Abstract
Mediator, a conserved multiprotein complex in animals, plants, and fungi, is a cofactor of RNA Polymerase II (Pol II). It is known to promote basal Pol II-mediated transcription as well as bridge sequence-specific transcriptional regulators and Pol II to integrate regulatory information. Pol II transcribes not only protein-coding genes but also intergenic regions to generate noncoding RNAs such as small RNAs (microRNAs and small interfering RNAs) and long noncoding RNAs. Intriguingly, two plant-specific polymerases, Pol IV and Pol V, have evolved from Pol II and play a role in the production of small interfering RNAs and long noncoding RNAs at heterochromatic regions to maintain genome stability through transcriptional gene silencing (TGS). Recent studies have defined the composition of the plant Mediator and evaluated its role in noncoding RNA production in relationship to Pol II, Pol IV and Pol V. Here, we review the functions of Mediator and that of noncoding RNAs generated by Pol II, Pol IV and Pol V in plants, and discuss a role of Mediator in epigenetic regulation via noncoding RNA production.
Keywords: small RNA, noncoding RNA, Mediator, Pol II, Pol IV, Pol V
Introduction
Dynamic changes in gene expression upon internal and external demands are first modulated at the transcription initiation step. Pol II, which is responsible for the transcription of protein-coding genes in eukaryotes, is recruited to promoters of genes (Sikorski and Buratowski, 2009). Biochemical studies show that Pol II alone is not sufficient to initiate transcription in vitro and imply the existence of co-factors for Pol II transcription initiation. One type of well-studied cofactors includes the basal initiation factors such as TFIIA, TFIIB, TFIID, TFIIE, TFIIF and TFIIH (Reese, 2003; Thomas and Chiang, 2006). In the presence of these factors, which are so-called general transcription factors (GTFs), Pol II supports basal transcription in vitro. The assembly of the pre-initiation complex (PIC) including Pol II and GTFs on the core promoter is a critical step in transcription initiation. Genetic and biochemical studies have revealed another cofactor that plays an important role in transcription initiation: a multiple protein complex termed Mediator. It has been thought that Mediator bridges sequence-specific transcriptional regulators to the Pol II-containing PIC for transcription initiation in a passive mode (Kuras and Struhl, 1999; Struhl, 1996; Yudkovsky et al., 2000). Recently, Mediator is proposed to play a more active role as a signal integrator to transmit information from the input transcriptional regulators to the transcription machinery (Malik and Roeder, 2010).
Pol II transcribes not only protein-coding genes but also genomic regions that give rise to noncoding RNAs. In addition, two plant-specific polymerases, Pol IV and Pol V, produce noncoding RNAs from repeats and transposable elements. The Pol IV- and Pol V-dependant noncoding RNAs are involved in the maintenance of genome stability through small interfering RNA (siRNA)-mediated transcriptional gene silencing (TGS) at repeats and transposable elements (Chen, 2009). Recently, it has been shown that Pol II is also required for siRNA-mediated TGS at a subset of heterochromatic loci (Zheng et al., 2009). A recent study in our laboratory has evaluated the role of Mediator in Pol II-, Pol IV-, and Pol V-dependent noncoding RNA production and TGS (Kim et al., 2011). In this article, we first review the general molecular functions of Mediator and the role of Pol II, Pol IV- or Pol V-dependant noncoding RNAs in TGS. Then we discuss roles of Mediator in noncoding RNA production and TGS and propose a working model.
Structure of the Mediator complex
Mediator is a large protein complex composed of 20-30 subunits (Table 1). It is functionally and structurally conserved in all eukaryotes although species-specific subunits exist ((Casamassimi and Napoli, 2007); Table 1). Mediator was first identified in yeast, Saccharomyces cerevisiae, an organism in which it remains to be the best studied (Carlson et al., 1981; Neigeborn and Carlson, 1984; Simchen et al., 1984; Stern et al., 1984; Suzuki et al., 1988). Structural studies of the yeast Mediator complex revealed that it is composed of three subdomains (Head, Middle and Tail) and a separable kinase module (Dotson et al., 2000; Guglielmi et al., 2004; Sato et al., 2003).
Table 1.
Mediator composition from diverse eukaryotes
| Mammalian | S. cerevisiae | A. thaliana | Module |
|---|---|---|---|
| MED1 | MED1 | - | Middle |
| MED2 | MED2 | - | Tail |
| MED3 | POLYGLUTAMINE DOMAIN 1 (PGD1) |
- | Tail |
| MED4 | MED4 | MED4 | Middle |
| MED5 | NEGATIVE REGULATION OF URS TWO 1 (NUT1) |
- | Tail |
| MED6 | MED6 | MED6 | Head |
| MED7 | MED7 | MED7 | Middle |
| MED8 | MED8 | MED8 | Head |
| MED9 | CHROMOSOME SEGREGATION 2 (CSE2) |
MED9 | Middle |
| MED10 | NEGATIVE REGULATION OF URS TWO 2 (NUT2) |
MED10 | Middle |
| MED11 | MED11 | MED11 | Head |
| MED12 | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 8 (SRB8) |
CRYPTIC PRECOCIOUS (CRP) |
CDK |
| MED13 | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 9 (SRB9) |
MED13 | CDK |
| MED14 | RESISTANT TO GLUCOSE REPRESSION 1 (RGR1) |
STRUWWELPETER (SWP) |
Tail |
| MED15 | GALACTOSE METABOLISM 11 (GAL11) |
MED15 | Tail |
| MED16 | SWITCH INDEPENDENT 4 (SIN4) |
MED16 | Tail |
| MED17 | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 4 (SRB4) |
MED17 | Head |
| MED18 | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 5 (SRB5) |
MED18 | Head |
| MED19 | REPRESSOR OF HYPOXIC GENES 3 (ROX3) |
MED19 | Head |
| MED20 | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 2 (SRB2) |
MED20 | Head |
| MED21 | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 7 (SRB7) |
MED21 | Middle |
| MED22 | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 6 (SRB6) |
MED22 | Head |
| MED23 | - | MED23 | not identified |
| MED24 | - | - | not identified |
| MED25 | - | PHYTOCHROME AND FLOWERING TIME 1 (PFT1) |
not identified |
| MED26 | - | - | not identified |
| MED27 | - | MED27 | not identified |
| MED28 | - | MED28 | Head |
| MED29 | - | - | Head |
| MED30 | - | - | Head |
| MED31 | SUPPRESSOR OF HPR 1 (SOH1) |
MED31 | Middle |
| CDK8 | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 10 (SRB10) |
HUA ENHANCER 3 (HEN3) |
CDK |
| Cyclin C | SUPPRESSOR OF RNA POLYMERASE B MUTATIONS 11 (SRB11) |
- | CDK |
The head domain, composed of MED6, MED8, MED11, MED17, MED18, MED19, MED20 and MED22, interacts with a Pol II-TFIIF complex in vitro (Takagi et al., 2006) and constitutes the most extensive Pol II-interacting interface in Mediator. Disruption of the head domain results in dissociation of Mediator from transcriptionally active promoters (Lariviere et al., 2006). The middle domain, which includes MED1, MED4, MED7, MED9, MED10, MED21 and MED31, directly interacts with the C-terminal domain (CTD) of the largest subunit in Pol II (Rbp1) (Kang et al., 2001). It is believed that the middle domain transmits input signals from transcriptional regulators to the head domain. The tail domain consists of MED2, MED3, MED5, MED14, MED15 and MED16 and interacts with the DNA-bound transcriptional regulators (Han et al., 2001; Lee et al., 1999; Park et al., 2000). The detachable kinase module is composed of four proteins: MED12, MED13, cyclin-dependant kinase 8 (CDK8) and Cyclin C (CycC). Mediators containing the kinase module are referred as large Mediators, whereas variants without this module are called small Mediators (Malik and Roeder, 2000; Mittler et al., 2001; Naar et al., 2002; Sun et al., 1998; Taatjes et al., 2004). The CDK8 module is mainly involved in transcriptional repression probably through its kinase activity, which phosphorylates the Rbp1 CTD heptads, some Mediator subunits, GTFs, and transcriptional regulators (Chi et al., 2001; Hallberg et al., 2004; Hengartner et al., 1998; Hirst et al., 1999; Liu et al., 2004; Nelson et al., 2003; van de Peppel et al., 2005; Vincent et al., 2001).
Mediator is not a fixed complex - several isoforms or alternative forms exist in cells (Casamassimi and Napoli, 2007). The identification of large and small Mediators based on the presence of the CDK8 module has uncovered the functional flexibility of Mediator as either an activator or a repressor (Malik and Roeder, 2000; Mittler et al., 2001; Naar et al., 2002; Sun et al., 1998; Taatjes et al., 2004). In addition, new isoforms in several subunits have been identified and differences in the composition of complexes in the mammalian Mediator have been found (Mittler et al., 2001). It is not clear how many alternative Mediator forms exist in organisms. However, it is thought that the structural arrangement and complexity allow it to integrate a multitude of regulatory inputs.
Molecular functions of Mediator
Studies with the yeast srb4 mutant suggest that Mediator is a GTF. srb4 was isolated as a temperature sensitive mutant through a genetic screen aimed at the identification of regulators of transcription. Later, SRB4 was found to be MED17, one of the head subunits of Mediator. In the srb4/med17 mutant, the levels of more than 90% of Pol II-dependent transcripts are decreased under the restrictive temperature (Holstege et al., 1998; Thompson and Young, 1995). This suggests that Mediator acts as a general factor in Pol II transcription. Consistently, it has been shown that the head domain of Mediator stimulates basal transcription in the absence of activators in vitro (Baek et al., 2006; Mittler et al., 2001).
However, controversy exists over the role of Mediator in transcription initiation. A genome-wide analysis shows that Mediator occupancy is not tightly correlated with that of Pol II at many highly active Pol II promoters in yeast (Fan et al., 2006), thereby arguing against a role of Mediator as a GTF. Moreover, another study suggests that Mediator is associated with promoters in an activator- rather than Pol II-dependent manner (Fan and Struhl, 2009). In contrast to these results, another study argues that Mediator is associated with constitutively active genes and is required for the recruitment of Pol II as a GTF (Ansari et al., 2009). Therefore, it appears that further investigations are needed to better understand the functions of Mediator.
Besides the general role of Mediator in PIC formation, several pieces of evidence indicate that Mediator also acts at the chromatin level through interactions with chromatin modification factors such as histone acetyltransferases and methyltransferases. Mediator recruits the histone acetyltransferase p300 to a promoter bound by a transcription factor through direct interaction with p300 to allow acetylation of the local chromatin. Subsequent dissociation of p300 from the DNA promotes TFIID binding followed by PIC formation (Black et al., 2006). There is also evidence that MED12 mediates ternary complex formation with two other proteins, the silencing transcription factor REST and the methyltransferase G9a. The deposition of the H3K9 dimethylation repressive mark at target genes by G9a is thought to play a role in REST-mediated neuronal gene silencing in non-neuronal cells (Ding et al., 2008; Ooi and Wood, 2007).
Mediator in plants
Recently, the Arabidopsis Mediator was biochemically characterized and found to contain 21 subunits conserved in eukaryotes and six plant-specific subunits (Backstrom et al., 2007). Although the CDK8 module was not co-purified with Mediator in the experiment, Arabidopsis has homologs to MED12, MED13, CDK8 and CycC genes encoding subunits of the CDK8 module. Prior to this purification of the Arabidopsis Mediator complex, several subunits had been studied genetically.
PHYTOCHROME and FLOWERING TIME1 (PFT1), now known as MED25, was identified as a factor of a Phytochrome B (phyB) signaling pathway that promotes flowering in response to shade (Cerdan and Chory, 2003). It was thought to be a transcriptional coactivator on the basis of its nuclear localization, the presence of a glutamine-rich domain and its transcription activation activity in yeast when fused to the LexA DNA-binding domain. A recent study suggested that PFT1 negatively regulates the phytochrome signaling pathway rather than acting as a component in the pathway (Wollenberg et al., 2008).
STRUWWELPETER (SWP)/MED14 was reported as a nuclear protein playing a role in defining the duration of cell proliferation (Autran et al., 2002). An swp mutant exhibits dwarfism with an abnormal architecture such as a fascinated stem and abnormal floral structures; some of the phenotypes being attributable to reduced cell numbers. Consistently, ectopic expression of SWP caused increased cell numbers. It was reported that the repressive activity of LEUNIG (LUG), a transcriptional corepressor, involves its interaction with SWP/MED14 and HUA ENHANCER3 (HEN3)/CDK8 (Gonzalez et al., 2007). HEN3 was identified as a weak regulator of AG, which is a target of LUG (Liu and Meyerowitz, 1995; Wang and Chen, 2004). It is possible that LUG negatively regulates AG expression through the larger Mediator complex containing the HEN3/CDK8 module that has repressor activity.
Recent studies have suggested that Mediator acts as an integrator in response to environmental cues in Arabidopsis (Kidd et al., 2009). Another function of PFT1/MED25 is that it is required for Jasmonic Acid (JA)-dependant defense gene expression and resistance to leaf-infecting necrotrophic fungal pathogens (Kidd et al., 2009). In addition to being late flowering, an atmed8 mutant showed delayed symptom development upon infection by a root-infecting hemibiotrophic fungal pathogen (Kidd et al., 2009), indicating that MED8 is a regulator of disease resistance and flowering time. In another study, it was shown that MED21 is required for resistance to necrotrophic fungal pathogens (Dhawan et al., 2009). Interestingly, MED21 interacts with HISTONE MONOUBIQUITINATION1 (HUB1) that is also involved in defense against necrotrophic fungal pathogens, implying that MED21 integrates pathogen-infection signaling through chromatin modifications.
Noncoding RNAs and Pol II, IV and V in plants
In recent years, small noncoding RNA-mediated gene silencing has been increasingly recognized to play crucial roles in a multitude of biological processes in plants and animals. Small RNAs of 20 - 30 nt in size serve as sequence-specific repressors of target gene expression. In plants, there are two major types of small RNAs: microRNAs (miRNAs; Fig. 1A) and small interfering RNAs (siRNAs; Fig. 1B) (Chen, 2009). Most plant miRNA genes (MIR) are located in intergenic regions and have their own promoters. It is thought that Pol II is responsible for MIR gene transcription in plants on the basis of common features between pri-miRNAs and mRNAs as well as the presence of TATA boxes in the promoters of MIR genes (Xie et al., 2005). Using a partial loss of function allele in the second largest subunit of Pol II (Zheng et al., 2009), we showed that miRNA accumulation indeed requires Pol II (Kim et al., 2011). We also showed that Pol II is present at the promoters of MIR genes (Kim et al., 2011), thus solidifying a role of Pol II in the transcription of MIR genes. In addition, several pieces of evidence support the transcriptional regulation of miRNA gene expression via transcription factor activity in plants (Bari et al., 2006; Kawashima et al., 2009; Megraw et al., 2006; Yamasaki et al., 2009).
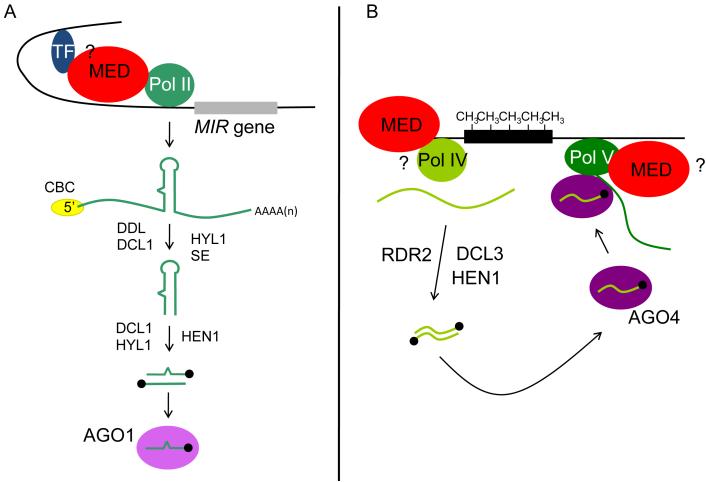
Figure 1.
Roles of Mediator in noncoding RNA production in plants. (A) A diagram of the miRNA biogenesis pathway in Arabidopsis. Mediator recruits Pol II to promoters of microRNA genes to promote the transcription of MIR genes possibly by bridging the interaction between transcription factors and Pol II. A MIR gene is transcribed by Pol II into a capped and polyadenylated primary precursor, which undergoes processing to give rise to a small RNA duplex containing the miRNA and the antisense miRNA*. The duplex is methylated on the 2′ OH of the 3′ terminal nucleotides, and the miRNA strand is bound by AGO1, the miRNA effector protein. Known factors in miRNA biogenesis are indicated. TF, transcription factor; CBC, cap-binding complex; DDL, DAWDLE; DCL1, DICER-LIKE1; HYL1, HYPONASTIC LEAVES1; SE, SERRATE; HEN1, HUA ENHANCER1; AGO1, ARGONAUTE1. (B) A diagram of siRNA biogenesis and siRNA-mediated transcriptional gene silencing at repeats and transposable elements. The black rectangle represents a repeats- or transposable element-containing locus that harbors DNA methylation (CH3). Pol IV presumably transcribes the locus into a single-stranded noncoding RNA, which eventually gives rise to 24 nt siRNAs through the activities of RNA-DEPENDENT RNA POLYMERASE2 (RDR2), DICER-LIKE3 (DCL3), and HEN1. The siRNAs are bound by AGO4, a major siRNA effector protein. The AGO4/siRNA complexes are thought to be recruited back to homologous chromatin by noncoding scaffold transcripts produced by Pol V, and at some loci, Pol II. It is not known whether Mediator assists Pol V in the production of noncoding RNAs, but it is required for noncoding RNA production by Pol II. The small black dots in (A) and (B) indicate the methyl group on the 3′ terminal ribose of small RNAs.
Heterochromatic-siRNAs (hc-siRNA) are derived from repeats and transposable elements and represent the great majority of endogenous siRNAs in plants. Plants have two specialized polymerases, Pol IV and Pol V, which are required for the biogenesis and function of hc-siRNAs. Pol IV and Pol V are probably derived from Pol II because they are composed of 12 subunits that are paralogous or identical to those of Pol II (Huang et al., 2009; Lahmy et al., 2009; Ream et al., 2009). More than 90% of hc-siRNAs are Pol IV-dependent (Mosher et al., 2008; Zhang et al., 2007). Pol IV is presumed to transcribe transposable elements and repeated sequences into RNAs that serve as precursors to siRNAs (Herr et al., 2005; Kanno et al., 2005; Mosher et al., 2008; Onodera et al., 2005; Zhang et al., 2007). An in vivo transcriptional activity of Pol V is supported by the identification of Pol V-dependent long noncoding RNAs from some heterochromatic loci and by the presence of Pol V at these loci (Wierzbicki et al., 2008).
Biological functions of Pol II-, Pol IV- and Pol V-dependent noncoding RNAs in TGS
hc-siRNAs maintain genome stability by causing TGS of homologous sequences. The production of hc-siRNAs requires Pol IV, which is thought to transcribe heterochromatic loci into single-stranded noncoding transcripts that are subsequently converted into double-stranded RNAs (dsRNAs) by RDR2 (Xie et al., 2004). The dsRNAs are diced into 24 nt siRNAs by DCL3 and the small RNAs are methylated by HEN1 (Li et al., 2005; Xie et al., 2004). One strand of the hc-siRNA duplex is incorporated into an effector complex containing one of the AGO4-clade of argonaute proteins (Havecker et al., 2010; Zheng et al., 2007; Zilberman et al., 2003). Pol V also transcribes heterochromatic loci into long noncoding transcripts, also known as scaffold transcripts, which are thought to recruit the AGO4/siRNAs to homologous chromatin through base-pairing with siRNAs (El-Shami et al., 2007; Li et al., 2006; Wierzbicki et al., 2008). The AGO4/siRNAs in turn recruit chromatin-modifying factors such as the DNA methyltransferase DRM2 and histone modification enzymes to deposit repressive chromatin marks to result in TGS. Loss-of-function mutations in Pol IV, Pol V, or other genes in the pathway cause the transcriptional de-repression of repeats and transposable elements. Pol II is also required for endogenous siRNA-mediated TGS at some intergenic, low-copy-number loci. Like Pol V, Pol II generates noncoding scaffold transcripts, which recruit AGO4/siRNAs to homologous loci (Zheng et al., 2009). In addition, Pol II transcription recruits Pol IV and Pol V to different locations at heterochromatic loci to promote siRNA biogenesis and scaffold RNA production, respectively (Zheng et al., 2009).
Roles of Mediator in noncoding RNA production
Mediator is required for the transcription of protein-coding genes by Pol II. However, the function of Mediator in noncoding RNA production is largely unknown. A recent study shows that Mediator regulates the transcription of a subset of Pol II-dependant small nuclear RNA (snRNA) genes in mouse (Krebs et al., 2010), therefore revealing a role of Mediator in noncoding RNA production by Pol II. In plants, Pol II, Pol IV and Pol V are required for endogenous siRNA-mediated TGS to maintain genome stability through generating noncoding RNAs, raising the question of whether Mediator is required for Pol II, Pol IV or Pol V activities in noncoding RNA production. A recent study from our laboratory has addressed this question by analyzing Arabidopsis mutants in three Mediator genes, MED17, MED18, and MED20a (Kim et al., 2011). This study reveals that Mediator plays a role in MIR gene expression as well as TGS of repeats and transposons to maintain genome stability by promoting Pol II activity in Arabidopsis. Mediator promotes the transcription of MIR genes by recruiting Pol II to their promoters. In addition, Mediator is required for Pol II-mediated intergenic transcription to produce long noncoding scaffold RNAs that recruit siRNAs to chromatin in TGS.
Perspective
Several studies have begun to reveal functions of Mediator in noncoding RNA production in animals and plants. In Arabidopsis, promoters of MIR genes contain cis-regulatory elements (Megraw et al., 2006) that may be bound by transcription factors. Given the established role of Mediator in recruiting Pol II to the promoters of MIR genes (Kim et al., 2011), it is possible that Mediator bridges the interaction between Pol II and transcription factors that bind the cis-elements (Fig. 1A). Mediator also plays a role in silencing repeats and transposons since several loci known to be silenced through siRNA-mediated DNA methylation are de-repressed in med17, med18, and med20a mutants (Kim et al., 2011). Mediator does so by recruiting Pol II to some of these loci to produce long noncoding RNAs that serve to recruit siRNAs to chromatin. However, it is not clear how broadly Mediator acts in the production of noncoding RNAs in TGS. The similarities in subunit composition among Pol II, Pol IV, and Pol V raise the possibility that Mediator also acts with Pol IV or Pol V to produce noncoding RNAs (Fig. 1B). But Pol IV- or Pol V-dependent noncoding RNAs were not affected in med17, med18, or med20a mutants (Kim et al., 2011). Given that these are not null mutants, a potential role of Mediator in Pol IV or Pol V transcription cannot be ruled out. Further studies are necessary to address the generality and specificity of the functions of Mediator in noncoding RNA production in TGS.
References
- Ansari SA, He Q, Morse RH. Mediator complex association with constitutively transcribed genes in yeast. Proc Natl Acad Sci U S A. 2009;106:16734–16739. doi: 10.1073/pnas.0905103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autran D, Jonak C, Belcram K, Beemster GT, Kronenberger J, Grandjean O, Inze D, Traas J. Cell numbers and leaf development in Arabidopsis: a functional analysis of the STRUWWELPETER gene. EMBO J. 2002;21:6036–6049. doi: 10.1093/emboj/cdf614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom S, Elfving N, Nilsson R, Wingsle G, Bjorklund S. Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol Cell. 2007;26:717–729. doi: 10.1016/j.molcel.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Baek HJ, Kang YK, Roeder RG. Human Mediator enhances basal transcription by facilitating recruitment of transcription factor IIB during preinitiation complex assembly. J Biol Chem. 2006;281:15172–15181. doi: 10.1074/jbc.M601983200. [DOI] [PubMed] [Google Scholar]
- Bari R, Pant B. Datt, Stitt M, Scheible WR. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JC, Choi JE, Lombardo SR, Carey M. A mechanism for coordinating chromatin modification and preinitiation complex assembly. Mol Cell. 2006;23:809–818. doi: 10.1016/j.molcel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Carlson M, Osmond BC, Botstein D. Mutants of yeast defective in sucrose utilization. Genetics. 1981;98:25–40. doi: 10.1093/genetics/98.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassimi A, Napoli C. Mediator complexes and eukaryotic transcription regulation: an overview. Biochimie. 2007;89:1439–1446. doi: 10.1016/j.biochi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Cerdan PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–885. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- Chen X. Small RNAs and their roles in plant development. Annu Rev Cell Dev Biol. 2009;25:21–44. doi: 10.1146/annurev.cellbio.042308.113417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Huddleston MJ, Zhang X, Young RA, Annan RS, Carr SA, Deshaies RJ. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 2001;15:1078–1092. doi: 10.1101/gad.867501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan R, Luo H, Foerster AM, Abuqamar S, Du HN, Briggs SD, Scheid O. Mittelsten, Mengiste T. HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell. 2009;21:1000–1019. doi: 10.1105/tpc.108.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Zhou H, Esteve PO, Chin HG, Kim S, Xu X, Joseph SM, Friez MJ, Schwartz CE, Pradhan S, et al. Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation. Mol Cell. 2008;31:347–359. doi: 10.1016/j.molcel.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson MR, Yuan CX, Roeder RG, Myers LC, Gustafsson CM, Jiang YW, Li Y, Kornberg RD, Asturias FJ. Structural organization of yeast and mammalian mediator complexes. Proc Natl Acad Sci U S A. 2000;97:14307–14310. doi: 10.1073/pnas.260489497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shami M, Pontier D, Lahmy S, Braun L, Picart C, Vega D, Hakimi MA, Jacobsen SE, Cooke R, Lagrange T. Reiterated WG/GW motifs form functionally and evolutionarily conserved ARGONAUTE-binding platforms in RNAi-related components. Genes Dev. 2007;21:2539–2544. doi: 10.1101/gad.451207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Chou DM, Struhl K. Activator-specific recruitment of Mediator in vivo. Nat Struct Mol Biol. 2006;13:117–120. doi: 10.1038/nsmb1049. [DOI] [PubMed] [Google Scholar]
- Fan X, Struhl K. Where does mediator bind in vivo? PLoS One. 2009;4:e5029. doi: 10.1371/journal.pone.0005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez D, Bowen AJ, Carroll TS, Conlan RS. The transcription corepressor LEUNIG interacts with the histone deacetylase HDA19 and mediator components MED14 (SWP) and CDK8 (HEN3) to repress transcription. Mol Cell Biol. 2007;27:5306–5315. doi: 10.1128/MCB.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi B, van Berkum NL, Klapholz B, Bijma T, Boube M, Boschiero C, Bourbon HM, Holstege FC, Werner M. A high resolution protein interaction map of the yeast Mediator complex. Nucleic Acids Res. 2004;32:5379–5391. doi: 10.1093/nar/gkh878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg M, Polozkov GV, Hu GZ, Beve J, Gustafsson CM, Ronne H, Bj√∂rklund S. Site-specific Srb10-dependent phosphorylation of the yeast Mediator subunit Med2 regulates gene expression from the 2-microm plasmid. Proc Natl Acad Sci U S A. 2004;101:3370–3375. doi: 10.1073/pnas.0400221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Lee JS, Kang JS, Kim YJ. Med9/Cse2 and Gal11 modules are required for transcriptional repression of distinct group of genes. J Biol Chem. 2001;276:37020–37026. doi: 10.1074/jbc.M105596200. [DOI] [PubMed] [Google Scholar]
- Havecker ER, Wallbridge LM, Hardcastle TJ, Bush MS, Kelly KA, Dunn RM, Schwach F, Doonan JH, Baulcombe DC. The Arabidopsis RNA-directed DNA methylation argonautes functionally diverge based on their expression and interaction with target loci. Plant Cell. 2010;22:321–334. doi: 10.1105/tpc.109.072199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner CJ, Myer VE, Liao SM, Wilson CJ, Koh SS, Young RA. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell. 1998;2:43–53. doi: 10.1016/s1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- Herr AJ, Jensen MB, Dalmay T, Baulcombe DC. RNA polymerase IV directs silencing of endogenous DNA. Science. 2005;308:118–120. doi: 10.1126/science.1106910. [DOI] [PubMed] [Google Scholar]
- Hirst M, Kobor MS, Kuriakose N, Greenblatt J, Sadowski I. GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol Cell. 1999;3:673–678. doi: 10.1016/s1097-2765(00)80360-3. [DOI] [PubMed] [Google Scholar]
- Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- Huang L, Jones AM, Searle I, Patel K, Vogler H, Hubner NC, Baulcombe DC. An atypical RNA polymerase involved in RNA silencing shares small subunits with RNA polymerase II. Nat Struct Mol Biol. 2009;16:91–93. doi: 10.1038/nsmb.1539. [DOI] [PubMed] [Google Scholar]
- Kang JS, Kim SH, Hwang MS, Han SJ, Lee YC, Kim YJ. The structural and functional organization of the yeast mediator complex. J Biol Chem. 2001;276:42003–42010. doi: 10.1074/jbc.M105961200. [DOI] [PubMed] [Google Scholar]
- Kanno T, Huettel B, Mette MF, Aufsatz W, Jaligot E, Daxinger L, Kreil DP, Matzke M, Matzke AJ. Atypical RNA polymerase subunits required for RNA-directed DNA methylation. Nat Genet. 2005;37:761–765. doi: 10.1038/ng1580. [DOI] [PubMed] [Google Scholar]
- Kawashima CG, Yoshimoto N, Maruyama-Nakashita A, Tsuchiya YN, Saito K, Takahashi H, Dalmay T. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J. 2009;57:313–321. doi: 10.1111/j.1365-313X.2008.03690.x. [DOI] [PubMed] [Google Scholar]
- Kidd BN, Edgar CI, Kumar KK, Aitken EA, Schenk PM, Manners JM, Kazan K. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell. 2009;21:2237–2252. doi: 10.1105/tpc.109.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zheng B, Yu Y, Won SY, Mo B, Chen X. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J. 2011 doi: 10.1038/emboj.2011.3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs AR, Demmers J, Karmodiya K, Chang NC, Chang AC, Tora L. ATAC and Mediator coactivators form a stable complex and regulate a set of non-coding RNA genes. EMBO Rep. 2010;11:541–547. doi: 10.1038/embor.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras L, Struhl K. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature. 1999;399:609–613. doi: 10.1038/21239. [DOI] [PubMed] [Google Scholar]
- Lahmy S, Pontier D, Cavel E, Vega D, El-Shami M, Kanno T, Lagrange T. PolV(PolIVb) function in RNA-directed DNA methylation requires the conserved active site and an additional plant-specific subunit. Proc Natl Acad Sci U S A. 2009;106:941–946. doi: 10.1073/pnas.0810310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere L, Geiger S, Hoeppner S, R√∂ther S, Str√§sser K, Cramer P. Structure and TBP binding of the Mediator head subcomplex Med8-Med18-Med20. Nat Struct Mol Biol. 2006;13:895–901. doi: 10.1038/nsmb1143. [DOI] [PubMed] [Google Scholar]
- Lee YC, Park JM, Min S, Han SJ, Kim YJ. An activator binding module of yeast RNA polymerase II holoenzyme. Mol Cell Biol. 1999;19:2967–2976. doi: 10.1128/mcb.19.4.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CF, Pontes O, El-Shami M, Henderson IR, Bernatavichute YV, Chan SW, Lagrange T, Pikaard CS, Jacobsen SE. An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell. 2006;126:93–106. doi: 10.1016/j.cell.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Li J, Yang Z, Yu B, Liu J, Chen X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr Biol. 2005;15:1501–1507. doi: 10.1016/j.cub.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kung C, Fishburn J, Ansari AZ, Shokat KM, Hahn S. Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol Cell Biol. 2004;24:1721–1735. doi: 10.1128/MCB.24.4.1721-1735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Meyerowitz EM. LEUNIG regulates AGAMOUS expression in Arabidopsis flowers. Development. 1995;121:975–991. doi: 10.1242/dev.121.4.975. [DOI] [PubMed] [Google Scholar]
- Malik S, Roeder RG. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem Sci. 2000;25:277–283. doi: 10.1016/s0968-0004(00)01596-6. [DOI] [PubMed] [Google Scholar]
- Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet. 2010;11:761–772. doi: 10.1038/nrg2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw M, Baev V, Rusinov V, Jensen ST, Kalantidis K, Hatzigeorgiou AG. MicroRNA promoter element discovery in Arabidopsis. RNA. 2006;12:1612–1619. doi: 10.1261/rna.130506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler G, Kremmer E, Timmers HT, Meisterernst M. Novel critical role of a human Mediator complex for basal RNA polymerase II transcription. EMBO Rep. 2001;2:808–813. doi: 10.1093/embo-reports/kve186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher RA, Schwach F, Studholme D, Baulcombe DC. PolIVb influences RNA-directed DNA methylation independently of its role in siRNA biogenesis. Proc Natl Acad Sci U S A. 2008;105:3145–3150. doi: 10.1073/pnas.0709632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar AM, Taatjes DJ, Zhai W, Nogales E, Tjian R. Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev. 2002;16:1339–1344. doi: 10.1101/gad.987602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigeborn L, Carlson M. Genes affecting the regulation of SUC2 gene expression by glucose repression in Saccharomyces cerevisiae. Genetics. 1984;108:845–858. doi: 10.1093/genetics/108.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C, Goto S, Lund K, Hung W, Sadowski I. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature. 2003;421:187–190. doi: 10.1038/nature01243. [DOI] [PubMed] [Google Scholar]
- Onodera Y, Haag JR, Ream T, Nunes PC, Pontes O, Pikaard CS. Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell. 2005;120:613–622. doi: 10.1016/j.cell.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Ooi L, Wood IC. Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet. 2007;8:544–554. doi: 10.1038/nrg2100. [DOI] [PubMed] [Google Scholar]
- Park JM, Kim HS, Han SJ, Hwang MS, Lee YC, Kim YJ. In vivo requirement of activator-specific binding targets of mediator. Mol Cell Biol. 2000;20:8709–8719. doi: 10.1128/mcb.20.23.8709-8719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream TS, Haag JR, Wierzbicki AT, Nicora CD, Norbeck AD, Zhu JK, Hagen G, Guilfoyle TJ, Pasa-Toliƒá L, Pikaard CS. Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II. Mol Cell. 2009;33:192–203. doi: 10.1016/j.molcel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese JC. Basal transcription factors. Curr Opin Genet Dev. 2003;13:114–118. doi: 10.1016/s0959-437x(03)00013-3. [DOI] [PubMed] [Google Scholar]
- Sato S, Tomomori-Sato C, Banks CA, Sorokina I, Parmely TJ, Kong SE, Jin J, Cai Y, Lane WS, Brower CS, et al. Identification of mammalian Mediator subunits with similarities to yeast Mediator subunits Srb5, Srb6, Med11, and Rox3. J Biol Chem. 2003;278:15123–15127. doi: 10.1074/jbc.C300054200. [DOI] [PubMed] [Google Scholar]
- Sikorski TW, Buratowski S. The basal initiation machinery: beyond the general transcription factors. Curr Opin Cell Biol. 2009;21:344–351. doi: 10.1016/j.ceb.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G, Winston F, Styles CA, Fink GR. Ty-mediated gene expression of the LYS2 and HIS4 genes of Saccharomyces cerevisiae is controlled by the same SPT genes. Proc Natl Acad Sci U S A. 1984;81:2431–2434. doi: 10.1073/pnas.81.8.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Jensen R, Herskowitz I. Five SWI genes are required for expression of the HO gene in yeast. J Mol Biol. 1984;178:853–868. doi: 10.1016/0022-2836(84)90315-2. [DOI] [PubMed] [Google Scholar]
- Struhl K. Chromatin structure and RNA polymerase II connection: implications for transcription. Cell. 1996;84:179–182. doi: 10.1016/s0092-8674(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang Y, Cho H, Rickert P, Lees E, Lane W, Reinberg D. NAT, a human complex containing Srb polypeptides that functions as a negative regulator of activated transcription. Mol Cell. 1998;2:213–222. doi: 10.1016/s1097-2765(00)80131-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nogi Y, Abe A, Fukasawa T. GAL11 protein, an auxiliary transcription activator for genes encoding galactose-metabolizing enzymes in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:4991–4999. doi: 10.1128/mcb.8.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taatjes DJ, Schneider-Poetsch T, Tjian R. Distinct conformational states of nuclear receptor-bound CRSP-Med complexes. Nat Struct Mol Biol. 2004;11:664–671. doi: 10.1038/nsmb789. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Calero G, Komori H, Brown JA, Ehrensberger AH, Hudmon A, Asturias F, Kornberg RD. Head module control of mediator interactions. Mol Cell. 2006;23:355–364. doi: 10.1016/j.molcel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Chiang CM. The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol. 2006;41:105–178. doi: 10.1080/10409230600648736. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Young RA. General requirement for RNA polymerase II holoenzymes in vivo. Proc Natl Acad Sci U S A. 1995;92:4587–4590. doi: 10.1073/pnas.92.10.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Peppel J, Kettelarij N, van Bakel H, Kockelkorn TT, van Leenen D, Holstege FC. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol Cell. 2005;19:511–522. doi: 10.1016/j.molcel.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Vincent O, Kuchin S, Hong SP, Townley R, Vyas VK, Carlson M. Interaction of the Srb10 kinase with Sip4, a transcriptional activator of gluconeogenic genes in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:5790–5796. doi: 10.1128/MCB.21.17.5790-5796.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chen X. HUA ENHANCER3 reveals a role for a cyclin-dependent protein kinase in the specification of floral organ identity in Arabidopsis. Development. 2004;131:3147–3156. doi: 10.1242/dev.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki AT, Haag JR, Pikaard CS. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell. 2008;135:635–648. doi: 10.1016/j.cell.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg AC, Strasser B, Cerd√°n PD, Amasino RM. Acceleration of flowering during shade avoidance in Arabidopsis alters the balance between FLOWERING LOCUS C-mediated repression and photoperiodic induction of flowering. Plant Physiol. 2008;148:1681–1694. doi: 10.1104/pp.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Allen E, Fahlgren N, Calamar A, Givan SA, Carrington JC. Expression of Arabidopsis MIRNA genes. Plant Physiol. 2005;138:2145–2154. doi: 10.1104/pp.105.062943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:E104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T. SQUAMOSA Promoter Binding Protein-Like7 Is a Central Regulator for Copper Homeostasis in Arabidopsis. Plant Cell. 2009;21:347–361. doi: 10.1105/tpc.108.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkovsky N, Ranish JA, Hahn S. A transcription reinitiation intermediate that is stabilized by activator. Nature. 2000;408:225–229. doi: 10.1038/35041603. [DOI] [PubMed] [Google Scholar]
- Zhang X, Henderson IR, Lu C, Green PJ, Jacobsen SE. Role of RNA polymerase IV in plant small RNA metabolism. Proc Natl Acad Sci U S A. 2007;104:4536–4541. doi: 10.1073/pnas.0611456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Wang Z, Li S, Yu B, Liu JY, Chen X. Intergenic transcription by RNA polymerase II coordinates Pol IV and Pol V in siRNA-directed transcriptional gene silencing in Arabidopsis. Genes Dev. 2009;23:2850–2860. doi: 10.1101/gad.1868009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Zhu J, Kapoor A, Zhu JK. Role of Arabidopsis AGO6 in siRNA accumulation, DNA methylation and transcriptional gene silencing. EMBO J. 2007;26:1691–1701. doi: 10.1038/sj.emboj.7601603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Cao X, Jacobsen SE. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science. 2003;299:716–719. doi: 10.1126/science.1079695. [DOI] [PubMed] [Google Scholar]