Abstract
Adhesion of epithelial cells to basement membranes (BM) occurs through 2 major structures: actin-associated focal contacts and keratin-associated hemidesmosomes, both of which form on laminin-332 (Ln-332). In epithelial-derived cancer cells, additional actin-linked structures with putative adhesive properties, invadopodia, are frequently present and mediate BM degradation. A recent study proposed that BM invasion requires a proper combination of focal contacts and invadopodia for invading cells to gain traction through degraded BM, and suggested that these structures may compete for common molecular components such as Src kinase. In this study, we tested the role of the Ln-332 in regulating invadopodia in 804G rat bladder carcinoma cells, a cell line that secretes Ln-332 and forms all 3 types of adhesions. Expression of shRNA to Ln-332 γ2 chain (γ2-kd) led to increased numbers of invadopodia and enhanced extracellular matrix degradation. Replating γ2-kd cells on Ln-332 or collagen-I fully recovered cell spreading and inhibition of invadopodia. Inhibition of α3 or β1, but not α6 or β4, phenocopied the effect of γ2-kd, suggesting that α3β1-mediated focal contacts, rather than a6β4-mediated hemidesmosome pathways, intersect with invadopodia regulation. γ2-kd cells exhibited alterations in focal contact-type structures and in activation of focal adhesion kinase (FAK) and Src kinase. Inhibition of FAK also increased invadopodia number, which was reversible with Src inhibition. These data are consistent with a model whereby actin-based adhesions can limit the availability of active Src that is capable of invadopodia initiation and identifies Ln-332-β1 interactions as a potent upstream regulator that limits cell invasion.
Keywords: invadopodia, integrin, laminin-332
INTRODUCTION
Metastasis is one of the hallmarks of cancer and the leading cause of cancer mortality (Hanahan and Weinberg, 2000). To metastasize, cancer cells must degrade and migrate through tissue barriers defined as basement membranes (BM). It has previously been shown that many highly invasive cancer cell lines form actin-rich subcellular protrusions on their basal surface, known as invadopodia, which are specialized to degrade BM (Weaver, 2006; Weaver, 2008). Similar structures, known as podosomes, are also found on Src transformed cells, monocyte-derived cells, smooth muscle cells, and osteoclasts, all of which must cross BM or remodel extracellular matrix (ECM) to fulfill their normal functions (Buccione et al., 2004).
Invadopodia can be induced in vitro by plating cancer cells on gelatin cushions (Weaver, 2006). Together with ECM degradation, colocalization of F-actin cores and invadopodia specific molecular markers, such as Tks5 or cortactin, are used to distinguish invadopodia from other actin-rich subcellular structures (Seals et al., 2005; Clark et al., 2007). In vivo, invadopodia-like structures have been imaged by multiphoton microscopy (Yamaguchi et al., 2005), though it is less well characterized. Considerable progress has been made toward understanding the basic structural and molecular components of invadopodia and podosomes in vitro. Four categories of molecules have been identified in invadopodia and podosomes: actin-assembly proteins, membrane trafficking proteins, signaling proteins, and transmembrane proteinases (Weaver, 2006; Weaver, 2008).
Invadopodia are thought to be regulated by integrins because several integrins (e.g., α5β1, α3β1, αVβ1) have been identified on or around these structures (Mueller et al., 1999; Nakahara et al., 1998), though the species and localization of integrins varies across cell lines (Buccione et al., 2004). However, only a few studies have tested specific roles of individual integrins in invadopodia or podosome regulation (Mueller et al., 1999; Nakahara et al., 1998; Nakahara et al., 1996). Interestingly, in addition to regulating invadopodia, integrins also mediate other cell-matrix adhesion structures: actin-associated focal contacts and keratin-associated hemidesmosomes, which are also substrate dependent (DiPersio et al., 1995; Nievers et al., 1999).
It is established that interactions between integrins and their specific substrates activate focal adhesion kinase (FAK) and mediate focal adhesions and other types of adhesive structures (Chan et al., 2009; Webb et al., 2004). Interestingly, a recent study by Chan et al. showed that suppression of FAK increases invadopodia number on breast cancer cells (Chan et al., 2009). They also found that suppression of FAK leads to redistribution of concentrated common molecules from focal adhesions to invadopodia. Therefore, they propose that focal adhesions and invadopodia may compete for common molecular components such as Src kinase (Chan et al., 2009). It is interesting to pursue the model presented by Chan et al. in the context of the FAK upstream effectors: the interaction between integrins and their substrates.
To this end, in this study we test the specific role of laminin-332 (Ln-332) and its integrin receptors in regulating invadopodia formation in the 804G rat bladder carcinoma cells, a cell line that secretes Ln-332 (Spinardi et al., 1995) and forms invadopodia, focal contacts, and hemidesmosomes (Kurpakus, 1991; Riddelle, 1991; Spinardi, 2004). Ln-332 is frequently secreted in an autocrine manner by remodeling epithelia (Rousselle et al., 1991). Ln-332 is also commonly associated with cancer progression (Guess et al., 2009; Guess and Quaranta, 2009; Martin et al., 1998), and is a unique BM component because it is known to promote formation of both focal contacts and hemidesmosomes by ligation of β1 or β4 integrins, respectively (Carter et al., 1991; Niessen et al., 1994; Spinardi et al., 1995).
To determine if Ln-332 plays a role in invadopodia formation in 804G cells, Ln-332 expression was suppressed by knocking down its γ2 chain. To our surprise, the number of invadopodia and invadopodia-associated ECM degradation significantly increased for Ln-332 knockdown cells. Further experiments revealed that blocking integrin α3β1, but not integrin α6β4, had a similar effect, suggesting that focal contacts mediated by integrin α3β1, rather than hemidesmosomes mediated by α6β4, intersect with invadopodia regulation. Interestingly, Ln-332 knockdown cells also exhibited alterations in activation of FAK and Src kinase. Taken together, these data are consistent with a model whereby actin-based adhesions can limit the availability of active Src that is capable of invadopodia initiation (Chan et al., 2009) and identifies Ln-332-β1 interactions as an upstream regulator that limits the potential of invasive cancer cells.
Experimental Procedures
Antibodies and chemicals
Monoclonal anti-cortactin antibody 4F11 was purchased from Upstate (Placid, NY). Monoclonal anti–β-actin (AC-74, Sigma, St. Louis, MO) and polyclonal anti Ln-332 γ2 antibody 2778 has been described previously (Koshikawa et al., 2004). Anti-FAK antibody (610087), anti-paxillin (P13520), integrin β1 blocking antibody Ha2/5, and IgM control are from BD transduction laboratories (Caribbean, CA). Integrin α6 blocking antibody GOH3 (sc-19622) and integrin α3 blocking antibody Ralph 3.2 (sc-7019) were purchased from Santa Cruz (Santa Cruz, CA). Mouse IgG (10400C) and anti-FAK p397 (44-624G) was purchased from Invitrogen (Carlsbad, CA). Anti-p-Src 416 (2101s, Cell Signaling), anti-p-Src 527 (2105s, Cell Signaling), and anti-total-Src (05-184, Upstate) were gifts from the Dr. Dennis Hallahan (Vanderbilt University). Anti-TKS5 was a gift from Dr. Sara Courtneidge (Burnham Institute for Medical Research). Anti-vinculin antibody (9131) was purchased from Sigma. All fluorescently labeled secondary antibodies for immunostaining, phalloidin, and Hoechst 33342 were from Invitrogen.
For western blotting, secondary antibodies were Alexa Fluor 680 anti–rabbit IgG (Invitrogen) and IRDye 800 anti–mouse IgG (Li-Cor Biosciences, Lincoln, NE), or HRP conjugated anti-mouse IgG (NA931V) and anti-rabbit IgG (NA4934v; GE Healthcare). PP2 inhibitor was a gift from Dr. Albert Reynolds (Vanderbilt University). pFAK inhibitor PF573,228 was a gift from Dr. Pierre Massion (Vanderbilt University).
Ln-332 was purified from 804G conditioned medium as previously described (Tripathi et al., 2008) . Vitronectin (VN; V8379) was purchased from Sigma. Rat tail collagen-I (Coll-I; 354236, BD) was a gift from Dr. Roy Zent (Vanderbilt University). To coat the MatTek dishes, matrix proteins were left on the dishes overnight at 4C with indication concentrations: Ln-332 (1 µg/ml), Coll- I (10 µg/ml), and VN (5µg/ml).
To inhibit Src kinase activity, cells were cultured on glass or FITC-gelatin for ~16 h, then treated with PP2 (1 µM) or DMSO diluents for 1 h before immunostaining. To inhibit FAK kinase activity, cells were treated with chemical compound PF573,228 (5 µM) overnight before immunostaining.
Integrin blocking antibodies were added after cells were attached on MatTek dishes (~2.5 hrs after plating). Blocking antibodies concentrations were used as previously described: IgM and Ha2/5 (10 µg/ml) (Tsiper and Yurchenco, 2002); IgG, GoH3, and Ralph3.2 (20 µg/ml) (Zent et al., 2001).
Construction of small interfering RNA and retroviral expression constructs
The pSuper.Retro.puro vector was a gift from Dr. Albert Reynolds (Vanderbilt University). These retroviral vectors are commercially available from Oligoengine. Rat knockdown and scrambled sequences specific to Ln-332 γ2 and integrin β4 were designed using siRNA wizard (http://www.sirnawizard.com/index.php). PAGE purified shRNA oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA) and inserted in vector HindIII and EcoRI restriction sites. The Phoenix retroviral gene transduction system, created in Dr. Garry Nolan’s lab (Stanford University), was used to generate viral to knock-down cells as previously described (Ireton et al., 2002). Briefly, Phoenix retroviral packaging cells were transfected with pSuper.Retro vectors containing knock-down or control scrambled sequences using Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions, producing retroviruses for 804G cell infection. Viral infected 804G cells were selected with 5 µg/ml puromycin (Research Products International Corp., Mt. Prospect, IL) for ~7 days or 700 µg/ml neomycin (Amresco, Solon, Ohio) for ~30 days in a tissue-culture incubator.
Cell culture
All cell lines were maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Gemini, Irvine, CA) and 1% glutamine/ penicillin/streptomycin (p/s/g; Life Technologies, Invitrogen). All 804G-derived cell lines were selected and maintained in 5 µg/ml puromycin (Research Products International Corp.).
Immunostaining
Immunofluorescence assays were performed as previously described (Jourquin et al., 2006). Cells were fixed in 3.7% formaldehyde (Sigma) in PBS for 10 min and permeabilized with 0.3% Triton X-100 (MP Biomedicals, Solon, OH) in PBS. Cells were then blocked with 10% goat serum and 1% BSA in PBS for 40 min and incubated with appropriate primary antibodies for 1 h, followed by secondary antibodies and/or fluorescent phalloidin for 1 h at room temperature.
In vitro matrix degradation assay
FITC labeling of gelatin was performed according a protocol obtained from Dr. Sara Courtneidge’s laboratory (Seals et al., 2005) (Burnham Institute for Medical Research). Briefly, gelatin (#G-1890, Sigma) was dissolved in borate buffer at 37°C to the concentration of 2.0%. [0.17 mol/L borate, 0.075 mol/L NaCl (pH 9.3); FITC (#46950, Fluka, Sigma) was added to the gelatin solution to the final concentration 0.02% and mixed well by stirring at 37°C for 2 h. The FITC gelatin solution was dialyzed extensively against pre-warmed PBS for 3 to 4 days. FITC-gelatin was aliquot after adding sucrose (Sigma) to a concentration of 2.0%.
The matrix degradation assay was performed as previously described (Alexander et al., 2008). Briefly, MatTek dishes were coated with 150 µl pre-warmed (37°C) FITC-gelatin Excess gelatin solution was removed after 1 min of polymerization in plates. The gelatin film on dishes was subsequently air dried for 30 min, followed by cross-linking with ice-cold 0.5% glutaraldehyde in PBS. Dishes was sterilized with 70% ethanol, washed with DMEM, and equilibrated with complete medium (DMEM supplemented with 10% FBS and p/s/g) for 1 h before addition of cells. For invadopodia assays, 20 × 104 cells were suspended in 2 mL of complete medium and added to plates for 16–18 h of incubation.
Microscopy and image analysis
Overhead and Z-section images were captured using a Zeiss LSM510 Meta with a x60 Plan-NeoFluar 1.3 NA objective confocal microscope. Invadopodia were identified as actin puncta that were also positive for either cortactin or Tks5 as previously described (Alexander et al., 2008; Seals et al., 2005). The number of invadopodia formed by single cells was manually counted in images.
Cell area for functional invadopodia degradation analysis was determined using ImageJ by manually outlining cells’ “footprints” β-actin channel (Clark et al., 2007) and applying the “measure tool” to calculate cell area (in pixels). Degradation area was also determined using ImageJ by performing an inclusive threshold of the FITC channel to include the degraded areas and again using the “measure tool”. Data were collected at the single-cell level and analyzed to calculate the ratio of digested area per cell area.
Focal contact (by paxillin) number and area were quantified using particle analysis tool in ImageJ. Briefly, images were thresholded to include paxillin staining, which were then analyzed by the particle analysis tool. Background particles reading were manually excluded according to objective observation.
Transwell migration and invasion assay
Transwell plates (0.8 µm pores, #3422) were purchased from Corning (Corning life Sciences, Wilkes Barre, PA). For invasion assays, 100 µl 10% Matrigel (#356232, BD Biosciences) was coated on the upper surface of inserts at room temperature for 1 h. Unbound Matrigel was then aspirated gently from inserts, inserts were washed with serum-free DMEM, and 500 µl of complete media (DMEM with FBS) were added to the bottom of wells. Approximately 200,000 cells in 200 µl serum-free media were plated per insert on top of Matrigel. After 16 h of incubation at 37°C, cells were fixed and stained with crystal violet for visualization. Cells that did not penetrate filters were cleared using cotton swabs and a gentle washing step. Membranes were cut from inserts and placed on microscope slides bottom side down. Images of 10 randomly chosen areas were obtained for each membrane using a 40x objective of a Zeiss Axiovert 220M microscope. Numbers of invaded cells were counted manually.
Western blot analysis
For western blots of whole-cell lysates, cells were lysed in lysis buffer (50 mM Tris-HCl, 2mM EDTA, 250 mM NaCl, 10% glycerol, 0.1% NP-40) with protease inhibitor cocktail (Sigma) and phosphotase inhibitors I and II (p5726 and p2850, Sigma) for 30 min on ice. Cell lysates were briefly sonicated and centrifuged. Supernatants were boiled with 4X sample buffer (Invitrogen) and 50 mM DTT at 100°C for 15 min. Protein samples were then separated by electrophoresis on 4–12% NuPAGE or 10% BisTris Gel (Invitrogen).
Proteins were transferred to PVDF and blocked in 5% non-fat milk TBST or blocking buffer (LI-COR Biotechnology, Lincoln, NE). After indicated antibody incubations, blots were developed by enhanced chemi-luminescence and exposed to X-ray film or scanned using the Odyssey Infrared Scanner (LI-COR).
Statistical analysis
Statistical analysis was performed using SPSS version 17 (SPSS Inc., Chicago, IL). Normality of data was examined using Shapiro Wilks W tests. For normally distributed data (Supp. Fig. 2A, B only), differences were examined using Students t-tests, and were considered significant when P < 0.05. Parametric data are presented in column plots as means γγstandard deviation of all experiments performed (N=2). For nonparametric data, differences between treatments were examined using Kolmogorov–Smirnov 2-test, and were considered significant when P < 0.05. Nonparametric data are presented in box-and-whisker plots overlaid with scatter plots that show medians (dark horizontal line), 25th and 75th quartiles (box), 95% confidence intervals (whiskers), and outliers (scatter) for all experiments performed (N=2–4).
RESULTS
Knocking down Ln-332 increases invadopodia number
To determine the role of Ln-332 in invadopodia formation, 804G cells were retrovirally transduced with a vector expressing shRNA specific for the Ln-332 γ2 chain (γ2-kd). This chain is essential for the assembly and secretion of Ln-332 (Matsui et al., 1995) and knocking it down results in loss of all 3 subunits (Supp. Fig. 1). In addition to focal contacts and hemidesmosomes (Kurpakus and Jones, 1991; Riddelle et al., 1991), 804G cells have previously been shown to form “podosome-like” invadopodia structures on glass (Spinardi et al., 2004), which we verified by colocalization of actin filaments (F-actin) and cortactin in punctate structures near the cell-coverslip interface by confocal microscopy (Fig. 1A). Interestingly, compared with scrambled oligo-expressing control cells (γ2-ctrl), γ2-kd cells contained significantly more invadopodia per cell (Fig. 1B; medians = 0 and 6, respectively).
Figure 1. Suppression of Ln-332 increases invadopodia number.
(A) Representative overhead and Z-section slice (Z) confocal images of γ2-ctrl and γ2-kd cells cultured for 16 h on glass, fixed, and stained with antibodies against F-actin (blue), cortactin (green), and Ln-332 γ2 chain (red). Colocalization between F-actin and cortactin is turquoise in merged images. Scale bar = 10 µm and arrows indicate examples of invadopodia. (B) Box-and-whisker plot shows the number of invadopodia counted per cell. γ2-kd cells had significantly more invadopodia than control cells (N≥3; p<0.001). (C) Representative confocal images from in vitro matrix degradation assay. Cells were cultured for 16 h on cross-linked FITC-gelatin (green), fixed, and stained with antibodies against F-actin (red) and cortactin (blue) to identify invadopodia. Pink regions in merged images show colocalization between F-actin and cortactin; yellow shows colocalization between F-actin and FITC-gelatin; turquoise shows colocalization between F-actin and FITC-gelatin; and white shows colocalization of all three markers. Scale bar = 10 µm and arrows indicate examples of invadopodia. (D) Box-and-whisker plots show number of invadopodia per cell. Again, γ2-kd cells formed significantly more invadopodia per cell than control cells (N=3; p<0.001). (E) Matrix-degrading assays show γ2-kd degraded more ECM per cell than control cells (N=3; p<0.001).
Since the most important function of invadopodia is to digest underlying ECM, both cell types were also tested for the ability to degrade FITC-labeled cross-linked gelatin coated on glass coverslips, as previously described (Seals et al., 2005). As expected, the number of invadopodia counted per γ2-kd cell was significantly greater than that of control cells (Fig. 1C, D; medians = 4 and 12, respectively), consistent with earlier results on glass. Degradation area per cell was also significantly increased in γ2-kd cells compared to γ2-ctrl cells (by 2.0-fold; Fig. 1E). Collectively, these data indicate that suppression of autocrine-secreted Ln-332 leads to increased numbers of functional invadopodia, at least for 804G cells. We also tested whether suppression of Ln-332 secretion affects invasion in traditional transwell invasion assays. However, γ2-kd cells were significantly less invasive than control cells using this method (Supp. Fig. 2A), probably due to decreased cellular motility (Supp. Fig. 2B).
Invadopodia number is increased by blocking integrin α3β1
Integrin adhesion receptors are thought to regulate invadopodia, however few studies have tested specific roles of individual integrins (Mueller et al., 1999; Nakahara et al., 1998; Nakahara et al., 1996). Previous studies have demonstrated that the major integrin subunits expressed in 804G cells are α2, α3, α6, β1, and β4 (Spinardi et al., 1995). Further, very low levels of αV are also expressed and α1, α4, and α5 are not detectable for this cell line (Spinardi et al., 1995). Thus, the major integrin heterodimers that 804G cells presumably possess are α6β4, α6β1, α3β1, and α2β1. Ln-332 can mediate different effects in cells, depending on its integrin partner: α6β4 is essential for the formation of intermediate filament-connecting hemidesmosomes (Borradori and Sonnenberg, 1999), whereas α3β1 and α6β1 form connections to the actin cytoskeleton and associated signaling molecules (Delwel et al., 1994).
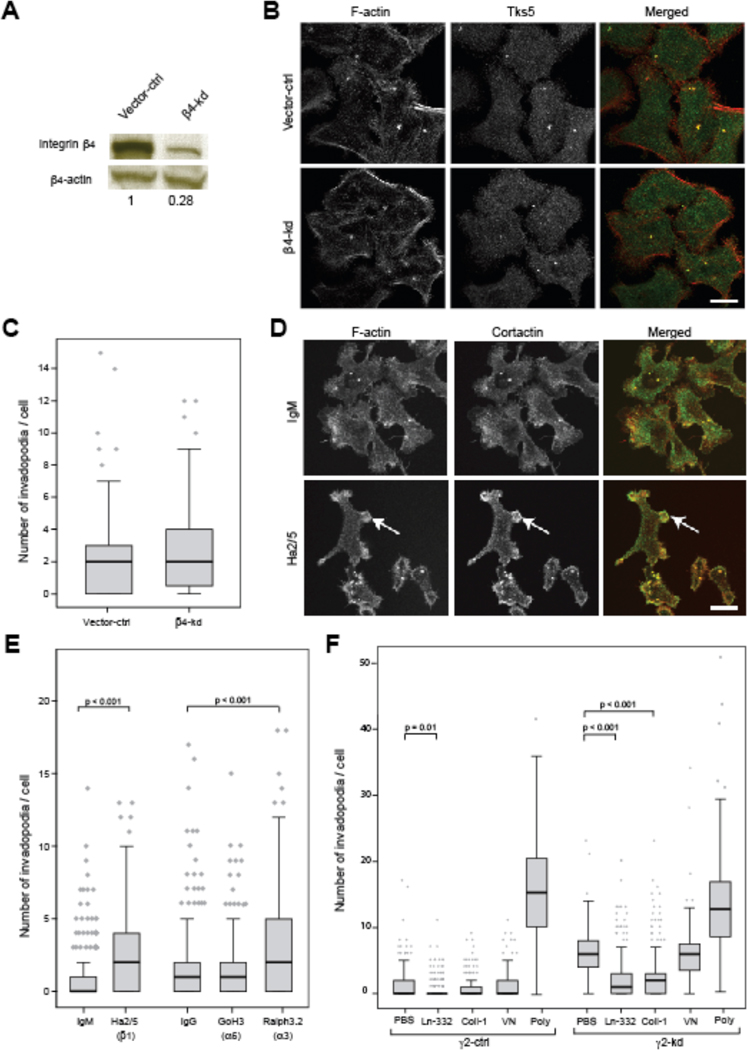
To investigate the involvement of α6β4 in invadopodia regulation by Ln-332, integrin β4 chain was knocked down in 804G cells using stable shRNA expression (β4-kd). Despite ~70% knockdown efficiency (Fig. 2A), there was no difference in number of invadopodia per cell between vector-ctrl and β4-kd cells (Fig. 2B, C, medians = 2 and 2, respectively, p = 0.141). Further, the number of invadopodia per β4-kd cell was also similar to the number counted for γ2-ctrl cells in earlier experiments (Fig. 1B; medians = 2 and 2.5, respectively). Inhibition of α6 integrin subunit (via GoH3) likewise had no effect on invadopodia number (Fig. 2E). Since α6β4 is essential for hemidesmosome formation (Spinardi et al., 1995), these data suggest that hemidesmosomes do not compete with or otherwise regulate invadopodia.
Figure 2. Invadopodia number is increased by blocking integrin β1 but not β4.
(A) Western blotting confirms that β4 was knocked down in ITGB4-kd cells by ~70%. (B) Representative images of vector-ctrl and β4-kd cells cultured on glass for 16 h, fixed, and stained with antibodies against TKS-5 (green) and F-actin (red). Yellow in merged images show colocalization of markers. Scale bar = 10 µm. (C) Box-and-whisker plots show number of invadopodia formed per cell. Knockdown of β4 had little effect on invadopodia formation compared to vector-ctrl cells (N=2; p=0.162). (D) Representative images of γ2-ctrl cells cultured on glass for 3 h, treated with Ha2/5 (integrin β1 blocking antibody; 10 µg/ml) or IgM (10 µg/ml) for 16 h, fixed, and stained with antibodies against cortactin (green) and F-actin (red). Yellow in merged images show colocalization of markers. (E) Blocking integrin β1 by Ha2/5 significantly increased number of invadopodia in γ2-ctrl cells (p<0.001). Scale bar is equal to 10 µm and arrows show podsome-like structure. (F) Plots show number of invadopodia formed per cell type plated on either Ln-332 (1 µg/ml), collagen I (Coll-I; 10 µg/ml), vitronectin (VN; 10 µg/ml), or Poly-D-lysine (Poly; 0.1mg/ml), or PBS. Addition of Ln-332 significantly reduced the formation of invadopodia on both cell types, and Coll-I significantly reduced invadopodia formation of γ2-kd cells (N≥3; P<0.001).
To investigate the involvement of β1 integrins in invadopodia regulation by Ln-332, we blocked the interaction between Ln-332 and β1 integrins by treating 804G cells with Ha2/5, a β1-blocking antibody before staining for invadopodia markers, F-actin and cortactin (Fig. 2D). As shown in Fig. 2E, the number of invadopodia formed per cell was significantly increased by treatment with Ha2/5 compared to IgM control (medians = 2 and 0, respectively), although it was slightly lower than the number observed for 804G γ2-kd cells in previous experiments (medians = 2 and 2.5, respectively). Furthermore, inhibition of integrin α3 with a function-blocking antibody (Ralph3.2) resulted in increased invadopodia number on γ2-ctrl cells (Fig. 2E; medians = 2 and 0, respectively). Taken together, these results indicate that α3β1, but not α6β1 or α6β4, is the relevant Ln-332 receptor that regulates invadopodia.
To determine if other β1 ligands can also regulate invadopodia number, γ2-kd cells were replated on glass coverslips coated with various ligands (Fig. 2F). As expected, replating cells on Ln-332 rescued the effect of γ2-kd, leading to increased spreading (Supp. Fig. 3) and decreased invadopodia formation (Fig. 2F). The number of invadopodia counted per cell for γ2-ctrl was also somewhat decreased by Ln-332 coating, suggesting tuning by Ln-332 concentration. Interestingly, replating on the β1 ligand Coll-I also fully rescued the phenotype of γ2-kd cells (increased spreading and invadopodia suppression), indicating that the effect of Ln-332 is not unique and can be substituted by other β1 ligands (Fig. 2F, Supp. Fig. 3). In contrast, the integrin αV ligand vitronectin (VN) did not rescue cell spreading or invadopodia. The non-integrin adhesive substrate poly-D-lysine (Poly) also did not rescue invadopodia phenotype, despite promoting cellular spreading (Fig. 2F, Supp. Fig. 3), and in fact enhanced invadopodia formation in both control and γ2-kd cells (Fig. 2F). These data indicate that ligation of β1 integrins can suppress invadopodia formation; in the case of 804G cells, Ln-332 is the major relevant ligand due to autocrine secretion (Langhofer et al., 1993).
Ln-332 acts upstream of Src
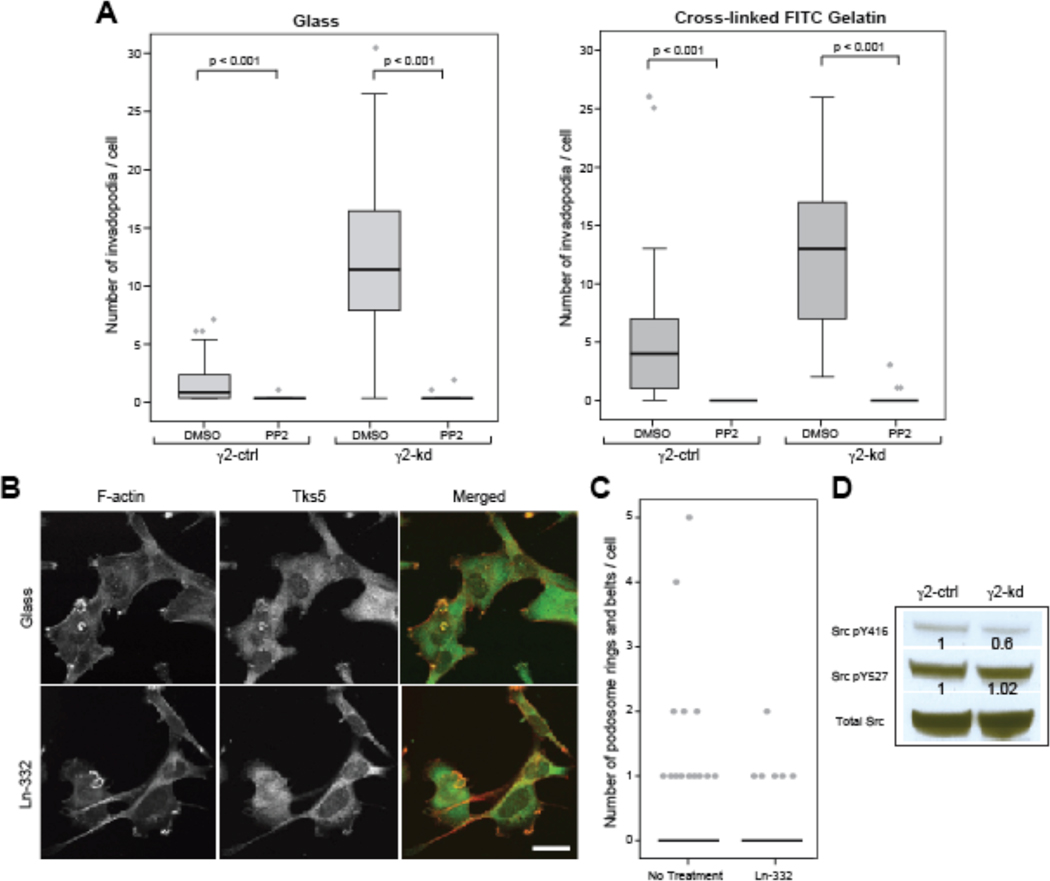
Src kinase activity is pivotal for the formation and function of both invadopodia and podosomes (David-Pfeuty and Singer, 1980; Destaing et al., 2008; Gimona et al., 2008; Linder and Aepfelbacher, 2003; Symons, 2008; Tarone et al., 1985) and can be regulated by β1 integrins (Playford and Schaller, 2004). Interestingly, some cells treated with the β1-blocking antibody Ha2/5 exhibited podosome-like rings (Fig. 2D), which suggests increased Src kinase activity (David-Pfeuty and Singer, 1980; Tarone et al., 1985). To investigate the role of Src kinase in Ln-332-mediated invadopodia regulation, γ2-ctrl and γ2-kd cells were incubated overnight on either uncoated or cross-linked FITC-gelatin coated glass coverslips and then incubated with PP2, a chemical inhibitor of Src kinase, or DMSO diluent for control. In all cases, PP2 treatment led to disappearance of invadopodia, consistent with a requirement for ongoing Src kinase activity for invadopodia maintenance and/or formation (Fig. 3B, C). We also tested whether fibroblasts expressing constitutively active Src kinase (Y527F) were sensitive to Ln-332 by plating them overnight on uncoated or Ln-332-coated glass coverslips and counting the number of podosome belts and rings that formed (Fig. 3C, D). Although there was some decrease in the number of podosomes per cell, it was not statistically significant, suggesting that Ln-332 is more likely to inhibit invadopodia upstream than downstream of Src kinase. However, since at the whole cell level we find that Src kinase activity (pY416) is decreased in γ2-kd cells (western blot in Fig. 3E), it seems likely that Ln-332 spatially regulates the availability of active Src kinase.
Figure 3. Ln-332 acts upstream of Src.
(A) Box-and-whisker plots show number of invadopodia formed per cell in the presence of Src kinase inhibitor PP2, or DMSO for control, on glass (left) or on cross-linked FITC-collagen (right). (B) Representative images of SrcMEF cells plated on uncoated or Ln-332 (10 µg/ml) coated dishes, fixed, and stained with antibodies against Tks5 (green) and phalloidin (red). (C) Plot shows number of podosome structures (half ring) formed by SrcMEF cells. Ln-332 had some, but not a significant, impact on the number of ring structures formed by SrcMEF cells (N=1; p=0.057). (D) Representative western blotting of total Src, Src p416, and Src p527 is shown. Src p416 was decreased in γ2-kd cells by ~50% compared to γ2-ctrl, while total Src expression level and Src p527 was not altered.
Inhibition of FAK increases invadopodia number
Another molecule activated downstream of β1 integrins is FAK. Chan et al. recently proposed a model in which FAK can negatively regulate invadopodia by sequestration of active Src kinase at focal contacts via binding to the autophosphorylation PY397 site on FAK (Chan et al., 2009). Therefore, we examined whether Ln-332 regulates phosphorylation of Y397-FAK by western blot analysis. As shown in Fig. 4A, pY397-FAK was decreased ~40% in γ2-kd cells, compared to control cells, indicating that Ln-332 - β1 interactions may repress the formation of invadopodia by altering Src and FAK activities. On the other hand, pY397-FAK was not altered in β-kd cells, compared to vector-ctrl cells. Consistent with this idea, inhibition of pFAK with the chemical compound PF573,228 (Supp. Fig. 4) led to significantly increased numbers of invadopodia per cell (Fig. 4B, C; medians = 0 and 6 for DMSO and PF573,228, respectively) and decreased whole cell Src kinase activity (Supp. Fig. 4), similar to the effect of γ2-kd in earlier experiments (Fig. 1B and Fig. 3E). The increase in invadopodia induced by PF573,228 treatment could be prevented by coincubation with the Src kinase inhibitor PP2 (Supp. Fig. 5), indicating that Src kinase activity is still required for invadopodia formation in FAK-inhibited cells. These data are consistent with a model in which Ln-332-β1 interactions inhibit invadopodia by spatial regulation of FAK and Src kinase.
Figure 4. Suppression of Ln-332 decreases pFAK Y397 and alters adhesion.
(A) Representative western blot of pFAK Y397 and total FAK reveals that pFAK Y397 was decreased in γ2-kd cells compared to γ2-ctrl cells, but not in integrin β4-kd cells, while total FAK expression level was not altered. (B) Representative images of γ2-ctrl cells treated with PF573,228 (pFAK inhibitor; 5 µM) or DMSO for ~16 h, fixed, and stained with antibodies against cortactin (green), F-actin (red), and Hoechst (blue). Yellow in merged image shows colocalization between F-actin and cortactin. (C) Box-and-whisker plot shows that blocking FAK phosphorylation by PF573,228 increased the number of invadopodia formed by γ2-ctrl (N=3; p<0.001). (D) Representative F-actin (red), paxillin (left, green), and vinculin staining (right, green) are shown in images of γ2-ctrl and γ2-kd. (E) Quantification of the number of focal contacts per cell (left; N=2; p<0.001) and size of focal contacts (right; N=2; p=0.247) of γ2-ctrl and γ2-kd.
Most invadopodia components localize to several cellular locations. Critical to this study, β1 integrins, FAK, and Src localize to both focal contacts and invadopodia (Alexander et al., 2008; Mueller et al., 1999; Spinardi et al., 2004). To determine the effect of Ln-332 on adhesions, control and γ2-kd cells were cultured overnight on glass coverslips and immunostained with the markers paxillin and vinculin. The control cells exhibited two different adhesion phenotypes. In ~55% of the ctrl cells, few obvious focal contacts were evident (Fig. 4D, zoom). In ~5% of the cells, large peripheral linear focal contacts were seen (Fig. 4D, zoom). In contrast, γ2-kd cells were more uniform, containing numerous small focal contacts arranged at the cell periphery (Fig. 4D, zoom). Quantitation of the number and size of focal contacts indicated that the major change was in the number of focal contacts, with γ2-kd cells exhibiting ~9-fold more focal contacts than control cells (Fig. 4E, left; medians = 0 and 9, respectively); focal contact size was not significantly different between control and γ2-kd cells (Fig. 4E, right). The lack of morphologically distinct adhesions in the majority of Ln-332-expressing cells is consistent with previous reports comparing adhesion morphologies in cells plated on Ln-332 versus other ECM substrates (DiPersio et al., 1995; Dogic et al., 1998). Because γ2-kd cells do not spread well (Fig. 6B) and have decreased overall levels of active Src and FAK (Figs. 3E, 4A), these data suggest that the increased number of paxillin- and vinculin- focal contacts in γ2-kd cells represent adhesions that cannot fully compensate for the loss of Ln-332, and are probably forming on ECM substrates other than Ln-332.
DISCUSSION
Taken together, our data indicate that the BM component Ln-332 can suppress the number of invadopodia that form by interacting with α3β1 integrin and altering the cellular balance of FAK and Src kinase activity. In this study, we investigated the role of Ln-332 in regulating invadopodia number in 804G rat bladder carcinoma cells, a cell line that secretes Ln-332 and forms all three types of adhesions (focal contacts, hemidesmosomes, and invadopodia)(Kurpakus and Jones, 1991; Riddelle et al., 1991; Spinardi et al., 2004), by knocking down the γ2 chain of the heterotrimeric Ln-332 structure. Interestingly, expression of shRNA to Ln-332 γ2 chain in 804G cells (γ2-kd) led to significantly increased numbers of invadopodia and enhanced invadopodia-associated ECM degradation using single cell-based in vitro assays (Fig. 1). Collectively, these data indicate that suppression of autocrine-secreted Ln-332 leads to increased numbers of functional invadopodia, at least for 804G cells. Of note, using classic transwell invasion assays γ2-kd cells were significantly less invasive than control cells (Supp. Fig. 2A), probably due to decreased cellular motility (Supp. Fig. 2B). These seemingly conflicting results are not altogether surprising, given that Chan et al. (Chan, Cortesio et al. 2009) also found that invadopodia activity did not correlate with transwell invasion in breast cancer cells expressing siRNA to FAK.
Not surprisingly, replating γ2-kd cells on purified Ln-332 fully recovered both cellular spreading (Supp. Fig. 3) and inhibition of invadopodia formation (Fig. 2F). Interestingly, plating cells on non-pepsinized Coll-I, another β1 ligand, had a similar effect. In contrast, replating cells on the RGD ligand VN had almost no effect. These results indicate that BM may actively inhibit invasion of tumor cells through engagement of β1 integrins. Antibody blocking experiments using 804G control cells confirmed that inhibition of integrins α3 and β1, but not α6 or β4, phenocopied the effect of γ2-kd on invadopodia formation (Fig. 2E), suggesting that a3β1-mediated focal contacts, rather than a6β4-mediated hemidesmosome pathways, intersect with invadopodia regulation. In line with this idea, knocking down β4 integrin subunit in 804G cells had almost no effect on invadopodia number (Fig. 2B, C). Further, γ2-kd cells exhibited alterations in the number of focal contact-type structures and in activation of FAK and Src kinase (Figs. 3, 4). Inhibition of FAK led to an increase in invadopodia number similar to γ2-kd, which was reversible with Src inhibition. These data are consistent with a model whereby actin-based adhesions can limit the availability of active Src that is capable of invadopodia initiation (Chan et al., 2009) and identifies Ln-332-β1 interactions as a potent upstream regulator that limits the invasive potential of cells.
Since phosphorylation of FAK Y397 creates a binding site for Src kinase (Schaller et al., 1994), it is possible that increased activation of FAK at β1 adhesions could lead to sequestration of Src kinase away from invadopodia sites, consistent with the model of Chan et al.(Chan et al., 2009). However, FAK-bound Src is also known to promote FAK autophosphorylation (Caron-Lormier and Berry, 2005), presumably starting a signaling amplification loop. Thus, in order to release active Src for invadopodia formation, it is reasonable to assume the existence of negative feedback loops that prevent FAK from fully monopolizing Src (Zaidel-Bar et al., 2007). Further studies are needed to identify critical feedback loops and how they lead to invadopodia formation. At any rate, the status of β1 adhesions appears to be a critical point of regulation for the invasive ability of cancer cells. Because overexpression of FAK frequently occurs in cancer (Cance et al., 2000; Gabarra-Niecko et al., 2003; Lark et al., 2005; Owens et al., 1995) and can also lead to increased invasiveness of cancer cells and invadopodia formation, it is possible that FAK is a rate limiting factor in cells that is sensitive to the status of β1 adhesions as well as ECM rigidity (Alexander et al., 2008; Hauck et al., 2002).
In vivo, Ln-332 is a major component of BM. Thus, in early cancer lesions with intact BM (carcinoma in situ), Ln-332 - α3β1 interactions may serve to inhibit cellular invasiveness by sequestration of active Src at β1 adhesions. Ln-332 expression is frequently lost in cancer and has been associated with poor patient prognosis and increased tumor formation and invasiveness in mouse models (Guess et al., 2009; Guess and Quaranta, 2009; Yuen et al., 2005). Our data suggest that the increased aggressiveness associated with Ln-332 loss may be in part due to increased invadopodia formation.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge the following funding sources for support of this work: U54-CA113007, 2R01-CA47858-17A2, and 5R01-GM067221-03l awarded to VQ, 1R01-GM075126 awarded to AMW, and DOD fellowship W81XWH-06-1-0308 awarded to SL. We would also like to thank Drs. Donna Webb and Roy Zent (Vanderbilt University) for critical reading of the manuscript and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18(17):1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112(4):411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nature reviews. 2004;5(8):647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6(6):2417–2423. [PubMed] [Google Scholar]
- Caron-Lormier G, Berry H. Amplification and oscillations in the FAK/Src kinase system during integrin signaling. Journal of theoretical biology. 2005;232(2):235–248. doi: 10.1016/j.jtbi.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991;65(4):599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185(2):357–370. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67(9):4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980;77(11):6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DL, Kuikman I, Lindblom A, Paulsson M, Timpl R, Sonnenberg A. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5(2):203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, De Camilli P, Baron R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19(1):394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Shah S, Hynes RO. alpha 3A beta 1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. Journal of cell science. 1995;108(Pt 6):2321–2336. doi: 10.1242/jcs.108.6.2321. [DOI] [PubMed] [Google Scholar]
- Dogic D, Rousselle P, Aumailley M. Cell adhesion to laminin 1 or 5 induces isoform-specific clustering of integrins and other focal adhesion components. Journal of cell science. 1998;111(Pt 6):793–802. doi: 10.1242/jcs.111.6.793. [DOI] [PubMed] [Google Scholar]
- Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22(4):359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Current opinion in cell biology. 2008;20(2):235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Guess CM, Lafleur BJ, Weidow BL, Quaranta V. A decreased ratio of laminin-332 beta3 to gamma2 subunit mRNA is associated with poor prognosis in colon cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1584–1590. doi: 10.1158/1055-9965.EPI-08-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guess CM, Quaranta V. Defining the role of laminin-332 in Carcinoma. Matrix Biol. 2009 doi: 10.1016/j.matbio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Ilic D, Schlaepfer DD. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. J Biol Chem. 2002;277(15):12487–12490. doi: 10.1074/jbc.C100760200. [DOI] [PubMed] [Google Scholar]
- Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159(3):465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourquin J, Yang N, Kam Y, Guess C, Quaranta V. Dispersal of epithelial cancer cell colonies by lysophosphatidic acid (LPA) J Cell Physiol. 2006;206(2):337–346. doi: 10.1002/jcp.20470. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Schenk S, Moeckel G, Sharabi A, Miyazaki K, Gardner H, Zent R, Quaranta V. Proteolytic processing of laminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. Faseb J. 2004;18(2):364–366. doi: 10.1096/fj.03-0584fje. [DOI] [PubMed] [Google Scholar]
- Kurpakus MA, Jones JC. A novel hemidesmosomal plaque component: tissue distribution and incorporation into assembling hemidesmosomes in an in vitro model. Exp Cell Res. 1991;194(1):139–146. doi: 10.1016/0014-4827(91)90143-i. [DOI] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JC. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. Journal of cell science. 1993;105(Pt 3):753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Lark AL, Livasy CA, Dressler L, Moore DT, Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ, Cance W. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol. 2005;18(10):1289–1294. doi: 10.1038/modpathol.3800424. [DOI] [PubMed] [Google Scholar]
- Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13(7):376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Martin KJ, Kwan CP, Nagasaki K, Zhang X, O'Hare MJ, Kaelin CM, Burgeson RE, Pardee AB, Sager R. Down-regulation of laminin-5 in breast carcinoma cells. Mol Med. 1998;4(9):602–613. [PMC free article] [PubMed] [Google Scholar]
- Matsui C, Wang CK, Nelson CF, Bauer EA, Hoeffler WK. The assembly of laminin-5 subunits. J Biol Chem. 1995;270(40):23496–23503. doi: 10.1074/jbc.270.40.23496. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Ghersi G, Akiyama SK, Sang QX, Howard L, Pineiro-Sanchez M, Nakahara H, Yeh Y, Chen WT. A novel protease-docking function of integrin at invadopodia. J Biol Chem. 1999;274(35):24947–24952. doi: 10.1074/jbc.274.35.24947. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273(1):9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen WT. A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. The Journal of biological chemistry. 1996;271(44):27221–27224. doi: 10.1074/jbc.271.44.27221. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Hogervorst F, Jaspars LH, de Melker AA, Delwel GO, Hulsman EH, Kuikman I, Sonnenberg A. The alpha 6 beta 4 integrin is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211(2):360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Nievers MG, Schaapveld RQ, Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999;18(1):5–17. doi: 10.1016/s0945-053x(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55(13):2752–2755. [PubMed] [Google Scholar]
- Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23(48):7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Riddelle KS, Green KJ, Jones JC. Formation of hemidesmosomes in vitro by a transformed rat bladder cell line. J Cell Biol. 1991;112(1):159–168. doi: 10.1083/jcb.112.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114(3):567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14(3):1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7(2):155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. A recombinant tail-less integrin beta 4 subunit disrupts hemidesmosomes, but does not suppress alpha 6 beta 4-mediated cell adhesion to laminins. J Cell Biol. 1995;129(2):473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinardi L, Rietdorf J, Nitsch L, Bono M, Tacchetti C, Way M, Marchisio PC. A dynamic podosome-like structure of epithelial cells. Exp Cell Res. 2004;295(2):360–374. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Symons M. Cell biology: watching the first steps of podosome formation. Curr Biol. 2008;18(19):R925–R927. doi: 10.1016/j.cub.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159(1):141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Tripathi M, Nandana S, Yamashita H, Ganesan R, Kirchhofer D, Quaranta V. Laminin-332 is a substrate for hepsin, a protease associated with prostate cancer progression. J Biol Chem. 2008;283(45):30576–30584. doi: 10.1074/jbc.M802312200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiper MV, Yurchenco PD. Laminin assembles into separate basement membrane and fibrillar matrices in Schwann cells. Journal of cell science. 2002;115(Pt 5):1005–1015. doi: 10.1242/jcs.115.5.1005. [DOI] [PubMed] [Google Scholar]
- Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23(2):97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- Weaver AM. Invadopodia. Curr Biol. 2008;18(9):R362–R364. doi: 10.1016/j.cub.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6(2):154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Current opinion in cell biology. 2005;17(5):559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Yuen HW, Ziober AF, Gopal P, Nasrallah I, Falls EM, Meneguzzi G, Ang HQ, Ziober BL. Suppression of laminin-5 expression leads to increased motility, tumorigenicity, and invasion. Exp Cell Res. 2005;309(1):198–210. doi: 10.1016/j.yexcr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. Journal of cell science. 2007;120(Pt 1):137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- Zent R, Bush KT, Pohl ML, Quaranta V, Koshikawa N, Wang Z, Kreidberg JA, Sakurai H, Stuart RO, Nigam SK. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev Biol. 2001;238(2):289–302. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.