Abstract
BACKGROUND
There were numerous efforts in the United States during the previous decade to concentrate selected surgical procedures in high-volume hospitals. It remains unknown whether referral patterns for high-risk surgery have changed as a result and how operative mortality has been affected.
METHODS
We used national Medicare data to study patients undergoing one of eight different cancer and cardiovascular operations from 1999 through 2008. For each procedure, we examined trends in hospital volume and market concentration, defined as the proportion of Medicare patients undergoing surgery in the top decile of hospitals by volume per year. We used regression-based techniques to assess the effects of volume and market concentration on mortality over time, adjusting for case mix.
RESULTS
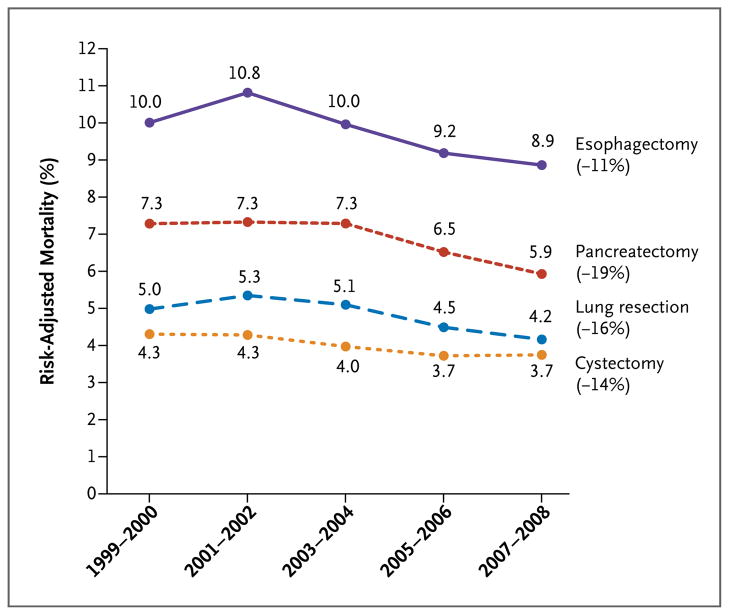
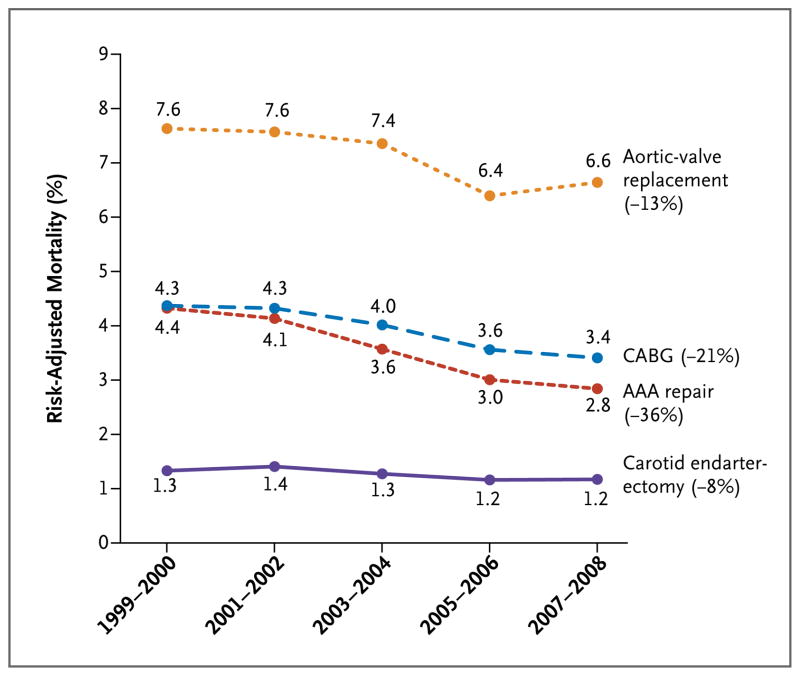
Median hospital volumes of four cancer resections (lung, esophagus, pancreas, and bladder) and of repair of abdominal aortic aneurysm (AAA) rose substantially. Depending on the procedure, higher hospital volumes were attributable to an increasing number of cases nationwide, an increasing market concentration, or both. Hospital volumes rose slightly for aortic-valve replacement but fell for coronary-artery bypass grafting and carotid endarterectomy. Operative mortality declined for all eight procedures, ranging from a relative decline of 8% for carotid endarterectomy (1.3% mortality in 1999 and 1.2% in 2008) to 36% for AAA repair (4.4% in 1999 and 2.8% in 2008). Higher hospital volumes explained a large portion of the decline in mortality for pancreatectomy (67% of the decline), cystectomy (37%), and esophagectomy (32%), but not for the other procedures.
CONCLUSIONS
Operative mortality with high-risk surgery fell substantially during the previous decade. Although increased market concentration and hospital volume have contributed to declining mortality with some high-risk cancer operations, declines in mortality with other procedures are largely attributable to other factors. (Funded by the National Institute on Aging.)
Fueled by a growing number of studies reporting inverse relationships between hospital volume and surgical mortality,1–3 there was considerable interest in the United States during the previous decade in concentrating selected operations in high-volume hospitals. The Leapfrog Group, a consortium of large corporations and public agencies that purchase health care, has been among the most prominent advocates of volume-based referral. In 2000, it established minimum volume standards for several surgical procedures as part of a broader, value-based purchasing initiative.4 Private payers and professional organizations in the United States have also established minimum volume standards as part of Centers of Excellence accreditation programs for a variety of operations.5
Whether such efforts have altered referral patterns for high-risk surgery remains uncertain, however. There are still many barriers to regionalization, including patient preferences for local care,6 financial incentives for smaller hospitals to retain surgical cases,7,8 and lack of access to high-volume centers in some regions.9 Despite increasing numbers of surgical patients in high-volume hospitals, the net effects on operative mortality are difficult to predict. Although hospital volume of a few high-risk cancer procedures (e.g., pancreatectomy) is a strong predictor of operative risk, relationships between volume and outcome are considerably weaker for most operations.2,10
In this study, we used data from national Medicare claims to evaluate trends in the use of high-volume hospitals for major cancer resections and cardiovascular surgery. We also examined concurrent trends in operative mortality rates associated with these procedures and the extent to which decreases in mortality could be associated with a concentration of surgical care in high-volume hospitals.
METHODS
STUDY DESIGN
We based this study on national Medicare Provider Analysis and Review (MEDPAR) files, which contain all hospital discharge abstracts for fee-for-service, acute care hospitalizations for Medicare recipients. Using appropriate procedure codes from the International Classification of Diseases, Ninth Revision (ICD-9),11 we identified all patients from 65 to 99 years of age who underwent one of the following eight cancer and cardiovascular operations from 1999 through 2008: esophagectomy, pancreatectomy, lung resection, cystectomy, repair of abdominal aortic aneurysm (AAA), coronary-artery bypass grafting (CABG), carotid endarterectomy, and aortic-valve replacement (for a full list of ICD-9 codes, see the Supplementary Appendix, available with the full text of this article at NEJM.org). Six of these procedures have been targeted for volume-based referral by the Leapfrog Group.4,12 We also included lung resection and cystectomy, two procedures that have been cited as potential candidates for regionalization.13–15
Each year, hospitals were ranked according to the volume of Medicare patients for each procedure, adjusting for the proportion of Medicare patients covered by fee-for-service plans. In assessing changes in hospital volumes over time, we sought to distinguish between the effects of “volume creep” (which occurs when more patients who undergo these high-risk procedures are distributed among the same hospitals) and market concentration (which occurs when patients are redistributed to a smaller number of higher-volume hospitals). To quantify market concentration, we determined the proportion of Medicare patients undergoing one of the eight procedures in the top decile and top quintile of hospitals by volume for each year.
Operative mortality, determined from the Medicare eligibility file, was defined as death before discharge or within 30 days after the operation. In creating cohorts for analysis of operative mortality, we used several limitations to enhance the homogeneity of our study cohorts and reduce confounding due to changes in case mix over time. For cancer resections, we excluded patients without an accompanying diagnosis code for cancer. Patients who underwent AAA repair were excluded if there was a diagnosis code or procedure code indicating rupture of the aneurysm, the presence of a thoracoabdominal aneurysm, or both. For patients who underwent CABG, we excluded those who had simultaneous valve replacement or repair.
STATISTICAL ANALYSIS
We used chi-square tests to determine the significance of trends in the proportion of Medicare patients undergoing surgery at high-volume hospitals and logistic-regression models with robust standard errors, adjusted for clustering at the hospital level, to evaluate temporal trends in risk-adjusted mortality. We determined risk-adjusted mortality using logistic-regression models to adjust for patient characteristics, including age, sex, race, admission acuity (elective, urgent, or emergency), coexisting conditions, and a composite measure of socioeconomic status according to ZIP Code.16 Coexisting conditions were identified from the surgical-admission data, as well as from data from any other admissions during the previous 6 months, with the use of methods described by Elixhauser et al.17 With the use of a stepwise logistic-regression model (P value at entry, <0.1), all significant coexisting conditions were identified and included in procedure-specific risk-adjustment models. For AAA repair, we adjusted for the type of repair (endovascular vs. open).
To understand the effects of hospital volume and market concentration on mortality over time, we examined the differences in mortality between the period from 1999 through 2000 and the period from 2007 through 2008 using a regression-based decomposition approach developed by Blinder18 and Oaxaca.19 The Blinder–Oaxaca method is a labor-economics technique that was initially developed to examine the contribution of factors responsible for wage disparities according to race and sex19 but has more recently been applied in research related to health services.20 Because our analysis examines a dichotomous outcome (mortality), we used a modification of this technique that was designed for use with nonlinear models.21 The decomposition method allows for partitioning of the relative contribution of component factors to outcomes.
We first used this technique to determine how much of the difference in mortality between the period from 1999 through 2000 and the period from 2007 through 2008 could be explained by changes in average hospital volumes for each procedure. In the analysis, death was the dependent variable, with patient factors and volume of hospital procedures (number of procedures per year for Medicare patients) modeled as independent variables. We then assessed the proportion of the effect of hospital volume that could be attributed to volume creep and market concentration. To determine the effect of volume creep, we compared risk-adjusted mortality from 1999 through 2000 for each procedure with the mortality that would have been predicted for 2007 through 2008, had each hospital increased its volume by the same degree (i.e., by the ratio of the total number of Medicare patients from 2007 through 2008 to the total number of patients from 1999 through 2000 undergoing these high-risk procedures). Calculating the predicted mortality on the basis of volume in turn required assessing the strength of the relationship between volume and mortality for each procedure, with the use of methods described previously.2 The proportion of the overall effect of hospital volume that could not be attributed to volume creep was attributed to market concentration.
All analyses were performed with the use of Stata statistical software, version 10.0. All tests were two-sided, and P values of less than 0.05 were considered to indicate statistical significance. The study protocol was approved by the institutional review board at the University of Michigan. The requirement for informed consent was waived.
RESULTS
VOLUME TRENDS
From 1999 through 2008, more than 3.2 million Medicare patients underwent one of eight cancer operations or cardiovascular procedures at hospitals in the United States. Median hospital volumes increased substantially for the four cancer procedures and AAA repair, and to a lesser extent for aortic-valve replacement (Table 1, and the Supplementary Appendix). In contrast, for CABG and carotid endarterectomy, hospital volumes declined sharply during the 10-year period. The total number of Medicare patients undergoing CABG decreased by more than one third, even though the number of hospitals performing this procedure increased, from 1073 to 1195.
Table 1.
Trends in Hospital Volumes of Medicare Patients and the Proportion of Medicare Patients Who Underwent Cancer and Cardiovascular Operations in High-Volume Hospitals, from 1999 through 2008.*
| Variable | Period | ||||
|---|---|---|---|---|---|
| 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | |
|
Esophagectomy
| |||||
| No. of hospitals | 1734 | 1632 | 1524 | 1403 | 1309 |
|
| |||||
| No. of patients | 8805 | 8864 | 8694 | 8674 | 8719 |
|
| |||||
| Hospital volume
| |||||
| Median | 4 | 5 | 5 | 7 | 7 |
|
| |||||
| Interquartile range | 2–10 | 2–11 | 2–14 | 3–16 | 3–17 |
|
| |||||
| Market concentration (%) | 39 | 40 | 43 | 45 | 44 |
|
| |||||
|
Pancreatectomy
| |||||
| No. of hospitals | 1308 | 1303 | 1110 | 1016 | 978 |
|
| |||||
| No. of patients | 6633 | 7486 | 8215 | 8932 | 10,350 |
|
| |||||
| Hospital volume
| |||||
| Median | 5 | 6 | 10 | 13 | 16 |
|
| |||||
| Interquartile range | 2–14 | 2–17 | 3–22 | 4–28 | 5–35 |
|
| |||||
| Market concentration (%) | 42 | 46 | 49 | 51 | 52 |
|
| |||||
|
Lung resection
| |||||
| No. of hospitals | 2408 | 2285 | 2200 | 2096 | 2045 |
|
| |||||
| No. of patients | 49,056 | 49,723 | 51,362 | 53,456 | 56,307 |
|
| |||||
| Hospital volume
| |||||
| Median | 18 | 19 | 21 | 23 | 25 |
|
| |||||
| Interquartile range | 9–32 | 10–35 | 10–37 | 11–42 | 12–49 |
|
| |||||
| Market concentration (%) | 39 | 40 | 39 | 41 | 41 |
|
| |||||
|
Cystectomy
| |||||
| No. of hospitals | 1817 | 1723 | 1607 | 1505 | 1366 |
|
| |||||
| No. of patients | 11,258 | 11,593 | 11,953 | 12,278 | 13,167 |
|
| |||||
| Hospital volume
| |||||
| Median | 5 | 6 | 6 | 8 | 10 |
|
| |||||
| Interquartile range | 2–10 | 3–13 | 3–16 | 3–22 | 4–27 |
|
| |||||
| Market concentration (%) | 35 | 40 | 43 | 47 | 47 |
|
| |||||
|
AAA repair
| |||||
| No. of hospitals | 2339 | 2159 | 2035 | 1949 | 1860 |
|
| |||||
| No. of patients | 56,333 | 66,909 | 64,645 | 67,288 | 71,170 |
|
| |||||
| Hospital volume
| |||||
| Median | 22 | 32 | 31 | 33 | 32 |
|
| |||||
| Interquartile range | 11–42 | 15–62 | 16–56 | 17–58 | 17–57 |
|
| |||||
| Market concentration (%) | 40 | 44 | 42 | 40 | 37 |
|
| |||||
|
CABG
| |||||
| No. of hospitals | 1073 | 1095 | 1142 | 1172 | 1195 |
|
| |||||
| No. of patients | 393,043 | 376,016 | 337,430 | 284,252 | 256,716 |
|
| |||||
| Hospital volume
| |||||
| Median | 244 | 214 | 179 | 148 | 130 |
|
| |||||
| Interquartile range | 139–408 | 129–366 | 110–304 | 90–249 | 80–216 |
|
| |||||
| Market concentration (%) | 32 | 31 | 31 | 31 | 30 |
|
| |||||
|
Carotid endarterectomy
| |||||
| No. of hospitals | 2635 | 2550 | 2502 | 2415 | 2341 |
|
| |||||
| No. of patients | 232,388 | 232,103 | 222,168 | 190,906 | 178,070 |
|
| |||||
| Hospital volume
| |||||
| Median | 77 | 78 | 75 | 67 | 64 |
|
| |||||
| Interquartile range | 40–131 | 40–130 | 40–125 | 36–109 | 35–105 |
|
| |||||
| Market concentration (%) | 39 | 38 | 37 | 37 | 35 |
|
| |||||
|
Aortic-valve replacement
| |||||
| No. of hospitals | 1013 | 1064 | 1105 | 1139 | 1161 |
|
| |||||
| No. of patients | 74,541 | 80,223 | 85,556 | 87,421 | 95,033 |
|
| |||||
| Hospital volume
| |||||
| Median | 53 | 54 | 57 | 53 | 60 |
|
| |||||
| Interquartile range | 28–99 | 29–105 | 30–104 | 29–102 | 31–108 |
|
| |||||
| Market concentration (%) | 37 | 37 | 37 | 37 | 38 |
Hospital volume is the number of Medicare patients per year. Market concentration is defined as the proportion of Medicare patients undergoing surgery in the top decile of hospitals by volume per 2-year period. AAA denotes abdominal aortic aneurysm, and CABG coronary-artery bypass grafting.
The reasons for increased hospital volumes varied according to procedure. For esophagectomy, increases in hospital volume were entirely attributable to market concentration, since the total number of cases remained relatively flat even though far fewer hospitals were performing them. Conversely, increasing volumes for aortic-valve replacement were explained exclusively by growth in the total number of procedures performed. For the remaining four procedures, increasing hospital volume occurred as a result of both volume creep and market concentration. For example, median hospital volumes increased from 5 cases of pancreatectomy per year to 16, because the total number of Medicare patients undergoing the procedure increased by 50% and the number of hospitals performing the procedure decreased by approximately 25% (from 1308 to 978).
Risk-adjusted operative mortality rates declined significantly for all eight procedures during the 10-year study period (P<0.001 for all procedures) (Fig. 1 and 2). From 1999 through 2000 and from 2007 through 2008, mortality for all cancer operations declined between 11% (for esophagectomy) and 19% (for pancreatectomy). For cardiovascular procedures, mortality fell between 8% (for carotid endarterectomy) and 36% (for AAA repair). Declining mortality could not be attributable to changes in the case mix, since predicted mortality rates remained relatively flat during the study period (Table 2, and the Supplementary Appendix).
Figure 1. Risk-Adjusted Mortality Associated with Cancer Resections among Medicare Patients, 1999 through 2008.
Risk-adjusted mortality was determined with the use of logistic-regression models to adjust for patient characteristics, including age, sex, race, admission acuity, coexisting conditions, and socioeconomic status.
Figure 2. Risk-Adjusted Mortality Associated with Cardiovascular Operations among Medicare Patients, 1999 through 2008.
AAA denotes abdominal aortic aneurysm, and CABG coronary-artery bypass grafting. Risk-adjusted mortality was determined with the use of logistic- regression models to adjust for patient characteristics, including age, sex, race, admission acuity, coexisting conditions, and socioeconomic status.
Table 2.
Procedure-Specific Characteristics, Major Coexisting Conditions, and Predicted Mortality Rates among Medicare Patients, from 1999 through 2008.*
| Variable | Period | ||||
|---|---|---|---|---|---|
| 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | |
|
Esophagectomy
| |||||
| Age — yr | 72.3±8.1 | 72.1±8.2 | 72.0±8.4 | 71.8±8.4 | 71.7±8.3 |
|
| |||||
| ≥3 Coexisting conditions — % | 28.1 | 29.8 | 31.5 | 34.2 | 30.0 |
|
| |||||
| Predicted mortality — % (95% CI) | 9.7 (9.5–9.9) | 9.5 (9.3–9.7) | 9.3 (9.1–9.5) | 9.6 (9.4–9.9) | 9.3 (9.1–9.5) |
|
| |||||
|
Pancreatectomy
| |||||
| Age — yr | 71.5±9.4 | 71.6±9.3 | 71.8±9.3 | 71.8±9.3 | 71.8±9.2 |
|
| |||||
| ≥3 Coexisting conditions — % | 25.0 | 27.2 | 30.4 | 33.4 | 33.4 |
|
| |||||
| Predicted mortality — % (95% CI) | 6.8 (6.7–7.1) | 6.7 (6.6–7.0) | 6.6 (6.4–6.7) | 6.4 (6.3–6.6) | 6.3 (6.1–6.4) |
|
| |||||
|
Lung resection
| |||||
| Age — yr | 72.2±7.1 | 72.2±7.2 | 72.3±7.4 | 72.3±7.6 | 72.3±7.7 |
|
| |||||
| ≥3 Coexisting conditions — % | 26.6 | 30.0 | 32.7 | 36.4 | 36.5 |
|
| |||||
| Predicted mortality — % (95% CI) | 4.8 (4.8–4.9) | 4.7 (4.7–4.8) | 4.7 (4.6–4.7) | 4.7 (4.7–4.8) | 4.7 (4.6–4.7) |
|
| |||||
|
Cystectomy
| |||||
| Age — yr | 73.4±7.9 | 73.3±8.1 | 73.4±8.0 | 73.5±8.1 | 73.6±8.0 |
|
| |||||
| ≥3 Coexisting conditions — % | 20.8 | 23.2 | 27.1 | 30.9 | 35.3 |
|
| |||||
| Predicted mortality — % (95% CI) | 3.7 (3.7–3.8) | 3.6 (3.6–3.7) | 3.6 (3.6–3.7) | 3.9 (3.9–4.0) | 4.2 (4.1–4.3) |
|
| |||||
|
AAA repair
| |||||
| Age — yr | 74.4±6.3 | 74.8±6.5 | 75.0±6.7 | 75.2±6.9 | 75.4±7.1 |
|
| |||||
| ≥3 Coexisting conditions — % | 33.5 | 34.4 | 36.9 | 40.3 | 37.0 |
|
| |||||
| Predicted mortality — % (95% CI) | 3.5 (3.5–3.6) | 3.4 (3.4–3.4) | 3.3 (3.3–3.4) | 3.6 (3.6–3.7) | 3.6 (3.6–3.7) |
|
| |||||
|
CABG
| |||||
| Age — yr | 72.4±7.4 | 72.3±7.6 | 72.2±7.8 | 72.1±7.9 | 71.9±8.0 |
|
| |||||
| ≥3 Coexisting conditions — % | 30.6 | 34.6 | 38.9 | 44.7 | 37.5 |
|
| |||||
| Predicted mortality — % (95% CI) | 3.8 (3.8–3.8) | 3.8 (3.8–3.8) | 3.9 (3.9–3.9) | 4.4 (4.4–4.4) | 4.3 (4.2–4.3) |
|
| |||||
|
Carotid endarterectomy
| |||||
| Age — yr | 74.6±7.0 | 74.7±7.0 | 74.7±7.3 | 74.7±7.4 | 74.7±7.6 |
|
| |||||
| ≥3 Coexisting conditions — % | 22.5 | 25.0 | 28.8 | 33.8 | 33.4 |
|
| |||||
| Predicted mortality — % (95% CI) | 1.2 (1.2–1.2) | 1.2 (1.2–1.2) | 1.2 (1.2–1.3) | 1.3 (1.3–1.3) | 1.3 (1.3–1.3) |
|
| |||||
|
Aortic-valve replacement
| |||||
| Age — yr | 74.7±8.0 | 74.8±8.1 | 74.8±8.3 | 74.9±8.3 | 75.1±8.1 |
|
| |||||
| ≥=3 Coexisting conditions — % | 39.4 | 43.0 | 46.6 | 51.7 | 35.2 |
|
| |||||
| Predicted mortality — % (95% CI) | 6.8 (6.8–6.8) | 6.9 (6.8–6.9) | 6.9 (6.9–7.0) | 7.6 (7.6–7.7) | 7.0 (7.0–7.1) |
Plus–minus values are means ±SD. AAA denotes abdominal aortic aneurysm, CABG coronary-artery bypass grafting, and CI confidence interval. Procedure-specific predicted mortality rates were determined for each patient with the use of an analysis of Medicare data from 1999 through 2000 and a logistic-regression model, clustering at the hospital level and controlling for patient characteristics, including age, sex, race, admission acuity, coexisting conditions, and socioeconomic status.
Higher hospital volumes explained a large portion of the decline in mortality associated with pancreatectomy (67%), cystectomy (37%), and esophagectomy (32%) (Table 3). Market concentration explained the majority of this effect for each of these three procedures. A smaller proportion of declines in mortality could be attributed to increasing hospital volume for lung resection (16%), AAA repair (11%), and aortic-valve replacement (9%). Hospital volume had no role in declining mortality associated with CABG and carotid endarterectomy.
Table 3.
Role of Hospital Volume in Explaining Declining Mortality among Medicare Patients Who Underwent High-Risk Procedures from 1999 through 2000 and from 2007 through 2008.
| Procedure | Proportion of the Difference in Mortality Explained by Increased Hospital Volume | ||
|---|---|---|---|
| More Patients Nationwide (“Volume Creep”) | Redistribution of Patients to Higher-Volume Hospitals (Market Concentration) | Overall Effect | |
| percent | |||
| Esophagectomy | 0 | 32 | 32 |
|
| |||
| Pancreatectomy | 18 | 49 | 67 |
|
| |||
| Lung resection | 16 | 0 | 16 |
|
| |||
| Cystectomy | 17 | 20 | 37 |
|
| |||
| AAA repair | 7 | 4 | 11 |
|
| |||
| CABG | 0 | 0 | 0 |
|
| |||
| Carotid endarterectomy | 0 | 0 | 0 |
|
| |||
| Aortic-valve replacement | 6 | 3 | 9 |
AAA denotes abdominal aortic aneurysm, and CABG coronary-artery bypass grafting.
DISCUSSION
This analysis of national Medicare data shows that average hospital volumes in the United States have increased for several high-risk operations, particularly complex cancer resections. In most cases, rising hospital volumes were driven not only by an overall increase in the number of procedures performed nationally but also by a higher concentration of procedures in a smaller number of hospitals. In addition to patients’ being referred from lower- to higher-volume centers, hundreds of U.S. hospitals stopped performing major cancer resections and AAA repair. For esophagectomy, the increase in average hospital volumes was explained entirely by market concentration. For CABG and carotid endarterectomy, hospital volumes decreased dramatically, largely as a result of fewer patients nationwide undergoing treatment.
Previous studies have described increasing regionalization of high-risk cancer resections.15,22,23 In California, the proportion of patients undergoing esophagectomy, pancreatectomy, and hepatectomy at high-volume hospitals increased by 17%, 31%, and 23%, respectively, from 1990 through 2004.22 Hollenbeck et al. noted increasing concentration of radical cystectomies in high-volume teaching hospitals from 1988 through 2000.15 These studies suggest that trends toward consolidating high-risk cancer resections at high-volume hospitals were under way well before the period of this analysis and, more specifically, before the efforts of the Leapfrog Group, which started in 2000.
It is not surprising that some procedures have become more concentrated in high-volume centers than have others. In our analysis, trends toward an increasing concentration of procedures in high-volume hospitals were most pronounced for pancreatectomy, esophagectomy, and cystectomy, which are procedures with particularly strong direct relationships between volume and outcome. These procedures are also relatively uncommon, thus the financial penalty is minimized for smaller hospitals that refer patients to higher-volume centers. At the same time, the number of hospitals performing CABG procedures increased, although the overall volume of the procedure declined. This proliferation of hospitals may be related to both the financial incentives for hospitals to be involved in cardiac surgery and their need to provide backup for interventional cardiologists.
From 1999 through 2008, risk-adjusted operative mortality fell between 8% and 36% for the eight procedures that we examined, which is consistent with several previous studies reporting trends toward declining mortality in association with high-risk surgery. For example, mortality for CABG and carotid endarterectomy fell steadily during the 1990s.24–27 Operative mortality associated with elective AAA repair has fallen, largely because of the increasing use of endovascular surgery.23,28 Finally, other studies also have documented declines in operative mortality in association with major cancer operations during the previous decade.22,29
In this study, the contribution of increasing hospital volume to declining mortality varied considerably according to procedure. Not surprisingly, the greatest increases in hospital volume during the study period were attributed to pancreatectomy, esophagectomy, and cystectomy procedures, which are associated with particularly strong direct relationships between volume and outcome. Conversely, hospital volume played little role for cardiovascular procedures, for which direct associations between hospital volume and outcome are considerably weaker.
For most procedures examined in this study, factors other than hospital volume were responsible for trends toward declining mortality. Some of these factors may be specific to the procedure. For example, the increasing use of the endovascular approach to AAA repair accounted for 60% of the associated observed decline in mortality. The national outcomes registries and improvement initiatives of the Society of Thoracic Surgeons, which has been in place in the majority of hospitals in the United States for many years, may have contributed to the observed reduction in operative mortality in association with CABG and aortic-valve replacement.30 Other efforts, such as public reporting initiatives31 and regional quality-improvement collaboratives,32 may also have played a role in declines in mortality.
The fact that mortality for all eight procedures declined during the 10-year study period suggests that there are factors common to all these procedures that contributed to mortality reduction. Technological advances and the use of checklists in the operating room and improvements in perioperative care, particularly intensive care, have most likely enhanced operative safety. In addition, in the wake of the Institute of Medicine study To Err Is Human,33 published in 1999, hospitals may be striving to improve their safety cultures, staffing, and other factors related to adverse outcomes after surgery. Finally, pay-for-performance programs and other efforts by payers to improve hospital compliance with evidence-based practices related to perioperative care may have contributed to improvements in surgical outcomes.34 Since most such programs have been implemented only recently, however, they cannot explain improvements in mortality starting more than 10 years ago.
Our study has several limitations. First, because we used administrative data, we cannot rule out the possibility that mortality is declining as a result of changes in case mix over time. However, studies based on large clinical registries suggest a trend toward older, sicker patients with many high-risk procedures.24,35 Second, because our analysis was based on fee-for-service Medicare patients, our results may not be broadly generalizable. However, patients over 65 years of age account for more than half of all patients undergoing the operations we studied and an even larger proportion of perioperative deaths.11 It seems unlikely that trends toward safer surgery would apply only to the elderly. Medicare fee-for-service patients tend to be older and have more coexisting conditions than Medicare patients enrolled in risk-bearing managed-care organizations (who account for approximately 14% of Medicare recipients), but this difference does not confound our finding that operative mortality has declined in the Medicare fee-for-service patients. An additional limitation related to our use of Medicare data is that, for specific procedures, Medicare volume is only a proxy of total hospital volume, and therefore, we may have misclassified the true volume of the hospitals. Although we believe this bias to be small, random misclassification of volume would tend to cause us to underestimate trends toward market concentration and its effect on mortality. Finally, because most ongoing policy initiatives have focused on hospital volume, we did not examine potential changes in surgeon volume over time. Thus, it is possible that trends toward declining operative mortality are attributable in part to increasing surgeon specialization within hospitals.
Although trends toward safer surgery are encouraging, tens of thousands of patients in the United States still die every year undergoing inpatient surgery. Wide variations in outcomes across hospitals suggest further opportunities for improvement. For a small number of procedures associated with particularly strong direct volume–outcome relationships, such as pancreatectomy and esophagectomy, referral to high-volume centers should continue to be encouraged. For most high-risk procedures, however, strategies such as operating-room checklists, outcomes-measurement and feedback programs, and collaborative quality-improvement initiatives are likely to be more effective than volume-based referral. Payers, policy-makers, and professional organizations should prioritize programs that have the potential to reduce mortality in all contexts.
Supplementary Material
Acknowledgments
Supported by a grant (P01AG019783-07S1) from the National Institute on Aging.
We thank Jonathan S. Skinner, Ph.D., the John Sloan Dickey Third Century Professor in Economics at Dartmouth College, for his advice regarding the statistical analysis.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Committee on Quality of Health Care in America and the National Cancer Policy Board. Interpreting the volume–outcome relationship in the context of health care quality. Washington, DC: Institute of Medicine; 2000. [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Dudley RA, Johansen KL, Brand R, Rennie DJ, Milstein A. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA. 2000;283:1159–66. doi: 10.1001/jama.283.9.1159. [DOI] [PubMed] [Google Scholar]
- 4.Milstein A, Galvin RS, Delbanco SF, Salber P, Buck CR., Jr Improving the safety of health care: the Leapfrog Initiative. Eff Clin Pract. 2000;3:313–6. [Erratum, Eff Clin Pract 2001;4:94.] [PubMed] [Google Scholar]
- 5.American College of Surgeons Bariatric Surgery Center Network. home page( http://www.acsbscn.org/Public/index.jsp.) [PubMed]
- 6.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF., Jr Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204–9. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Chappel AR, Zuckerman RS, Finlayson SR. Small rural hospitals and high-risk operations: how would regionalization affect surgical volume and hospital revenue? J Am Coll Surg. 2006;203:599–604. doi: 10.1016/j.jamcollsurg.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Ward MM, Jaana M, Wakefield DS, et al. What would be the effect of referral to high-volume hospitals in a largely rural state? J Rural Health. 2004;20:344–54. doi: 10.1111/j.1748-0361.2004.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 9.Dimick JB, Finlayson SR, Birkmeyer JD. Regional availability of high-volume hospitals for major surgery. Health Aff (Millwood) 2004;(Suppl Web Exclusives):VAR45–VAR53. doi: 10.1377/hlthaff.var.45. [DOI] [PubMed] [Google Scholar]
- 10.Peterson ED, Coombs LP, DeLong ER, Haan CK, Ferguson TB. Procedural volume as a marker of quality for CABG surgery. JAMA. 2004;291:195–201. doi: 10.1001/jama.291.2.195. [DOI] [PubMed] [Google Scholar]
- 11.Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Eff Clin Pract. 2001;4:172–7. [Erratum, Eff Clin Pract 2001;4:235.] [PubMed] [Google Scholar]
- 12.The Leapfrog Group. Evidence based hospital referral. ( http://www.leapfroggroup.org/for_hospitals/leapfrog_hospital_survey_copy/leapfrog_safety_practices/evidence-based_hospital_referral.)
- 13.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 14.Cheung MC, Hamilton K, Sherman R, et al. Impact of teaching facility status and high-volume centers on outcomes for lung cancer resection: an examination of 13,469 surgical patients. Ann Surg Oncol. 2009;16:3–13. doi: 10.1245/s10434-008-0025-9. [DOI] [PubMed] [Google Scholar]
- 15.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Montie JE, Wei JT. The regionalization of radical cystectomy to specific medical centers. J Urol. 2005;174:1385–9. doi: 10.1097/01.ju.0000173632.58991.a7. [DOI] [PubMed] [Google Scholar]
- 16.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Blinder AS. Wage discrimination: reduced form and structural estimates. J Hum Resour. 1973;8:436–55. [Google Scholar]
- 19.Oaxaca R. Male-female wage differentials in urban labor markets. Int Econ Rev. 1973;14:693–709. [Google Scholar]
- 20.Kirby JB, Taliaferro G, Zuvekas SH. Explaining racial and ethnic disparities in health care. Med Care. 2006;44:I-64–I-72. doi: 10.1097/01.mlr.0000208195.83749.c3. [DOI] [PubMed] [Google Scholar]
- 21.Fairlie R. Discussion paper no. 873. New Haven, CT: Economic Growth Center, Yale University; 2003. An extension of the Blinder-Oaxaca decomposition technique to logit and probit models. ( http://www.econ.yale.edu/growth_pdf/cdp873.pdf.) [Google Scholar]
- 22.Gasper WJ, Glidden DV, Jin C, Way LW, Patti MG. Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference? A follow-up analysis of another decade. Ann Surg. 2009;250:472–83. doi: 10.1097/SLA.0b013e3181b47c79. [DOI] [PubMed] [Google Scholar]
- 23.Hill JS, McPhee JT, Messina LM, Ciocca RG, Eslami MH. Regionalization of abdominal aortic aneurysm repair: evidence of a shift to high-volume centers in the endovascular era. J Vasc Surg. 2008;48:29–36. doi: 10.1016/j.jvs.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson TB, Jr, Hammill BG, Peterson ED, DeLong ER, Grover FL. A decade of change — risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Ann Thorac Surg. 2002;73:480–9. doi: 10.1016/s0003-4975(01)03339-2. [DOI] [PubMed] [Google Scholar]
- 25.Goodney PP, Siewers AE, Stukel TA, Lucas FL, Wennberg DE, Birkmeyer JD. Is surgery getting safer? National trends in operative mortality. J Am Coll Surg. 2002;195:219–27. doi: 10.1016/s1072-7515(02)01228-0. [DOI] [PubMed] [Google Scholar]
- 26.Holmes JS, Kozak LJ, Owings MF. Use and in-hospital mortality associated with two cardiac procedures, by sex and age: national trends, 1990–2004. Health Aff (Millwood) 2007;26:169–77. doi: 10.1377/hlthaff.26.1.169. [DOI] [PubMed] [Google Scholar]
- 27.Matsen SL, Chang DC, Perler BA, Roseborough GS, Williams GM. Trends in the in-hospital stroke rate following carotid endarterectomy in California and Maryland. J Vasc Surg. 2006;44:488–95. doi: 10.1016/j.jvs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg. 2009;49:543–50. doi: 10.1016/j.jvs.2008.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho V, Heslin MJ, Yun H, Howard L. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol. 2006;13:851–8. doi: 10.1245/ASO.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Shahian DM, O’Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1 — coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(Suppl):S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 31.Steinbrook R. Public report cards — cardiac surgery and beyond. N Engl J Med. 2006;355:1847–9. doi: 10.1056/NEJMp068222. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor GT, Plume SK, Olmstead EM, et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. JAMA. 1996;275:841–6. [PubMed] [Google Scholar]
- 33.Kohn KT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 34.Rowe JW. Pay-for-performance and accountability: related themes in improving health care. Ann Intern Med. 2006;145:695–9. doi: 10.7326/0003-4819-145-9-200611070-00013. [Erratum, Ann Intern Med 2007; 146:151.] [DOI] [PubMed] [Google Scholar]
- 35.Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–76. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.