Abstract
Chagas disease, induced by Trypanosoma cruzi, is a common cause of infectious myocarditis. Recent clinical treatment trials and vaccine studies indicate that chagasic immunopathology is directed against the parasite and not self-antigens. Therefore, vaccines may have the potential to protect against disease progression. Certain combinations of mouse and parasite strains produce significant histopathology and can be used for safety analyses of new vaccination strategies. The goals of this study were to determine: (1) if the development of Chagasic cardiomyopathy in the murine model could be identified by ECG; and (2) whether these potential Chagasic ECG changes would correlate with histopathologic findings. Groups of BALB/c, C57BL/6, and C3H mice were infected with different parasite strains (Tulahuén, Brazil or Sylvio-X10/4) and evaluated weekly by ECG. Selected tissues from subsets of mice were harvested periodically for blinded histologic evaluation. Significantly increased proportions of BALB/c mice infected with Brazil and Tulahuén strain parasites displayed prolonged QT intervals. Prolonged mean QT intervals detected in infected BALB/c mice significantly correlated with chagasic histopathologic changes. These results indicate that ECG can be used as a non-invasive method to screen for immune-mediated damage resulting in Chagasic cardiomyopathy in the murine model.
Chagas disease, caused by infection with the parasite Trypanosoma cruzi, is the most common cause of infectious myocarditis. More than 11 million people are infected worldwide with this protozoan parasite (CDC, 2007). Currently, there are no highly effective treatments once an individual has proceeded past acute infection, and vaccine work is still in the research stage.
Human infection begins after being inoculated with T. cruzi parasites, usually via the fecal matter of triatomine insects (`kissing bugs') through an insult to the dermis or via mucosal membranes, such as the eye. The acute phase of infection is characterized by mild clinical symptoms (fever and malaise) in spite of high parasitemia. Patients usually recover from this acute illness and enter the latent (or indeterminate) stage of the infection with low-level parasite persistence which can last from 10 to > 40 yr. An estimated 30% of infected individuals develop the symptomatic chronic stage of infection, which is characterized by myocarditis or megacolon (CDC, 2007). Recent studies report progress of drug intervention during chronic infection, but are more often successful in reducing progression towards disease when given during acute infection (Braga et al., 2000; Lauria-Pires et al., 2000; Cancado, 2002; Andrade et al., 2004; Garcia et al., 2005; Viotti et al., 2006). However, with detection of acute infection being low, treatment is often not given.
Previous studies have indicated that infection of certain mouse strains with specific parasite strains can induce significant histological changes in both skeletal muscle and cardiac tissue (Postan et al., 1986; Postan et al., 1987; Garg et al., 2002; Marinho et al., 2004). ECG studies have been sporadic and results often unconvincing. In our current study, we aim to lay the groundwork for future murine investigation by establishing a disease model which can be measured non-invasively. This would not only be valuable in providing a better understanding of the natural progression of Chagas disease, but could also prove valuable in evaluation of future vaccination strategies.
MATERIALS AND METHODS
Parasites and Mice
Six- to 8-wk-old female SCID, BALB/c, C57BL6, and C3H mice purchased from Charles River/NCI (Frederick, Maryland) were used in these studies. Different T. cruzi strains and life stages (Tulahuén insect-derived metacyclic trypomastigotes [IMT], Brazil tissue culture-derived trypomastigotes [TCT], and Sylvio-X10/4 blood-form trypomastigotes [BFT]) were frozen in 7.5% DMSO and stored in liquid nitrogen until use. BFT were obtained from highly parasitemic SCID mice and IMT collected from excreta of infected reduviid insects as previously described (Hoft, 1996). TCT were propagated in BALB/c 3T3 cells using DMEM supplemented with 2% serum. We chose the life stage and number of parasites for infection of mice based upon the level of virulence of each parasite strain. For example, as few as 100 Tulahuén BFT injected into BALB/c mice leads to significant mortality; thus, we decided to use generally non-lethal IMT as the challenge parasite. Sylvio-X10/4 BFT rarely lead to death in C3H mice, so we chose a larger challenge dose. Groups of 10 BALB/c mice were infected intraperitoneally with 2,000 Tulahuén IMT, 5,000 Brazil TCT, or 25,000 Sylvio-X10/4 BFT. Age and sex matched non-infected mice served as controls. Similarly, groups of X10/4 strain infection only) were studied.
Electrocardiograms
Each wk, starting with wk 1 and continuing to wk 8, then once again at wk 13, all of the mice were anesthetized and had 1-min telemetry recordings taken in ECG leads 1, 2, and 3. Mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg of body weight). Two to 3 min later, after anesthesia had taken effect, ECG leads were attached to the digits of anesthetized mice, readings taken using a Powerlab 4/25 + Animal Bio Amp and collected into Chart software with ECG analysis module (AD Instruments, Colorado Springs, Colorado). Artifactual and aberrant sections of tracings were removed and then the average RR interval, PR interval, QRS interval, and QT interval analyzed and recorded for each mouse in each respective lead. Typically, 150 – 200 successive beats were analyzed from each tracing. For the entire telemetry strip, the Chart software generates averages for each successive 4 beats. We then exported all 4 successive beat averages from an entire telemetry strip into Microsoft Excel and generated average intervals for these data. Then data derived from 4 – 10 individual mice for each experimental group were analyzed to generate group means and standard errors as well as group ranges for non-parametric statistics. Additionally, Bazett and Mitchell corrected QT (QTc) values were obtained in a subset of mice using Chart Software. This same software package was used to average 20 successive beats from individual naïve and infected mice in order to demonstrate automated measurements of electrocardiogram intervals. Data were compiled and statistics performed (Wilcoxon match pairs, Fisher Exact, Mann-Whitney U-test and Spearman Rank tests) using Statistica software (Statsoft, Inc, Tulsa, Oklahoma).
Histologic evaluation of T. cruzi-infected mice
Heart and skeletal muscle tissue from groups of mice were harvested after obtaining ECG readings on wk 4, 8, and 13 (N=3 to 4 mice per group). Hearts and skeletal muscle (quadriceps) were collected, fixed overnight using 10% neutral buffered formalin, transferred into PBS and later trimmed, processed, and embedded in paraffin. Serial 5-μm tissue sections were cut and adjacent sections were stained with hematoxylin and eosin (H&E). Samples were blinded using numerical identifiers specific to each individual mouse and evaluated microscopically using a 4 point grading system as previously described (Postan et al., 1986). These data were compiled into a Statistica database for further analyses.
RESULTS
Prolongation of QRS and QT intervals in infected mice
Trypanosoma cruzi seropositive humans exhibit significantly increased QRS, QT, and PR intervals as compared to seronegative subjects, and these differences increase with age (Williams-Blangero et al., 2007). In the murine model, only some mouse strains infected with certain parasite strains have been shown to develop cardiac pathology, and most of these studies report histological endpoints rather than electrical changes measured by ECG. A noninvasive method of monitoring for cardiac disease development in experimentally infected mice would facilitate pre-clinical safety testing of new T. cruzi drugs and vaccines. We infected multiple mouse strains with parasite isolates previously reported to induce histopathology. Age and sex matched naïve mice from each mouse strain were used as controls in all studies. We infected BALB/c mice with Brazil, Tulahuén, or Sylvio-X10/4 strain parasites; B6 mice with Brazil strain parasites; and C3H mice with Sylvio-X10/4 strain parasites. The limited reports detailing ECG changes in T. cruzi infected animals published previously used relatively subjective ECG analysis methods. To prevent possible investigator bias, automated recordings and software analysis modules were used to collect and analyze ECG tracings.
Weekly ECG recordings were obtained from each mouse (wk 1–8 and again at wk 13) in leads 1, 2, and 3, and RR, PR, QRS, and QT intervals determined using non-subjective software analysis. ECG segment intervals from each mouse group at wk 2–13 were compared with wk 1 measurements (prior to the development of systemic infection detectable as parasitemia). As seen in Table I, RR and PR intervals did not increase after infection with T. cruzi in any of the combinations tested. No ECG abnormalities could be detected in C3H mice infected with Sylvio-X10/4 strain parasites, despite the fact that this mouse/parasite strain combination has been reported previously to result in significant cardiac inflammation. In addition, Brazil strain infection of B6 mice did not induce significant ECG segment changes, although a trend towards widened QT could be detected in both leads 1 and 2. Only BALB/c mice infected with Tulahuén parasites developed significantly widened QRS as detected in lead 2; however, all infected BALB/c groups (regardless of parasite strain used) exhibited significantly prolonged QT intervals in both leads 1 and 2 (P<0.05 by Wilcoxon matched pairs test).
Table I.
ECG analysis of Trypanosoma cruzi infected mice.
| Mouse-parasite combination | RR | PR L1 | QRS L1 | QRS L2 | QT L1 | QT L2 |
|---|---|---|---|---|---|---|
| BALB/c – Brazil | NS | NS | NS | S | S | S |
| BALB/c - Tulahuén | NS | NS | NS | NS | S | S |
| BALB/c – Sylvio-X10/4 | NS | NS | NS | NS | S | S |
| B6 – Brazil | NS | NS | NS | NS | T | T |
| C3H – Sylvio-X10/4 | NS | NS | NS | NS | NS | NS |
L1, Lead 1 ECG; L2, Lead 2 ECG; NS, No Significance; S, At least 1 time point significantly different (P<0.05, (Wilcoxon match pairs test – compared all wk to wk 1), at P<0.05; T – Trend for differences
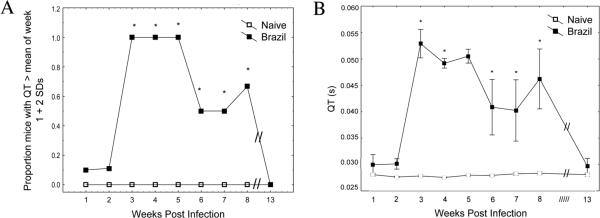
Lead 2 QT analyses are shown in further detail in Table II and Figure 1A, demonstrating that significantly increased proportions (P<0.05 by Fisher's exact test) of infected BALB/c mice were found to display prolonged QT intervals (defined as greater than the mean of wk 1 responses + 2 standard deviations) between wk 3–5 post-infection (PI). Brazil strain infection of BALB/c mice induced more persistent QT prolongation than any other combinations tested. Specifically, all Brazil infected BALB/c mice had increased QT intervals throughout weeks 3–5, and significantly increased proportions had prolonged QT intervals persisting through wk 8.
Table II.
Proportion of BALB/c mice positive for QT elongation by wk.
| Group | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W13 |
|---|---|---|---|---|---|---|---|---|---|
| Naive | 0/10 | 0/10 | 0/10 | 0/10 | 0/7 | 0/7 | 0/7 | 0/7 | 0/4 |
| Brazil | 1/10 | 1/9 | 9/9* | 7/7* | 7/7* | 3/6* | 3/6* | 4/6* | 0/4 |
| Tulahuén | 0/10 | 0/10 | 3/5* | 3/5* | 1/4 | 0/4 | 0/4 | 1/4 | 0/4 |
| Sylvio-X10/4 | 0/10 | 0/10 | 3/10 | 1/10 | 0/7 | 0/7 | 1/7 | 1/7 | 0/4 |
Shown are the proportions of mice with lead 2 QT intervals > 0.035s, corresponding to mean+2SD of all wk 1 QT intervals.
P<0.05 by Fisher Exact test comparing experimental group to naïve control group.
FIGURE 1.
Kinetics of QT elongation following Trypanosoma cruzi infection. BALB/c mice were infected i.p. with 5,000 Brazil strain TCT, then lead 2 ECG tracings recorded at various time points (wk 1–8 and wk 13). QT intervals from individual mice were analyzed from 100–200 beats using an automated software package. (A) Baseline recordings from all mice were used to define an elongated QT interval as any value > QT(mean) + 2 standard deviations (0.035s). Significantly higher proportions of T. cruzi infected animals displayed elongated QT intervals wk 3–8 as compared to uninfected controls (P<0.05, Fisher Exact test). (B) Actual QT intervals obtained from individual mice were also significantly higher in T. cruzi infected animals than controls during wk 3–8 PI (P<0.05, Mann-Whitney U-test).
Figure 1B demonstrates that Brazil strain infected BALB/c mice displayed significantly increased mean QT duration compared with control mice during wk 3 to 8 PI (P<0.05 by Mann-Whitney U-test). In fact, peak QT intervals at wk 3 PI were almost twice as long as the QT intervals measured at wk 1 baseline measurements (0.53s and 0.030s, respectively). QT intervals in these infected mice started to decrease substantially starting at wk 5, and by wk 13 were back to near baseline.
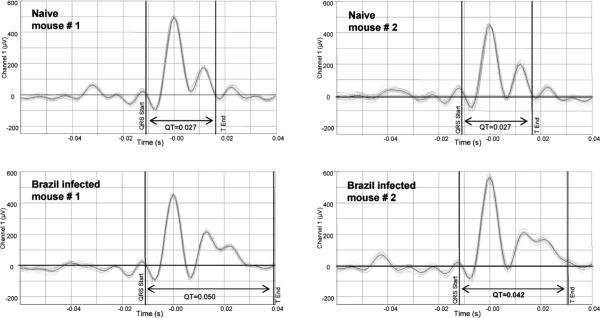
Figure 2 demonstrates representative changes seen in T. cruzi infected mice. Twenty successive beats from 4 individual mice were compiled and averaged for display. Two random samples of BALB/c mice infected 3 wk prior with Brazil parasites developed much longer QT intervals (0.050s and 0.042s) compared with naïve mice (0.027s and 0.027s). Also, it can be seen that individual beats (light grey lines) closely mirrored the patterns and intervals shown for the averaged data.
FIGURE 2.
QT interval analysis of Trypanosoma cruzi infected mice. ECG recordings were obtained from individual naïve and T. cruzi infected BALB/c mice (3 wk PI with 5,000 Brazil strain TCT i.p.). An automated software package was used to plot and average 20 consecutive beats, and cursers placed to denote points used to analyze the QT interval. Shown are example tracings and QT intervals obtained from 2 random naïve (0.027s and 0.027s) and T. cruzi infected BALB/c mice (0.050s and 0.042s).
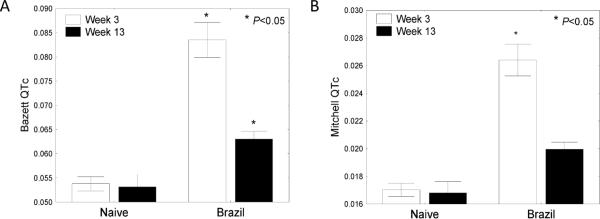
QT interval is dependent on heart rate and QT intervals corrected for heart rate (QTc) can be used for normalization to more accurately assess the significance of QT prolongation. We further analyzed ECG tracings in Brazil strain infected BALB/c mice at wk 1, 3, and 13 PI using Chart software to obtain corrected QT values using 2 common QTc methods (Mitchell and Bazett QTc formulas). As seen in Figure 3, T. cruzi infected mice yielded significantly higher QTc values than control animals during both acute (3wpi) and chronic (13wpi) time points.
FIGURE 3.
Corrected QT values in Trypanosoma cruzi infected mice. BALB/c mice were infected with 5,000 Brazil strain TCT i.p. and ECG tracings recorded 3 and 13 wk PI. Over 100 individual beats from each mouse were analyzed and Bazett and Mitchell corrected QT (QTc) values generated. Trypanosoma cruzi-infected animals displayed significantly increased QTc values as determined using Bazett (A) and Mitchell (B) formula calculations during both acute (wk 3) and chronic (wk 13) time points (P<0.05, Mann-Whitney U-test).
T. cruzi induced inflammation in heart and skeletal muscle
In addition to ECG monitoring of mice after T. cruzi infection, representative mice were periodically killed during the study for histopathological evaluation. Skeletal and heart specimens from naïve and T. cruzi infected mice (30, 60, and 90 days PI) were collected and processed. Hematoxylin and eosin (H&E) sections were scored in a blinded fashion (scale 0 – 4) by a veterinary pathologist as described previously (Postan et al., 1986). Inflammatory lesions were qualitatively similar in affected mice, regardless of the strain of mouse or parasite. Inflammatory lesions were non-suppurative in nature, being characterized by intramyocardial infiltration of variable numbers of primarily lymphocytes and lesser numbers of macrophages. Lesions tended to be concentrated within the atrial myocardium and in the ventricular myocardium near the atrioventricular junctions. Inflammatory lesions were distributed evenly between left and right atria and ventricles, as well as in the interventricular myocardium. The character of the inflammatory cell infiltration was similar in affected skeletal muscle tissue. Lesions tended to exhibit primarily a perivascular distribution; however, extension along fascial planes and between individual myofibers was also observed.
Despite the fact that Brazil strain infected B6 and Sylvio-X10/4 strain infected C3H mice failed to develop significant changes as detected by ECG, heart and skeletal muscle collected from these animals at 1 to 3 mo PI displayed significant inflammation (P<0.05 by Mann-Whitney U-test compared with uninfected controls). Based on our findings of significantly prolonged QT intervals (and in BALB/c mice infected with Brazil strain parasites widened QRS), we expected to find substantial inflammation in hearts and skeletal muscle sections of all infected BALB/c mice. Since BALB/c mice are relatively susceptible to T. cruzi infection, we also expected to observe death of some mice infected with aggressive strains of T. cruzi. None of the BALB/c mice infected with the least virulent Sylvio-X10/4 parasites succumbed to infection. However, in the case of more virulent infections with both Brazil and Tulahuén strains, BALB/c mice suffered significant mortality, i.e., Brazil: 8/10 survived; Tulahuén: 5/10 survived. Therefore, some early time point studies could not be performed in BALB/c mice infected with Brazil and Tulahuén parasites.
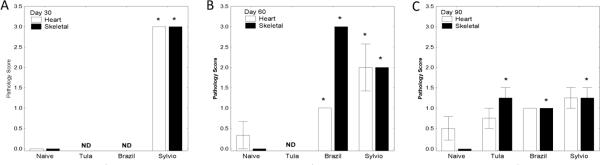
As shown in Figure 4, BALB/c mice infected with Sylvio-X10/4 strain parasites were found to have significantly increased levels of both cardiac and skeletal inflammation when compared with infected controls at both day 30 and 60 PI. Inflammation decreased some by day 90, although significantly increased inflammation of skeletal muscle persisted (P<0.05 by Mann-Whitney U-test). Similar cardiac and skeletal inflammation was detected in BALB/c mice infected with more virulent T. cruzi challenges. Although early time points were not obtained in the Tulahuén-infected BALB/c group, significantly increased inflammation compared with uninfected controls was seen in the skeletal muscles of infected animals 90 days PI. Hearts obtained from Brazil-infected BALB/c mice displayed significantly increased inflammation on day 60 PI, and this inflammation persisted in some Brazil infected BALB/c mice on day 90. All parasite strains used to infect BALB/c mice resulted in significant skeletal muscle inflammation at all time points.
FIGURE 4.
Trypanosoma cruzi-induced inflammation. BALB/c mice were infected with Tulahuén, Brazil, or Sylvio-X10/4 strain parasites. H&E sections from hearts and quadriceps (skeletal muscle source) obtained from representative mice 30 (A), 60 (B), and 90 (C) days PI were examined microscopically and inflammation graded on a scale of 0 – 4. BALB/c mice infected with Sylvio-X10/4 parasites exhibited significant inflammation in heart sections at 30 and 60 days PI, as did Brazil strain infected mice at 60 days PI. BALB/c mice infected with each of the 3 strains of T. cruzi exhibited significantly more inflammation in skeletal muscle during all time points analyzed as compared to uninfected controls. (ND – Not done, * P<0.05, Mann-Whitney U-test as compared to naïve mice)
QT prolongation correlates with chronic pathology
Based on our findings that infection of BALB/c mice with Brazil strain parasites resulted in both electrical abnormalities (prolonged QT and QRS) and significantly increased inflammation in heart and skeletal muscle, we next investigated whether QT prolongation and histologic effects were correlated using Spearman rank tests. As seen in Table III, correlations were detected: (1) between cardiac and skeletal muscle inflammation at 60 + 90 days; and (2) between QT intervals and day 90 skeletal muscle inflammation (Spearman Rank >0.90, P<0.05). In addition, a strong trend was seen for a correlation between QT interval and cardiac inflammation at day 90 (Spearman Rank >0.50, P<0.17).
Table III.
QT prolongation predicts chronic pathology.
| Pair of variables | Valid N | Spearman rank | P-level |
|---|---|---|---|
| Skeletal pathology and cardiac pathology (D60+D90) | 13 | 0.5918 | 0.0331 |
| QT L1 and D90 skeletal pathology | 8 | 0.956 | 0.0002 |
| QT L2 and D90 skeletal pathology | 8 | 0.936 | 0.0006 |
| QT L1 and D90 cardiac pathology | 8 | 0.552 | 0.1559 |
| QT L2 and D90 cardiac pathology | 8 | 0.541 | 0.1666 |
Shown are spearman rank correlations using naïve and Brazil infected BALB/c mice. QT L1, Maximum QT interval obtained from lead 1 ECG tracing; QT L2, Maximum QT interval obtained from lead 2 ECG tracing, i.e., D 60, Day 60 PI; D 90, Day 90 PI.
DISCUSSION
Although there appears to be decreasing numbers of newly acquired T. cruzi infections worldwide likely due to vector control initiatives, there are still more than 11 million individuals chronically infected with T. cruzi (CDC, 2007). Benznidazole and nifurtimox have shown varying degrees of success in reducing the rates of progression towards disease and death, but both drugs produce serious side effects and contraindications (Braga et al., 2000; Lauria-Pires et al., 2000; Cancado, 2002; Andrade et al., 2004; Garcia et al., 2005; Castro et al., 2006; Viotti et al., 2006). In the mouse model, vaccines have been shown to prevent disease and death when given both prophylactically and therapeutically (Garg et al., 2002; Dumonteil et al., 2004). Most data published to date have relied exclusively on microscopic examination and inflammatory grading of skeletal and cardiac muscle sections. We have shown here that QT prolongation in acute stages of infection correlates with chronic inflammation as identified by histology. Thus, QT interval measured during acute infection may predict chronic inflammation and chronic disease progression in the murine model. The noninvasive ECG method presented here will provide a valuable tool in future studies of experimental vaccinations and chronic disease progression.
To our knowledge, this is the first systematic serial evaluation of murine ECG measurements investigating changes detectable from early acute T. cruzi infection and chronic PI time points. Previous reports have suggested that bradycardia and widened QRS intervals can be detected during murine T. cruzi infection. We found that heart rate was highly variable depending on environmental conditions and did not detect significant heart rate changes due to infection. We also found that murine electrocardiograms did not allow us to easily detect reproducible QRS intervals because the curve does not always define discrete S segment initiation, probably due to the rapid murine heart rate. Instead, the QT interval, representing both depolarization and repolarization is more reproducibly measured.
The timing of QT elongation coincides with the onset of peak parasitemia, but persists for quite some time after resolution of acute infection (up to 13 wk PI). In the case of BALB/c mice infected with Brazil strain parasites, QT elongation could be detected in many cases from 3 wk PI through our endpoint tracings recorded 13 wk PI. Perhaps not only the maximum QT interval achieved, or the duration of increased QT intervals detected, but possibly a combination of QT increase and duration (area under the curve analysis) may correlate best with level of histologic disease. This possibility should be explored in future studies.
The QT interval represents the duration of activation and recovery of the ventricular myocardium. Myocarditis of all types have been shown to cause a number of ECG abnormalities, including ST segment changes, T wave changes, and prolongation of the QT interval. Abnormal ventricular repolarization, reflected by a prolonged QTc interval, could be due to cardiac autonomic dysfunction which is frequent in Chagasic heart disease, or to the relentless myocarditis process itself, with its consequent myocardial fibrosis and dilatation. Concomitant altered ventricular repolarization with LV dysfunction and myocardial fibrotic areas along with myocardial electrical instability, can create the conditions that predispose Chagas disease patients to serious ventricular arrhythmias and sudden death. The length of the QTc interval along with LV systolic function have been shown to be important mortality risk predictors in patients with Chagas disease (Salles et al., 2003). Further mechanistic studies are required to determine the specific effects of T. cruzi infection on activation and for recovery of the ventricular myocardium.
Several parasite and mouse strain combinations have been previously reported to induce acute and/or chronic inflammation detected by histologic evaluation and, in some cases, ECG abnormalities including increased PQ (Bustamante et al., 2002), PR (Postan et al., 1987; Garcia et al., 2005), QRS (Bustamante et al., 2002), or QT (Garcia et al., 2005) intervals. Two of the most common mouse strain / parasite strain combinations used for murine pathologic studies reported to date are C3H mice infected with Sylvio-X10/4 parasites, and B6 mice infected with Brazil strain parasites. Significant inflammation starting at day 14 PI and persisting out to > 1 yr can be detected in hearts of C3H mice infected with Sylvio-X10/4 parasites (Postan et al., 1986; Marinho et al., 2004). Significant alterations of electrocardiographic tracings have also been seen in this strain combination 1 yr PI, the most significant of which reported was prolonged PR interval (Postan et al., 1987). B6 mice infected with Brazil strain parasites develop significant chronic inflammation in striated / skeletal muscle sections and have been used as a model to evaluate protective efficacy of vaccines during both acute and chronic stages of infection (Garg et al., 2002). However, the C3H / Sylvio-X10/4 and B6 / Brazil models are not without their limitations, i.e., the B6 mouse strain is relatively resistant to T. cruzi infection, and the Sylvio-X10/4 strain of T. cruzi rarely leads to detectable parasitemia, even after robust challenges in most mouse strains. We have focused on the BALB/c mouse model in our previous T. cruzi vaccine development and immune studies because it is a naturally susceptible mouse strain and, therefore, can be used as a model for development of more universally effective vaccines (Hoft and Eickhoff, 2002; Schnapp et al., 2002; Giddings et al., 2006; Hoft et al., 2007). To this end, it is fortunate that ECG abnormalities including elongated QT and QRS intervals were seen most consistently in BALB/c mice infected with Brazil strain parasites (summarized in Table IV). The results obtained in this study will allow us to utilize the common BALB/c mouse model to explore pathologic consequences of protective vaccines.
Table IV.
Significant findings summary.
| Mouse strain | Parasite strain | ECG findings | Chronic inflammation |
|---|---|---|---|
| BALB/c | Brazil | ↑QRS, ↑QT | ↑Heart, ↑Skeletal |
| BALB/c | Tulahuén | ↑QT | ↑Skeletal |
| BALB/c | Sylvio-X10/4 | ↑QT | ↑Heart, ↑Skeletal |
| B6 | Brazil | ↑TQT | ↑Skeletal |
| C3H | Sylvio-X10/4 | ↑Heart, ↑Skeletal |
Shown are significant findings (P<0.05) of ECG abnormalities and chronic histopathology in T. cruzi infected mice. ↑QRS, increased QRS intervals as compared to uninfected control mice, ↑QT, increased QT intervals as compared to uninfected control mice, ↑TQT, non-significant, but a strong trend of increased QT intervals as compared to uninfected control mice, ↑Heart, significant inflammation detected in cardiac tissues at 60 and/or 90 days PI compared to uninfected control mice, ↑Skeletal, significant inflammation detected in skeletal muscle tissues detected at 60 and/or 90 days PI compared to uninfected control mice.
LITERATURE CITED
- Andrade AL, Martelli CM, Oliveira RM, Silva SA, Aires AI, Soussumi LM, Covas DT, D.T., Silva LS, Andrade JG, Travassos LR, Almeida IC. Short report: benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. American Journal of Tropical Medicine and Hygiene. 2004;71:594–597. [PubMed] [Google Scholar]
- Braga MS, Lauria-Pires L, Arganaraz ER, Nascimento RJ, Teixeira AR. Persistent infections in chronic Chagas disease patients treated with anti-Trypanosoma cruzi nitroderivatives. Revista do Instituto de Medicina Tropical de Sao Paulo. 2000;42:157–161. doi: 10.1590/s0036-46652000000300009. [DOI] [PubMed] [Google Scholar]
- Bustamante JM, Rivarola HW, Fernandez AR, Enders JE, Fretes R, Palma JA, Paglini-Oliva PA. Trypanosoma cruzi reinfections in mice determine the severity of cardiac damage. International Journal for Parasitology. 2002;32:889–896. doi: 10.1016/s0020-7519(02)00023-1. [DOI] [PubMed] [Google Scholar]
- Cancado JR. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Revista do Instituto de Medicina Tropical de Sao Paulo. 2002;44:29–37. [PubMed] [Google Scholar]
- Castro JA, de Mecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas disease (American trypanosomiasis) Human & Experimental Toxicology. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- CDC Blood donor screening for Chagas disease--United States, 2006–2007. Morbidity and Mortality Weekly Report. 2007;56:141–143. [PubMed] [Google Scholar]
- Dumonteil E, Escobedo-Ortegon J, Reyes-Rodriguez N, Arjona-Torres A, Ramirez-Sierra MJ. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice. Infection & Immunity. 2004;72:46–53. doi: 10.1128/IAI.72.1.46-53.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia S, Ramos CO, Senra JF, Vilas-Boas F, Rodrigues MM, Campos-de-Carvalho AC, Ribeiro-Dos-Santos R, Soares MB. Treatment with benznidazole during the chronic phase of experimental Chagas disease decreases cardiac alterations. Antimicrobial Agents and Chemotherapy. 2005;49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N, R.L., Tarleton Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infection & Immunity. 2002;70:5547–5555. doi: 10.1128/IAI.70.10.5547-5555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings OK, Eickhoff CS, Smith TJ, Bryant LA, Hoft DF. Anatomical route of invasion and protective mucosal immunity in Trypanosoma cruzi conjunctival infection. Infection & Immunity. 2006;74:5549–5560. doi: 10.1128/IAI.00319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft DF. Differential mucosal infectivity of different life stages of Trypanosoma cruzi. American Journal of Tropical Medicine and Hygiene. 1996;55:360–364. doi: 10.4269/ajtmh.1996.55.360. [DOI] [PubMed] [Google Scholar]
- Hoft DF, Eickhoff CS. Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges. Infection & Immunity. 2002;70:6715–6725. doi: 10.1128/IAI.70.12.6715-6725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft DF, Eickhoff CS. Type 1 immunity provides both optimal mucosal and systemic protection against a mucosally invasive, intracellular pathogen. Infection & Immunity. 2005;73:4934–4940. doi: 10.1128/IAI.73.8.4934-4940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft DF, Eickhoff CS, Giddings OK, Vasconcelos JR, Rodrigues MM. Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic Trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. Journal of Immunology. 2007;179:6889–6900. doi: 10.4049/jimmunol.179.10.6889. [DOI] [PubMed] [Google Scholar]
- Lauria-Pires L, Braga MS, Vexenat AC, Nitz N, Simoes-Barbosa A, Tinoco DL, Teixeira AR. Progressive chronic Chagas heart disease ten years after treatment with anti-Trypanosoma cruzi nitroderivatives. American Journal of Tropical Medicine and Hygiene. 2000;63:111–118. doi: 10.4269/ajtmh.2000.63.111. [DOI] [PubMed] [Google Scholar]
- Marinho CR, Bucci DZ, Dagli ML, Bastos KR, Grisotto MG, Sardinha LR, Baptista CR, Goncalves CP, Lima MR, Alvarez JM. Pathology affects different organs in two mouse strains chronically infected by a Trypanosoma cruzi clone: a model for genetic studies of Chagas disease. Infection & Immunity. 2004;72:2350–2357. doi: 10.1128/IAI.72.4.2350-2357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postan M, Bailey JJ, Dvorak JA, McDaniel JP, Pottala EW. Studies of Trypanosoma cruzi clones in inbred mice III. Histopathological and electrocardiographical responses to chronic infection. American Journal of Tropical Medicine and Hygiene. 1987;37:541–549. doi: 10.4269/ajtmh.1987.37.541. [DOI] [PubMed] [Google Scholar]
- Postan M, Cheever AW, Dvorak JA, McDaniel JP. A histopathological analysis of the course of myocarditis in C3H/He mice infected with Trypanosoma cruzi clone Sylvio-X10/4. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986;80:50–55. doi: 10.1016/0035-9203(86)90193-8. [DOI] [PubMed] [Google Scholar]
- Salles G, Xavier S, Sousa A, Hasslocher-Moreno A, Cardoso C. Prognostic value of QT interval parameters for mortality risk stratification in Chagas disease: results of a long-term follow-up study. Circulation. 2003;108:305–312. doi: 10.1161/01.CIR.0000079174.13444.9C. [DOI] [PubMed] [Google Scholar]
- Schnapp AR, Eickhoff CS, Sizemore D, Curtiss R, III, Hoft DF. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infection & Immunity. 2002;70:5065–5074. doi: 10.1128/IAI.70.9.5065-5074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Annals of Internal Medicine. 2006;144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- Williams-Blangero S, Magalhaes T, Rainwater E, Blangero J, Correa-Oliveira R, Vandeberg JL. Electrocardiographic characteristics in a population with high rates of seropositivity for Trypanosoma cruzi infection. American Journal of Tropical Medicine and Hygiene. 2007;77:495–499. [PubMed] [Google Scholar]