Abstract
Interactions between the multi-kinase inhibitor sorafenib and MEK1/2 inhibitors were investigated in DLBCL cells. Sorafenib (3 – 10µM) triggered apoptosis in multiple GC and ABC lymphoma cells. Unexpectedly, sorafenib did not cause sustained ERK1/2 inactivation, and in SUDHL-6 and -16 cells, triggered ERK1/2 activation. Marginally toxic MEK1/2 inhibitor concentrations (5µM PD184352) abrogated ERK1/2 activation in sorafenib-treated cells and synergistically potentiated apoptosis. MEK1 shRNA transfection also significantly increased sorafenib-mediated lethality. Sorafenib/PD184352 co-administration accelerated Mcl-1 down-regulation without upregulating BimEL. Finally, ectopic Mcl-1 expression attenuated sorafenib/PD184352-mediated apoptosis. Together, these findings provide a theoretical basis for potentiating sorafenib anti-lymphoma activity by MEK1/2 inhibitors.
Keywords: Lymphoma, sorafenib, PD184352, MEK1/2/ERK1/2, Mcl-1
INTRODUCTION
Diffuse large-B-cell lymphoma (DLBCL) is the most common aggressive form of non-Hodgkin’s lymphoma (NHL) in adults. It accounts for 30 to 40 percent of the total incidence of NHL [1]. Based upon gene expression profiling, patients with DLBCL have been subdivided into three groups: germinal-center B-cell-like DLBCL (GCB-DLBCL), activated B-cell-like DLBCL (ABC-DLBCL) and mediastinal or unclassified type [2, 3]. These sub-types vary significantly with respect to prognosis, response to chemotherapy, and dependence upon survival signaling pathways, particularly NF-κB [4–7]. In addition to the subset of patients eligible for allogeneic or autologous bone marrow transplantation, combination chemotherapy represents a potentially curative option for patients with DLBCL. However, the potential for cure of patients with DLBCL treated with cytotoxic chemotherapy (e.g., R-CHOP) varies considerably, and depends upon multiple factors, including stage of disease and genetic profile, among other factors [8–12]. In particular, patients with the ABC-DLBCL appear to have a significantly worse prognosis than other sub-types [2, 12, 13]. Such considerations have prompted the search for new and more effective agents.
The Ras/Raf/MEK1/2 (mitogen activated kinase 1/2)/ERK1/2 (extracellular signal-regulated kinase 1/2) or MAPK (mitogen-activated protein kinase) pathway is one of the most frequently dysregulated signaling cascades in cancer. Activating mutations of Ras and Raf occur frequently in both solid tumors and hematologic malignancies, leading to activation of their downstream targets MEK1/2 and ERK1/2 [14–16]. However, there is accumulating evidence that cross-talk between the MEK1/2/ERK1/2 and various other signaling pathways exists [17]. The Raf1/MEK1/2/ERK1/2 pathway is involved in multiple cellular processes, including cell proliferation, differentiation, and transformation. These findings stimulated the clinical development of small molecule inhibitors targeting specific components of the MAPK cascade, including farnesyltransferase inhibitors (e.g., tipifarnib), Raf-1 inhibitors (e.g., sorafenib), and MEK1/2 inhibitors (e.g., AZD6244) [18]. The MEK1/2/ERK1/2 pathway regulates the activity of multiple proteins involved in cell survival decisions, particularly members of the Bcl-2 family. For example, the expression and function of the anti-apoptotic multidomain Bcl-2 family protein Mcl-1 has been shown to be dependent upon an intact MEK1/2/ERK1/2 pathway in both hematopoietic and non-hematopoietic cells [19–21]. Mcl-1 plays a particularly important role in cell survival, particularly in hematopoietic cells [20]. In addition, transcriptional and post-translational regulation of Bim, a BH3-only pro-apoptotic direct-activator protein, is also mediated in part by MEK1/2/ERK1/2 signaling [22, 23].
The multi-kinase inhibitor BAY 43–9006 (sorafenib) was initially developed as a Raf-1 inhibitor, but has subsequently been shown to inhibit multiple other kinases, including FLT3, PDGFR, VEGFR1 and VEGFR2, among others [24, 25]. Sorafenib has been approved for the treatment of advanced renal cell carcinoma [26, 27] and more recently, hepatocelluar carcinoma [28, 29]. These considerations prompted us to investigate the activity of sorafenib, administered at pharmacologically relevant total concentrations of bound and free drug, in a variety of DLBCL cells, as well as other NHL cell types. Here, we report that exposure of such cells to sorafenib effectively induces mitochondrial injury and apoptosis in multiple NHL cell types. However, contrary to expectations, sorafenib did not lead to sustained ERK1/2 inactivation; instead, under some circumstances and in some cells, it triggered a time- and dose-dependent induction of ERK1/2 phosphorylation. Notably, in sorafenib-treated cells, ERK1/2 inactivation by pharmacologic MEK1/2 inhibitors such as PD184352 led to the highly synergistic induction of apoptosis, an event associated with more rapid and complete down-regulation of Mcl-1, but not Bim. Genetic approaches (e.g., shRNA MEK1 knockdown or ectopic Mcl-1 expression) confirmed the functional contribution of ERK1/2 inactivation and Mcl-1 down-regulation in synergistic interactions between these agents. Together, these studies suggest that a therapeutic strategy combining sorafenib with MEK1/2 inhibitors warrants further investigation in ex vivo assays of DLBCL.
MATERIALS AND METHODS
Cell lines
SUDHL-6 and OCI-Ly10 cells were provided by Dr. Lisa Rimsza, University of Arizona. Raji is purchased from American Type Culture Collection (Rockville, MD). SUDHL-1, L428, KM-H2, and Karpas-299 were purchased from DSMZ - German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). SUDHL-16 cells were provided by Dr. Catherine Dumur, Department of Pathology, Virginia Commonwealth University. All cells, excluding OCI-Ly10 and OCI-Ly19, were cultured in RPMI supplemented with sodium pyruvate, non-essential amino acid, L-glutamate, penicillin, streptomycin and 10% fetal bovine serum. OCI-LY10 was cultured in RPMI as above supplemented with Herpes 10mM and 0.1% 2-mercaptoethanol (2ME).
Reagents
Sorafenib (BAY 43–9006; Bayer) was provided by the Cancer Treatment and Evaluation Program, National Cancer Institute/NIH (Bethesda, MD). PD184352 was chemically synthesized and formulated as described elsewhere [30]. Doxycyline were purchased from Sigma (St. Louis, MO). Z-VAD-fmk was purchased from MP Biomedicals (Irvine, CA).
Assessment of apoptosis
The extent of apoptosis was evaluated by either annexin V–fluorescein isothiocyanate (FITC) staining (BD Pharmigen) or 7-aminoactinomycin D (7-AAD-Sigma-Aldrich) by flow cytometry as described previously [30].
Immunoblot (Western blot)
Western blot analysis was performed as previously described [30]. The following were used as primary antibodies: phospho-MEK1/2 (Ser217/221), p-ERK1/2 (Thr42/Tyr44), Cleaved caspase-3, Bcl-xL (Cell Signaling Technology, Beverly, MA); ERK1/2, (Santa Cruz Biotechnology, Santa Cruz, CA); poly(ADP-ribose) polymerase (Biomol Research Laboratories, Plymouth Meeting, PA); Mcl-1 (Pharmigen); Bim (Calbiochem) and actin (Sigma-Aldrich). The blots were stripped and reprobed with actin antibodies to ensure equal loading and transfer of proteins.
Analysis of cytosolic released proteins
Cells (2 × 106) were lysed by incubating in digitonin lysis buffer (75 mmol/L NaCl, 8 mmol/L Na2HPO4, 1 mmol/L NaH2PO4, 1 mmol/L EDTA, 350 µg/mL digitonin and 250mM sucrose) for 15 minutes at room temperature. Whole cell lysates were centrifuged 1000 × g for 10 min and the lysated were collected and further centrifuged at 16,000 × g for 45 minutes. Subsequently, the supernatants were collected, quantified, and prepared in a final concentration in 1× NuPAGE LDS sample buffer (Invitrogen) and subjected to Western blot as described above. Cytochrome-c and apoptosis-inducing factor (AIF) antibodies (Santa Cruz Biotechnology) were used as primary antibodies.
Quantitative Real-Time Polymerase Chain Reaction
After treatment, cells were lysed, and total RNA was extracted using the RNeasy mini kit (QIAGEN) according to the manufacturer's protocol. Quantitative real-time PCR analysis was carried out on the ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) using the TaqMan One-Step PCR Master Mix Reagents kit as previously described [32].
Transfection and plasmids
Knockdown experiments involving stable transfection with short hairpin RNA (shRNA) directed against MEK1 were generated as follows. Two cDNA oligonucleotides containing the targeted sequence (5’-GCTTCTATGGTGCGTTCTACA-3’) were synthesized, annealed, and cloned into the inducible pSingle-tTS-shRNA (Clonetech) vector by using standard techniques. This construct was transfected into SUDHL-6 and SUDHL-16 cells using an Amaxa nucleofector (Koeln, Germany) with the human B cell kit and programs O-017. Stable clones were selected in the presence of 500 µg/ml geneticin (Invitrogen). Clones were cultured in doxycycline 500 ng/ml for 24–48 hrs to induce shRNA-MEK1 then screened by Western blot. Clones with reduced expression of p-ERK1/2 levels compared to those of control cells were selected and used for subsequent experiments. pCEP4/Mcl-1 construct was kindly provided by Dr. Ruth Craig (Dartmouth Medical School, Hanover). For transient transfection of Mcl-1, transfected cells were immediately transferred to regular medium and exposed to the indicated agents after 24 hr.
Statistical analysis
The significance of differences between experimental conditions was determined using the 2-tailed Student t test. Characterization of synergistic and antagonistic interactions in cells exposed to a range of sorafenib and PD184352 concentrations administered at a fixed ratio was performed using Median Dose Effect analysis in conjunction with a commercially available software program (CalcuSyn; Biosoft, Ferguson, MO). Combination Index values < 1.0 denote synergistic interactions.
RESULTS
Sorafenib induces apoptosis in the absence of sustained ERK1/2 inactivation in human lymphoma cells
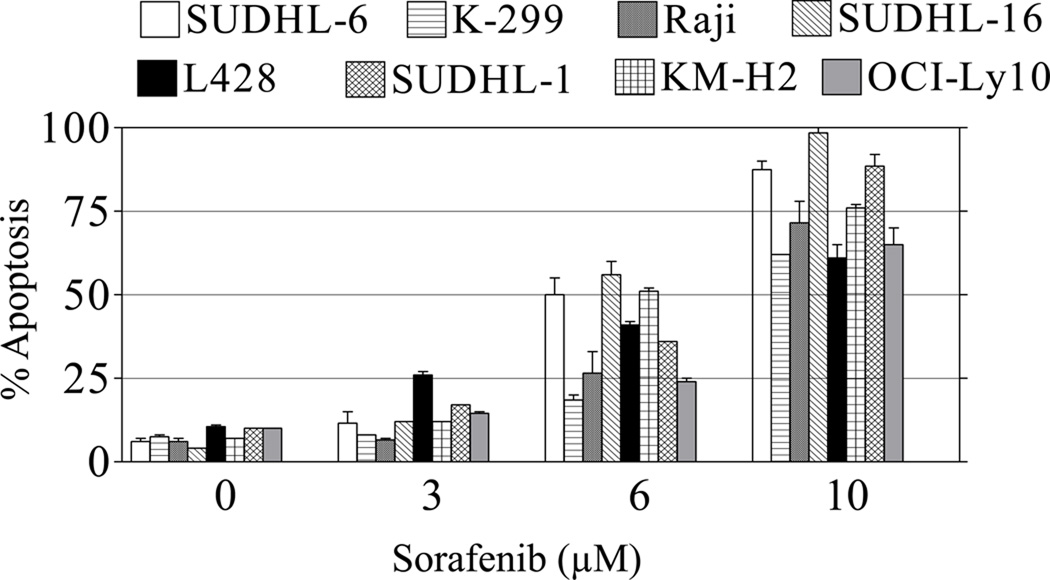
To characterize the ability of sorafenib to induce apoptosis in lymphoma cells, SUDHL-6, SUDHL-16, SUDHL-1 (GCB-DLBCL), Karpas-299 (Alk+ anaplastic large cell lymphoma), Raji (Burkitt’s B-cell lymphoma), L428, KM-H2 (Hodgkin’s lymphoma) and OCI-Ly10 (ABC- DLBCL) were exposed to sorafenib concentrations ranging from 3µM to 10 µM for 48 hr, and apoptosis monitored by 7-AAD staining. These concentrations of total drug (bound and free) have been previously shown to be pharmacologically achievable in the plasma of humans following oral administration of sorafenib [31]. As shown in Figure 1, while some variability was observed, all lymphomas were sensitive to sorafenib, particularly at the highest concentration (10 µM). Virtually identical results were obtained when apoptosis was monitored by annexin V/PI staining (data not shown).
Figure 1. Sorafenib induces apoptosis in lymphoma cells.
SUDHL-6, Karpas-299, Raji, SUDHL-16, L428, DHL-1, KM-H2, OCI-Ly10 cells were exposed to 3, 6 or 10 µM sorafenib (Sor) for 48 hr, after which viability was determined by flow cytometry and 7-AAD uptake as described in Materials and Methods. Values represent the means ± S.D. for triplicate determinations repeated on at least 3 occasions.
Previous reports from our laboratory and others indicated that sorafenib induced apoptosis in human leukemia cells (U937) primarily through down-regulation of Mcl-1, and that Raf/MEK/ERK inhibition did not contribute to this process [32]. Consequently, the effects of sorafenib on ERK1/2 activation in lymphoma cells were investigated. In SUDHL-16 cells, exposure (20 hr) to sorafenib concentrations of 2.5–7.5 µM failed to inactivate ERK1/2, as we previously observed in the case of U937 human leukemia cells [32]; instead, clear increases in expression of phospho-ERK were observed at concentration up to 7.5 µM (Fig 2A). This increase in ERK phosphorylation appeared to be bi-phasic since at higher sorafenib concentrations (e.g., 10 µM) ERK1/2 phosphorylation was similar to basal levels in untreated cells. Time course analysis of cells exposed to 7.5 µM sorafenib revealed that the increase in ERK1/2 activation occurred after 16 hr of drug exposure and persisted over the ensuing 16 hr (Fig 2A). Similarly, in SUDHL-6 cells, sorafenib concentrations ≥ 2.5 µM increased levels of phospho-ERK1/2 at early intervals, a phenomenon that was sustained over 32 hr (Fig 2B). In separate studies, exposure of other DLBCL cells (e.g., OCI-Ly10) to sorafenib also failed to inactivate ERK1/2, although in these cells, sorafenib-mediated ERK1/2 activation was either not apparent or significantly less pronounced than that observed in SUDHL-6 and -16 cells (Supplement 1A). Together, these findings indicate that exposure of human cells of DLBCL to sorafenib at concentrations that induce apoptosis is not accompanied by sustained ERK1/2 inactivation, as previously observed in the case of human leukemia cells (U937); instead, in these cells, sorafenib exposure may under some circumstances induce ERK1/2 phosphorylation.
Figure 2. Sorafenib fails to inhibit ERK1/2 phosphorylation in DLBCL cells.
(A) SUDHL-16 and (B) SUDHL-6 cells were exposed to the indicated concentrations of sorafenib for 20 hr (left panels) or exposed to 7.5 µM sorafenib for the indicated intervals. The cells were then lysed and subjected to WB analysis to monitor p-ERK1/2 (Thr42/Tyr44) expression as described in Materials and Methods. (C) SUDHL-16 and SUDHL-6 cells were exposed (8 hr) to either 5 µM PD184352 (PD), 5 µM of sorafenib, or the combination, after which proteins were prepared and subjected to WB as described above to monitor p-ERK1/2 expression. For (A), (B), and (C), lanes were loaded with 25 µg of protein; blots were then stripped and re-probed with tubulin (Tub) to ensure equivalent loading and transfer. Representative results are shown; two additional studies yielded equivalent results.
MEK1/2 inhibitors inactivate ERK1/2 in untreated and sorafenib-treated DLBCL cells
Parallel studies were then performed to assess the effects of MEK1/2 inhibition on ERK1/2 activation in sorafenib-treated DLBCL cells. Exposure to 5.0 µM sorafenib (8 hr) induced ERK1/2 activation in SUDHL-16 and in SUDHL-6 cells (Fig 2C). However, co-administration of the MEK1/2 inhibitor PD184352 essentially abrogated expression of phospho-ERK1/2 in both untreated and sorafenib-treated SUDHL-16 and -6 cells. Similar findings were observed in other DLBCL lines, including both ABC (OCI-Ly10, OCI-Ly19) and GC sub-types whether or not sorafenib induced ERK1/2 activation (Supplement 1B). These findings indicate that in DLBCL cells, MEK1/2 inhibitors essentially abrogate basal ERK1/2 activation as well as sorafenib-mediated ERK1/2 activation.
PD184352 synergistically enhances the lethality of sorafenib in lymphoma cells
The impact of inhibiting ERK1/2 activation (e.g., by PD184352) on the response of DLBCL cells to sorafenib was then examined. Individual exposure (24 hr) of SUDHL-16 or SUDHL-6 cells to 5 µM PD184352 or 5 µM sorafenib only minimally induced apoptosis (Figure 3A). However, combined treatment resulted in a pronounced increase in apoptosis (e.g., to 65–70 % of cells). Median Dose Effect analysis of apoptosis induction, in which SUDHL-6 and SUDHL-16 cells were exposed to a range of sorafenib and PD184352 concentrations, alone and in combination, at a fixed concentration ratio (1:1), yielded combination index (CI) values significantly less than 1.0, indicating a highly synergistic interaction (Figure 3B). Comparable interactions were observed with another MEK1/2 inhibitor, U0126 (data not shown).
Figure 3. PD184352 synergistically enhances the lethality of sorafenib in lymphomas.
(A) SUDHL-16 and SUDHL-6 cells were exposed to 5 µM PD and 5 µM sorafenib alone or in combination for 24 hr, after which the percentage of apoptotic cells was determined by 7-AAD analysis and flow cytometry as described in Materials and Methods.
(B) SUDHL-16 (left) or SUDHL-6 (right) cells were exposed to varying concentrations of PD and sorafenib for 24 hr administered at a fixed ratio (1:1), after which combination index (CI) values were determined in relation to the Fractional Effect (FA) using CalcuSyn software. Combination index values less than 1.0 correspond to synergistic interactions.
(C) Left panel: SUDHL-16 cells were exposed to the designated concentration of sorafenib alone or in conjunction with 5 µM of PD for 24 hr, after which the percentage of apoptotic cells was determined by annexin V/PI analysis as described in Materials and Methods. Center panel: SUDHL-16 cells were exposed to the indicated concentrations of PD alone or in combination with 5 µM sorafenib (24 hr), after which apoptosis was determined as described above. Right panel: Cells were treated with PD (5 µM) or sorafenib (5 µM) individually or in combination for the indicated intervals, after which the extent of cell death was determined. Values represent the means ± S.D. for triplicate determinations repeated on at least 3 occasions.
(D) OCI-Ly10, OCI-Ly19 (ABC-DLBCL) cells, Karpas-299 (anaplastic large cell lymphoma) cells, and KM-H2 (Hodgkin’s lymphoma) cells were exposed to PD184352 (5 µM) and sorafenib (7 µM) alone or in combination for 48 hr. Raji cells were exposed to the 5 µM PD184352 ± sorafenib (10 µM) alone or in combination for 24 hr. At the end of the incubation periods, the percentage of apoptotic cells was determined by flow cytometry and 7-AAD uptake. Values represent the means ± SD for duplicate determinations performed on 3 separate occasions.
Dose-response analysis revealed that 5 µM PD184352 significantly increased the lethality of sorafenib in SUDHL-16 cells at concentration as low as 2.5 µM, whereas concentrations of PD184352 as low as 2.5 µM significantly increased the lethality of 5 µM sorafenib (Figure 3C). Time-course analysis in SUDHL-16 cells demonstrated that simultaneous exposure to 5 µM sorafenib and 5 µM PD184352 resulted in relatively small increases in apoptosis at 8 hours (~ 25% apoptosis), but that cell death increased substantially at 16 hr, and approached 65–70% at the 24 hr interval (Figure 3C-right).
Finally, similar synergistic interactions were observed when sorafenib and PD184352 were observed in multiple other malignant B-cell lines including OCI-Ly10, OCI-Ly19, Raji, Karpas-299 and KM-H2 cells (Figure 3D). These findings indicate that non- or minimally toxic concentrations of MEK1/2 inhibitors markedly potentiate the lethality of marginally toxic concentrations of sorafenib in human lymphoma cells.
PD184352 enhances sorafenib-mediated mitochondrial damage and caspase activation
The effects of sorafenib and PD184352 alone and in combination were then examined in relation to mitochondrial injury and activation of the caspase cascade in SUDHL-16 and SUDHL-6 cells. Exposure of cells to sorafenib or PD184352 individually for 8 hr had little or relatively modest effects on release of cytochrome-c and AIF into the cytosol. However, combined treatment increased AIF and cytochrome-c cytsolic release (Figure 4A). Consistent with these findings, treatment with PD184352 or sorafenib alone had either no or minimal effects on cleavage of caspase-3, caspase-9, or poly(ADP-ribose) polymerase (PARP), whereas combined treatment resulted in a pronounced increase in caspase-3 and -9 activation, and PARP degradation (Figure 4A). These findings indicate that MEK1/2 inhibition potentiates the ability of sorafenib to trigger mitochondrial injury and caspase activation in DLBCL cells.
Figure 4. Combined exposure of DLBCL cells to PD184352 and sorafenib results in a marked increase in mitochondrial injury, caspase activation, and Mcl-1 down-regulation.
(A) SUDHL-16 and SUDHL-6 cells were exposed to 5 µM sorafenib and 5 µM PD alone or in combination for 8 hr, after which mitochondria-free cytosolic fractions (S-100) were obtained and subjected to Western blot analysis to monitor release of cytochrome c and apoptosis inducing factor (AIF). Alternatively, whole-cell lysates were obtained and Western blot analysis employed to monitor caspase cleavage/activation and c-PARP degradation.
(B) SUDHL-16 and SUDHL-6 cells were exposed to PD184352 and sorafenib alone or in combination as described in (A) for 8 hr, after which protein were prepared and subjected to WB using the indicated primary antibodies.
(C) SUDHL-16 cells were exposed to 5 µM sorafenib ± 5 µM PD184352 for the indicated intervals, after which cells were lysed and expression of Mcl-1 was monitored by Western blot analysis as described above.
(D) SUDHL-16 cells were pre-treated with 20 µmol/L Z-VAD-fmk for 2 hr then treated with 5 µM sorafenib ± 5 µM PD184352 for 8 hr after which the cells were lysed and subjected to WB to monitor expression of Mcl-1 and the cleavage product of caspase-3. For these studies, lanes were loaded with 25 µg of protein; blots were stripped and re-probed with tubulin to ensure equivalent loading and transfer. Representative results are shown; two additional studies yielded equivalent results.
Co-administration of PD184352 and sorafenib results in more rapid and complete down-regulation of Mcl-1 in DLBCL cells
The effects of co-exposure of SUDHL-6 and SUDHL-16 cells to sorafenib and/or PD (5 µM each) were then examined in relation to effects on several survival signal proteins at an exposure interval (8 hr) prior to the extensive induction of cell death. As shown in figure 4B, individual or combined treatment did not induce discernible changes in the expression of Bcl-2, Bcl-xL or total levels of EKR1/2. Expression of p-AKT also did not change with drug treatment (data not shown). In addition, despite evidence that ERK1/2 activation status regulates the abundance of the pro-apoptotic BH3-only protein Bim [22, 23], neither individual nor combined treatment significantly altered expression of any of the Bim isoforms (Bim-EL, Bim-S, or Bim-L). In contrast, cells exposed to 5 µmol/L sorafenib or PD individually displayed only modest or no changes in Mcl-1 expression in both SUDHL-6 and SUDHL-16, cells. However, combined treatment resulted in a pronounced reduction in Mcl-1 expression (Figure 4B). Time course analysis of Mcl-1 expression was then monitored in SUDHL-16 cells. As shown in Fig 4C, exposure of cells to sorafenib alone (5 µM) had little effect on Mcl-1 expression at 6 hr, although at 16 hr down-regulation was clear but incomplete. PD184352 by itself had no discernible effect. However, the decline in expression of Mcl-1 was extensive in cells exposed to sorafenib/PD184352 as early as 2 hr after the addition of these agents and completely abrogated at 16 hr. Next, Mcl-1 mRNA was quantified using real-time PCR. Treatment with sorafenib or PD184352 alone resulted in a modest decline or unchange in Mcl-1 mRNA levels, however co-treatment of sorafenib/PD184352 results further decrease of Mcl-1 mRNA both at 8 h and 16 h time points (P < 0.01). This suggests that down-regulation of Mcl-1 in the combination partially attribute to transcription inhibition of Mcl-1 (Supplement Figure 2). To determine if Mcl-1 down-regulation represented a primary or secondary event (e.g., resulting from caspase cleavage), parallel studies were performed in the presence or absence of the pan-caspase inhibitor, Z-VAD-fmk (20 µmol/L) administered 2 hr prior to treatment with sorafenib/PD184352. Down-regulation of Mcl-1 in sorafenib/PD184352-treated cells was unchanged in the presence of Z-VAD-fmk despite clear inhibition of caspase-3 cleavage (Figure 4D). Similar results were obtained in SUDHL-6 and OCI-Ly19 cells (Supplement Figure 1B and data not shown). Together, these findings indicate that co-exposure of DLBCL cells to sorafenib in conjunction with PD184352 is associated with marked inactivation of ERK1/2, accompanied by the rapid and pronounced down-regulation of Mcl-1 through a caspase-independent mechanism.
Knockdown of MEK1 sensitizes lymphoma cells to sorafenib-induced apoptosis
To investigate the functional contribution of MEK1/2/ERK1/2 interruption to sorafenib-induced apoptosis, SUDHL-16 cells were stably transfected with an inducible shMEK1/pSingle-tTS plasmid. A SUDHL-16 clone expressing diminished phospho-ERK1/2 expression following doxycycline (Dox) treatment was obtained, and designated SUDHL16/cl7 (Figure 5A). In the presence of Dox, DHL16/cl7 cells displayed clearly diminished activation of ERK1/2 in response to 10 µM sorafenib (upper panel). Moreover, these cells were significantly more sensitive to sorafenib-mediated lethality compared to cells exposed to sorafenib in the absence of Dox (P < 0.05; Figure 5A, lower panel). Similar results were obtained in two SUDHL-6 clones stably transfected with a Dox-inducible MEK1 shRNA expression vector (SUDHL-6/cl6 and /cl12), which displayed diminished ERK activation in the presence of Dox (Figure 5B, upper panel). Both SUDHL-6/cl6 and /cl12 cells were significantly more sensitive to sorafenib-induced apoptosis in the presence of Dox than in its absence (P < 0.05). These findings argue that ERK1/2 inactivation plays a significant functional role in the potentiation of sorafenib lethality.
Figure 5. Inhibition of MEK/ERK1/2 sensitizes SUDHL-16 and SUDHL-6 cells to sorafenib-induced apoptosis.
(A) SUDHL-16 cells were stably transfected with inducible shMEK1/pSingle-tTS vector under the control of doxycycline (clone 7; cl7). After culturing for 24 hr in the presence of Dox, cl7 cells were exposed ± 7.5 µM sorafenib for 24 hr, after which cells were lysed and expression of phospho-ERK1/2 determined by Western blot analysis (upper panel). In parallel, sorafenib-induced cell death was determined by 7-AAD in the presence or absence of Dox as described previously (lower panel). (B) Two inducible SUDHL-6 knockdown clones (cl6 and cl12) expressing diminished levels of phospho-ERK1/2 in the presence of Dox were generated were generated (upper inset). These cells were cultured in the presence or absence of Dox for 24 hr and then treated with 7.5 µM sorafenib for 24 h. Cell death was then determined by 7-AAD. All values represent the means ± SD for duplicate determinations performed on 3 separate occasions. * = significantly greater than values obtained for cells cultured in the absence of Dox; P <0.05).
Mcl-1 down-regulation plays a functional role in the lethality of the sorafenib/PD184352 regimen
As described previously, the decline in expression of Mcl-1 was more rapid and extensive in cells exposed to sorafenib/PD184352 compared to cells exposed to the agents individually (Figure 3C). To characterize the functional significance of Mcl-1 down-regulation in sorafenib/PD184352-mediated lethality, SUDHL-16 cells were transiently transfected with pCEP/Mcl-1 (Figure 6, left panel). Such cells displayed approximately 1.7-fold increase in Mcl-1 protein levels compared to cells transfected with an empty-vector control (pCEP). Notably cells ectopically expressing Mcl-1 were significantly less susceptible to sorafenib/PD184352-mediated apoptosis compared to controls (Figure 6, right panel; P < 0.05). This finding supports the notion that Mcl-1 down-regulation plays a significant functional role in the lethality of the sorafenib/PD184352 regimen.
Figure 6. Mcl-1 plays a functional role in sorafenib/PD184352-mediated lethality.
SUDHL-16 cells were transiently transfected with empty vector (pCEP4) or a Mcl-1 construct (pCEP4/Mcl-1) as described in Materials and Methods. Twenty-four hours after transfection, Western blot analysis was performed and expression of Mcl-1 quantified (left panel). The value shown corresponds to the densitometric ratios of cells transfected with the Mcl-1 construct and the empty control, normalized to tubulin. Right panel: Empty vector and Mcl-1 cells were exposed to 5 µM PD184352 + 7.5 µM sorafenib for 20 hr, after which the percentage of apoptotic cells was determined by flow cytometry and 7-AAD uptake. Values represent the means ± SD for duplicate determinations performed on 3 separate occasions * = significantly less than values for cells transfected with empty-vector pCEP4 controls (P <0.05).
Discussion
The development of the Raf-1 and multi-kinase inhibitor sorafenib was prompted by evidence of deregulation of the Ras/Raf/MEK1/2/ERK1/2 pathway in diverse transformed cells, including those of hematopoietic origin [18]. The results of the present study indicate that sorafenib, administered at pharmacologically relevant concentrations [31], induces apoptosis in both ABC- and GC-type DLBCL cells, at least in vitro, and that this phenomenon is dramatically increased by pharmacologic MEK1/2 inhibitors. In addition to its effects on Raf-1, sorafenib inhibits multiple other protein kinases including Raf-1, PDGFR, VEGF receptor kinases, FLT-3 and c-Kit, among others, which could contribute to lethality independently of effects on the Raf-1 cascade [24, 25]. In fact, evidence indicates that at least in certain malignant hematopoietic cells (e.g., leukemia), sorafenib induces cell death independently of Raf/MEK/ERK inhibition [32–34]. Instead, sorafenib-mediated lethality toward human leukemia cells has been related to induction of ER stress, and downregulation of the anti-apoptotic protein Mcl-1 as a consequence of inhibition of translation [32, 33]. It is important to note that in human leukemia cells (U937), administration of sorafenib at concentrations that induced ER stress effectively inactivated ERK1/2 [32, 33]. These actions stand in contrast to actions in DLBCL cells, in which sustained sorafenib-mediated ERK1/2 inactivation was not observed, and in fact in some cases, ERK1/2 activation was induced. Such findings indicate that the effects of sorafenib on ERK1/2 activation are likely to be both cell type- and context-specific. Efforts to characterize the effects of sorafenib on the ER stress machinery in DLBCL cells, and their impact on ERK1/2 activation status and lethality, are currently in progress.
Aberrant activation of the Raf/MEK1/2/ERK1/2 pathway contributes to tumorigenesis through multiple mechanisms [35–38]. In addition, ERK1/2 activation, e.g., by growth factors, promotes cell survival through a variety of means, including induction of anti-apoptotic proteins, down-regulation of pro-apoptotic proteins, and inhibition of caspases, among others [15]. In addition to its contribution to transformation, activation of the Raf/MEK1/2/ERK1/2 pathway represents a general mechanism by which cells respond to environmental insults to maintain their integrity. For example, it has been shown that exposure of human leukemia or multiple myeloma cells to Chk1 inhibitors results in MEK1/2/ERK1/2 activation, and as observed in the present study, co-administration of MEK1/2 inhibitors dramatically increases lethality [39, 40]. In the case of sorafenib, it was tempting to speculate that simultaneous inhibition of Raf-1 and MEK1/2 would lead to a more complete, linear blockade of the Raf/MEK1/2/ERK1/2 cascade, thus accounting for enhanced lethality. However, the finding that sorafenib did not result in sustained ERK1/2 inactivation in DLBCL cells, and in some cases triggered ERK1/2 activation, argues against this view, and suggest that alternative mechanisms are operative. One plausible explanation is that, in this setting, maintenance or induction of ERK1/2 activation represents a cytoprotective response to sorafenib-mediated cellular insults, and that disabling this pathway lowers the threshold for sorafenib-related lethality.
The decision of a cell to undergo apoptosis or to survive following environmental stresses (eg, growth factor deprivation or exposure to cytotoxic agents) is largely determined by interactions between pro-apoptotic and anti-apoptotic members of the Bcl-2 family, which include multidomain members (BH1–4) that either mediate (e.g., Bax and Bak) or prevent (e.g., Bcl-2, Bcl-xL, Mcl-1) apoptosis [41–44]. In contrast, BH3-only members (Bim, Bad, Bid, Noxa, and Puma) are exclusively pro-apoptotic [41–44]. Up regulation of Mcl-1 has been shown to play a particularly important role in cell survival in leukemias [45, 46] and in certain forms of large cell lymphoma [47, 48]. It is therefore significant that synergistic interactions between sorafenib and PD184352 were associated with more rapid and pronounced inhibition of Mcl-1. Arguments for a functional role for Mcl-1 were reinforced by the finding that ectopic Mcl-1 expression significantly protected DLBCL cells from sorafenib/MEK1/2 inhibitor lethality. In this context, the MEK1/2/ERK1/2 pathway is known to regulate Mcl-1 stability through a phosphorylation-related mechanism, possibly reflecting diminished association with the pro-apoptotic protein Bim [22, 49, 50]. Mcl-1 is known to be regulated at the transcriptional level by a variety of transcription factors, including STATs, E2F1, CREB [51–53]. Thus, enhanced Mcl-1 down-regulation in sorafenib/MEK1/2 inhibitor treated cells may represent the consequence of cooperative actions e.g., inhibition of translation (i.e., by sorafenib) in conjunction with inhibition of Mcl-1 mRNA.
The findings that shRNA-mediated knockdown of MEK1 significantly potentiated sorafenib lethality in DLBCL cells, and that enforced expression of constitutively active MEK1 significantly protected cells from sorafenib/PD184352-mediated lethality, argues that enhanced ERK1/2 inactivation plays an important functional role in potentiation of cell death with this combination. ERK1/2 cytoprotective activity may involve multiple factors including, as noted above, enhanced Mcl-1 stability, diminished caspase-9 activation, activation of certain transcription factors (e.g. ELK1 and Ets) and inactivation of sensitizer proteins (e.g., Bad) [14, 15]. In addition, ERK1/2 activation results in phosphorylation and subsequent ubiquitination and proteasomal degradation of the pro-apoptotic direct activator protein Bim [22, 23]. Therefore, it seemed plausible that combined Raf-1 and MEK1/2 inhibition might lead to diminished Bim phosphorylation and resulting protection from degradation. However, DLBCL cells exposed to sorafenib ± PD184352 did not exhibit changes in expression of any of the Bim isoforms. While such findings do not rule out a contribution of Bim to sorafenib/PD184352-mediated lethality in DLBCL cells, they do argue against a primary role for Bim upregulation in synergistic interactions, at least in DLBCL cells.
Molecular profiling analysis has revealed that patients with the ABC-DLBCL sub-type have 5-year survival rates of approximately 30%, in contrast to patients with the GC- or PM-DLBCL sub-types, who have survival rates of approximately twice this figure [2, 12]. In contrast to GC-DLBCL, ABC-DLBCL and PM-DLBCL exhibit constitutive activation of NF-κB, and are more dependent on activation of this pathway for survival than the GC-DLBCL sub-type [5–7, 13]. Results of a very recent study indicate that patients with ABC-DLBCL are more likely to benefit from addition of the proteasome inhibitor bortezomib to chemotherapy than patients with GC-DLBCL [54]. It is noteworthy that OCI-Ly10 (ABC-DLBCL) cells were as sensitive to the sorafenib/MEK1/2 inhibitor regimen as their SUDHL-6 and -16 counterparts, which correspond to the GC-DLBCL sub-type. This could reflect induction of cell death through an NF-κB-independent process, or alternatively, circumvention of NF-κB cytoprotective effects e.g., through activation of downstream processes. Whatever the explanation for the equivalent lethality, these findings raise the possibility that a strategy combining sorafenib with MEK1/2 inhibitors may be effective in poor-prognosis DLBCL sub-types.
In summary, the present findings indicate that constitutive or stimulated MEK/ERK1/2 activation plays an important functional role of protecting DLBCL cells, including both the GC and ABC sub-types, from the multi-kinase inhibitor sorafenib. They also demonstrate that interruption of this pathway synergistically enhances sorafenib lethality in diverse NHL subtypes. Importantly, the present results suggest that in DLBCL cells, this interaction involves factors other than the linear inhibition of the Raf/MEK1/2/ERK1/2 cascade, but may instead reflect abrogation of cytoprotective ERK1/2 signaling in sorafenib-treated cells. Finally, these findings suggest that down-regulation of Mcl-1, rather than Bim up-regulation, plays an important functional role in this interaction. Collectively, these observations suggest that a strategy combining sorafenib with MEK1/2 inhibitors warrants further attention in DLBCL and potentially other lymphoid malignancies.
Supplementary Material
A. OCI-Ly10 cells were exposed to the indicated concentrations of sorafenib for 16 hr. The cells were then lysed and subjected to WB analysis to monitor p-ERK1/2 (Thr42/Tyr44) expression as described in Materials and Methods.
B. OCI-Ly19 cells were exposed to sorafenib (5 or 7 µM) ± 5 µM PD184352 for 20h, after which cells were lysed and expression of cleavage of PARP, caspase 3, p-ERK1/2, Mcl-1 was monitored by Western blot analysis as described above.
SUDHL-16 cells were exposed to 5 µM sorafenib ± 5 µM PD184352 for 8 and 16 h after which total RNAs were extacted and expression of Mcl-1 mRNA was monitored by real time RT-PCR as described in Material and Method. Values of each condition are expressed as the percentage of specific Mcl-1/18S mRNAs normalized untreated control cells (100%).
Abbreviations
- MEK1/2
mitogen-activated protein kinase kinase 1/2
- ERK1/2
extracellular signal- regulated kinase 1/2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coiffier B. Diffuse large cell lymphoma. Curr Opin Oncol. 2001;13(5):325–334. doi: 10.1097/00001622-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 3.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89(11):3909–3918. [PubMed] [Google Scholar]
- 4.Feuerhake F, Kutok JL, Monti S, Chen W, LaCasce AS, Cattoretti G, Kurtin P, Pinkus GS, de Leval L, Harris NL, Savage KJ, Neuberg D, Habermann TM, Dalla-Favera R, Golub TR, Aster JC, Shipp MA. NFkappaB activity, function, and target-gene signatures in primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma subtypes. Blood. 2005;106(4):1392–1399. doi: 10.1182/blood-2004-12-4901. [DOI] [PubMed] [Google Scholar]
- 5.Lam LT, Davis RE, Pierce J, Hepperle M, Xu Y, Hottelet M, Nong Y, Wen D, Adams J, Dang L, Staudt LM. Small molecule inhibitors of IkappaB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res. 2005;11(1):28–40. [PubMed] [Google Scholar]
- 6.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194(12):1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage KJ, Monti S, Kutok JL, Cattoretti G, Neuberg D, De Leval L, Kurtin P, Dal Cin P, Ladd C, Feuerhake F, Aguiar RC, Li S, Salles G, Berger F, Jing W, Pinkus GS, Habermann T, Dalla-Favera R, Harris NL, Aster JC, Golub TR, Shipp MA. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102(12):3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 8.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, Christian B, Lepage E, Tilly H, Morschhauser F, Gaulard P, Salles G, Bosly A, Gisselbrecht C, Reyes F, Coiffier B. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23(18):4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 9.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 10.Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005;23(26):6387–6393. doi: 10.1200/JCO.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Coiffier B. Rituximab and CHOP-like chemotherapy in good-prognosis diffuse large-B-cell lymphoma. Nat Clin Pract Oncol. 2006;3(11):594–595. doi: 10.1038/ncponc0638. [DOI] [PubMed] [Google Scholar]
- 12.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L, Wilson WH, Jaffe ES, Simon R, Klausner RD, Powell J, Duffey PL, Longo DL, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Montserrat E, Lopez-Guillermo A, Grogan TM, Miller TP, LeBlanc M, Ott G, Kvaloy S, Delabie J, Holte H, Krajci P, Stokke T, Staudt LM. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 13.Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, LeBlanc M, Chiorazzi M, Zhao H, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Staudt LM. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101(12):4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 15.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 16.Rapp UR, Gotz R, Albert S. BuCy RAFs drive cells into MEK addiction. Cancer Cell. 2006;9(1):9–12. doi: 10.1016/j.ccr.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 18.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4(12):937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 19.Huang HM, Huang CJ, Yen JJ. Mcl-1 is a common target of stem cell factor and interleukin-5 for apoptosis prevention activity via MEK/MAPK and PI-3K/Akt pathways. Blood. 2000;96(5):1764–1771. [PubMed] [Google Scholar]
- 20.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307(5712):1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 21.Leu CM, Chang C, Hu C. Epidermal growth factor (EGF) suppresses staurosporine-induced apoptosis by inducing mcl-1 via the mitogen-activated protein kinase pathway. Oncogene. 2000;19(13):1665–1675. doi: 10.1038/sj.onc.1203452. [DOI] [PubMed] [Google Scholar]
- 22.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. Embo J. 2007;26(12):2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci U S A. 2004;101(43):15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 26.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, Sridhara R, Garvey P, Justice R, Pazdur R. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12(24):7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 27.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 28.Simpson D, Keating GM. Sorafenib: in hepatocellular carcinoma. Drugs. 2008;68(2):251–258. doi: 10.2165/00003495-200868020-00007. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TK, Rahmani M, Harada H, Dent P, Grant S. MEK1/2 inhibitors sensitize Bcr/Abl+ human leukemia cells to the dual Abl/Src inhibitor BMS-354/825. Blood. 2007;109(9):4006–4015. doi: 10.1182/blood-2006-09-045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23(5):965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 32.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43–9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280(42):35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 33.Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, Grant S. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol Cell Biol. 2007;27(15):5499–5513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, Adjei AA. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43–9006. Oncogene. 2005;24(46):6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418(6901):934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 36.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18(3):813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 37.Pollock PM, Meltzer PS. A genome-based strategy uncovers frequent BRAF mutations in melanoma. Cancer Cell. 2002;2(1):5–7. doi: 10.1016/s1535-6108(02)00089-2. [DOI] [PubMed] [Google Scholar]
- 38.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 39.Dai Y, Yu C, Singh V, Tang L, Wang Z, McInistry R, Dent P, Grant S. Pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) kinase/MAPK cascade interact synergistically with UCN-01 to induce mitochondrial dysfunction and apoptosis in human leukemia cells. Cancer Res. 2001;61(13):5106–5115. [PubMed] [Google Scholar]
- 40.Dai Y, Landowski TH, Rosen ST, Dent P, Grant S. Combined treatment with the checkpoint abrogator UCN-01 and MEK1/2 inhibitors potently induces apoptosis in drug-sensitive and -resistant myeloma cells through an IL-6-independent mechanism. Blood. 2002;100(9):3333–3343. doi: 10.1182/blood-2002-03-0940. [DOI] [PubMed] [Google Scholar]
- 41.Willis S, Day CL, Hinds MG, Huang DC. The Bcl-2-regulated apoptotic pathway. J Cell Sci. 2003;116(Pt 20):4053–4056. doi: 10.1242/jcs.00754. [DOI] [PubMed] [Google Scholar]
- 42.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67(7):2908–2911. doi: 10.1158/0008-5472.CAN-07-0082. [DOI] [PubMed] [Google Scholar]
- 44.van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16(2):203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 45.Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S, Wang HG, Zhang X, Bullrich F, Croce CM, Rai K, Hines J, Reed JC. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998;91(9):3379–3389. [PubMed] [Google Scholar]
- 46.Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, Reed JC. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood. 1998;91(3):991–1000. [PubMed] [Google Scholar]
- 47.Michels J, O'Neill JW, Dallman CL, Mouzakiti A, Habens F, Brimmell M, Zhang KY, Craig RW, Marcusson EG, Johnson PW, Packham G. Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. Oncogene. 2004;23(28):4818–4827. doi: 10.1038/sj.onc.1207648. [DOI] [PubMed] [Google Scholar]
- 48.Rust R, Harms G, Blokzijl T, Boot M, Diepstra A, Kluiver J, Visser L, Peh SC, Lim M, Kamps WA, Poppema S, van den Berg A. High expression of Mcl-1 in ALK positive and negative anaplastic large cell lymphoma. J Clin Pathol. 2005;58(5):520–524. doi: 10.1136/jcp.2004.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Domina AM, Smith JH, Craig RW. Myeloid cell leukemia 1 is phosphorylated through two distinct pathways, one associated with extracellular signal-regulated kinase activation and the other with G2/M accumulation or protein phosphatase 1/2A inhibition. J Biol Chem. 2000;275(28):21688–21694. doi: 10.1074/jbc.M000915200. [DOI] [PubMed] [Google Scholar]
- 50.Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem. 2000;79(3):355–369. [PubMed] [Google Scholar]
- 51.Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19(9):6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Croxton R, Ma Y, Song L, Haura EB, Cress WD. Direct repression of the Mcl-1 promoter by E2F1. Oncogene. 2002;21(9):1359–1369. doi: 10.1038/sj.onc.1205157. [DOI] [PubMed] [Google Scholar]
- 53.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8(4):945–954. [PubMed] [Google Scholar]
- 54.Dunleavy K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, Shovlin M, Jaffe ES, Janik JE, Staudt LM, Wilson WH. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113(24):6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. OCI-Ly10 cells were exposed to the indicated concentrations of sorafenib for 16 hr. The cells were then lysed and subjected to WB analysis to monitor p-ERK1/2 (Thr42/Tyr44) expression as described in Materials and Methods.
B. OCI-Ly19 cells were exposed to sorafenib (5 or 7 µM) ± 5 µM PD184352 for 20h, after which cells were lysed and expression of cleavage of PARP, caspase 3, p-ERK1/2, Mcl-1 was monitored by Western blot analysis as described above.
SUDHL-16 cells were exposed to 5 µM sorafenib ± 5 µM PD184352 for 8 and 16 h after which total RNAs were extacted and expression of Mcl-1 mRNA was monitored by real time RT-PCR as described in Material and Method. Values of each condition are expressed as the percentage of specific Mcl-1/18S mRNAs normalized untreated control cells (100%).