Abstract
Growing evidence supports the role of TNF-α as a mediator of neurodegeneration in glaucoma. Glial production of TNF-α is increased and its death receptor is up-regulated on retinal ganglion cells (RGCs) and optic nerve axons in glaucomatous eyes. This multifunctional cytokine can induce RGC death through receptor-mediated caspase activation, mitochondrial dysfunction, and oxidative stress. Opposing these cell-death promoting signals, binding of TNF receptors can also trigger the activation of survival signals. A critical balance between a variety of intracellular signaling pathways determines the predominant in vivo bioactivity of TNF-α as best exemplified by differential responses of RGCs and glia. In addition to the direct neurotoxicity of TNF-α to RGCs and their axons, potential interplay of TNF-α signaling with other cellular events associated with glaucomatous neurodegeneration may also contribute to secondary neurodegenerative injury. This review focuses on the present evidence supporting the involvement of TNF-α signaling in glaucomatous neurodegeneration and possible treatment targets to provide neuroprotection.
Keywords: glaucoma, neurodegeneration, retinal ganglion cell, glia, tumor necrosis factor-alpha
Tumor necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine with multiple functions in the immune response. Since its initial discovery as a serum factor causing tumor necrosis, it has been clear that besides its overwhelming functions in the regulation of inflammatory processes, this potent immuno-mediator is also a major mediator of apoptosis. Thousands of studies over the last 30 years have implicated TNF-α in the pathogenesis of a wide spectrum of human diseases, including sepsis, diabetes, cancer, collagen tissue diseases, and neurodegenerative diseases (Chen and Goeddel, 2002; Locksley et al., 2001). In addition to macrophages, lymphoid cells, mast cells, endothelial cells, and fibroblasts, TNF-α is also produced by glial cells and may lead to neuronal cell death (Downen et al., 1999). Cytotoxic effects of TNF-α are largely associated with its ability to induce apoptosis signaling as well as its function as a potent activator of neurotoxic substances such as nitric oxide and excitotoxins. In addition, picogram concentrations of TNF-α known to be non-cytotoxic induce neuronal cell death through the silencing of survival signals (Venters et al., 2000). Several studies also implicate sphingomyelin hydrolysis and ceramide generation in TNF-α-induced cell death (Dressler et al., 1992). TNF-α is rapidly up-regulated in the brain after injury, suggesting an important role of this cytokine in modifying the neurodegenerative process. Its excessive synthesis after brain injury has been correlated with poor outcome, and its inhibition has been associated with reduced neuronal damage in various diseases of the central nervous system (McCoy et al., 2006; Shohami et al., 1999). Regarding the optic nerve, intravitreal injections of TNF-α have resulted in degeneration of optic nerve axons and delayed loss of retinal ganglion cell (RGC) bodies (Kitaoka et al., 2006).
Evidence supporting the role of TNF-α as a mediator of retinal ganglion cell death in glaucoma
Growing evidence supports that TNF-α, through the binding of TNF receptor-1 (TNF-R1), a death receptor, is involved in mediating RGC death during glaucomatous neurodegeneration. Glial production of TNF-α is increased in the retina and optic nerve head, and TNF-R1 is up-regulated in RGCs and their axons in glaucomatous human donor eyes (Tezel et al., 2001; Yan et al., 2000; Yuan and Neufeld, 2000). Findings of ongoing in vivo studies support that TNF-α and TNF-R1 are also up-regulated following experimental elevation of intraocular pressure (IOP), and TNF-α signaling is involved in the RGC death process during neurodegeneration in ocular hypertensive eyes. TNF-α secreted by stressed-glial cells during the neurodegenerative process of glaucoma can induce RGC death through receptor-mediated caspase cascade, mitochondrial dysfunction, and oxidative damage (Tezel and Wax, 2000a; Tezel and Yang, 2004).
An exciting finding of our earlier in vitro studies was the activation of retinal caspase-8 in response to different stress stimuli evident in glaucomatous eyes (Tezel and Wax, 1999; Tezel and Wax, 2000b). Since caspase-8 is associated with the apoptosis pathway triggered by TNF death receptor binding (Hsu et al., 1996), this finding stimulated interest in the role of TNF-α signaling in glaucoma. Following this initial observation, a series of experiments provided evidence that the TNF-α signaling is indeed involved in the death of RGCs during glaucomatous neurodegeneration. A direct evidence comes from primary co-culture experiments. These experiments proved that following exposure to different glaucomatous stimuli, glial cells adversely affect the survival of co-cultured RGCs through the increased production of TNF-α. A similar neurotoxic effect was replicated by the transfer of the conditioned medium obtained from stressed glia cultures, and inhibition of TNF-α bioactivity by treatment of co-cultures with a neutralizing antibody resulted in a decreased rate of apoptosis in RGCs (Tezel and Wax, 2000a).
Another series of in vitro experiments also revealed that in addition to caspase activity, mitochondrial dysfunction accompanies RGC death induced by TNF-α (Tezel and Yang, 2004). These studies demonstrated that the inhibition of caspase activity is not adequate to block RGC death in primary cultures exposed to TNF-α if the mitochondrial membrane potential is lost and mitochondrial cell death mediators, cytochrome c and apoptosis inducing factor, are released. RGCs exposed to TNF-α also accumulated reactive oxygen species (ROS) over time, and when combined with caspase inhibition, a free radical scavenger treatment reduced the production of ROS and provided an additional increase in RGC survival (Tezel and Yang, 2004). These findings support that in addition to receptor-mediated caspase cascade, RGC death induced by TNF-α also involves both caspase-dependent and caspase-independent components of the mitochondrial cell death pathway and generation of ROS (Figure 1).
Figure 1.
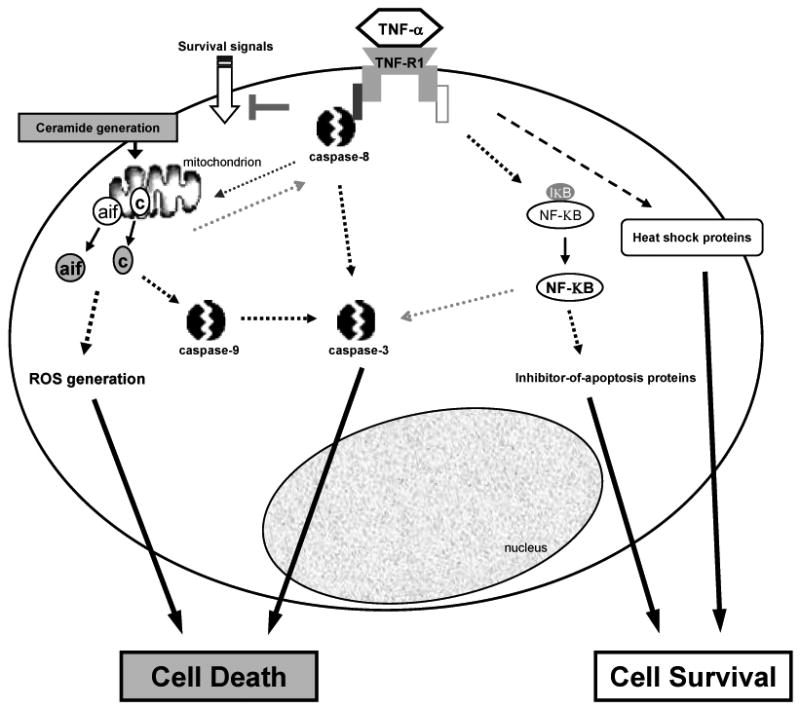
Binding of TNF-α to TNF-R1, a death receptor, can induce RGC death through receptor-mediated caspase activation, and caspase-dependent and -independent components of the mitochondrial cell death pathway, which includes increased generation of ROS leading to oxidative damage. In addition, ceramide generation and silencing of survival signals have been associated with TNF-α-mediated cell death. Opposing these cell-death promoting signals, binding of TNF-R1 can also trigger the activation of survival signals, which include the activation of a transcription factor, NF-KB, whose target genes include inhibitor-of-apoptosis proteins. Another intrinsic protection mechanism activated after TNF receptor binding involves heat shock proteins. A critical balance between a variety of intracellular signaling pathways determines whether a RGC will die or survive the exposure to TNF-α (TNF-α, tumor necrosis factor-alpha; TNF-R1, TNF death receptor; aif, apoptosis inducing factor; c, cytochrome c; ROS, reactive oxygen species; NF-KB, nuclear factor-kappaB; IKB, NF-KB inhibiting protein).
Parallel studies through immunohistochemical analysis of human donor eyes revealed an increased immunolabeling for TNF-α and TNF-R1 in the optic nerve head (Yan et al., 2000) and retina (Tezel et al., 2001) of glaucomatous eyes compared to age-matched controls. Using in situ hybridization, mRNA signals for TNF-α or TNF-R1 were also found to be similarly more intense in glaucomatous eyes relative to controls (Tezel et al., 2001). Up-regulation of TNF-α in the glaucomatous optic nerve head and retina was mostly detectable in glial cells. However, TNF-R1 up-regulation was also prominent in nerve bundles and RGC bodies. The up-regulation of TNF-α and TNF-R1 in glaucomatous tissues supports the association of TNF-α signaling with glaucomatous neurodegeneration. The predominant localization of TNF-R1, a death receptor, to RGCs and their axons indicates that they are sensitive targets for the cytotoxic effects of TNF-α.
A series of in vivo observations also support the involvement of TNF-α signaling in the neurodegenerative process of glaucoma. In vivo studies utilizing a chronic pressure-induced rat model of glaucoma demonstrated retinal caspase activation in ocular hypertensive eyes, which include the activation of caspase-8 (available at www.iovs.org, 2005; 46:E-Abstract 3772). Caspase-8 activation after IOP elevation was also detected by another study using two different rat glaucoma models (McKinnon et al., 2002). Activation of caspase-8 is an important early step following death receptor binding (Hsu et al., 1996), although there is in vitro evidence suggesting that caspase-8 activation may also occur downstream of mitochondria (Slee et al., 1999). The activation of not only the receptor-mediated caspase cascade, but also the TNF-α-mediated mitochondrial cell pathway has been associated with caspase-8 activation, since caspase-8 cleaves a pro-apoptotic member of the bcl-2 family of proteins, Bid, which then participates in the activation of the mitochondrial cell death pathway (Luo et al., 1998). Further studies also indicate Bid-independent mechanisms for the involvement of mitochondria during TNF-α-mediated cell death (Chen et al., 2007).
In vivo experiments also determined the expression and cellular localization of TNF-α and TNF-R1, and IOP-dependent regulation of these molecules in ocular hypertensive rat eyes. Findings of these experiments demonstrated an up-regulation of TNF-α signaling in ocular hypertensive eyes relative to controls. This up-regulation exhibited a close association with the cumulative IOP exposure and neuronal damage (available at www.iovs.org, 2005; 46:E-Abstract 3772). Similar to glaucomatous human eyes (Tezel et al., 2001; Yan et al., 2000), increased TNF-α immunolabeling in ocular hypertensive rat eyes compared with the controls was mainly localized to glial cells. However, retinal TNF-R1 immunolabeling in the ocular hypertensive eyes was most prominent on RGCs and their axons. These findings further support the association of TNF-α signaling with the experimental paradigm of glaucomatous neurodegeneration.
The increased gene expression for TNF-α and TNF-R1 detected in ocular hypertensive eyes is consistent with other studies using gene microarray analysis in experimental models of glaucoma, which have also identified differential regulation of genes associated with TNF-α signaling. For example, a study detected up-regulation of a transcription factor regulating TNF-α gene expression, Litaf, in the ocular hypertensive rat retina (Ahmed et al., 2004). Another study using Affymetrix analysis of rat retinal RNA identified multiple genes differentially regulated in eyes with ocular hypertension or optic nerve transaction, and its findings were also consistent with the participation of TNF-α in glaucomatous injury in association with JNK signaling (available at www.iovs.org, 2007; 48:E-Abstract 3279).
To assess the specific role of TNF death receptor signaling in the induction of RGC death, in vivo studies also utilized another experimental model, the optic nerve crush injury model, in TNF-R1 knockout mice (Tezel et al., 2004). Although not a perfect simulation of glaucomatous conditions, optic nerve degeneration in the crush injury model mimics many of the features of glaucomatous optic nerve degeneration. Most importantly, spreading of damage by secondary degeneration of RGCs is likely similar in crush injury and glaucomatous injury of the optic nerve. Counts of RGCs and their axons 6 weeks after the injury demonstrated that their loss was significantly less in TNF-R1 knockout mice compared with the controls. The most prominent decrease in neuronal loss detected in these animals was beyond the initial 2-week period after the injury. This time period was correlated with the period of glial activation and increased glial immunolabeling for TNF-α in these eyes. No further protection against neuronal loss was detectable in TNF-R1 knockout mice treated with D-JNKI1, which is a specific peptide inhibitor of JNK. However, anti-JNK treatment of control animals provided significant protection against neuronal loss during the same secondary degeneration period. Phospho-JNK immunolabeling of RGCs in control mice subjected to optic nerve crush significantly decreased following their treatment with D-JNKI1, and anti-JNK treatment protected RGCs from degeneration, similar to the lack of TNF-R1 (Tezel et al., 2004). These findings provide evidence that TNF death receptor signaling is involved in the secondary degeneration of RGCs following optic nerve injury and is associated with JNK signaling.
TNF-α has also been suggested to mediate oligodendrocyte death and delayed RGC loss in a mouse model of glaucoma (Nakazawa et al., 2006). Intravitreal TNF-α injections in normal mice mimicked these effects. Conversely, treatment with an antibody neutralizing TNF-α activity or deleting the genes encoding TNF-α or its receptor, TNF-R2, blocked the deleterious effects of ocular hypertension.
Optineurin gene mutations detected in glaucoma patients (Sarfarazi and Rezaie, 2003) provide another line of evidence supporting the role of TNF-α signaling in glaucoma. In addition to TNF-α gene polymorphism detected in different ethnic populations (Lin et al., 2003), a possible interaction between polymorphisms in optineurin and TNF-α genes has been suggested to increase the risk of glaucoma in the Japanese population (Funayama et al., 2004). Optineurin, which is expressed by RGCs (Wang et al., 2007), has been proposed to be associated with TNF-α signaling pathway leading to RGC death based on its direct interaction with adenovirus E3 14.7 kDa protein, which utilizes TNF receptor pathways to mediate apoptosis (Sarfarazi and Rezaie, 2003). However, a mutated form of optineurin, E50K, identified in normal-tension glaucoma patients, loses its ability to translocate to the nucleus and when over-expressed compromises the mitochondrial membrane integrity, resulting in cells that are less fit to survive under stress conditions (De Marco et al., 2006). In a recent study, a glaucoma-associated mutant of optineurin has been shown to selectively induce oxidative stress-mediated RGC death, and optineurin has been suggested to be a likely component of the TNF-α signaling pathway leading to RGC death (Chalasani et al., 2007). More recently, microRNA silencing of optineurin has resulted in markedly enhanced TNF-α-induced nuclear factor kappaB (NF-KB) activity, thereby supporting a physiologic role of optineurin in dampening TNF-α signaling in association with glaucoma (Zhu et al., 2007).
Interplay of TNF-α signaling with other cellular events associated with glaucomatous neurodegeneration
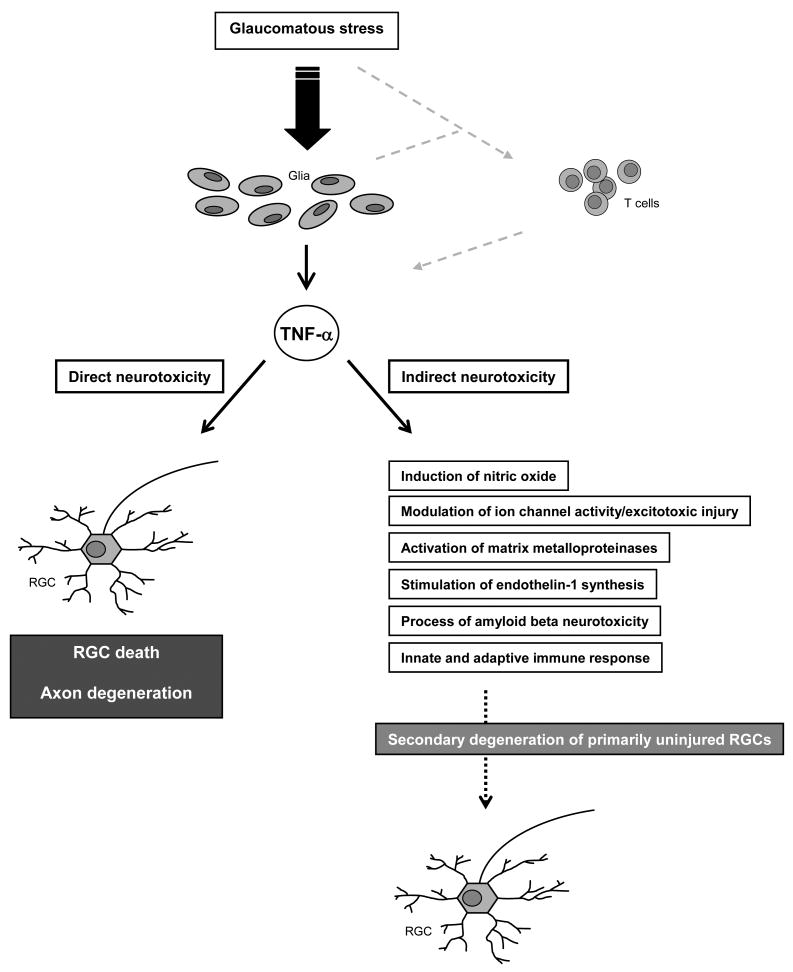
Multiple pathogenic mechanisms, possibly sequentially functioning and interplaying with each other, have been associated with RGC death and optic nerve degeneration in glaucoma. Various intrinsic adaptive/protective mechanisms are also known to be activated in response to glaucomatous stress, and life and death decisions are made by integrating a variety of cell death and survival signals in RGCs. Potential interplay of TNF-α signaling with other cellular events associated with glaucomatous neurodegeneration contributes to the complexity of the picture (Figure 2).
Figure 2.
Glial production of TNF-α is increased in the glaucomatous retina and optic nerve head. In addition to direct neurotoxicity to RGCs and their axons, potential interplay of TNF-α signaling with the other cellular events associated with glaucomatous neurodegeneration likely contributes to secondary degeneration of primarily uninjured RGCs. Glaucomatous tissue stress and activation of the immune-regulatory function of glial cells may also facilitate the initiation of an immune response. TNF-α is one of the most abundant cytokines produced by reactive T cells and appears to play a major role in T cell-mediated neurotoxicity.
Besides caspase activation through receptor-mediated apoptosis signaling and increased generation of ROS through mitochondrial dysfunction, a neurodestructive role of TNF-α in glaucoma may also be associated with its ability to induce glial nitric oxide production (Goureau et al., 1997). This highly potent secondary oxidant has been implicated in glaucomatous injury (Neufeld et al., 1999).
In addition, known interaction of cytokines with the glutamate system is of great importance among different mechanisms possibly involved in TNF-α-induced neurodegeneration. The role of TNF-α in various pathophysiological conditions of the central nervous system has been associated with its interaction with ion channels, as this cytokine can modulate ion channel activity, thereby regulating neuronal excitability, synaptic plasticity, and excitotoxic injury (Pickering et al., 2005). Similar to several pathological conditions which are largely dependent on excessive glutamate release and subsequent over-stimulation of the NMDA receptor, glutamate excitoxicity has been associated with glaucomatous neurodegeneration (Vorwerk et al., 1999).
TNF-α also activates matrix metalloproteinases (Gottschall and Yu, 1995), which are not only involved in tissue remodeling in the glaucomatous optic nerve head (Yan et al., 2000), but have also be associated with neurotoxicity. Retinal matrix metalloproteinase activity has been related to RGC death including that induced by IOP elevation (Guo et al., 2005). Although direct pathogenic role of matrix metalloproteinases in glaucoma is unclear, these proteases have been suggested to play a role in glutamate receptor processing thereby predisposing RGCs to damage. More relevant to TNF-α-mediated RGC death, the release of TNF-α from its membrane-bound precursor is also a matrix metalloproteinase-dependent process. TNF-α, which is produced as a biologically inactive trans-membrane precursor, pro-TNF-α, must be enzymatically cleaved to release the 17 kDa active form of TNF-α. Matrix metalloproteinases that catalyze the normal turnover of extracellular matrix molecules can also function as TNF-α-converting enzyme to release the soluble TNF-α from its membrane-bound precursor (Black et al., 1997).
Endothelin-1 (ET-1), a potent vasoactive peptide, which can produce optic nerve damage analogous to that in glaucoma, has been associated with glaucomatous neurodegeneration, since ET-1 levels are increased in aqueous and vitreous humor in patients with glaucoma and animal models of glaucoma (Prasanna et al., 2003). TNF-α is a potent stimulator of ET-1 synthesis and secretion in several ocular cell types, including optic nerve head astrocytes (Desai et al., 2004).
Proposed involvement of amyloid-beta in glaucomatous neurodegeneration, mimicking the Alzheimer's Disease at the molecular level, has also been linked with the TNF death receptor signaling. Since the activation of amyloid-beta requires caspase-8 activity by cross-linking with TNF family death receptors (Ivins et al., 1999), based on the observation of retinal caspase-8 activation and abnormal processing of the retinal amyloid precursor protein in ocular hypertensive rats (McKinnon et al., 2002), chronic amyloid-beta neurotoxicity has been associated with RGC death in glaucoma.
Another association of TNF-α with glaucomatous neurodegeneration appears to be related to its key role in immune response with many effects ranging from inflammation to apoptosis. Immune privilege of the central nervous system makes it crucial that glial cells are capable of responding rapidly to any injurious condition or damage. It is now generally accepted that these resident cells with immunoregulatory functions, particularly the microglia, play a central role in the initiation of immune response, which includes increased production of cytokines like TNF-α. Pertinent to its originally described function in the anti-tumoral response of the immune system, TNF-α is a mediator of the innate immune system. Cytokine response provides an intrinsic mechanism facilitating tissue repair; however, avoiding the initiation of an autoimmune neurodegenerative process is critical, since local production of cytokines, especially during injury and disease, makes the neuronal tissue more vulnerable to harmful effects of the innate immune response. Although innate immunity may initially participate in healing the injured tissue by eliminating cell debris and potentially toxic protein aggregates, uncontrolled and continued activity may lead to neurodestructive events. It is evident that the immune-regulatory function of optic nerve head and retinal glia is altered in glaucomatous eyes (Tezel et al., 2003; Yang et al., 2001), which can initiate an aberrant activity of the immune system, thereby facilitating primary and/or secondary degeneration of RGCs in glaucoma (Tezel and Wax, 2004). For example, in addition to direct neurototoxicity of cytokines secreted by reactive glia in glaucomatous eyes, cytokine-stimulated microglia also generate copious amounts of reactive oxygen and reactive nitrogen species, creating additional stress on neurons. Conversely, oxidants can stimulate pro-inflammatory gene transcription in glia, as well as serving as co-stimulatory molecules required for antigen presentation to the immune system (Tezel et al., 2007). TNF-α appears to be one of the key cytokines involved in the vicious cycle of the immune response with neurodestructive consequences. In respect to adaptive immune response evident in glaucoma patients, this most abundant cytokine produced by reactive T cells may also participate in T cell-mediated neuronal injury. There is evidence supporting that any neurotoxicity through an adaptive immune response mediated by T cells is likely be associated with TNF receptor family signaling, although related evidence needs further verification (available at www.iovs.org, 2006; 47:E-Abstract 1828).
Involvement of TNF-α in innate immune response may also have implications in axonal degeneration in glaucomatous eyes. Despite the fact that the initial site of injury is unclear, glaucomatous neurodegeneration exhibits widespread damage through a set of compartmentalized subcellular processes, which eventually involve RGC soma and dendrites in the retina and axons in the optic nerve. Axonal degeneration in glaucoma may evidently extend from the eye to synapses in the brain, possibly through an active and regulated self-destruction program, such as Wallerian degeneration. There is evidence supporting that TNF-α signaling may also be associated with axonal dysfunction and loss by disintegrating axons to initiate the innate immune response that characterizes Wallerian degeneration. TNF-α and its receptors are differentially regulated during Wallerian degeneration (Shamash et al., 2002). One of the functions of TNF-α during Wallerian degeneration has been suggested to be the induction of macrophage recruitment for debris removal (Liefner et al., 2000). TNF-α-induced matrix metalloproteinase activity has also been shown to promote macrophage recruitment into injured nerves during delayed axonal degeneration (Shubayev et al., 2006). Indeed, TNF-α knockout mice exhibit slower macrophage migration into the injured nerve and delayed myelin clearance during Wallerian degeneration (Liefner et al., 2000). However, another study utilizing TNF-α knockout mice has shown a significantly higher number of preserved axons within the degenerating distal nerve stump (Siebert and Bruck, 2003), thereby suggesting that despite the debris removal function of invading macrophages, TNF-α secreted by these cells may also mediate axonal damage. It is highly warranted to determine the pathogenic importance of TNF-α signaling for axon degeneration in glaucoma, since in addition to increased TNF-α production of neighboring cells, optic nerve axons prominently express TNF-R1, which is up-regulated in glaucoma (Tezel et al., 2001; Yan et al., 2000; Yuan and Neufeld, 2000).
Signaling pathways promoting neurodestructive or neuroprotective effects of TNF-α
TNF-α is generally assumed to be a cytokine exacerbating and sustaining neurodegenerative processes, while many other cytokines mostly promote regeneration, protection, and cell survival. Although TNF-R1 is able to signal cell death via its cytoplasmic death domain in a variety of cell types, including neurons, and despite massive evidence supporting numerous neurotoxic consequences of TNF-α signaling, this fascinating cytokine does not always cause neuronal cell death, but can also exert protective functions (Rath and Aggarwal, 1999; Wajant et al., 2003).
The prominent paradoxical role of TNF-α in the central nervous system has generally been attributed to neuromodulatory function of this cytokine in the normal brain, but neurotoxic features under injurious conditions. Bioactivity of this cytokine can be influenced considerably by the functional state and type of cells, and the concentration and duration of exposure. Diverse cellular effects of TNF-α may also be dependent on the combination of other cytokines present at the same time, and even the temporal sequence of several cytokines acting on the same cell. Besides these parameters determining the net in vivo effect of TNF-α, diverse actions of TNF-α have also been associated with the composition of TNF receptors. Constitutive expression of TNF receptors, which are present on most cell types throughout the brain, exhibits rapid up-regulation in response to injury, and the dual role of TNF-α has been associated with the opposing actions of TNF receptors. Two main subgroups of the TNF receptor superfamily, TNF-R1 (CD120a; p55/60) and TNF-R2 (CD120b; p75/80), bind both the membrane-bound and soluble forms of TNF-α, as well as another secreted molecule, lymphotoxin-α. However, soluble TNF-α predominantly stimulates TNF-R1 and has limited signaling capacities on TNF-R2. TNF-R1 is constitutively expressed in most tissues, whereas expression of TNF-R2 is typically found and highly regulated in cells of the immune system. In the vast majority of cells, TNF-R1 appears to be the key mediator of TNF-α signaling, whereas TNF-R2 seems to play a major role in the lymphoid system. The extracellular domains of these receptors can be proteolytically cleaved, yielding soluble receptor fragments, which are considered to be physiological mechanisms that scavenge excess TNF-α, and have been detected in the vitreous of normal subjects, as well as in patients with retinal diseases (Sippy et al., 1996). Regarding intracellular domains, TNF-R1 contains a protein-protein interaction domain, called death domain, which recruits different signaling proteins involved in the apoptotic cascade. After TNF-α-induced formation of a multiprotein signaling complex at the cell membrane, the cytoplasmic tail of this complex binds to a death domain adaptor protein (TRADD, TNF receptor-associated death domain protein), which then binds to a receptor-interacting protein (FLICE) containing a protease domain (caspase-8) and initiates the apoptotic caspase cascade directly. TNF-R1 is also a potent activator of gene expression via indirect recruitment of members of the TNF receptor-associated factor (TRAF) family. Interestingly, TNF-R2 directly recruits TRAF2, inducing gene expression through a cross-talk relation with TNF-R1.
Based on current understanding of TNF-α signaling, it is generally accepted that TNF-R1 signaling promotes neuronal cell death while TNF-R2 mediates proliferative and regulatory signals promoting cell survival (Shohami et al., 1999). Counteracting functions of these TNF receptors in neuronal tissues are also evident in the retina. For example, a recent study utilizing a murine model of retinal ischemia has demonstrated that TNF-R1 aggravates neuronal cell death, whereas TNF-R2 promotes neuroprotection via activation of PKB/Akt (Fontaine et al., 2002). However, growing evidence from many studies elucidating the mechanisms of TNF-α signaling pathways over the past three decades now supports that the diverse bioactivities of TNF-α can actually be mediated through each of these receptors. Evidently, TNF-α through TNF-R1 and also through the non-death domain-containing receptor, TNF-R2, can promote cell death, while binding any of these receptors can promote cell survival (Rath and Aggarwal, 1999; Wajant et al., 2003).
It is fascinating how the signaling through TNF-R2 not containing death domain can induce cell death. Many evidences now support that the stimulation of TNF-R2 does not directly engage the apoptotic program, but the induction of endogenously produced membrane-bound TNF-α can subsequently activate TNF-R1. In addition to such a TRADD-independent signaling mechanism, a TNF-R2-dependent process can lead to depletion of TRAF2 and TRAF2-associated protective factors. TNF-R2 may also compete with TNF-R1 for the recruitment of newly synthesized TRAF2-bound anti-apoptotic factors, thereby promoting the formation of a caspase activation rather than production of anti-apoptotic factors (Wajant et al., 2003). Furthermore, TNFR-2 signaling can potentiate programmed necrosis via TNFR-1 and caspase inhibition (Chan et al., 2003); however, the relevance of such a cell death mechanism to glaucomatous neurodegeneration is unclear.
Opposing these cell-death promoting signals, binding of two TNF receptors can also trigger the activation of survival signals (Figure 1). Although many details are yet unclear, an important transcription factor, NF-KB, appears to be a regulator of the neuronal survival programs induced by TNF-α signaling (Baeuerle and Baltimore, 1996). Target genes of this important transcription factor include those that encode inhibitor-of-apoptosis proteins (IAPs), which provide an intrinsic protection mechanism against caspase activity (Deveraux et al., 1997). NF-KB may also induce cell survival through the induction of some members of the Bcl-2 family and through the inhibition of JNK (Tang et al., 2001).
Another cell survival-promoting consequence of TNF-α signaling is the induction of heat shock proteins (HSPs) in cross-talk with NF-KB signaling (Nakano et al., 1996). A signaling mechanism similar to TNF-R1 and TNF-R2 cross-talk in inducing cell death has also been proposed for the induction of heat shock proteins as a survival-promoting consequence of TNF-α signaling. TNF-R1 has been shown to be required for stress-induced HSP70 expression, and an intracellular role of TNF-R1 through a TNF-α-independent pathway has been described for the production of HSP70 (Heimbach et al., 2001).
Thus, bioactivities of TNF-α are exerted by activating a cross-talk relationship between multiple signaling pathways (Shohami et al., 1999; Wajant et al., 2003). While the activation of caspases promotes cell death, activation of NF-KB-dependent genes inhibits apoptosis and promotes the survival and proliferation effects of TNF-α (Beg and Baltimore, 1996). These opposing pathways are inhibitory to each other, and a critical balance determines the predominant in vivo bioactivity of TNF-α.
Diverse consequences of TNF-α signaling in glaucoma
Differential cellular response to TNF-α is best exemplified by differential susceptibilities of RGC and glia to glaucomatous injury. Primary co-culture studies demonstrate that RGCs undergo TNF-α-mediated apoptosis following exposure to glaucomatous stimuli, but glial cells survive the same condition (Tezel and Wax, 2000a). This is also in agreement with in vivo and clinical observations that glaucoma is a selective disease of neurons, specifically the RGCs and their axons. Despite a selective vulnerability of RGCs to primary and/or secondary degeneration in glaucoma, glial cells not only survive the glaucomatous stressors, but also exhibit an activated phenotype (Tezel et al., 2003). However, the current understanding of factors determining diverse cellular responses to chronic and widespread tissue stress in glaucoma, such as the death of neurons but the sparing and activation of glia, are limited.
One explanation for relative protection of glia against TNF-α cytotoxicity could be differential expression of different receptor subtypes. However, although the expression level of TNF receptors may vary between RGCs and glial cells, they are expressed by both cell types (Tezel et al., 2001). Considerable evidence rather suggests that the diverse responses of RGCs and glial cells to TNF-α are associated with differential regulation of many signaling molecules involved in TNF-α signaling between these cell types. Since the functional activation of several adaptive/protective or pathogenic proteins involved in TNF-α signaling is known to require phosphorylation, kinase activity appears to be crucial in determining the ultimate cell fate in response to TNF-α. For example, similar to other cell types, functional activation of TNF-α-induced survival signaling through NF-KB in RGCs and glia is phosphorylation dependent (Tezel and Yang, 2005). On the other hand, JNK activity following optic nerve injury has been associated with the amplification of TNF-α-mediated cell death signaling, thereby switching the life balance toward cell death (Tezel et al., 2004). Findings of a recent histopathological study in human donor eyes support a prominent and persistent activation of glial ERK signaling in glaucomatous retinas, although the most prominent immunolabeling for phospho-JNK or phospho-p38 in these eyes is localized to RGCs (Tezel et al., 2003). Such a differential kinase activity between RGCs and glia appears to be associated with the different susceptibility of these cell types to TNF-α, since a critical balance between the survival promoting ERK pathway and the death promoting JNK and p38 pathways regulates the cell fate (Xia et al., 1995).
Consistent with immunohistochemical observations, a recent comparative gene array analysis revealed differential regulation of MAPK or NF-KB signaling pathways between RGCs and glial cells exposed to TNF-α (Tezel and Yang, 2005). Findings of this in vitro study support that phosphorylation cascades are important components of TNF-α signaling and that a crosstalk between death or survival promoting signals determines whether a RGC should live or die in response to TNF-α. Besides the differential activity of MAPK and NF-KB signaling between RGCs and glial cells exposed to TNF-α, differentially regulated genes in RGCs and glial cells exposed to TNF-α also included HSP27. Up-regulation of this chaperonin protein selectively in glial cells suggests that various intrinsic adaptive/protective mechanisms, including HSPs (as well as MAPK and NF-KB signaling pathways), are important for relative protection of these cells against TNF-α-mediated cell death. Better understanding of the factors determining diverse responses of RGCs and glia to TNF-α can provide clues for therapeutic manipulation of TNF-α signaling for the gain of RGC survival, similar to that naturally seen in glia.
Conclusions on neuroprotective treatment targets in glaucoma
Growing evidence supports that TNF-α-mediated neurotoxicity is a component of the neurodegenerative injury in glaucoma. It is clear that a critical balance between diverse signaling pathways determines RGC fate after increased exposure to TNF-α in glaucomatous eyes. Based on our current understanding of diverse bioactivities of TNF-α signaling, which can promote both cell death and survival, specific inhibition of cell death signaling and/or the amplification of survival signaling, rather than the inhibition of death receptor binding, is expected to accomplish RGC protection. Since such strategies are expected to not impede the survival promoting signaling triggered by TNF-α, their neuroprotective outcome should be superior. Present evidence of TNF-α-mediated cell death signaling in RGCs suggests that neuroprotective strategies targeting TNF-α signaling for RGC rescue should include tools to block the caspase cascade and also improve the ability of these neurons to survive cytotoxic consequences of mitochondrial dysfunction. In addition, several molecules involved in TNF-α signaling, such as MAPKs, NF-KB, and HSPs, appear to be promising treatment targets for manipulation of the life balance between cell death and survival signals. The neuroprotective ability of anti-TNF-α strategies in targeting axonal injury also needs further studies. Because the main source of TNF-α is the glial cells, targeting key glial activation pathways should also be determined for attenuation of the glia-associated component of neurodegeneration. Evidently, multiple neurodegenerative processes are involved in RGC death and current strategies are not expected to individually provide complete neuroprotection in glaucoma patients. Elucidation of specific signaling pathways is therefore crucial to define new treatment targets for effective neuroprotective interventions. Ongoing efforts utilizing targeted proteomic approaches are expected to identify signaling molecules associated with glaucomatous neurodegeneration. RNA interference technology also offers a powerful tool through which specific siRNAs can be used to determine functional significance of newly identified molecules as treatment targets. This technology can also serve as an intervention strategy along with other genomic or pharmacologic treatments to provide neuroprotection in glaucoma.
Acknowledgments
Dr. Tezel's work is supported by National Eye Institute (2R01 EY013813, 1R01 EY017131, R24 EY015636), Bethesda, MD; and an unrestricted grant to University of Louisville Department of Ophthalmology & Visual Sciences from Research to Prevent Blindness Inc., New York, NY.
References
- Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45:1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Chalasani ML, Radha V, Gupta V, Agarwal N, Balasubramanian D, Swarup G. A glaucoma-associated mutant of optineurin selectively induces death of retinal ganglion cells which is inhibited by antioxidants. Invest Ophthalmol Vis Sci. 2007;48:1607–1614. doi: 10.1167/iovs.06-0834. [DOI] [PubMed] [Google Scholar]
- Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Chen X, Ding WX, Ni HM, Gao W, Shi YH, Gambotto AA, Fan J, Beg AA, Yin XM. Bid-independent mitochondrial activation in tumor necrosis factor alpha-induced apoptosis and liver injury. Mol Cell Biol. 2007;27:541–553. doi: 10.1128/MCB.01166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco N, Buono M, Troise F, Diez-Roux G. Optineurin increases cell survival and translocates to the nucleus in a Rab8-dependent manner upon an apoptotic stimulus. J Biol Chem. 2006;281:16147–16156. doi: 10.1074/jbc.M601467200. [DOI] [PubMed] [Google Scholar]
- Desai D, He S, Yorio T, Krishnamoorthy RR, Prasanna G. Hypoxia augments TNF-alpha-mediated endothelin-1 release and cell proliferation in human optic nerve head astrocytes. Biochem Biophys Res Commun. 2004;318:642–648. doi: 10.1016/j.bbrc.2004.04.073. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Downen M, Amaral TD, Hua LL, Zhao ML, Lee SC. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia. 1999;28:114–127. [PubMed] [Google Scholar]
- Dressler KA, Mathias S, Kolesnick RN. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: Opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama T, Ishikawa K, Ohtake Y, Tanino T, Kurosaka D, Kimura I, Suzuki K, Ideta H, Nakamoto K, Yasuda N, Fujimaki T, Murakami A, Asaoka R, Hotta Y, Tanihara H, Kanamoto T, Mishima H, Fukuchi T, Abe H, Iwata T, Shimada N, Kudoh J, Shimizu N, Mashima Y. Variants in optineurin gene and their association with tumor necrosis factor-{alpha} polymorphisms in Japanese patients with glaucoma. Invest Ophthalmol Vis Sci. 2004;45:4359–4367. doi: 10.1167/iovs.03-1403. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Yu X. Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurochem. 1995;64:1513–1520. doi: 10.1046/j.1471-4159.1995.64041513.x. [DOI] [PubMed] [Google Scholar]
- Goureau O, Amiot F, Dautry F, Courtois Y. Control of nitric oxide production by endogenous TNF-alpha in mouse retinal pigmented epithelial and Muller glial cells. Biochem Biophys Res Commun. 1997;240:132–135. doi: 10.1006/bbrc.1997.7581. [DOI] [PubMed] [Google Scholar]
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach JK, Reznikov LL, Calkins CM, Robinson TN, Dinarello CA, Harken AH, Meng X. TNF receptor I is required for induction of macrophage heat shock protein 70. Am J Physiol Cell Physiol. 2001;281:C241–247. doi: 10.1152/ajpcell.2001.281.1.C241. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Thornton PL, Rohn TT, Cotman CW. Neuronal apoptosis induced by beta-amyloid is mediated by caspase-8. Neurobiol Dis. 1999;6:440–449. doi: 10.1006/nbdi.1999.0268. [DOI] [PubMed] [Google Scholar]
- Kitaoka Y, Kwong JM, Ross-Cisneros FN, Wang J, Tsai RK, Sadun AA, Lam TT. TNF-alpha-induced optic nerve degeneration and nuclear factor-kappaB p65. Invest Ophthalmol Vis Sci. 2006;47:1448–1457. doi: 10.1167/iovs.05-0299. [DOI] [PubMed] [Google Scholar]
- Liefner M, Siebert H, Sachse T, Michel U, Kollias G, Bruck W. The role of TNF-alpha during Wallerian degeneration. J Neuroimmunol. 2000;108:147–152. doi: 10.1016/s0165-5728(00)00262-9. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Tsai FJ, Chen WC, Shi YR, Hsu Y, Tsai SW. Association of tumour necrosis factor alpha -308 gene polymorphism with primary open-angle glaucoma in Chinese. Eye. 2003;17:31–34. doi: 10.1038/sj.eye.6700227. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, Merges CA, Pease ME, Kerrigan DF, Ransom NL, Tahzib NG, Reitsamer HA, Levkovitch-Verbin H, Quigley HA, Zack DJ. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci. 2002;43:1077–1087. [PubMed] [Google Scholar]
- Nakano M, Knowlton AA, Yokoyama T, Lesslauer W, Mann DL. Tumor necrosis factor-alpha-induced expression of heat shock protein 72 in adult feline cardiac myocytes. Am J Physiol. 1996;270:H1231–1239. doi: 10.1152/ajpheart.1996.270.4.H1231. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A. 1999;96:9944–9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Narayan S, Krishnamoorthy RR, Yorio T. Eyeing endothelins: a cellular perspective. Mol Cell Biochem. 2003;253:71–88. doi: 10.1023/a:1026005418874. [DOI] [PubMed] [Google Scholar]
- Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- Sarfarazi M, Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol Clin North Am. 2003;16:529–541. doi: 10.1016/s0896-1549(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10:119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31:407–415. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert H, Bruck W. The role of cytokines and adhesion molecules in axon degeneration after peripheral nerve axotomy: a study in different knockout mice. Brain Res. 2003;960:152–156. doi: 10.1016/s0006-8993(02)03806-4. [DOI] [PubMed] [Google Scholar]
- Sippy BD, Hofman FM, Wright AD, He S, Ryan SJ, Hinton DR. Soluble tumor necrosis factor receptors are present in human vitreous and shed by retinal pigment epithelial cells. Exp Eye Res. 1996;63:311–317. doi: 10.1006/exer.1996.0120. [DOI] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–3033. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- Tezel G, Li LY, Patil RV, Wax MB. Tumor necrosis factor-alpha and its receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42:1787–1794. [PubMed] [Google Scholar]
- Tezel G, Wax MB. Inhibition of caspase activity in retinal cell apoptosis induced by various stimuli in vitro. Invest Ophthalmol Vis Sci. 1999;40:2660–2667. [PubMed] [Google Scholar]
- Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000a;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J Neurosci. 2000b;20:3552–3562. doi: 10.1523/JNEUROSCI.20-10-03552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. The immune system and glaucoma. Curr Opin Ophthalmol. 2004;15:80–84. doi: 10.1097/00055735-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 2004;45:4049–4059. doi: 10.1167/iovs.04-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X. Comparative gene array analysis of TNF-alpha-induced MAPK and NF-kappaB signaling pathways between retinal ganglion cells and glial cells. Exp Eye Res. 2005;81:207–217. doi: 10.1016/j.exer.2005.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci. 2007;48:705–714. doi: 10.1167/iovs.06-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Yang J, Wax MB. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004;996:202–212. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Venters HD, Dantzer R, Kelley KW. A new concept in neurodegeneration: TNFalpha is a silencer of survival signals. Trends Neurosci. 2000;23:175–180. doi: 10.1016/s0166-2236(99)01533-7. [DOI] [PubMed] [Google Scholar]
- Vorwerk CK, Gorla MS, Dreyer EB. An experimental basis for implicating excitotoxicity in glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43 1:S142–150. doi: 10.1016/s0039-6257(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Wang JT, Kunzevitzky NJ, Dugas JC, Cameron M, Barres BA, Goldberg JL. Disease gene candidates revealed by expression profiling of retinal ganglion cell development. J Neurosci. 2007;27:8593–8603. doi: 10.1523/JNEUROSCI.4488-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yan X, Tezel G, Wax MB, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 2000;118:666–673. doi: 10.1001/archopht.118.5.666. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang P, Tezel G, Patil RV, Hernandez MR, Wax MB. Induction of HLA-DR expression in human lamina cribrosa astrocytes by cytokines and simulated ischemia. Invest Ophthalmol Vis Sci. 2001;42:365–371. [PubMed] [Google Scholar]
- Yuan L, Neufeld AH. Tumor necrosis factor-alpha: A potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50. [PubMed] [Google Scholar]
- Zhu G, Wu CJ, Zhao Y, Ashwell JD. Optineurin negatively regulates TNFalpha-induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr Biol. 2007;17:1438–1443. doi: 10.1016/j.cub.2007.07.041. [DOI] [PubMed] [Google Scholar]