Abstract
Background
Prolonged-release melatonin (PRM) 2 mg is indicated for insomnia in patients aged 55 years and older. A recent double-blind placebo-controlled study demonstrated 6-month efficacy and safety of PRM in insomnia patients aged 18–80 and lack of withdrawal and rebound symptoms upon discontinuation.
Objective
To investigate the efficacy, safety, and withdrawal phenomena associated with 6–12 months PRM treatment.
Methods
Data from a prospective 6–12-month open-label study of 244 community dwelling adults with primary insomnia, who had participated in a placebo-controlled, double-blind dose-ranging trial of PRM. Patients received PRM nightly, followed by a 2-week withdrawal period. Main outcome measures were patient-reported sleep quality ratings (diary), adverse events, vital signs, and laboratory tests recorded at each visit, and withdrawal symptoms (CHESS-84 [Check-list Evaluation of Somatic Symptoms]). Nocturnal urinary 6-sulfatoxymelatonin excretion, a measure of the endogenous melatonin production, was assessed upon discontinuing long-term PRM.
Results
Of the 244 patients, 36 dropped out, 112 completed 6 months of treatment, and the other 96 completed 12 months of treatment. The mean number of nights by which patients reported sleep quality as “good” or “very good” was significantly higher during PRM than before treatment. There was no evidence of tolerance to PRM. Discontinuation of PRM was not associated with rebound insomnia or withdrawal symptoms; on the contrary, residual benefit was observed. PRM was well tolerated, and there was no suppression of endogenous melatonin production.
Conclusion
Results support the efficacy and safety of PRM in primary insomnia patients aged 20–80 throughout 6–12 months of continuous therapy. PRM discontinuation even after 12 months was not associated with adverse events, withdrawal symptoms, or suppression of endogenous melatonin production.
Keywords: PRM, adverse events, sleep, insomnia, patients
Introduction
Insomnia is a common complaint characterized by difficulties to initiate or maintain sleep and/or poor quality of sleep. These complaints last at least 1 month and have negative effects on subsequent daytime functioning.1–3 Patients may suffer immensely from poor quality of sleep, even when their sleep quantity is within the normal limits.1,2 Poor sleep quality adversely affects physical and mental health, wellbeing, activities of daily living, driving skills, memory, productivity, and satisfaction with life.4–11 The prevalence of complaints on poor sleep quality increases with age.6,7,12 Insomnia is often chronic (lasting more than 3 months),13 and although benzodiazepines and Z drugs (zolpidem, zopiclone, zaleplon, and derivates) are considered inadequate for management of chronic insomnia patients, patients tend to use these drugs over extended periods of time varying from 4 weeks to several years.14
Melatonin (N-acetyl-5-methoxytryptamine) is produced at night by the pineal gland and signals darkness to the organism to facilitate synchronization of the circadian clock with the ambient day–night cycle.15 In addition, melatonin is an endogenous sleep promoter acting at brain networks involved in sleep regulation.16 A prolonged- release melatonin (PRM) 2 mg formulation (Circadin®, Neurim Pharmaceuticals, Tel- Aviv, Israel) is licensed in Europe and other countries for the treatment of primary insomnia in patients aged 55 years and over for a duration of up to 3 months. The approval was based on a series of double-blind placebo-controlled clinical trials of PRM (3 weeks) treatment in patients aged 55 years and older. The rationale for this age limit was based on the well documented age-associated decline in the capacity to produce the endogenous hormone,17–19 the decline in biological clock output, and the age-related increase in the incidence of poor sleep quality.10,20 PRM circumvents the fast clearance of the hormone and essentially mimics the physiological patterns of the endogenous secretion of melatonin.
In a recent double-blind, placebo-controlled trial, the long-term (6 months) efficacy and safety of PRM was demonstrated in patients with insomnia aged 18–80; patients aged 55 years and older were found to be those who benefit most from the drug.21,22 It was also found that efficacy was maintained and even enhanced during the 6 months of treatment with PRM, without evidence of tolerance. Discontinuation of PRM after either 3 weeks or 6 months was not associated with rebound insomnia or withdrawal symptoms.21–25 The present study aimed to demonstrate: (1) that the efficacy and safety of PRM (2 mg) in adults with insomnia who are permanently taking the medication is maintained for up to 12 months; (2) that there are no withdrawal symptoms associated with discontinuation of the treatment, even after 12 months; and (3) that normal endogenous melatonin levels are produced despite long-term administration (6 months) of PRM 2 mg.
Methods
Patients
Participants were community dwelling men and women aged 20–80 years, diagnosed as having primary insomnia according to the Diagnostic and Statistical Manual of Mental Disorders (revision IV) criteria. Exclusion criteria were respiratory related sleep disorders, circadian rhythm sleep disorders, dyssomnias not otherwise specified, sleep disorder secondary to medical conditions, significant psychiatric or neurological disorders (anxiety, depression, dementia, psychosis), or the use of hypnotic medications in the past 2 weeks.
Study design
The study was carried out in 30 general practitioners’ clinics in France and Israel. The study was conducted in accordance with the International Conference on Harmonisation (ICH) Guidelines and the provisions of the Declaration of Helsinki (18th World Medical Assembly 1964) and revisions (48th World Medical Assembly – South Africa 1996). In addition, this study was undertaken in accordance with current ICH Guidelines on Good Clinical Practice on the conducting and monitoring of clinical studies. The protocol and the statement of informed consent were approved by independent ethics committees (in France, Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale; and in Israel, the Central EC of the Wolfson Medical Center, Holon) prior to each center’s study initiation. All patients provided written informed consent prior to enrolment.
All participants had a 1 week run-in period with placebo (baseline) followed by a 6-week placebo-controlled dose-ranging study with PRM. Completers who gave their consent for the long-term study were allowed to enter the prospective 6–12-month open-label study. In this phase, all patients received (open-label) PRM 2 mg (Circadin®). Participants were instructed to take the study medication regularly after the evening meal, 1–2 hours before bedtime and preferably between 21:00–22:00. Treatment compliance was monitored in all subjects using a monthly tablet count. Patients with significant adverse events were to be withdrawn from the study at the discretion of the investigator. All hypnotics or treatments used to induce sleep (herbals, antihistamines, alcohol) were not allowed during the study. Use of hypnotic drugs for up to two times per week at any time during the study, were to be recorded as a protocol deviation, and using hypnotics three or more times per week led to withdrawal of the patient from the study.
Once the first 100 subjects had completed the 26-week (6 months) open-label period, they were not given the option to continue up to 52 weeks, as safety data for 26 weeks was requested by regulatory authorities. They therefore underwent the 2-week withdrawal period, their study was terminated, and their safety data were summarized. The remaining patients continued PRM treatment up to 52 weeks (12 months), with a 2-week follow-up period without study medication (withdrawal).
During the week before each follow-up visit (weeks 1, 7, 13, 23, 33, and 59 and withdrawal phase weeks 35/61), all participants were asked to complete a daily sleep diary comprising two questions: “How was your night?”, regarding their sleep quality in the previous night (QOS), and “How was your mood during the day?”, assessing their perceived mood during the previous day (QOD). QOS and QOD were rated on a five-grade severity scale (very bad, bad, fair, good, and very good). The diary was completed also during the 2-week withdrawal periods (week 35 for those who completed 26 weeks of PRM treatment, and week 61 for those who completed 52 weeks of PRM treatment). Physical examination, vital signs assessment, and evaluation of safety parameters, including adverse events and unusual events in the patient diary, were performed at each visit. Safety laboratory tests were performed on each visit. At the end of the withdrawal period, the patients were assessed by the mini mental state examination and CHESS-84 (Check-list Evaluation of Somatic Symptoms).26 This scale evaluates a list of somatic symptoms on digestive system, cardiovascular system, central nervous system, and specifically sleep and daytime parameters.
Endogenous melatonin production
It is widely accepted27 that the levels of the major melatonin metabolite 6-sulphatoxymelatonin (6SMT) in urine is a reliable measure of endogenous melatonin production. The levels of 6SMT in urine were determined in a group of 15 of the insomnia patients (mean ± standard deviation [SD]: 64 ± 6 years) who had been treated for 6 months with PRM. 6SMT was assessed 2 weeks after withdrawal of the drug. Patients were asked to collect all urine excreted between 20:00 and 8:00 hours (for nocturnal production) and between 8:00 and 20:00 hours (for daytime production) in separate containers provided by the investigator. To enhance compliance, no specific instructions were given regarding lighting conditions while sleeping. Urine was delivered to the local center or laboratory, the volume was measured and recorded, and a 1 mL sample from each collection was retained and frozen until measurement of urinary 6SMT. Determination of 6SMT was performed in duplicates as described in Arendt et al27 using the anti 6SMT antibody and 6SMT calibration standards provided by Stockgrand (Surrey, UK).
Statistical analyses
The number of nights per week rated “good” and “very good” in the sleep diary was calculated for the 1-week run-in period and for weeks 7, 13, 23, 33, and 59, and during the 2 weeks following withdrawal. The mean rate of “good” and “very good” nights per week was summarized using descriptive statistics. Two-sided t-tests were used to test the null hypothesis that the rates after treatment were equal to those at baseline. P-values were reported.
Descriptive statistics were also used for assessing adverse events, changes in laboratory parameters, changes in physical examination parameters, and vital signs. For withdrawal symptoms, the number of patients reporting somatic symptoms possibly, probably, or definitely related to treatment discontinuation during the withdrawal period was summarized using CHESS-84. Two-sided Fisher’s exact tests were used to compare adverse event incidence in the <55 and ≥55 years age groups.
Results
Study population
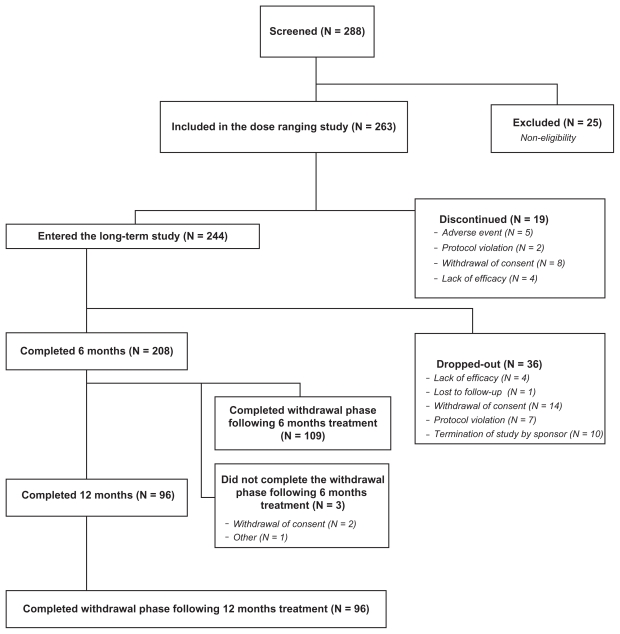
A total of 244 patients entered the prospective 6–12-month open-label study (safety population). Of those, 36 dropped out, 112 completed 26 weeks, and the other 96 completed 52 weeks of the open-label PRM treatment. Altogether, 208 patients were included in the full analysis set (FAS) population. Three patients who completed the 26 week treatment phase and did not undergo a withdrawal period were included in the FAS analyses, and their withdrawal data were missing (Figure 1). The main reasons for dropping out were withdrawal of consent (N = 16), termination of study by sponsor (N = 10), and protocol violation (N = 7). Mean ± SD age of the safety population was 55.3 ± 13.0 years (132 aged ≥ 55 years and 112 aged 20–54 years), and 69% were females. With the exception of one participant of Asian origin, all subjects were Caucasians. A total of 79% were taking concomitant medications at entry, and 94% took at least one dose of a concomitant medication during the study. The concomitant medications used in more than 5% of the 244 patients were paracetamol (acetaminophen) in 35/208 (14%), statins in 25 (10%), acetylsalicylic acid in 22 (9%), amoxycycline and ibuprofen in 17 (7%) each, levothyroxine sodium in 17 and thyroxine in 7 patients (altogether 10%), famotidine, glibenclamide, omeprazole, metformin, estradiol, ergynon in 12 (5%) patients each.
Figure 1.
Overall study patient disposition (Consolidated Standards for Reporting Trials diagram). The study comprised 1-week, single-blind placebo run-in followed by 6-week dose-ranging study with 3 doses of PRM or placebo. Completers were allowed to continue PRM treatment for 26 weeks (N = 100) or 52 weeks (all the rest) followed by 2 weeks run-out on placebo.
Abbreviation: PRM, prolonged-release melatonin.
Sleep quality during the long-term period
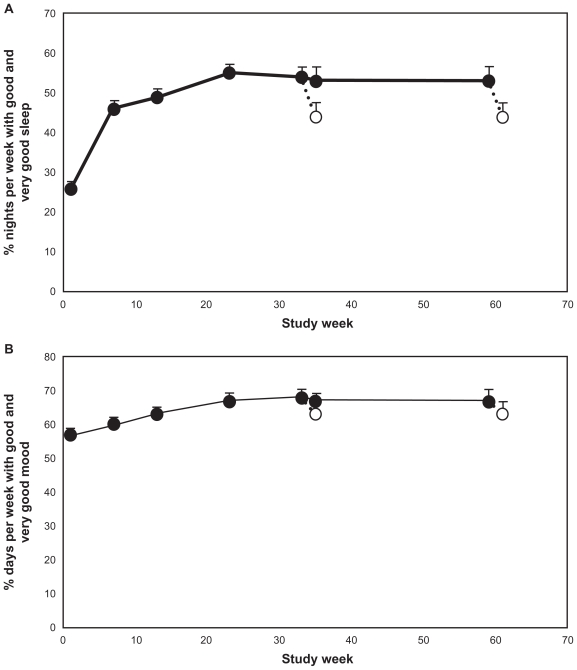
The mean (+standard error of the mean [SEM]) percentage of nights per week reported as “good sleep” or “very good sleep” as calculated from the night-time sleep diary in the last 7 nights before each visit is presented in Figure 2A (FAS population). The mean (+SEM) percentage of nights per week scored by the patients as “good” or “very good” increased progressively with treatment duration, reaching plateau levels between weeks 13 and 23. At the plateau level, 54%–56% of nights per week were scored as “good” or “very good” (ie, 3.8 nights per week) compared with 26% (ie, 1.8 nights per week) at baseline (P < 0.001, t-test), and this was maintained throughout the entire PRM treatment period. Following discontinuation of treatment and 2 weeks withdrawal, the percentage declined to around 44% (ie, 3.1 nights per week scored as “good” or “very good” on average), regardless of whether patients had been treated for 26 or 52 weeks (Figure 2). Although the percentage of nights per week scored as “good” or “very good” at withdrawal was lower than during the PRM treatment, it was still significantly better than at baseline before PRM treatment (P < 0.001 for both 26 and 52 weeks).
Figure 2.
Mean + standard error of the mean values for: (A) percentage of nights per week rated “good” or “very good” from the sleep diary during the baseline week and the last week preceding each visit during the treatment phase and withdrawal phase; and (B) percentage of days per week with mood rated “good” or “very good” from the sleep diary during the baseline week and the last week preceding each visit during the treatment phase and withdrawal phase. Blank circles indicate the values recorded at the withdrawal period following respective 26 or 52 weeks treatment with prolonged-release melatonin. The x-axis depicts the time since entering the dose ranging phase of the study. Week 1 is baseline, week 8 is the first week of the long-term period that lasted 26 weeks (ending on week 31) and 52 weeks (ending on week 59) followed by a 2-week withdrawal phase (between weeks 31–33 and 59–61 respectively).
At baseline, 57% of days (4.2 days per week) were scored as “good” or “very good” mood by the patients. This score increased progressively with PRM treatment, reaching plateau levels of 67%–68% (4.7 days per week) between weeks 13 and 23 (P < 0.002). This plateau level was maintained until the end of the treatment phase. However, following PRM discontinuation and the 2-week withdrawal period, the percentage of days scored as “good” or “very good” decreased to approximately baseline values.
Because PRM is licensed to patients aged 55 years and older, the long-term efficacy in patients younger and older than 55 years was compared. For patients younger than 55 years, the percentage of nights per week scored by the patients as “good” or “very good” increased progressively with treatment duration from 30% at baseline, reaching plateau levels of 58%–57% between weeks 13 and 23 respectively (P < 0.001), which was maintained for the rest of the treatment period. The percentage of days scored by patients as “good” or “very good” mood also increased, from 62% at baseline, reaching plateau levels of 68%–70% between weeks 13 and 23 respectively. Following PRM discontinuation and the 2-week withdrawal period, the percentage of nights scored as “good” or “very good” decreased to 50%, and the percentage of days scored as “good” or “very good” decreased to 68%; both were higher than baseline values.
Similarly, for patients who were 55 years and over, the percentage of nights per week scored by the patients as “good” or “very good” increased progressively with treatment duration from 23% at baseline, reaching plateau levels of 52%–50% between weeks 13 and 23 respectively (P < 0.001). The percentage of days scored by patients as “good” or “very good” mood also increased, from 52% at baseline, reaching plateau levels of 67%–66% between weeks 13 and 23 respectively (P < 0.001). Following PRM discontinuation and the 2-week withdrawal period, the percentage of nights scored as “good” or “very good” decreased to 38%, and the percentage of days scored as “good” or “very good” decreased to 57%, which were also still higher than baseline values.
Serious adverse events
No deaths were reported during any phase of the study. Nine adverse events were considered “serious”: one (pelvic fracture) was reported during run-in under placebo, and eight events during the 26–52 weeks open-label phase of the study. These included (one case each) duodenal sphincterectomy, dilatation of bile duct, elective surgery for worsening of venous insufficiency, fall with wrist fracture, fall from a ladder with loss of consciousness, acute subendocardial anteriolateral myocardial infarction, tibial fracture, syncope due to aortic stenosis, and cholecystitis due to gallstones. All of these events were considered to be “not related” to the study medication. No patients withdrew as a result of adverse events during the open-label phase of the study.
Safety parameters
Out of the 244 patients in the safety population, 48 patients (18%) reported a total of 63 adverse events during the run-in period on placebo. The total number of weeks on PRM therapy for the 244 patients was 8475.5. The mean ± SD treatment duration with PRM was 243.5 ± 109.90 days. Out of the 244 subjects patients in the safety population, 153 (63%) reported adverse events that emerged during the 26–52 weeks treatment phase of the study; the most commonly reported events were pharyngitis (12.4%), back pain (11.8%), asthenia (9.1%), upper respiratory tract infection (8.5%), bronchitis (7.8%), arthralgia and sinusitis (7.2% each), headache and rhinitis (5.9% each), and gastroenteritis (5.2%). In 7% of the patients, the adverse events were considered by the investigator to be definitely, probably, or possibly related to study medication. Of these, the most commonly reported adverse events were dizziness in four patients (1.6%) and headache in three patients (1.2%). No noticeable changes were found in hematologic and biochemical laboratory tests at any time-point during the study. Furthermore, there were no differences in the number and nature of changes from baseline in the physical examination between patients treated for the first 6 months and those treated for a whole year of single blind treatment (Table 1). In order to evaluate whether safety problems occur more with prolonged use, we have calculated the differences in the rate of changes from normal to abnormal status, between baseline to either week 33 or week 59. As can be seen in Table 1, the incidence of such changes was lower in the period between 33 and 59 than between baseline and week 33.
Table 1.
Number of patients demonstrating a change from normal to abnormal physical examination observations between baseline and study week 33 or 59 (safety population)
| Body system | Number of patients with changes (treatment)
|
Difference in incidence rates between baseline to weeks 59 and 33 | |
|---|---|---|---|
| Baseline to week 33 N = 112 |
Baseline to week 59 N = 99 |
||
| Eyes, ears, nose and throat | 8 (6.7%) | 9 (9.1%) | 2.4% |
| Cardiovascular | 2 (1.8%) | 0 | −2% |
| Respiratory | 3 (2.7%) | 3 (3.0%) | 0.3% |
| Abdomen | 3 (2.7%) | 3 (3.0%) | 0.3% |
| Urogenital | 0 | 3 (3.0%) | 3% |
| Neurological | 0 | 3 (3.0%) | 3% |
| Musculoskeletal | 6 (5.4%) | 6 (6%) | 0.6% |
| Lymph nodes | 0 | 0 | 0 |
| Total shifts | 25 (22.3%) | 38 (38.4%) | 16% |
Because PRM is licensed to patients aged 55 years and older, the long-term safety profiles in patients younger and older than 55 years were compared. The total rate of patients with adverse events per 100 patient-weeks of PRM therapy, and in the two age groups is presented in Table 2.
Table 2.
Summary of age group comparisons on adverse event incidence
| Body system
|
All (N = 244)
|
≥55 years (N = 133)
|
<55 years (N = 111)
|
P-valueb | |||
|---|---|---|---|---|---|---|---|
| Adverse event | No | Ratea | No | Ratea | No | Ratea | |
| Application site disorders | 3 | 0.04 | 2 | 0.04 | 1 | 0.03 | 1.000 |
| Body as a whole – general disorders | 43 | 0.51 | 24 | 0.54 | 19 | 0.48 | 0.867 |
| Central and peripheral nervous system disorder | 30 | 0.35 | 18 | 0.40 | 12 | 0.30 | 0.561 |
| Gastrointestinal system disorders | 35 | 0.41 | 20 | 0.45 | 15 | 0.38 | 0.855 |
| Metabolic and nutritional disorders | 11 | 0.13 | 5 | 0.11 | 6 | 0.15 | 0.555 |
| Musculoskeletal system disorders | 46 | 0.54 | 27 | 0.60 | 18 | 0.45 | 0.413 |
| Psychiatric disorders | 17 | 0.20 | 7 | 0.16 | 10 | 0.25 | 0.316 |
| Reproductive disorders, female | 7 | 0.08 | 2 | 0.04 | 5 | 0.13 | 0.251 |
| Resistance mechanism disorders | 22 | 0.26 | 8 | 0.18 | 14 | 0.35 | 0.115 |
| Respiratory system disorders | 64 | 0.76 | 36 | 0.80 | 28 | 0.71 | 0.771 |
| Secondary terms | 6 | 0.07 | 5 | 0.11 | 1 | 0.03 | 0.224 |
| Skin and appendages disorders | 13 | 0.15 | 6 | 0.13 | 7 | 0.18 | 0.579 |
| Urinary system disorders | 17 | 0.20 | 11 | 0.25 | 6 | 0.15 | 0.454 |
| Vascular (extracardiac) disorders | 3 | 0.04 | 1 | 0.02 | 2 | 0.05 | 0.594 |
| Vision disorders | 3 | 0.04 | 1 | 0.02 | 2 | 0.05 | 0.594 |
| Total | 153 | 1.81 | 83 | 1.85 | 69 | 1.74 | 1.000 |
Notes: Rate of patients with adverse event per 100 patient-weeks of therapy;
Two-sided Fisher’s exact test.
Patients younger than 55 years (N = 111) had 3968 weeks and those older than 55 (N = 132) had 4480 weeks of PRM exposure. There were no differences in rate and nature of adverse events between patients above and below 55 years of age (Table 2).
Withdrawal effects
Withdrawal effects after long-term administration (26 and 52 weeks) were assessed by comparing adverse event rates during the 2-week withdrawal phase and the long-term open-label phase using the CHESS-84 questionnaire. Out of 100 subjects who underwent a 2-week withdrawal following 26 weeks of treatment with PRM, 20% reported adverse events during the withdrawal phase. The most commonly reported adverse event during this period was sinusitis, reported by three patients. Among the 96 subjects who underwent a 2-week withdrawal following 52 weeks treatment with PRM, 13% reported adverse events during the withdrawal phase. The most commonly reported events were insomnia and urinary tract infections, each reported by two patients. No consistent changes in any laboratory finding were noted.
The number of somatic symptoms reported in CHESS-84 during withdrawal following 26 and 52 weeks of PRM treatment are summarized in Table 3. Overall, 24 patients (24%) of those treated for 33 weeks and 21 (22%) of those treated for 52 weeks reported somatic symptoms possibly, probably, or definitely related to study treatment. Most of those were relate to sleep.
Table 3.
Somatic symptoms during the withdrawal period following 26 and 52 weeks of open-label PRM treatmenta
| Number (% safety population) of patients with new symptomsb |
||
|---|---|---|
| 26 weeks N = 100 |
52 weeks N = 96 |
|
| Total patients reporting somatic symptoms | 24 (24%) | 21 (22%) |
| Body system | ||
| Nonrestorative sleep | 13 (13%) | 9 (9%) |
| Waking during the night | 11 (11%) | 14 (15%) |
| Difficulty in falling asleep | 11 (11%) | 10 (10%) |
| Waking early in the morning | 11 (11%) | 7 (7%) |
| Change in dream pattern | 6 (6%) | 3 (3%) |
| Drowsiness during the day | 5 (5%) | 4 (4%) |
| Dryness of the mouth | 4 (4%) | 2 (2%) |
| Excessive drowsiness | 3 (3%) | 4 (4%) |
| Constipation | 2 (2%) | 0 |
| Stomach aches, cramps | 0 | 2 (2%) |
| Memory problems increase | 0 | 2 (2%) |
Notes: Events possibly, probably, or definitely associated with treatment;
As reported in the CHES-84 questionnaire.
Abbreviations: CHES, Check-list Evaluation of Somatic Symptoms; PRM, prolonged-release melatonin.
Rebound insomnia is defined as a worsening of the insomnia parameters below pre-treatment values. Based on the CHESS-84 questionnaire, during the withdrawal period, 11% of patients reported difficulty in falling asleep, and in 25% of those they reported the difficulty to be moderate, severe, or extreme. At baseline, 53% of the patients reported a score for sleep latency of ≥3 (more than 30 minutes to more than 2 hours) in their sleep diary. Therefore, the number of patients having difficulties in falling asleep during withdrawal is apparently much lower than those having had difficulties in falling asleep at baseline.
Also, based on the CHESS-84 questionnaire, during the withdrawal period, 11% of the patients reported waking during the night at withdrawal and in 29% of those, the reported difficulty was defined as moderate, severe, or extreme. At baseline, 24% of the patients reported on waking up more than three times a night. Therefore, the number of patients waking during the night at withdrawal did not appear to differ greatly from that reported in the patients’ diaries at baseline. In addition, there was no evidence of new somatic symptoms not related to sleep, experienced during withdrawal that had not been previously experienced at baseline. Altogether, the CHESS-84 score for discontinuation of PRM after 33 or 52 weeks was 1.37.
The score of all subjects who completed the Mini-Mental State Examination were within the normal range in subjects of all age groups. There was no apparent decline in the cognitive status following PRM treatment for 33 or 52 weeks.
Endogenous melatonin production
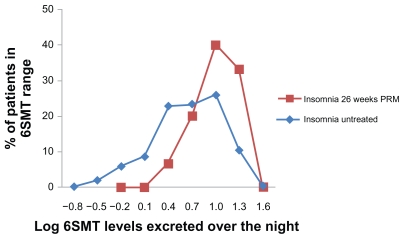
The overnight 6SMT excretion (between 22:00 and 10:00) in 15 patients who completed 6 months of daily PRM administration and the following 2 weeks withdrawal of treatment was mean ± SD of 15.3 ± 7.7 μg, median 15 μg, range 4–30 μg. These levels were significantly higher than those of a large reference population of patients with insomnia of the same age group (mean ± SD of 9.5 ± 7.9 μg, range 0–47 μg/12-hour night; t-test, P < 0.01) and similar to those without insomnia of the same age group (mean ± SD of 18.1 ± 12.7 μg per 12-hour night) (Figure 3).17,19 Fourteen out of the 15 patients were considered to be within the normal range of the nocturnal 6SMT (2.9–29.0 μg 6SMT/12-hour night).19 A clear diurnal rhythm in melatonin production was evident in these patients, with levels of 6SMT over daytime hours (10:00–22:00) of mean ± SD 9.80 ± 5.06, range 3–19 μg/12-hour day, which is significantly lower than the night-time levels (mean ± SD of 15.3 ± 7.7 μg, median 15 μg, range 4–30 μg; t-test, P < 0.01).
Figure 3.
Frequency distribution (in percentage of patients) of 6SMT excreted over the night by patients completing 26 weeks of PRM treatment followed by 2 weeks of withdrawal (big squares; N = 15) compared with that in a historical sample 17,19 of untreated insomnia patients of the same age group (small squares; N = 384).
Abbreviations: 6SMT, 6-sulphatoxymelatonin; PRM, prolonged-release melatonin.
Discussion
Out of 244 patients aged 18–80 years who entered the open-label phase of the study, 112 patients completed the 6-month treatment period, and 96 patients completed the 12-month treatment period. This enabled comparison between the efficacy and safety in the second 6 months to the first 6 months of treatment, thereby extending our knowledge about long-term effects of the drug. A significant improvement in quality of night sleep from baseline value was observed with PRM. The response evolved gradually, reaching plateau levels between weeks 13 and 21 (approximately 54%–56% of nights were defined as “good” or “very good” compared with around 26% at baseline), and this improvement was maintained throughout the 12 months of PRM treatment. Because this was an open-label phase, it was pertinent to ask whether the apparent increase in efficacy during treatment periods of up to 13–21 weeks is a drug-related clinical benefit or instead linked to spontaneous remission of insomnia. A similar enhancement in effects of PRM with treatment of up to week 13 was also demonstrated in a double-blind placebo-controlled study of PRM in insomnia patients.21,22 In agreement with the results of other short- and long-term placebo-controlled trials with this drug, PRM did not ameliorate and even improved patients’ perceived wellbeing during the day.21–25,28 The enhanced efficacy over a number of sleep and daytime variables was attributed to improvement in the adjustment of the circadian system to the day–night cycle.21,22 It can therefore be concluded that there is no tolerance to PRM even upon extended use. Rather, some added benefit is seen with prolonged treatment duration for 3–4 months, with no signs of tolerance even after 12 months.
A good safety profile was observed during treatment with PRM 2 mg in the long-term (6- and 12-month) treatment period for adult patients, including the elderly with similar safety profiles for patients aged 55 years and older and younger patients. In addition, no major safety concerns followed withdrawal of the study medication. During the PRM treatment period, 63% of patients reported adverse events; this figure dropped to 20% during the 2-week withdrawal period after 26 weeks, and to 13% after 52 weeks of PRM treatment. No patients reported serious adverse events during the withdrawal period following 26 weeks and 52 weeks of treatment.
During withdrawal, the amount of days rated as “good” and “very good” declined but did not reach baseline levels recorded before start of treatment. Daytime mood, which was quite high at baseline, also decreased but was still higher (not significantly) than baseline levels after 2 weeks of withdrawal. Thus, PRM does not cause rebound insomnia; on the contrary there are signs of lasting effects of the drug after discontinuation. A number of previous studies have demonstrated that indeed some effects of PRM do not fade away as treatment is discontinued (eg, quality of sleep, daytime psychomotor function.24,29 The lasting effect of PRM, by which is meant that patients continued to have benefit beyond the treatment phase, has not been seen with other hypnotic drugs and may represent reinforcement of the sleep–wake cycle.
Based on the CHESS-84 questionnaire, during the withdrawal period, 11% of patients reported difficulty in falling asleep and was considered by the investigator to be possibly, probably, or definitely related to study medication; in 25% of those, the difficulty in falling asleep was rated as moderate, severe, or extreme. At baseline, 53% of the study patients reported in their sleep diary a score for sleep latency of ≥3 (more than 30 minutes to more than 2 hours). Combined with the diary-recorded rating of the quality of sleep, which indicated that there were no rebound effects, the difficulties of falling asleep during withdrawal probably reflects insomnia relapse in these patients.
Also, based on the CHESS-84 questionnaire, during the withdrawal period, 11% of patients reported waking, which was considered by the investigator to be possibly, probably or definitely related to study medication; in 29% of those, the difficulty in waking during the night was rated as moderate, severe, or extreme. At baseline 24% of the patients reported waking up more than three times a night. Therefore, combined with the diary-recorded rating of the quality of sleep that indicated that there were no rebound effects, the difficulties with mid-sleep awakening after withdrawal probably indicate insomnia relapse in these patients.
In addition, there was no evidence of new somatic symptoms (whether possibly, probably, or definitely related to study treatment) that were not related to sleep during withdrawal that had not been previously experienced at baseline.
During the withdrawal period following 6 months of open-label treatment, 24 patients (24%) reported somatic symptoms thought to be possibly, probably, or definitely related to study treatment, compared with 21 patients (22%) following 12 months of open-label treatment. This indicates that the use of PRM 2 mg over an additional 6 months does not increase the incidence of somatic symptoms on withdrawal of the drug. The most commonly reported somatic symptoms on PRM withdrawal were related to sleep. This is not clinically remarkable given the therapeutic area under study.
PRM has a much lower score for somatic symptoms at withdrawal compared with other drugs used for sleep promotion. The CHESS-84 withdrawal scores obtained for the discontinuation of PRM following 6 months of continuous use (1.37) were much lower than that reported for discontinuation after 8 weeks of treatment with lorazepam (7.06).26 It should be noted that patients did not receive any study medication during the withdrawal period and were aware of the fact that they had stopped the treatment drug. Therefore, the symptoms recorded on the CHESS-84 are not masked by placebo effects. These data are in line with previous observations from short- and long-term studies indicating that PRM is not associated with significant withdrawal phenomena or rebound insomnia even following prolonged periods of use.
The long-term administration of PRM did not cause suppression of endogenous melatonin production as evident by the rhythm in 6SMT in the urine. The presence of such rhythm also confirms that the 6SMT was endogenous rather than a metabolite of the residual PRM discontinued 2 weeks before. The reported observations are confirmatory and compatible with earlier reports in the literature on the lack of the suppressing effect of exogenous melatonin on its endogenous production.30–32
Traditional hypnotics (eg, benzodiazepines) have been associated with marked rebound insomnia and withdrawal symptoms leading to unwarranted intake of these drugs for long-term periods.33,34 PRM has been shown to facilitate benzodiazepine discontinuation.35 The lack of rebound and withdrawal symptoms upon discontinuation of PRM use suggests that this drug will potentially contribute to lowering of the inappropriate long-term intake of insomnia drugs.
Conclusion
It may be concluded that PRM 2 mg maintained efficacy during long-term treatment. PRM 2 mg was well tolerated, and there were no obvious differences in safety parameters between patients who had been treated for 6 months and those treated for 12 months. In addition, there appears to be no major safety concerns during PRM administration and following withdrawal of study medication up to 1 year of continuous use.
Footnotes
Disclosure
PL and DG were the primary investigators of the study sponsored by Neurim Pharmaceuticals Ltd, the manufacturers of PRM (Circadin®) for insomnia and declare no conflicts of interest. ML and TN are employees of Neurim Pharmaceuticals Ltd. NZ is the founder and Chief Scientific Officer of Neurim Pharmaceuticals Ltd.
References
- 1.American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. Washington, DC: APA; 1994. [Google Scholar]
- 2.World Health Organization (WHO) Tenth Revision of the International Classification of Diseases (ICD-10) Geneva: WHO; 1992. Mental and behavioural disorders. Clinical descriptions and diagnostic guidelines; p. 182/184. [Google Scholar]
- 3.Harvey A, Stinson K, Whitaker K, Moskovitz D, Virk H. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31:383–393. doi: 10.1093/sleep/31.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students. J Psychosom Res. 1997;42:583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 5.Pilcher JJ, Ott ES. The relationships between sleep and measures of health and well-being in college students: a repeated measures approach. Behav Med. 1998;23:170–178. doi: 10.1080/08964289809596373. [DOI] [PubMed] [Google Scholar]
- 6.Zeitlhofer J, Schmeiser-Rieder A, Tribl G, et al. Sleep and quality of life in the Austrian population. Acta Neurol Scand. 2000;102:249–257. doi: 10.1034/j.1600-0404.2000.102004249.x. [DOI] [PubMed] [Google Scholar]
- 7.Hajak G. Epidemiology of severe insomnia and its consequences in Germany. Eur Arch Psychiatry Clin Neurosci. 2001;251:49–56. doi: 10.1007/s004060170052. [DOI] [PubMed] [Google Scholar]
- 8.Rombaut N, Maillard F, Kelly F, Hindmarch I. The quality of life of insomniacs questionnaire (QOLI) Med Sci Res. 1990;18:845–847. [Google Scholar]
- 9.Ohayon M, Zulley J. Correlates of global sleep dissatisfaction in the German population. Sleep. 2001;24:780–787. [PubMed] [Google Scholar]
- 10.Buysse D, Reynolds C, III, Monk T, Berman S, Kupfer D. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll HC, Serody L, Patrick S, et al. Sleeping well, aging well: a descriptive and cross-sectional study of sleep in “successful agers” 75 and older. Am J Geriatr Psychiatry. 2008;16:74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nugent AM, Gleadhill I, McCrum E, Patterson CC, Evans A, MacMahon J. Sleep complaints and risk factors for excessive daytime sleepiness in adult males in Northern Ireland. J Sleep Res. 2001;10:69–74. doi: 10.1046/j.1365-2869.2001.00226.x. [DOI] [PubMed] [Google Scholar]
- 13.Chesson A, Hartse K, Anderson WM, et al. Practice parameters for the evaluation of chronic insomnia. Sleep. 2000;23:237–241. [PubMed] [Google Scholar]
- 14.Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs. 2009;23:19–34. doi: 10.2165/0023210-200923010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 16.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64:1174–1186. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haimov I, Laudon M, Zisapel N, et al. Sleep disorders and melatonin rhythms in elderly people. BMJ. 1994;309:167. doi: 10.1136/bmj.309.6948.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennaway DJ, Lushington K, Dawson D, Lack L, van den Heuvel C, Rogers N. Urinary 6-sulfatoxymelatonin excretion and aging: new results and a critical review of the literature. J Pineal Res. 1999;27:210–220. doi: 10.1111/j.1600-079x.1999.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 19.Leger D, Laudon M, Zisapel N. Nocturnal 6-sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapy. Am J Med. 2004;116:91–95. doi: 10.1016/j.amjmed.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 21.Wade AG, Ford I, Crawford G, et al. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010;16:51. doi: 10.1186/1741-7015-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wade AG, Crawford G, Ford I, et al. Prolonged release melatonin in the treatment of primary insomnia: evaluation of the age cut-off for short- and long-term response. Curr Med Res Opin. 2011;27:87–98. doi: 10.1185/03007995.2010.537317. [DOI] [PubMed] [Google Scholar]
- 23.Lemoine P, Nir T, Laudon M, Zisapel N. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res. 2007;16:372–380. doi: 10.1111/j.1365-2869.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 24.Luthringer R, Muzet M, Zisapel N, Staner L. The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int Clin Psychopharmacol. 2009;24:239–249. doi: 10.1097/YIC.0b013e32832e9b08. [DOI] [PubMed] [Google Scholar]
- 25.Wade A, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years. Current Med Res Opinion CMRO. 2007;23:2597–2605. doi: 10.1185/030079907X233098. [DOI] [PubMed] [Google Scholar]
- 26.Bourin M, Malinge M. Controlled comparison of the effects and abrupt discontinuation of buspirone and lorazepam. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:567–575. doi: 10.1016/0278-5846(95)00102-2. [DOI] [PubMed] [Google Scholar]
- 27.Arendt J, Bojkowski C, Franey C, Wright J, Marks V. Immunoassay of 6-hydroxymelatonin sulfate in human plasma and urine: abolition of the urinary 24-hour rhythm with atenolol. J Clin Endocrinol Metab. 1985;60:1166–1173. doi: 10.1210/jcem-60-6-1166. [DOI] [PubMed] [Google Scholar]
- 28.Garfinkel D, Laudon M, Nof D, Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet. 1995;346:541–544. doi: 10.1016/s0140-6736(95)91382-3. [DOI] [PubMed] [Google Scholar]
- 29.Lemoine P, Nir T, Laudon M, Zisapel N. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res. 2007;16:372–380. doi: 10.1111/j.1365-2869.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 30.Mallo C, Zaidan R, Faure A, Brun J, Chazot G, Claustrat B. Effects of a four-day nocturnal melatonin treatment on the 24 h plasma melatonin, cortisol and prolactin profiles in humans. Acta Endocrinol (Copenh) 1988;119:474–480. doi: 10.1530/acta.0.1190474. [DOI] [PubMed] [Google Scholar]
- 31.Lissoni P, Rovelli F, Pittalis S, et al. Therapy with melatonin does not suppress its endogenous production in healthy volunteers. Recenti Prog Med. 1999;90:84–85. [PubMed] [Google Scholar]
- 32.Matsumoto M, Sack RL, Blood ML, Lewy AJ. The amplitude of endogenous melatonin production is not affected by melatonin treatment in humans. J Pineal Res. 1997;22:42–44. doi: 10.1111/j.1600-079x.1997.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 33.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;19:1169–1176. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kales A, Bixler EO, Tan TL, Scharf MB, Kales JD. Chronic hypnotic-drug use. Ineffectiveness, drug-withdrawal insomnia, and dependence. JAMA. 1974;227:513–517. [PubMed] [Google Scholar]
- 35.Garfinkel D, Zisapel N, Wainstein J, Laudon M. Facilitation of benzodiazepine discontinuation by melatonin: a new clinical approach. Arch Intern Med. 1999;159:2456–2460. doi: 10.1001/archinte.159.20.2456. [DOI] [PubMed] [Google Scholar]