacerbate drug-induced QT prolongation in the setting of blockade of the delayed rectifier potassium ion current IKR 6. Thus, the combination of a drug such as methadone, which blocks IKR, plus lofexidine, which inhibits autonomic output, could result in synergistic delay in cardiac repolarization. Moreover, both drugs slow cardiac conduction, which may also pose a cardiac safety concern, given that torsade de pointes is a pause-dependent arrhythmia26. Although it is conceivable that lofexidine has independent effects on repolarization, our literature search did not identify reports suggesting that either lofexidine or clonidine blocks IKR in vitro or is associated clinically with torsade de pointes. Finally, we think it unlikely that there is a pharmacokinetic interaction at the level of P450 metabolism, because lofexidine is neither a substrate nor an inducer/inhibitor of P450 metabolic pathways, the principal pathways for methadone metabolism. For the participants who developed a prolonged QTc interval, the abnormal measure resolved after lofexidine was discontinued, which suggests a causal relationship27.
Limitations of the current study notwithstanding, our findings suggest that the combination of methadone and lofexidine may induce QTc interval prolongation. Our results suggest that if lofexidine is prescribed to patients maintained on methadone, they should be closely monitored for QTc interval prolongation. Furthermore, because the participants with the largest changes in QTc interval in our study were female, women may be at highest risk.
Acknowledgments
This research was supported by funding from the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, U.S. Department of Health and Human Services. Lofexidine and matching placebo was provided by Forum Products, Inc. Redhill Surry, United Kingdom.
References
- 1.Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment, Office of Pharmacologic and Alternative Therapies. [Accessed May 29, 2007]; Available from: http://dpt.samhsa.gov/ppt_slices/metadone/frame.htm.
- 2.Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose-related effects of methadone on QT prolongation in a series of patients with Torsade de Pointes. Pharmacotherapy. 2003;23:802–805. doi: 10.1592/phco.23.6.802.32186. [DOI] [PubMed] [Google Scholar]
- 3.Krantz MJ, Mehler PS. QTc prolongation: methadone’s efficacy-safety paradox. Lancet. 2006;368:356–357. doi: 10.1016/S0140-6736(06)69173-3. [DOI] [PubMed] [Google Scholar]
- 4.Katchman AN, McGroary KA, Kilborn MJ, Kornick CA, Manfredi PL, Woosley RL, Ebert SN. Influence of opioid agonists on cardiac human ether-a-go-go related gene K+ currents. Journal Of Pharmacology And Experimental Therapeutics. 2002;303:688–694. doi: 10.1124/jpet.102.038240. [DOI] [PubMed] [Google Scholar]
- 5.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 6.Smith AH, Norris KJ, Roden DM, Kannankeril PJ. Autonomic tone attenuates drug-induced QT prolongation. Journal of Cardiovascular Electrophysiology. 2007;18(9):960–964. doi: 10.1111/j.1540-8167.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 7.Akhurst JS. The use of lofexidine by drug dependency units in the United Kingdom. European Addiction Research. 1999;5:43–49. doi: 10.1159/000018962. [DOI] [PubMed] [Google Scholar]
- 8.Gowing L, Farrell M, Ali R, White J. Alpha2 adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2004;8:CD002024. doi: 10.1002/14651858.CD002024.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Glasper A, Reed LJ, de Wet CJ, Gossop M, Bearn J. Induction of patients with moderately severe methadone dependence onto buprenorphine. Addiction Biology. 2005;10:149–155. doi: 10.1080/13556210500123126. [DOI] [PubMed] [Google Scholar]
- 10.Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- 11.Highfield D, Yap J, Grimm JW, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 12.Lê AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- 13.Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- 14.Martell BA, Arnsten JH, Krantz MJ, Gourevitch MN. Impact of methadone treatment on cardiac repolarization and conduction in opioid users. American Journal Of Cardiology. 2005;95:915–918. doi: 10.1016/j.amjcard.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Berkowitz BA. Calcium antagonist activity of methadone, l-acetylmethadol and l-pentazocine in the rat aortic strip. Journal of Pharmacology and Experimental Therapeutics. 1977;202:646–653. [PubMed] [Google Scholar]
- 16.Rendig SV, Amsterdam EA, Henderson GL, Mason DT. Comparative cardiac contractile actions of six narcotic analgesics: morphine, meperdine, pentazocine, fentanyl, methadone, and l-alpha-acetylmethadol (LAAM) Journal of Pharmacology and Experimental Therapeutics. 1980;215:259–265. [PubMed] [Google Scholar]
- 17.Graf E, Sieck A, Wenzl H, Winkelmann J. Animal experiments on the safety pharmacology of lofexidine. Arzneimittelforschung. 1982;32:931–940. [PubMed] [Google Scholar]
- 18.Schmittner J, Schroeder JR, Epstein DH, Preston KL. QT interval increased after single dose of lofexidine. British Medical Journal. 2004;329:1075. doi: 10.1136/bmj.329.7474.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder JR, Schmittner J, Bleiberg J, Epstein DE, Krantz MJ, Preston KL. Hemodynamic and cognitive effects of lofexidine and methadone co-administration. Pharmacotherapy. 2007;27:1111–1119. doi: 10.1592/phco.27.8.1111. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell S, Shinderman MS. Optimizing long-term response to methadone maintenance treatment: an 152-week follow-up using higher-dose methadone. Journal of Addiction Disease. 2002;21:1–12. doi: 10.1300/J069v21n03_01. [DOI] [PubMed] [Google Scholar]
- 21.Krantz MJ, Baker WA, Schmittner JS. Cocaine and Methadone: parallel effects on the QTc interval. American Journal of Cardiology. 2006;98:1121. doi: 10.1016/j.amjcard.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Center for Drug Evaluation and Research, Food and Drug Administration; Rockville, Maryland: 2005. [Google Scholar]
- 23.Seyler DE, Borowitz JL, Maickel RP. Calcium channel blockade by certain opioids. Fundam Appl Toxicol. 1983;3:536–42. doi: 10.1016/s0272-0590(83)80101-8. [DOI] [PubMed] [Google Scholar]
- 24.Eikenburg DC, Stickney JL. Anti-cholinesterase activity of l-alpha-acetylmethadol: relationship to bradycardia. Gen Pharmacol. 1979;10:195–200. doi: 10.1016/0306-3623(79)90089-2. [DOI] [PubMed] [Google Scholar]
- 25.Overholser BR, Zheng X, Tisdale JE. Catecholaminergic effects on ventricular repolarization during inhibition of the rapid component of the delayed rectifier potassium current in a perfused heart model. Pharmacotherapy. 2008;28(11):1315–1324. doi: 10.1592/phco.28.11.1315. [DOI] [PubMed] [Google Scholar]
- 26.Kurita T, Ohe T, Shimizu W, Hotta D, Shimomura K. Early afterdepolarization in a patient with complete atrioventricular block and torsades de pointes. Pacing Clin Electrophysiol. 1993;16:33–8. doi: 10.1111/j.1540-8159.1993.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 27.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domencq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]