Abstract
The synaptic signaling mechanisms mediating the behavioral effects of ethanol (EtOH) remain poorly understood. Post-synaptic density 95 (PSD-95, SAP-90, Dlg4) is a key orchestrator of N-methyl-D-aspartate receptors (NMDAR) and glutamatergic synapses, which are known to be major sites of EtOH’s behavioral actions. However, the potential contribution of PSD-95 to EtOH-related behaviors has not been established. Here, we evaluated knockout (KO) mice lacking PSD-95 for multiple measures of sensitivity to the acute intoxicating effects of EtOH (ataxia, hypothermia, sedation/hypnosis), EtOH drinking under conditions of free access and following deprivation, acquisition and long-term retention of EtOH conditioned place preference (CPP) (and lithium chloride-induced conditioned taste aversion), and intoxication-potentiating responses to NMDAR antagonism. PSD-95 KO exhibited increased sensitivity to the sedative/hypnotic, but not ataxic or hypothermic, effects of acute EtOH relative to wild-type controls (WT). PSD-95 KO consumed less EtOH than WT, particularly at higher EtOH concentrations, although increases in KO drinking could be induced by concentration-fading and deprivation. PSD-95 KO showed normal EtOH CPP 1 day after conditioning, but showed significant aversion 2 weeks later. Lithium chloride-induced taste aversion was impaired in PSD-95 KO at both time points. Finally, the EtOH-potentiating effects of the NMDAR antagonist MK-801 were intact in PSD-95 KO at the dose tested. These data reveal a major, novel role for PSD-95 in mediating EtOH behaviors, and add to growing evidence that PSD-95 is a key mediator of the effects of multiple abused drugs.
Keywords: Alcohol, alcoholism, glutamate, MAGUK, mouse, synapse
INTRODUCTION
Ethanol (EtOH) acts an allosteric inhibitor of N-methyl-D-aspartate receptors (NMDAR) in vitro at intoxicating doses, likely via direct receptor occupancy and actions on gating, and receptor phosphorylation (Lovinger, White & Weight 1989; Woodward 2000). Behaviorally, NMDAR antagonists mimic the subjective feelings of intoxication in humans, substitute for the discriminative stimulus effects and exacerbate acute intoxicating effects of EtOH in rodents (Gass & Olive 2008). With chronic exposure to EtOH, there is an upregulation of NMDAR protein, synaptic NMDAR clustering and NMDAR-mediated synaptic currents (Gass & Olive 2008).
The downstream signaling events that mediate EtOH’s acute and chronic actions at NMDARs have not been fully elucidated (Chandler 2003; Ron 2004; Newton & Messing 2006). NMDARs form macromolecular complexes at the post-synaptic density with multiple intracellular proteins. The cytoplasmic C-terminal tail of NMDAR subunits binds to proteins within the density, notably the membrane-associated guanylate kinase (MAGUK) family including PSD-95 post-synaptic density (Dlg4) (Coba et al. 2009). PSD-95 supports the localization, clustering and subunit expression profile of NMDARs, and plays an important role in both NMDAR-mediated and L-alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid ionotropic receptors (AMPAR)-mediated synaptic plasticity (Kim & Sheng 2004; Malenka & Bear 2004; Elias & Nicoll 2007).
These functions of PSD-95 are notable in the context of current theories that mechanisms supporting synaptic plasticity are co-opted by drugs of abuse (Everitt & Robbins 2005; Kalivas & O’Brien 2008). Interestingly, there is evidence that PSD-95 might contribute to the actions of various classes of abused drugs. PSD-95 mRNA and protein was reduced in mouse striatum following repeated cocaine treatment, while gene knockout (KO) of PSD-95 potentiated the hyperactivity-induced effects of acute cocaine and prevented the development of cocaine locomotor sensitization (Yao et al. 2004). In addition, PSD-95 KO exhibited attenuation of the [(2,5-dimethoxy-4-iodophenyl)-2-aminopropane] (DOI)-induced head twitch response; a behavioral measure of the effects of hallucinogens in rodents (Abbas et al. 2009). Carpenter-Hyland and Chandler have demonstrated that EtOH application increased synaptic clustering of PSD-95 in rat hippocampus cultures in a manner that was prevented by dispersal of synaptic PSD-95 (via blockade of palmitate cycling), or by co-application of NMDA (Carpenter-Hyland & Chandler 2006). However, while these findings demonstrate that PSD-95 is a target for certain drugs of abuse, including EtOH, the potential role of PSD-95 in mediating EtOH’s behavioral actions has not been directly studied.
The lack of pre-clinical research on PSD-95 is in part due to the absence of pharmacological compounds with selective actions at PSD-95 to utilize as research tools in vivo. Therefore, in the present study we employed a gene mutant approach. Using a recently generated mutant mouse with complete functional excision of PSD-95, we examined the role of PSD-95 in mediating behavioral effects of EtOH. We phenotyped PSD-95 KO for sensitivity to the acute intoxicating effects of EtOH. We then tested for reward-related properties of EtOH, by measuring basal and deprivation-induced EtOH drinking in PSD-95 KO, and by examining the ability of the KOs to form and retain EtOH conditioned place preference (CPP) [and lithium chloride-induced conditioned taste aversion (LiCl CTA) for comparison]. Next, to explore possible mechanisms underlying altered EtOH behaviors in PSD-95 KO, we tested for in vivo responses to the EtOH potentiating effects of NMDAR antagonism (Shen & Phillips 1998; Gass & Olive 2008; Chen & Holmes 2009).
MATERIALS AND METHODS
Subjects
PSD-95 KO mice were generated by targeted deletion of the guanylate kinase (GK) domain. This mutation results in near total absence of PSD-95 mRNA and loss of any detectable PSD-95 protein (Arbuckle and Grant, unpublished observations and Yao et al. 2004). For the current study, the mutant mice were backcrossed >10 generations onto a C57BL/6 background (~95% congenicity confirmed by genome scan: Feyder et al. 2010). To avoid potential parental genotype influences on early development (Millstein et al. 2006), mice were bred from heterozygous mutant (HET) × HET parents at The Jackson Laboratory (Bar Harbor, ME, USA) and transported to the NIH at ~8 weeks of age. Males and females were tested (age range 9–32 weeks), and as there were no sex differences observed, data were collapsed across sex. Procedures were approved by the NIAAA Animal Care and Use Committee and followed NIH guidelines described in ‘Using Animals in Intramural Research’.
EtOH intoxication
Mice were tested for EtOH-induced ataxia, hypothermia and sedation/hypnosis, in that order, with an interval of at least 1 week between tests, as previously described (Chen & Holmes 2009). This regimen is not expected to produce long-term tolerance to EtOH’s effects (Crabbe et al. 2008).
EtOH-induced ataxia
EtOH-induced ataxia was assessed using the accelerating rotarod as previously described (Rustay, Wahlsten & Crabbe 2003; Hefner & Holmes 2007). The apparatus was a Med Associates Inc. (St Altons, VT, USA) rotarod typically used for testing rats (model ENV-577). The 7-cm-diameter dowel was covered with 320 grit sandpaper to provide a uniform surface that prevented mice gripping the rubberized dowel. Mice were placed on the rotarod dowel that was then accelerated at a constant rate of 8 rpm/minute up to 40 rpm. The latency to fall to the floor 10.5 cm below was automatically recorded by photocell beams, with a maximum cutoff latency of 300 seconds. Mice first received 10 consecutive training trials separated by 30-second inter-trial intervals. Genotype × trial effects were analyzed using analysis of variance (ANOVA), with repeated measures for trial, and Newman Keuls post hoc tests.
One day after training, there was a baseline acclimation trial followed by two additional baseline trials (average = pre-drug performance). Mice were then injected intraperitoneally (i.p.) with 1.75 g/kg EtOH. For this (and the two assays below), EtOH (200 proof) was prepared in 0.9% saline to produce 20% (v/v) solutions and injected i.p. with the dose determined by manipulating the volume of injection. Thirty minutes after EtOH injection, there was one acclimation trial followed by two test trials (average = post-drug performance). The dependent measure was the difference in pre- versus post-drug performance (= delta latency) and genotypes were compared using Student’s t-test.
EtOH-induced hypothermia
EtOH-induced hypothermia was tested as previously described (Hefner & Holmes 2007). Ambient room temperature was 23°C. Basal core body temperature was first measured by inserting a Thermalert TH-5 thermometer (Physitemp, Clifton, NJ, USA) 2 cm into the rectum until a stable reading was obtained. Mice were then injected with 3.0 g/kg EtOH and temperature was measured 30, 60, 90 and 120 minutes later to provide an average post-EtOH measure. The difference between pre-EtOH and post-EtOH temperature was taken as the dependent measure (= delta temperature) and genotypes were compared using Student’s t-test.
EtOH- and pentobarbital-induced sedation/hypnosis
EtOH-induced sedation/hypnosis was assessed as previously described (Daws et al. 2006). Mice were injected with 3.0 g/kg EtOH and immediately placed into the supine position in a ‘V’-shaped chamber. Duration of the loss of the righting reflex (LORR) was measured as the time from injection to recovery (turning onto all four paws twice in 30 seconds after initial self-righting) and genotypes were compared using Student’s t-test. The sedative/hypnotic effects of 30 mg/kg of pentobarbital (Sigma, St. Louis, MO, USA) were tested in the same way. This dose was chosen as an effective sedative/hypnotic dose based upon our previous findings in C57BL/6J mice (Boyce-Rustay, Cameron & Holmes 2007).
EtOH metabolism
EtOH metabolism was assessed by measuring blood ethanol concentrations (BECs) 5, 30, 60 and 240 minutes following injection with 3.5 g/kg EtOH. To avoid trauma to any one region and to conform to local ACUC regulations, blood samples were taken from the submandibular vein at 5 and 30 minutes, from the tail at 60 minutes, and from the trunk (after rapid cervical dislocation and rapid decapitation) at 240 minutes. BECs were measured using the Analox AM1 Alcohol Analyzer (Analox Instruments USA Inc, Lunenburg, MA, USA). Genotype × timepoint effects were analyzed using ANOVA, with repeated measures for timepoint.
Basal and deprivation-induced EtOH drinking
Basal EtOH drinking (two-bottle choice)
Two-bottle free choice EtOH drinking was measured as previously described (Boyce-Rustay & Holmes 2006). Mice were individually housed in ‘Space Saver’ cages (Model 1145T, Tecniplast, Buguggiate, Italy) with lids fitted for two fluid bottles [Model #1145T482SUDB—Polysulfone cage-top to accommodate two water bottles (top flow design; Tecniplast)]. Mice were offered a choice between two bottles for 12 days, one containing a 10% EtOH solution and the other containing tap water. Food was available ad libitum throughout all drinking experiments. Drinking was measured every 2 days, correcting for evaporation and spillage (i.e. the average loss of fluid measured from bottles in an empty ‘dummy’ cage was subtracted from the amount ‘consumed’). The left/right position of the bottles was switched every 2 days to control for any side bias. These data were used to calculate EtOH consumption and percent preference for the EtOH-containing solution (EtOH consumption/total fluid consumption × 100). Genotypes were compared using Student’s t-test.
In another set of mice, we measured two-bottle EtOH drinking across increasing EtOH concentrations. Two bottles were offered, as above, with the EtOH bottle containing increasing concentrations of EtOH: 3% (4 days), 6% (4 days), 9% (4 days), 11% (6 days), 13% (6 days) and 15% (6 days). Drinking and preference was measured as above. Genotype × concentration effects were analyzed using ANOVA, with repeated measures for concentration, and Newman Keuls post hoc tests.
Taste preference for bitter (quinine) and sweet (saccharin) tastants was tested as previously described (Boyce-Rustay et al. 2006). One week after the (increasing concentration) EtOH drinking experiment, mice were offered a choice between a given tastant versus water, each for 4 days in the following order: 0.033% saccharin, 0.066% saccharin, 0.015 mM quinine and 0.06 mM quinine. To control for possible tastant order effects, another group underwent the same procedure but with quinine preceding the saccharin. Genotype × tastant effects were analyzed using ANOVA and Newman Keuls post hoc tests.
Deprivation-induced EtOH drinking
We tested for an alcohol deprivation effect (ADE) based on a previously described procedure (Khisti et al. 2006). EtOH naïve mice were offered water or 10% EtOH bottles (as above) for 18 hours/day, beginning at the start of the dark cycle, for 7 days. Water only was available during the other 6 hours/day. The concentration of EtOH was increased to 13% for another 7 days. The EtOH bottle was then removed for a 4-day deprivation period. At the beginning of the dark cycle, mice were again offered the choice between water and a 13% EtOH bottle for 18 hours/day for 4 days to measure deprivation-induced drinking. EtOH consumption was expressed as (g/kg/18 hours) and EtOH preference as (EtOH consumption/total fluid consumption × 100). Days post-deprivation were compared with the day before deprivation using paired t-tests. Data were analyzed using repeated measures ANOVA and Newman Keuls post hoc tests.
EtOH conditioned place preference
EtOH conditioned place preference was measured as previously described (Karlsson et al. 2008). The apparatus consisted of two equally illuminated compartments (each 17 × 13 × 13 cm): one compartment had black walls with a grid floor (3.2-mm rods) and the other had white walls with a wire mesh floor housed in a sound-attenuated chamber (customized from Med Associates Inc.). Photo-beams were located 1.2 cm apart across the full length of the apparatus to measure time spent and locomotor activity within each compartment.
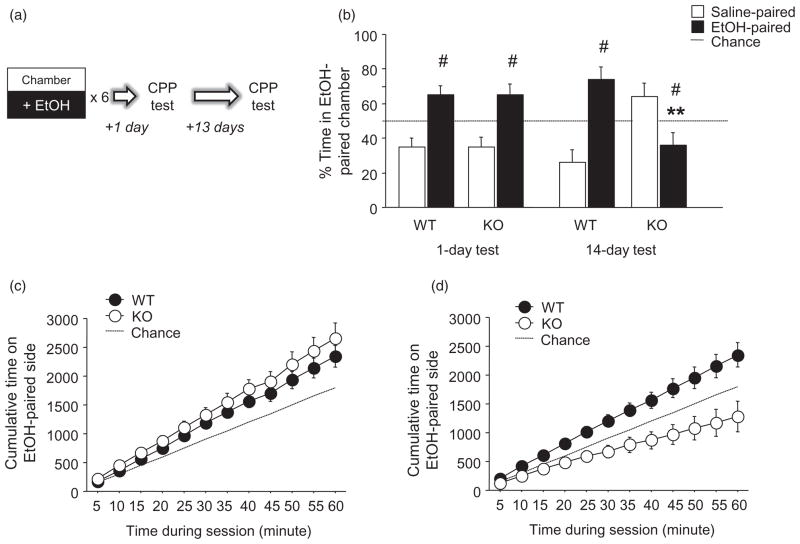
Mice first had a 5-minute habituation free-exploration session (floors covered with solid Plexiglas to prevent exposure) after a saline injection to reduce the novelty of the apparatus and the stress of i.p. injection. Conditioning began 1 day later, using an unbiased design in which half the mice had EtOH paired with the black walls/grid floor compartment and the other half with the white walls/wire mesh floor compartment. Mice were injected with 2.0 g/kg EtOH [conditioned stimulus] (CS+ trials) or saline (CS− trials) and immediately placed into the appropriate compartment for 5 minutes. CS+ trials and CS−trials were alternated daily, and the order of CS+ versus CS− trials counterbalanced within genotype. One day after the last of six CS+ and six CS− conditioning trials, mice received a preference test in which they were injected with saline and placed between compartments to freely explore the whole apparatus for 60 minutes. We used a longer probe test session than we have previously employed (Karlsson et al. 2008) in order to allow for a more detailed examination of within-session patterns in behavior (as in, e.g. Cunningham, Henderson & Bormann 1998). Fourteen days later mice were given another drug-free preference test (for schematic, see Fig. 3a). Time spent in the EtOH-paired side was compared between genotypes using Student’s t-test.
Figure 3.
PSD-95 KO show long-term loss of EtOH conditioned place preference. (a) Schematic of study design. (b) WT and KO showed significant preference for the EtOH-paired compartment 1 day after conditioning. When tested 14 days after conditioning, WT retained a significant preference for the EtOH-paired side while KO showed a significant aversion to the EtOH-paired side. (c) Both genotypes preferred the EtOH-paired side, as measured by cumulative time spent in that compartment, 1 day after conditioning. (d) WT preference for the EtOH-paired side was intact 14 days post-conditioning, while KO showed an increasing aversion to the EtOH-paired side, as measured by cumulative time spent in the EtOH-paired side (n = 10/genotype). Data are Means ± SEM. #P < 0.05 versus 50% chance/same genotype, **P < 0.01 versus WT/same test day
LiCl conditioned taste aversion
A separate cohort of mice was examined for LiCl induced CTA as described previously (Hefner et al. 2008). Singly-housed mice were first water-deprived and habituated to drinking from two water-filled sipper tubes offered in the cage for 30 minutes twice per day (9:00 am and 4:00 pm) for 6 days. The following day, mice were offered one tube, containing 0.5% saccharin, at 9:00 am for 30 minutes. Thirty minutes later, mice were given an i.p. injection of 0.15 M LiCl in volume of 20 ml/kg (= 0.30 M) and observed for signs of malaise. Malaise was defined as protracted periods of non-sleeping immobility, piloerection, contraction of the flanks, prostrate, flat belly, and elongated body posture (mice displaying malaise were given a score of 1.0). Mice were offered water during the 4:00 pm presentation to prevent excessive dehydration.
To mimic the design of the CPP experiment, CTA was probed 1 day and again 14 days after conditioning. On each probe, mice were offered two tubes (one containing 0.5% saccharin, the other tap water), for 40 minutes, with the left/right position of the tubes counterbalanced across experimental groups (but the same across probes for a given mouse). An aversion index was calculated as fluid consumed from water-contained tube/total fluid consumed from both tubes; with an index score of 1.0 indicating maximum aversion.
MK-801-potentiation of EtOH intoxication
Mice were injected i.p. with 0.2 mg/kg MK-801 (in a volume of 10 ml/kg body weight) or saline vehicle, and then, 30 minutes later, injected with EtOH and tested for EtOH-induced ataxia, hypothermia and sedation/hypnosis (1 week apart, as above). Trunk blood was taken at awakening (as above) to ascertain BECs. The dose of MK-801 was chosen as an effective EtOH-potentiating dose in C57BL/6J mice (dose based on Palachick et al. 2008). Genotype × treatment effects were analyzed using ANOVA and Newman Keuls post hoc tests.
Statistical analysis
All analyses were performed using ANOVA or Student’s t-test, as detailed in the relevant methods. The threshold for statistical significance was set at P < 0.05.
RESULTS
PSD-95 KO show hypersensitivity to EtOH intoxication
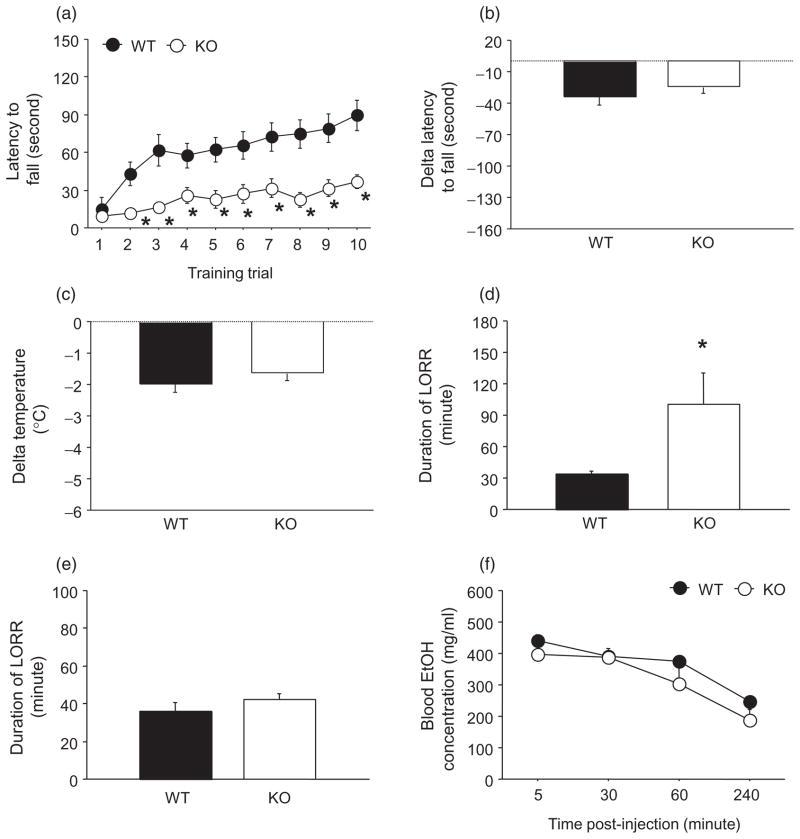
The acute intoxicating effects of EtOH challenge were assessed using 3 separate endpoint measures: EtOH-induced ataxia, hypothermia and sedation/hypnosis. EtOH-induced ataxia was assessed using the accelerating rotarod. During rotarod training prior to EtOH challenge, there was a significant genotype-trial interaction for latency to fall (F9,189 = 2.76, P < 0.01). While both WT and KO showed a significant increase in latencies across training trials, post hoc analyses showed that latencies in KO were lower than WT on all but the first trial (all P < 0.05) (Fig. 1a). After EtOH challenge, the latency to fall was reduced (= negative delta latency) to a similar degree across genotypes (Fig. 1b).
Figure 1.
PSD-95 KO show hypersensitivity to the sedative/hypnotic effects of EtOH. (a) Baseline rotarod performance was impaired in KO relative to WT, as demonstrated by lower latencies to fall on all trials but the first (n = 10–13/genotype). (b) Injection of 1.75 g/kg EtOH caused ataxia (as indicated by negative delta latency to fall) that was no different between genotypes (n = 10–15/genotype). (c) Injection of 3.0 g/kg EtOH caused hypothermia (as indicated by negative delta temperature) that was no different between genotypes (n = 7–14/genotype). (d) Injection of 3.0 g/kg EtOH produced a significantly prolonged sedative/hypnotic response in KO relative to WT (n = 8–11/genotype). (e) Injection of 30 mg/kg pentobarbital produced a similar sedative/hypnotic response in both genotypes (n = 6–8/genotype). (f) Blood EtOH levels following injection with 3.5 g/kg EtOH did not differ between genotypes (n = 5–11/genotype). Data are Means ± SEM. *P < 0.05 versus WT
Next, we tested for EtOH-induced hypothermia and found that the hypothermic effects EtOH (as demonstrated by a negative delta temperature) were similar in WT and KO (Fig. 1c). On a third assay, EtOH-induced sedation/hypnosis, we found that the sedative/hypnotic effects of EtOH were significantly greater in KO than WT (t = 2.77, d.f. = 16, P < 0.05) (Fig. 1d). By contrast, the sedative/hypnotic effects of pentobarbital were similar between WT and KO (Fig. 1e).
Finally, to discount potential EtOH pharmacokinetic abnormalities in PSD-95 KO mice, we examined BECs at various timepoints after EtOH challenge. There was a significant effect of timepoint (F3,39 = 92.11, P < 0.01) but not genotype and no timepoint-genotype interaction for BECs, as WT and KO showed similar BECs at all timepoints (Fig. 1f).
PSD-95 KO show reduced EtOH drinking
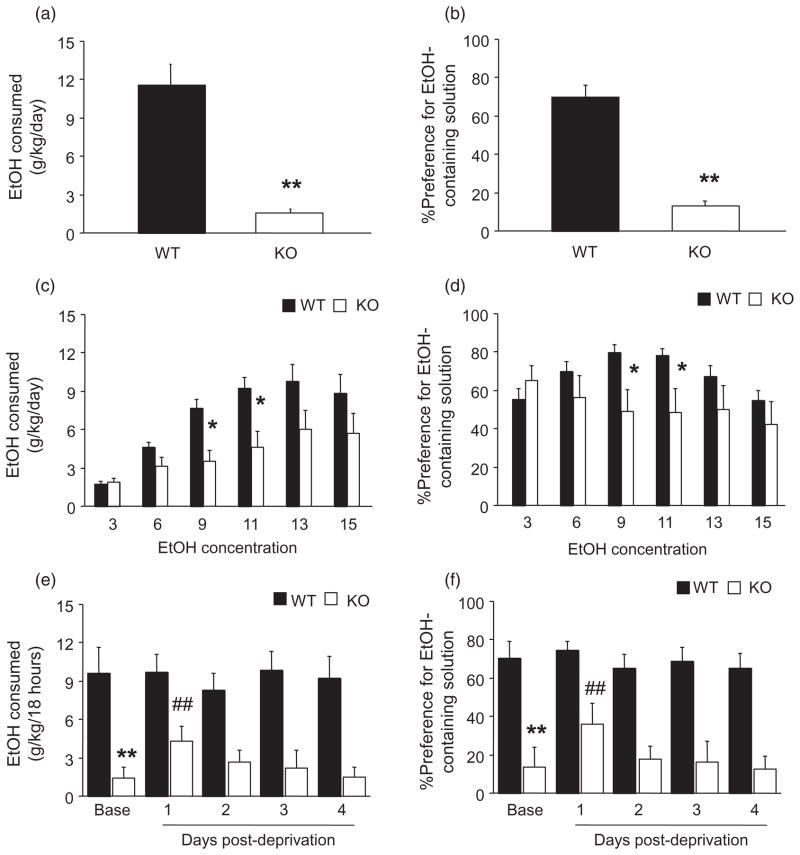
We first tested two-bottle free choice EtOH drinking of high (15%) EtOH concentration, and found that KO showed significantly lower scores both for EtOH dose consumed (t = 5.44, d.f. = 15, P < 0.01) (Fig. 2a), and preference (t = 7.86, d.f. = 15, P < 0.01) (Fig. 2b). In a separate cohort, we offered mice gradually increasing (3–15%) concentrations of EtOH. Here, there was a significant genotype-concentration interaction for both EtOH dose consumed (F5,110 = 3.52, P < 0.01) and preference (F5,110 = 5.90, P < 0.01). Post hoc tests showed that KO consumed less EtOH (Fig. 2c), and had lesser preference (Fig. 2d), at the 9% and 11%, but not the 3, 6, 13 or 15% EtOH concentrations, relative to WT. Total fluid consumption did not differ between genotypes regardless of concentration (data not shown).
Figure 2.
PSD-95 KO show reduced EtOH drinking. (a) When offered a single (15%) concentration of EtOH, KO consumed significantly less EtOH and (b) had a significantly lesser preference for EtOH than WT (n = 8–9/genotype). (c) When offered increasing concentrations of EtOH, KO consumed significantly less EtOH at 9% and 11% concentrations and (d) had significantly lesser preference at 9% and 11% concentrations relative to WT (n = 12/genotype). (e) Prior to EtOH deprivation, KO consumed significantly less (15%) EtOH than WT. KO, but not WT, consumed significantly more EtOH 1 day after deprivation, relative to pre-deprivation baseline, and this elevation returned to baseline over the next 3 days. (f) KO showed significantly lesser preference for EtOH than WT during pre-deprivation baseline, and KO, but not WT, showed a significant increase in preference 1 day (but not 2–4 days) post-deprivation, relative to pre-deprivation baseline (n = 8/genotype). Data are Means ± SEM. **P < 0.01, *P < 0.05 versus WT/same concentration or period, ##P < 0.01 versus KO baseline
To exclude confounding effects of general taste abnormalities on EtOH drinking in PSD-95 KO, we tested preference for bitter (quinine) and sweet (saccharin) tastants. Genotypes did not significantly differ in preference for either concentration of saccharin (low concentration: WT = 89 ± 4%, KO = 72 ± 11%, high concentration: WT = 98 ± 1%, KO = 81 ± 11%), although KO drank more of the low (WT = 47 ± 8%, KO = 82 ± 4%), but not high (WT = 44 ± 7%, KO = 43 ± 8%) concentration of quinine, relative to WT.
PSD-95 KO show deprivation-induced drinking
We next tested PSD-95 KO for ADE. Although the procedure used does not produce significant ADE in C57BL/6J mice (the genetic background of the PSD-95 KO) (Khisti et al. 2006), we employed it to test whether low EtOH consumption we observed in PSD-95 KO under free-choice conditions could be increased under conditions of deprivation. During the pre-deprivation baseline period, KO again drank less (t = 3.26, d.f. = 14, P < 0.01) (Fig. 2e) and had lesser preference (t = 3.36, d.f. = 14, P < 0.01) (Fig. 2f) than WT. On the first day of deprivation, KO (t = 4.77, d.f. = 7, P < 0.01), but not WT, showed a significant increase in both EtOH consumption (Fig. 2e) and EtOH preference (t = 4.11, d.f. = 7, P < 0.01) (Fig. 2f), relative to their pre-deprivation baseline. By day 2, elevated drinking in the KO had returned to baseline.
PSD-95 KO show long-term loss of EtOH CPP
To evaluate reward-related effects of EtOH, we measured CPP for EtOH. During the habituation phase prior to conditioning, there was no effect of genotype on time spent in either compartment (WT = 45 ± 5% time in to-be-conditioned compartment, KO = 41 ± 12%). During conditioning trials, locomotor activity in the drug-paired compartment did not differ as a function of either genotype or trial-type.
A first CPP probe, 1 day after the completion of conditioning, was conducted to determine successful acquisition and expression of a EtOH-context association. There was a significant effect of compartment (F1,16 = 6.00, P < 0.05) but no compartment-genotype interaction for compartment time. There was preference for the EtOH-paired over the saline-paired compartment in both genotypes (Fig. 3b). Cumulative time spent in the EtOH-paired compartment illustrates strong preference for the EtOH-paired compartment expressed over the course of the test in both genotypes (Fig. 3c).
We then conducted a second CPP probe, 14 days after conditioning, to assess ‘remote’ retention of the EtOH-context association. Here, there was a significant compartment-genotype interaction for compartment time (F1,27 = 6.75, P < 0.01). Post hoc tests showed that KO spent less time in the EtOH-paired compartment than WT (Fig. 3b), due to WT spending more time in the EtOH-paired than saline-paired compartment (t = 2.57, d.f. = 9, P < 0.05), and KO spending more time in the saline-paired than EtOH-paired compartment (t = 2.34, d.f. = 9, P < 0.05) (Fig. 3b). Cumulative time analysis revealed a developing preference for the EtOH-paired compartment over the session in WT, and an increasing aversion to the EtOH-paired compartment in KO (Fig. 3d).
PSD-95 KO show impaired LiCl CTA
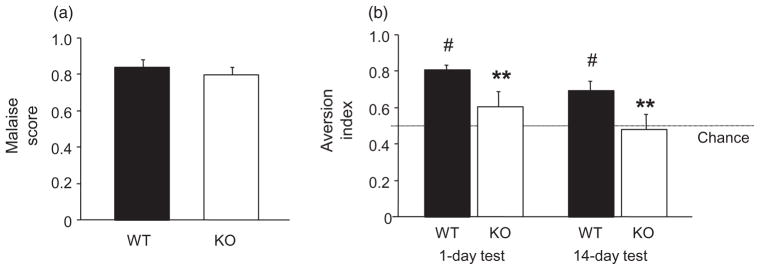
To provide a comparison with EtOH CPP, we also examined LiCl induced CTA. This is another type of Pavlovian association but, in contrast to CPP, involves a learned aversion rather than preference. Results showed that WT and KO had high and similar malaise scores after LiCl injection, demonstrating treatment was equally effective in inducing sickness (Fig. 4a). KO had a significantly lower aversion index than WT during the CTA probe test 1 day after conditioning (t = 2.21, d.f. = 14, P < 0.05). The aversion index was significantly above chance in WT (t = 10.56, d.f. = 7, P < 0.01), but not in KO (Fig. 4b). CTA index scores were again lower in KO than WT on the 14-day probe test (t = 2.20, d.f. = 14, P < 0.05). WT retained an aversion index significantly above chance (t = 3.77, d.f. = 7, P < 0.01), while KO again showed no aversion (Fig. 4b).
Figure 4.
PSD-95 KO show impaired lithium chloride conditioned taste aversion. (a) WT and KO showed high, similar malaise scores following LiCl injection. (b) KO had a significantly lower aversion index than WT during probe tests 1 and 14 days after conditioning. The aversion index was significantly above chance in WT, not KO, on both test days (n = 8–10/genotype). Data are Means ± SEM. #P < 0.05 versus 50% chance/same genotype, **P < 0.01 versus WT/same test day
Unaltered MK-801-potentiation of EtOH intoxication
We next examined whether the ability of pharmacological blockade of NMDARs to potentiate acute intoxicating effects of EtOH was altered in PSD-95 KO (Shen & Phillips 1998; Boyce-Rustay & Holmes 2005; Gass & Olive 2008). Mice were challenged with the uncompetitive NMDAR antagonist, MK-801/dizocilpine [(+)-5-methyl-10,11-dihydro-SH-dibenzo(a,d)cyclohepten-5,10-imine maleate] prior to EtOH and then given separate tests for EtOH-induced ataxia, hypothermia and sedation/hypnosis.
During rotarod training prior to EtOH challenge, there was a significant genotype-trial interaction for latency to fall (F9,405 = 9.52, P < 0.01). As in the cohort described above, KO showed lower latency to fall than WT across trials (data not shown). Following MK-801 pre-treatment and EtOH challenge, there was a significant MK-801-EtOH interaction for delta latency (F5,43 = 7.77, P < 0.01). Delta latency was significantly greater in MK-801 pre-treated mice than vehicle pre-treated counterparts, both in WT and KO (Fig. 5a). Although magnitude of the MK-801 effect was greater in WT than KO, this was likely due to ‘floor effect’ caused by a lower baseline latency in KO.
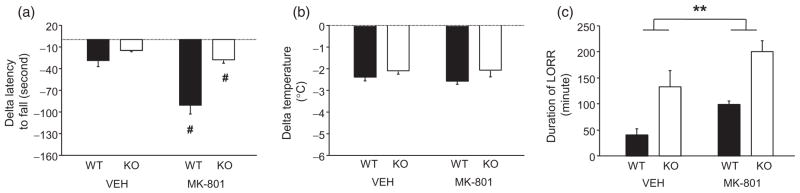
Figure 5.
PSD-95 KO show intact EtOH-potentiating effects of MK-801. (a) WT and KO pre-treated with MK-801 showed a greater ataxic response to EtOH than mice pre-treated with vehicle (VEH) (n = 9–13/genotype/treatment). (b) MK-801 pre-treatment did not alter the hypothermic response to EtOH, regardless of genotype (n = 8–13/genotype/treatment). (c) WT and KO pre-treated with 0.2 mg/kg MK-801 showed a greater sedative/hypnotic response to EtOH than mice pre-treated with vehicle (n = 8–11/genotype). Data are Means ± SEM. #P < 0.05 versus VEH/same genotype, ##P < 0.01 versus VEH/same genotype, **P < 0.01 WT versus KO
Measuring the hypothermic effects of EtOH, we found that neither genotype nor MK-801 pre-treatment affected this measure (Fig. 5b). By contrast, both factors significantly affected LORR duration (main effect of genotype: F1,36 = 11.91, P < 0.01, main effect of MK-801 pre-treatment: F1,36 = 28.73, P < 0.01), but did not significantly interact. Specifically, the sedative/hypnotic effects of EtOH were significantly greater in KO than WT, regardless of MK-801 pre-treatment, and greater in MK-801 pre-treated mice relative to vehicle pre-treated mice, regardless of genotype (Fig. 5c). Reflecting these longer LORR times, BECs at awakening were significantly lower (F1,33 = 10.76, P < 0.01) in KOs than WTs, regardless of MK-801 treatment (WT saline = 346 ± 17, KO saline = 252 ± 39, WT MK-801 = 286 ± 9, KO MK-801 = 246 ± 22).
DISCUSSION
The findings of the current study reveal a major role for PSD-95 in regulating the intoxicating effects of EtOH and the propensity to consume EtOH, as well as in the long-term (remote) maintenance of a learned, reward-related response to EtOH.
Using a battery of measures of acute responses to EtOH, we found that PSD-95 KO mice displayed a prolonged sedative/hypnotic response to relatively high (3.0–3.5 g/kg) doses of EtOH. By contrast, the sedative/hypnotic effects of the barbiturate, pentobarbital, were normal in KO mice, indicating an exaggerated response specifically to EtOH, rather than sedative/hypnotic drugs in general. We also found that KO mice had a normal hypothermic response to EtOH, which speaks to a selective alteration to the sedative/hypnotic effects and also discounts the possibility that this exaggerated response is a pharmacokinetic artifact (e.g. slower EtOH clearance). The latter interpretation was reinforced by the fact that BECs at awakening were lesser in KO than WT mice (as expected if clearance rates were equivalent between genotypes, as opposed to slower in KO mice) and the finding that BECs were not different between genotypes at various time points up to four hours after injection of a sedation/hypnosis-inducing EtOH dose. On a third measure of intoxication, motor ataxia to lower doses of EtOH (1.75–2.0 g/kg), genotypes did not differ, although a caveat to these data is that KO mice exhibited impaired accelerating rotarod motor coordination at baseline (as previously reported, Feyder et al., 2010)—making it difficult to detect possible hypersensitivity on this measure. Collectively, these results indicate a significant and selective hypersensitivity to the sedative/hypnotic effects of EtOH in PSD-95 KO mice, and provide an interesting parallel with the recent finding that acute locomotor responses to cocaine are also exaggerated in these mice (Yao et al. 2004).
In human subjects, sensitivity to acute EtOH challenge is a predictor of risk for alcohol abuse (Newlin & Thomson 1990; Schuckit 1994). Along related lines, recent conceptual models of alcoholism highlight the contribution of insensitivity to the unpleasant subjective effects of intoxication, such as ataxia and sedation, to risk for the disease (Krystal et al. 2003). This relationship is supported in rodent preclinical studies by numerous instances in which there is an association between increased sensitivity to EtOH-induced sedation/hypnosis and decreased EtOH consumption; although this relationship certainly does not occur in all cases (reviewed in Crabbe et al. 2006). Consistent with a relationship between high sensitivity to intoxication and low drinking, we found that PSD-95 KO mice had markedly lower levels of EtOH drinking and preference than WTs in a two-bottle choice paradigm. Given we have previously shown that the KO mice exhibit normal levels of home cage locomotor activity (Feyder et al. 2010), their reduced drinking in a similar setting seems unlikely to be an artifact of the aforementioned motor incoordination.
Moreover, the reduced EtOH drinking phenotype was most pronounced when mice were offered the choice between water and a 15% EtOH solution from the start of the experiment. When concentrations of EtOH were gradually increased from 3%, reduced EtOH drinking in the KO mice was less pronounced than it was in the group offered only 15%. Moreover, lesser drinking was only seen at the higher concentrations, where consumption is likely to be primarily motivated by pharmacodynamic effects, rather than by taste, calorie intake, etc. (George et al. 2008). In addition, when mice were EtOH deprived after 2 weeks of free-choice drinking, KO mice showed a transient, 1 day increase in drinking and preference for a 15% solution, even though (as previously reported for C57BL/6J, Khisti et al. 2006) these deprivation conditions were insufficient to produce a demonstrable increase in WT drinking (possibly due a ‘ceiling’ effect of an already high baseline). Thus, while PSD-95 KO mice have a reduced propensity to drink EtOH, they are not fully protected against at least two manipulations that can increase drinking: deprivation and concentration-fading.
While CPP provides a valuable measure of learned, context-associated preference for EtOH, it is not synonymous with motivation to drink. Indeed, certain EtOH-averse mouse strains, such as DBA/2J, exhibit stronger EtOH CPP than EtOH-preferring strains such as C57BL/6J (Cunningham et al. 1992). We found that PSD-95 KO mice showed intact EtOH CPP when probed for preference 1 day after conditioning. Interestingly however, while WT controls retained preference when probed again 2 weeks later, PSD-95 KO lost this preference and actually showed a significant aversion to the EtOH-paired chamber. To provide a comparison with the CPP data, we examined malaise-induced CTA, which is an aversive, rather than appetitive, form of associative memory. Here, however, we found a KO deficit on both the recent and remote test, consistent with the disruption of rat taste learning produced by PSD-95 knockdown in gustatory cortex (Elkobi et al. 2008).
Our data demonstrate that PSD-95 is not necessary for acquisition or expression of EtOH CPP at a recent time point, but is necessary for retention and/or expression of CPP at a more remote time point. This time-dependent preference-for/aversion switch in EtOH CPP has very few precedents in the literature. Pertinently, however, Cunningham and colleagues found that non-mutant mice repeatedly pre-treated with naloxone showed preference 1 day after conditioning, but aversion 9 days after conditioning (Cunningham et al. 1998). While we do not claim that the same mechanisms (e.g. opioid receptors) necessarily underlie the switch in PSD-95 KO mice, the Cunningham et al. study serves to demonstrate how the relative balance between preference and aversion (processes likely learned in parallel during EtOH CPP) can differentially manifest at recent and remote CPP testing. Given our intoxication and drinking findings, one possibility is that the aversive component of CPP was more strongly acquired in the PSD-95 KO mice and this latent aversion was unmasked at the remote timepoint, perhaps when preference has sufficiently weakened. Another interesting, and not necessarily exclusive, possibility is that the KO mice have a deficit in retaining CPP at the more remote timepoint. This would be broadly congruent with the key role PSD-95 is known to play in synaptic functions, including the stabilization of glutamatergic molecules, including AMPARs and NMDARs, mediating memory (Kim & Sheng 2004; Malenka & Bear 2004; Elias & Nicoll 2007). However, it not yet clear why a PSD-95-related loss of synaptic stability would manifest as memory loss only at remote timepoints and further studies will be needed to test these ideas.
The precise mechanisms underlying the EtOH hypersensitivity in the PSD-95 KO mice also remains to be determined. The effects of deleting PSD-95 on EtOH intoxication and drinking phenocopies the effects produced by treatment with an NMDAR antagonist in non-mutant mice (Gass & Olive 2008; Palachick et al. 2008; Chen & Holmes 2009), and as just mentioned, PSD-95 has a critical function in the trafficking, stabilization and signal transduction of NMDARs. On this basis, we reasoned that the PSD-95 KO phenotype might reflect functional impairment of the NMDAR, at least in the receptor’s response to EtOH, and that such an alteration could be interrogated by measuring sensitivity to NMDAR antagonist effects on EtOH intoxication. However, we found that while treatment with the uncompetitive NMDAR antagonist MK-801 produced robust increases in acute behavioral responses to EtOH, these effects were unaltered in the KO mice. While these data may suggest that the altered EtOH behavioral responses in these mice are not driven by a major deficit in the NMDAR-mediated component of the response to EtOH, this should be taken as a preliminary conclusion in lieu of future work to elucidate the mechanistic basis of the marked changes in EtOH-related behaviors caused by loss of PSD-95 function.
In this context, our findings raise the intriguing possibility that other post-synaptic proteins also modulate EtOH behaviors. The multi-protein complexes formed by protein interactions in the post-synaptic proteome include many proteins underlying a variety of behaviors affected both acutely and chronically by EtOH (Fernandez et al. 2009). In humans, many of the PSD-95-associated complex proteins have already been implicated in a number of neuropsychiatric diseases, as evidenced by association with gene mutations and pathological changes in protein/gene expression (Grant et al. 2005; Fernandez et al. 2009) (http://www.genes2cognition.org). A key future avenue will be to examine whether variation in genes encoding PSD-95 complexes affect, protect or predispose to alcoholism, as the current data in mice suggest they might. Another interesting question for additional studies will be to examine responses to chronic EtOH exposure in PSD-95 KO mice. PSD-95 has recently been found to contribute to synaptic clustering of NMDARs produced by chronic exposure to EtOH in vitro (Carpenter-Hyland & Chandler 2006), and PSD-95 KO mice fail to develop locomotor sensitization to chronic treatment with another abused drug, cocaine (Yao et al. 2004). Given these findings, loss of PSD-95 might be predicted to alter the neural and behavioral adaptations to chronic EtOH. While we have not examined this in the current study, examining responses to chronic EtOH in PSD-95 KO mice will be a key avenue for future work.
In summary, current findings reveal a novel role for PSD-95 in mediating EtOH behaviors. Specifically, functional deletion of PSD-95 produced hypersensitivity to some of EtOH’s putatively negative acute intoxicating effects, reduced but did not completely abolish EtOH drinking and time-dependently destabilized CPP for EtOH. PSD-95 KO mice did not show abnormal EtOH responses to NMDAR antagonist challenge. Taken together, these data add to a nascent literature implicating PSD-95 as a key mediator of the behavioral effects of various abused drugs. Elucidating this role could have implications for understanding the pathophysiology and treatment of alcoholism and other addictions.
Acknowledgments
We are very grateful to Christina Gremel for helpful discussions. Research supported by the National Institute of Alcohol Abuse and Alcoholism Intramural Research Program (Z01-AA000411) and the Wellcome Trust Genes to Cognition Programme.
Footnotes
Authors Contribution
AH was responsible for the study concept and design, analyzed data and interpretation of findings and drafted the manuscript. MCC contributed to the acquisition of animal data, assisted with data analysis and assisted with writing. MF, RMK and YCC contributed to the acquisition of animal data and assisted with data analysis. MPC analyzed data and assisted with writing. SGNG supplied the mutant mice, assisted with data analysis and assisted with writing. JI, BP, BH and BN contributed to the acquisition of animal data. All authors critically reviewed manuscript content and approved the final version for publication.
References
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Functional roles of NMDA receptor NR2A and NR2B subunits in the acute intoxicating effects of ethanol in mice. Synapse. 2005;56:222–225. doi: 10.1002/syn.20143. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur J Neurosci. 2006;24:3496–3506. doi: 10.1111/j.1460-9568.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Chen YC, Holmes A. Effects of topiramate and other anti-glutamatergic drugs on the acute intoxicating actions of ethanol in mice: modulation by genetic strain and stress. Neuropsychopharmacology. 2009;34:1454–1466. doi: 10.1038/npp.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coba MP, Pocklington AJ, Collins MO, Kopanitsa MV, Uren RT, Swamy S, Croning MD, Choudhary JS, Grant SG. Neurotransmitters drive combinatorial multistate postsynaptic density networks. Sci Signal. 2009;2:ra19. doi: 10.1126/scisignal.2000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe J, Cameron A, Munn E, Bunning M, Wahlsten D. Overview of mouse assays of ethanol intoxication. Curr Protoc Neurosci. 2008;(suppl 42):9.26.1–9.26.19. doi: 10.1002/0471142301.ns0926s42. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology (Berl) 1998;139:62–70. doi: 10.1007/s002130050690. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Elkobi A, Ehrlich I, Belelovsky K, Barki-Harrington L, Rosenblum K. ERK-dependent PSD-95 induction in the gustatory cortex is necessary for taste learning, but not retrieval. Nat Neurosci. 2008;11:1149–1151. doi: 10.1038/nn.2190. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MD, Zografos L, Armstrong JD, Choudhary JS, Grant SG. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol Syst Biol. 2009;5:269. doi: 10.1038/msb.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyder M, Karlsson RM, Mathur P, Lyman M, Bock R, Momezan R, Munasinghe J, Scattoni ML, Ihne J, Camp M, Graybeal C, Strathdee DJ, Begg A, Alvarez VA, Kirsch P, Rietschel M, Cichon S, Walter H, Meyer-Lindenberg A, Grant SGN, Holmes A. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism-spectrum disorders and Williams’ (e.g. Williams’) syndrome. Am J Psychiatry 2010. 2010;167:1508–1517. doi: 10.1176/appi.ajp.2010.10040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Grant SG, Marshall MC, Page KL, Cumiskey MA, Armstrong JD. Synapse proteomics of multiprotein complexes: en route from genes to nervous system diseases. Hum Mol Genet. 2005;14(Spec No 2):R225–R234. doi: 10.1093/hmg/ddi330. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 2008;200:117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol. 2006;40:119–126. doi: 10.1016/j.alcohol.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Ralph RJ, Yang RJ, Holmes A. Effects of repeated maternal separation on prepulse inhibition of startle across inbred mouse strains. Genes Brain Behav. 2006;5:346–354. doi: 10.1111/j.1601-183X.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Palachick B, Chen YC, Enoch AJ, Karlsson RM, Mishina M, Holmes A. Role of major NMDA or AMPA receptor subunits in MK-801 potentiation of ethanol intoxication. Alcohol Clin Exp Res. 2008;32:1479–1492. doi: 10.1111/j.1530-0277.2008.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D. Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist. 2004;10:325–336. doi: 10.1177/1073858404263516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav Brain Res. 2003;141:237–249. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Shen EH, Phillips TJ. MK-801 potentiates ethanol’s effects on locomotor activity in mice. Pharmacol Biochem Behav. 1998;59:135–143. doi: 10.1016/s0091-3057(97)00389-4. [DOI] [PubMed] [Google Scholar]
- Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]