Abstract
DNA vaccines contribute to a promising new approach for the generation of cytotoxic T lymphocytes (CTL). DNA vaccines do have several disadvantages, including poor immunogenicity and oncogene expression. We used the natural killer T-cell (NKT) ligand α-galactosylceramide (α-GalCer) as an adjuvant to prime initial DNA vaccination; and used the potent immune-stimulatory tumor antigen-expressing dendritic cells (DCs) as a booster vaccination. A DNA vaccine expressing human papillomavirus (HPV) type 16 E7 (pcDNA3-CRT/E7) was combined with α-GalCer at the prime phase, and generated a higher number of E7-specific CD8+ T-cells in vaccinated mice than vaccine used at boost phase. Therefore, priming with a DNA vaccine in the presence of α-GalCer and boosting with E7-pulsed DC-1 led to a significant enhancement of E7-specific CD8+ effector and memory T-cells as well as significantly improved therapeutic and preventive effects against an E7-expressing tumor model (TC-1) in vaccinated mice. Our findings suggested that the potency of a DNA vaccine combined with α-GalCer could be further enhanced by boosting with an antigen-expressing DC-based vaccine to generate anti-tumor immunity.
Keywords: DNA vaccine, α-Galactosylceramide, Anti-tumor immunity
1. Introduction
Cancer is a worldwide leading cause of death, and several malignancies are incurable by conventional therapies. Therefore, new anti-tumor immunotherapies are necessary to improve the outcome of patients with advanced cancer. DNA vaccines are a potentially valuable form of antigen-specific immunotherapy because of their safety, ease of production, stability, and expression of the tumor-specific antigens for longer time periods than RNA or protein-based vaccines [1]. Further, DNA vaccines do not elicit neutralizing antibody production in the patients, unlike live vector vaccines, and can therefore be repeatedly boostered [2]. However, DNA vaccines also have several significant disadvantages. Some DNA vaccines may express oncogenes which could potentially integrate into host genomes [3]. DNA vaccines also result in relatively poor immunogenicity [4].
Dendritic cells (DCs) can pick up antigens and activate naïve and memory CD4+ and CD8+ T-cells, and this characteristic may allow them to trigger specific anti-tumor immunity [5]. DCs present antigen and prime T-cells, and therefore vaccines utilizing DCs should stimulate superior protective and therapeutic immune responses in cancer patients when compared to other vaccination strategies [6]. Further, DC-based vaccines may circumvent tumor-mediated immune suppression [7,8]. However, individualized DC-based vaccine are difficult to prepare and costly; vaccine preparation requires harvesting of DCs from patients and in vitro exposure to tumor antigens in large-scale culture. Furthermore, the route of administration is likely to be important for DC-based vaccination because the DCs must home to the lymphoid organs to interact with the majority of naïve T-cells [9].
Several alternate immunostimulants and adjuvants have been developed and applied, including CpG-oligodeoxynucleotide (ODN) [10], polyactide-co-glycolide (PLG) [11], and the NKT-cell ligand α-galactosylceramide (α-GalCer) [12]. α-GalCer is a glycolipid originally extracted from marine sponges, and is presented by the CD1d molecule on DCs [13]. Several studies have reported that α-GalCer may be used as a systemically delivered vaccine adjuvant for the induction of potent natural killer cell-dependent anti-tumor cytotoxic responses [14,15]. α-GalCer enhanced anti-tumor immunity in mice when administered in combination with various types of vaccines [16–18]. Previous studies have demonstrated that α-GalCer and tumor cells are cross-presented by DCs in vivo to induce T-cell-mediated immunity [16] and can stimulate splenic DCs maturation, not DCs from bone marrow progenitors in mice [19]. These data suggest that α-GalCer may function as a potent adjuvant for DNA and DC-based vaccines.
We co-administered DNA vaccines or tumor antigen-loaded DC vaccines with α-GalCer in the present study to start early immunotherapy and improve anti-tumor efficacy. We examined several vaccine protocols to determine which combination of DNA-or DC-based vaccines and α-GalCer would most effectively prime naïve CD8+ T-cells to generate and maintain E7-specific CD8+ T-cell immune responses after boosting. Our data suggested that priming with a DNA vaccine and α-GalCer followed by boosting with peptide-pulsed DC most effectively induced E7-specific CD8+ T-cell immune responses. These data suggested that initial co-administration of a DNA vaccine and α-GalCer and a subsequent booster with a DC-based vaccine might generate robust anti-tumor immunity.
2. Materials and methods
2.1. Antibodies(Abs), peptide, α-GalCer, cell line and mice
The HPV-16E7 (RAHYNIVTF) peptide was synthesized at ≥ 90% purity by Macromolecular Resources (Denver, CO, USA). Anti-CD8 (PE-conjugated, clone Ly-1) and anti-IFN-γ (FITC-conjugated, clone XMG1.2) antibodies were purchased from BD Pharmingen. α-GalCer (2S, 3S, 4R-1-O [a-galactopyranosyl]-2[N-hexacosanoylam-ino]-1,3,4-octadecanetriol) was purchased from Toronto Research Chemicals (Ontario, Canada) and diluted in phosphate-buffered saline. HPV-16 E7-expressing murine tumor cells (the TC-1 cell line) were used for the tumor model [20], and the DC-1 cell line was used as a dendritic cell model. All cells were maintained in RPMI medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 0.1 mM MEM non-essential amino acids, 50 uM β-mercaptoethanol, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA). Female C57BL/6 mice (6–8 weeks of age) were purchased from the Chung-Ang Laboratory Animal Service (Seoul, Korea), and housed the Animal Facility of the Pre-Clinical Research Center in Chung-Ang University. All animals were maintained under specific pathogen-free conditions. All procedures were performed according to previously approved protocols and in accordance with the recommendations for the proper use and care of laboratory animals of the Ethics Committee of the College of Medicine, Chung-Ang University.
2.2. Plasmid DNA construct and DNA preparation
The generation of pcDNA3-CRT/E7 has been described previously [21]. Plasmid constructs were confirmed by DNA sequencing. Amplification and purification of DNA were previously described [22].
2.3. DNA vaccination and DC immunization
Intramuscular (i.m.) DNA vaccination was performed with 100 μg of pcDNA3-CRT/E7 DNA/mouse; mice received booster vaccines 1 week later. DC-1 cells were pulsed with HPV-16 E7 (aa 49–57) peptide (RAHYNIVTF, 10 μg/ml) at 37 °C for 3 h. DC-1 cells were washed with RPMI-1640, supplemented with 10% FBS and Hank’s balanced salt solution, and re-suspended in Hank’s balanced salt solution at final concentration of 1 × 107 cells/ml. DC-1 cells (100 μl/mouse) were injected into the footpads of mice. The mice were boosted once with the same immunization regimen 1 week later. α-GalCer (2 μg) was mixed with either a DNA construct or E7-pulsed DC-1 cells and injected as previously described.
2.4. Maturation of splenic DCs with α-GalCer
DCs were isolated from spleen of mouse administered several adjuvant using previous study methods [19]. Splenocytes from mice administrated with α-GalCer (2 μg) or LPS (25 μg) were harvested by homogenization followed by treatment with collagenase. Collagenase-treated splenocytes were suspended with sterile PBS in the presence of 5% BSA. We separated CD11c+ fractions using anti-CD11c coated magnetic beads (Miltenyi Biotech, Auburn, CA, USA). Then we stained cells with PE-conjugated anti-CD40, CD80, CD86, Db, I-Ab. All antibodies were purchased from BD Pharmingen (San Diego, CA, USA). Flow cytometry was performed on a Becton-Dickinson FACSCalibur with CELLQuest software (Becton Dickinson Immunocytometry System, Mountain View, CA, USA).
2.5. Intracellular cytokine staining and flow cytometric analysis
Splenocytes were harvested from mice (n = 5 per group) either 1 week or 60 days after the last vaccination. A total of 5 × 106/mouse of pooled splenocytes from each vaccination group were incubated for 16 h with E7 peptide (1 μg/ml, aa 49–57, RAHYNIVTF) [23] containing an MHC class I epitope for the detection of E7-specific CD8+ T-cell precursors in the presence of GolgiPlug (BD Pharmingen). Stimulated splenocytes were washed twice with FACS buffer (PBS containing 5% BSA); cell surface marker staining for CD8 and intracellular cytokine staining for IFN-γ were performed using previously described conditions [22,24]. Flow cytometry was performed on a Becton-Dickinson FACSCalibur with CELLQuest software (Becton Dickinson Immunocytometry System, Mountain View, CA, USA).
2.6. In vivo tumor treatment experiment using TC-1 tumor cells
C57BL/6 mice (n = 5 per group) were challenged with 2 × 105 TC-1 tumor cells/mouse by subcutaneous (s.c.) injection in the right hind leg for the in vivo tumor treatment experiment. Mice were vaccinated in various protocols twice with a one-week interval between the injections starting at the third day after challenge with TC-1 tumor cells. Mice were monitored for evidence of tumor growth twice per week by inspection and palpitation, as previously described [20]. Tumor volumes were measured starting at day 7 after tumor challenge.
2.7. In vivo tumor protection and antibody depletion experiment
To identify the subset of lymphocytes that are important for the anti-tumor effects, an in vivo antibody depletion experiment was performed. The in vivo antibody depletions were started immediately after last vaccination. MAb GK1.5 was used for CD4 depletion, MAb 2.43 was used for CD8 depletion, and MAb PK136 was used for NK1.1 depletion. Flow cytometry analysis revealed that the >95% of the appropriate lymphocyte subset were depleted with a normal level of other subsets. All Antibodies were purchased from Harlan (Indianapolis, IN, USA). C57BL/6 mice (n = 5 per group) were subcutaneously challenged with 2 × 105 TC-1 tumor cells/mouse in the right hind leg 1 week after last vaccination. Mice were monitored for evidence of tumor growth twice per week by inspection and palpitation, as previously described [20].
2.8. Long-term in vivo tumor protection experiment
Mice (n = 5 per group) were vaccinated with various vaccination protocols for long-term tumor protection experiments; mice were boosted with the same protocol as the initial vaccination after 1 week. Mice were challenged with TC-1 tumor cells (5 × 105/mouse) s.c. in the right hind leg at day 60 after booster vaccination. Tumor growth was monitored by twice weekly visual inspection and palpation, as previously described [20].
2.9. Tumor measurement and conditional survival
Three-dimensional tumor sizes were measured three times per week with Vernier calipers. Tumor sizes were approximated by multiplication of measured lengths. Tumors were measured every other day from day 25 after tumor cell challenge, and mice with tumor sizes >17 mm in diameter or with projected tumor volumes >10% body weight or >2500 mm3 were sacrificed. Tumor volumes were calculated using the following formula: V = (L × W × D); V is tumor volume, L is length, W is width, and D is depth. All procedures were performed according to approved protocols and in accordance with recommendations of the ethics committee of College of Medicine, Chung-Ang University, for the proper use and care of laboratory animals.
2.10. Statistical analysis
All data were expressed as mean ± standard deviation (SD) values and were representative of at least two different experiments. Data for intracellular cytokine staining, flow cytometric analysis, and tumor treatment experiments were evaluated by analysis of variance. Comparisons between individual data points were made using the Student’s t-test. Statistical significance was defined at P-values <0.05.
3. Results
3.1. Co-administration of pcDNA3-CRT/E7 with a-GalCer at prime phase enhances E7-Specific CD8+ T-cell responses
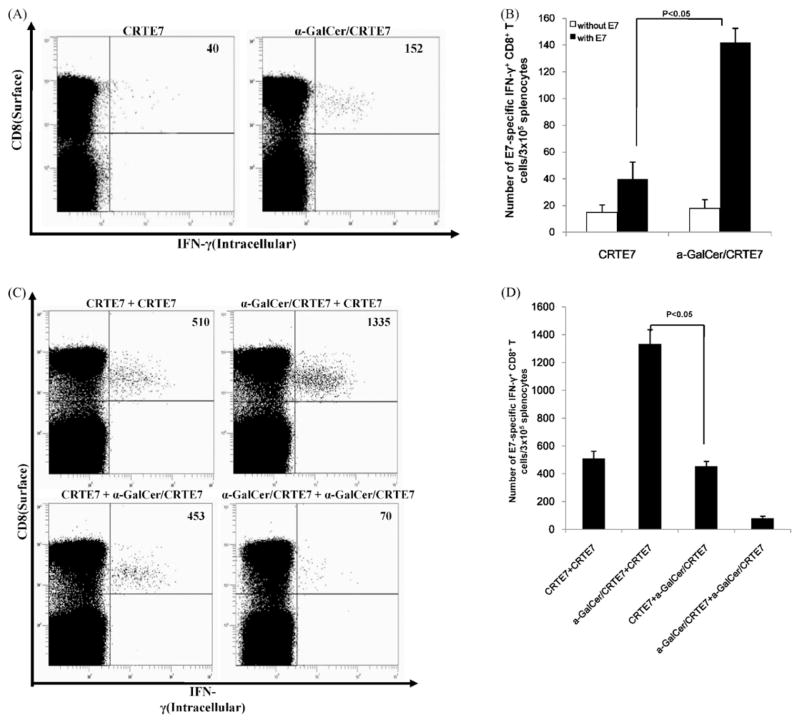
DNA vaccination is capable of inducing antigen-specific CD8+ T-cell responses. However, the overall immunogenicity of DNA vaccination is relatively low. DNA vaccines frequently require adjuvants and multiple applications of DNA or heterogonous boosting protocols [25,26]. We measured E7-specific CD8+ T-cell immune responses in vaccinated mice using intracellular IFN-γ staining and flow cytometric analysis after priming to determine the effects of α-GalCer on the quantity of E7-Specific CD8+ T-cell precursors generated by co-administration of the CRT/E7 vaccine construct with α-GalCer. Mice vaccinated with CRT/E7 and α-GalCer generated more E7-specific CD8+ T-cell precursors compared to mice vaccinated with CRT/E7 alone (Fig. 1A and B). We also tested the effects of α-GalCer as an adjuvant at either the prime or boost phase. Mice were vaccinated with CRT/E7 in the presence of α-GalCer as prime or boost regimen; intracellular cytokine staining and flow cytometry were performed 1 week after the last vaccination. Mice primed with CRT/E7 and α-GalCer and boosted with CRT/E7 generated the strongest E7-Specific CD8+ T-cell responses compared to mice primed and boosted with CRT/E7 or mice primed with CRT/E7 and boosted with α-GalCer + CRT/E7 (Fig. 1C and D). We investigated the effects of α-GalCer as an adjuvant on dendritic cell (DC)-based vaccines. Mice were vaccinated with E7-pulsed DC-1(DC-1-E7) with or without α-GalCer and boosted 1 week post-priming. Mice who received DC-1-E7 with α-GalCer at the prime or boost phases generated greater E7-Specific CD8+ T-cell responses compared to mice vaccinated without α-GalCer. However, mice were vaccinated DC-1-E7 with α-GalCer at both prime and boost phase did not enhance E7-Specific CD8+ T-cell immune responses (Supplemental Fig. 1). Previous studies have shown that α-GalCer stimulate the splenic DC maturation [19]. We also examined the splenic DC responses to α-GalCer after intramuscular administration. Splenic DC from mice injected α-GalCer (2 μg) or LPS (25 μg) expressed significant high level of CD40, CD86 on the surface compared with mice non-injected control group. However, CD80, Db (MHC class I) and I-Ab (MHC class II) expression were not significantly changed (Supplemental Fig. 2).
Fig. 1.
Characterization of E7-specific CD8+ T-cell immune responses in mice primed with DNA vaccine and α-GalCer. (A) C57BL/6 mice (n = 5 per group) were immunized with combination DNA vaccines (100 μg of DNA/mouse), either with or without α-GalCer (2 μg per injection). Splenocytes were harvested 1 week post-vaccination and were tested for E7-specific CD8+ T-cells by intracellular IFN-γ staining followed by flow cytometry. (B) Bar graphs depicting numbers of E7-specific IFN-γ-secreting CD8+ T-cells per 3 × 105 splenocytes after administration of a single vaccination (mean ± SD values). (C) C57BL/6 mice (5 mice per group) were immunized with the DNA vaccine (100 μg/mouse) either with or without in α-GalCer (2 μg per injection). Mice received vaccination boosters of both the DNA vaccine and α-GalCer. Representative flow cytometry data for the E7-specific CD8+ T-cells are also described. Numbers in the upper right-hand corner represent the number of E7-specific IFN-γ-secreting CD8+ T-cells per 3 × 105 pooled splenocytes. (D) Bar graphs depicted the numbers of E7-specific IFN-γ-secreting CD8+ T-cells per 3 × 105 splenocytes (mean ± SD values). The data presented in this figure are from one of two representative experiments.
Our data therefore suggested that CRT/E7 co-administration with α-GalCer at the prime phase generated greater E7-specific CD8+ T-cell precursors after CRT/E7 boosting through the stimulation of maturation DCs.
3.2. Mice primed with pcDNA3-CRT/E7 vaccine and α-GalCer and boosted pcDNA3-CRT/E7 vaccine alone generated potent anti-tumor effects
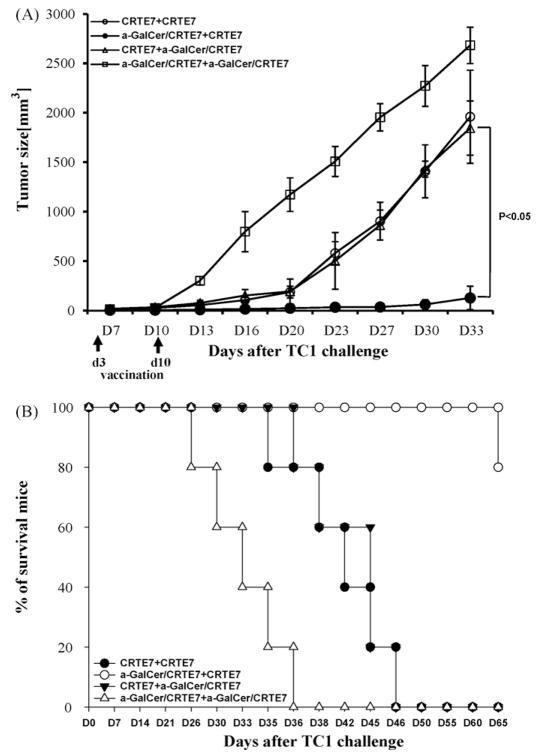
We performed in vivo tumor treatment experiments using the previously characterized E7-expressing tumor model, TC-1 [20] to determine whether the enhanced E7-specific CD8+ T-cells generated by the combination of CRT/E7 and α-GalCer could be translated into therapeutic anti-tumor effects. C57BL/6 mice were initially challenged with TC-1 cells (2 × 105/mouse) by subcutaneous injection into the right hind leg. Mice were vaccinated i.m. twice at 1 week intervals with various CRT/E7 and α-GalCer combinations three days after tumor challenge. Treated mice were monitored for tumor growth. We observed that mice primed with α-GalCer + CRT/E7 and boosted with CRT/E7 generated the best therapeutic anti-tumor effects among all tested vaccination regimens tested (Fig. 2A). The mice treated with the α-GalCer + CRT/E7 prime and CRT/E7 booster regimen demonstrated prolonged survival in vaccinated mice (Fig. 2B). Therefore, our data suggested that the therapeutic anti-tumor effects of the DNA vaccines in tumor-challenged mice could be significantly improved by the addition of α-GalCer at the prime phase.
Fig. 2.
In vivo tumor treatment experiments. C57BL/6 mice (n = 5 per group) were initially challenged with TC-1 tumor cells (1 × 105/mouse) by s.c. injection. Mice were injected with DNA (100 μg/mouse) with or without added α-GalCer (2 μg per injection) three days after tumor challenge. The mice were vaccinated 1 week later with various vaccine combination regimens, and were monitored twice weekly for evidence of tumor growth by inspection and palpation. Tumor volumes were measured starting at day 7 after tumor challenge. (A) Line graph depicting the tumor volumes in mice of different tumor treatments (means ± SD). (B) Kaplan–Meier survival analysis of tumor treatment experiments in mice. Data shown are from one of two representative experiments.
3.3. Priming with α-GalCer + CRT/E7 and boosting with E7-pulsed DC-1 further enhances E7-specific CD8+ T-cell immune responses in vaccinated mice
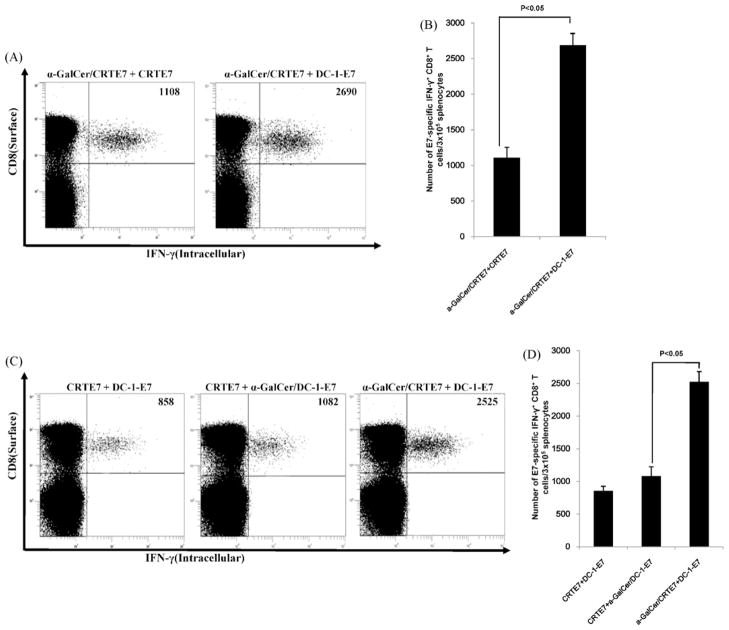
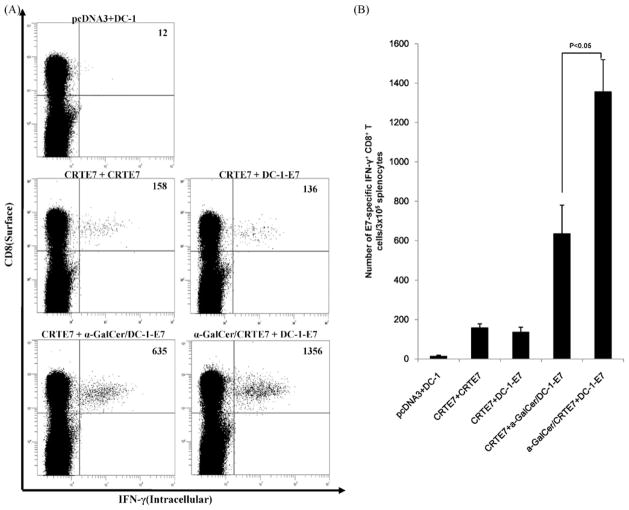
DNA- and DC-based vaccines have advantages and disadvantages, as previously discussed. Mice were vaccinated with various combinations of the DNA vaccines, α-GalCer and DC-1-E7 to identify the best vaccine protocol. As shown in Fig. 3A and B, Mice primed with α-GalCer + CRT/E7 and boosted with DC-1-E7 generated two times the numbers of E7-specific CD8+ T-cells compared to the mice primed with α-GalCer + CRT/E7 and boosted with CRT/E7 (Fig. 3A and B). Furthermore, we investigated the effects of α-GalCer on a DNA vaccine prime-dendritic cell vaccine boost regimen. Mice primed with α-GalCer + CRT/E7 and boosted with DC-1-E7 generated more E7-specific CD8+ T-cells than the mice primed with CRT/E7 and boosted with α-GalCer + DC-1-E7 (Fig. 3C and D). Our data suggested that the α-GalCer and DNA vaccine prime and DC-1-E7 boost regimen further enhanced E7-specific CD8+ T-cell immune responses in vaccinated mice.
Fig. 3.
Characterization of E7-specific CD8+ T-cell immune responses in mice primed with a α-GalCer + DNA vaccine and boosted with DC-1-E7. C57BL/6 mice (n = 5 per group) were immunized with various combination DNA vaccines (100 μg DNA/mouse) with α-GalCer (2 μg per injection). Mice received booster vaccinations 1 week later with various vaccine combination regimens. Splenocytes were harvested 1 week after the last vaccination and were tested for E7-specific CD8+ T-cells by intracellular IFN-γ staining and flow cytometry. (A and C) Representative flow cytometry data for E7-specific CD8+ T-cells. Numbers in the upper right-hand corner represent the number of E7-specific IFN-γ-secreting CD8+ T-cells per 3 × 105 pooled splenocytes. (B and D) Bar graphs depicting numbers of E7-specific IFN-γ-secreting CD8+ T-cells per 3 × 105 splenocytes (means ± SD). Data presented are from one of two representative experiments.
3.4. Therapeutic anti-tumor effects of α-GalCer + CRT/E7 can be further improved by boosting with E7-pulsed DC-1
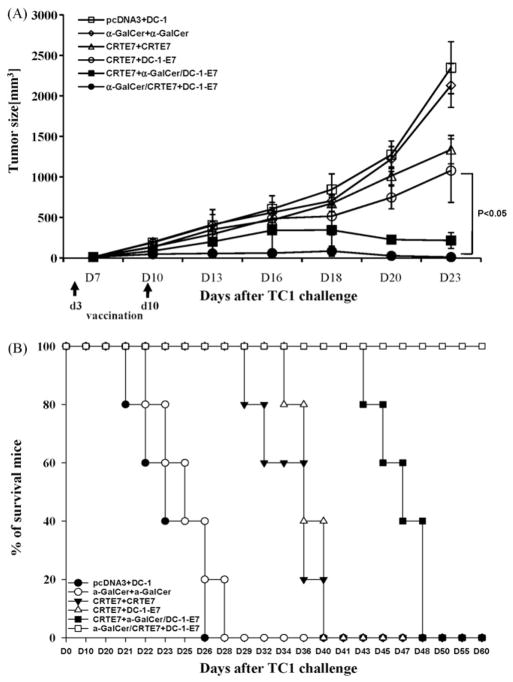
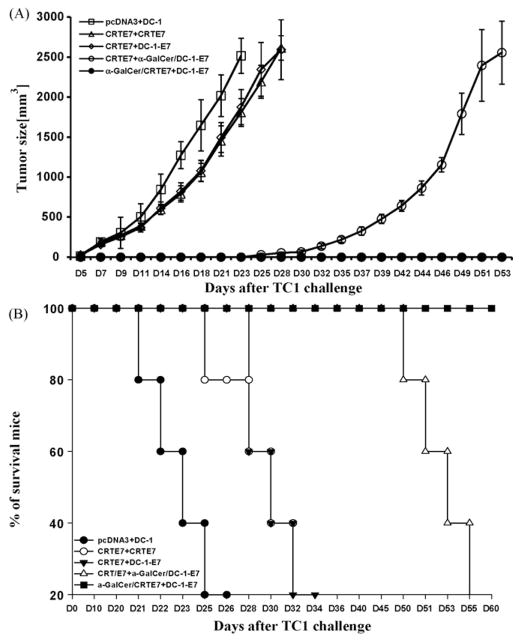
We performed in vivo tumor treatment experiments using the previously characterized E7-expressing tumor model, TC-1, to determine whether the enhanced E7-specific CD8+ T-cells generated by priming with α-GalCer + CRT/E7 and boosting with DC-1-E7 could be translated into improved anti-tumor effects [20]. C57BL/6 mice were initially challenged with TC-1 cells (2 × 105/mouse) subcutaneously. Mice were vaccinated twice i.m. at 1 week intervals with various combinations three days after tumor challenge. The α-GalCer + CRT/E7 prime-DC-1-E7 boost combination generated the best therapeutic anti-tumor effects among all tested combinations (Fig. 4A). Mice primed with CRTE7 and boosted with DC-1-E7 in the presence of α-GalCer also resulted in significantly prolonged survival; however, these mice began to die on the 43rd day after tumor challenge (Fig. 4B). These data suggested that the DNA vaccines in the presence of α-GalCer followed by antigen-expressing dendritic cells led to enhanced anti-tumor effects and prolonged survival in tumor-bearing mice through generation of E7-specific CD8+ T-cells.
Fig. 4.
In vivo tumor treatment experiments. C57BL/6 mice (n = 5 per group) were initially challenged with a s.c. injection of TC-1 tumor cells (2 × 105/mouse). Mice received DNA injections (100 μg of DNA/mouse) three days after tumor challenge with or without added α-GalCer (2 μg/injection). The mice were boosted 1 week later with various vaccine combination regimens, including DNA vaccine or E7-pulsed DC-1 in the presence or absence of α-GalCer. The mice vaccinated with pcDNA3 or α-GalCer was used as controls. The mice were monitored for evidence of tumor growth by twice weekly inspection and palpation. Tumor volumes were measured starting at day 7 after tumor challenge. (A) Line graph depicting tumor volumes in mice of different tumor treatments (mean ± SD values). (B) Kaplan–Meier survival analysis in mice from the tumor treatment experiments. The data shown here are from one of two representative experiments.
3.5. CD8+ T-cells are important for the anti-tumor effect in mice primed with α-GalCer + CRT/E7 and boosted with E7-pulsed DC-1
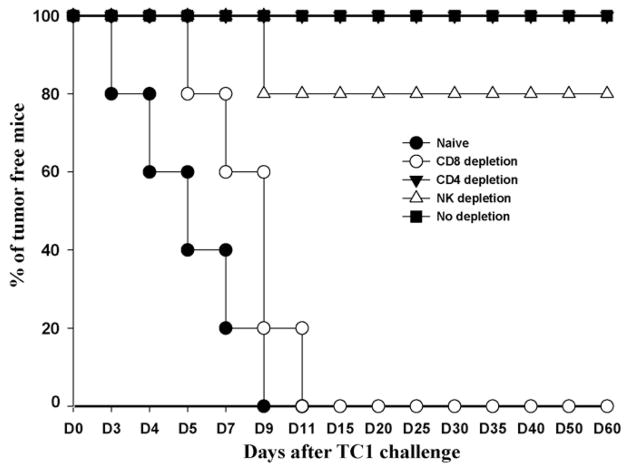
Several studies have shown that α-GalCer presented by CD1d molecule expressing on dendritic cells efficiently stimulates NKT cells implicated in innate immunity [27,28]. We performed an in vivo antibody depletion experiment to determine the contribution of various lymphocyte subsets to tumor protection and treatment generated in mice vaccinated α-GalCer + CRT/E7 prime-DC-1-E7 boost combination. CD4, CD8, and NK depletion were initiated after vaccination. As shown in Fig. 5, mice immunized with α-GalCer + CRT/E7 prime-DC-1-E7 boost completely were protected tumor growth after TC-1 challenge, In contrast, 100% of nonvaccinated(naïve) mice and mice depleted of CD8+ T-cells grew tumor within 2 weeks after TC-1 challenge. None of the mice depleted of CD4+ T-cells and 80% of mice depleted of NK cells remained tumor-free conditions at day 60 after TC-1 challenge. These data suggested that CD8+ T-cells are the major contributors to the observed anti-tumor effects generated by α-GalCer + CRT/E7 prime-DC-1-E7 boost combination.
Fig. 5.
In vivo tumor protection experiment and in vivo antibody depletion experiment in α-GalCer + DNA vaccine primed and DC-1-E7 boosted mice. An in vivo tumor protection experiment was performed to determine if the observed enhancement in E7-specific CD8+ T-cells mediated by α-GalCer + DNA vaccine prime-DC-1-E7 boost combination also led to a significant E7-specific anti-tumor protection response. Mice (n = 5 per group) were primed with 100 μg of DNA vaccine in the presence of α-GalCer and boosted with E7-pulsed DC-1. In vivo antibody depletion was performed to determine the effect of lymphocyte subsets on tumor protection. CD4, CD8 or NK depletion was initiated after last vaccination. At 1 week after the last vaccination, mice were challenged with 2 × 105 TC-1 tumor cells/mouse in the right hind leg. Tumor growth was monitored by visual inspection and palpation twice or thrice weekly. The data shown here are from one of two representative experiments.
3.6. Priming by CRT/E7 with α-GalCer and boosting with E7-pulsed dendritic cells leads to enhanced E7-specific CD8+ memory T-cells in vaccinated mice
Therapeutic anti-tumor vaccines can generate long-term memory immune responses. Mice were vaccinated with various combinations of α-GalCer and DC-1-E7 to determine the effects of α-GalCer on the generation of long-term E7-specific CD8+ memory T-cells. Control mice were also immunized with pcDNA3 and DC-1 alone. Splenocytes were collected and characterized for the presence of E7-specific CD8+ memory T-cells by intracellular IFN-γ cytokine staining followed by flow cytometric analysis two months later. Mice primed with α-GalCer + CRT/E7 and boosted with DC-1-E7 generated the highest number of E7-specific CD8+ memory T-cells. CRT/E7 priming followed by DC-1-E7 boosting in the presence of α-GalCer also produced significant levels of E7-specific CD8+ memory T-cells in vaccinated mice (Fig. 6A and B). Our data therefore suggested that the addition of α-GalCer to the DNA vaccine led to increased E7-specific CD8+ memory T-cell responses after boosting with antigen-expressing dendritic cells in vaccinated mice.
Fig. 6.
Characterization of long-term E7-specific CD8+ T-cell immune responses in mice primed with α-GalCer + DNA vaccine and boosted DC-1-E7. C57BL/6 mice (n = 5 per group) were immunized with various DNA vaccine combinations (100 μg of DNA/mouse) and α-GalCer (2 μg/injection). The mice were boosted at 1 week later with various vaccine combination protocols. Splenocytes from vaccinated mice were harvested 2 months after the last vaccination and tested for E7-specific CD8+ T-cells by staining for intracellular IFN-γ followed by flow cytometry. (A) Representative flow cytometric data for the E7-specific CD8+ T-cells. Numbers in the upper right-hand corner represent the number of E7-specific IFN-γ-secreting CD8+ T-cells per 3 × 105 pooled splenocytes. (B) Bar graphs depicting the numbers of E7-specific IFN-γ-secreting CD8+ T-cells per 3× 105 splenocytes (mean ± SD values). The data presented in this figure are from one of two representative experiments.
3.7. α-GalCer + CRT/E7 prime-E7-pulsed dendritic cells boost leads to long-term protection against TC-1 tumors in vaccinated mice
We performed long-term in vivo tumor protection experiments to determine whether the observed increases in E7-specific CD8+ memory T-cells generated by priming with α-GalCer + CRT/E7 and boosting with DC-1-E7 could be translated into long-term protective anti-tumor effects. C57BL/6 mice were vaccinated with various combinations of the DNA vaccines, α-GalCer and DC-1-E7. Immunized mice were challenged s.c. with TC-1 tumor cells (5 × 105/mouse) two months after the last vaccination and were monitored for tumor growth. Mice primed with α-GalCer + CRT/E7 and boosted with DC-1-E7 demonstrated complete inhibition of tumor growth compared with mice vaccinated with other regimens (Fig. 7A). We also observed significantly prolonged survival in these mice. Mice primed with CRT/E7 and boosted with DC-1-E7 in the presence of α-GalCer also demonstrated time-limiting prevention of tumor generation in vaccinated mice for a period of time; tumors grew at day 25 after tumor challenge (Fig. 7B). These data suggested that mice primed with α-GalCer + CRT/E7 and boosted with E7-pulsed dendritic cells could further enhance the generation of E7-specific CD8+ memory T-cells against the TC-1 tumor model in vaccinated mice.
Fig. 7.
Long-term in vivo tumor protection experiments. C57BL/6 mice (n = 5 per group) were immunized with various DNA vaccine combinations (100 μg of DNA/mouse) in the presence or absence of α-GalCer (2 μg/injection). The mice were boosted at 1 week later with various vaccine combination regimens. Mice were challenged by s.c. injection of 5 × 105 of TC-1 cells/mouse two months after the last vaccination. The mice were monitored for evidence of tumor growth by twice weekly inspection and palpation. Tumor volumes were measured starting at the 7th day after tumor challenge. (A) Line graph depicting tumor volumes in mice challenged with TC-1 cells (mean ± SD values). (B) Kaplan–Meier survival analysis in mice challenged with TC-1 cells. The data shown here are from one of two representative experiments.
4. Discussion
We demonstrated that the heterogenous α-GalCer + CRT/E7 prime and E7-pulsed DC-1 boost regimen generated the highest number of E7-specific CD8+ effectors and memory T-cells and the best anti-tumor effects in vaccinated mice compared with other protocols in the present study. DNA vaccination represents an attractive strategy for cancer immunotherapy by combining vaccine stability, cost-effectiveness and safety. Additionally, enhancement of immune responses after plasmid DNA immunization has been noted in mouse models. Despite these advantages, DNA vaccines alone have not been sufficient to induce complete or sustained immunity and also displayed suboptimal potency in non-human primate models and in clinical trials [29].
The primary mechanism of α-GalCer to enhance and maintain immune response to DNA vaccination may associate to several steps in antigen uptake and presentation. While the mechanism of antigen recruitment and presentation after intramuscular injection of DNA is not entirely defined, previous studies agree that the likely pathway responsible for antigen presentation is either the direct transfection of muscle cells or transfection of dendritic cells (DCs) in vivo [26]. The NKT-cell ligand, α-GalCer, stimulates the full maturation of DCs in situ after a single injection and results in induction of CD8+ T-cell immunity against co-administered proteins [19]. We also observed that splenic DC from mice injected α-GalCer (2 μg) expressed significant high level of CD40, CD86 on the surface compared with mice non-injected control group. However, several clinical trials using or combined with α-GalCer only focused on enhancement of the NKT cell-specific immune responses. Motohashi et al. [30] showed that the administration of α-GalCer-pulsed IL-2/GM-CSF-cultured peripheral monocytes was well tolerated and was accompanied by the successful induction of NKT cell-dependent immune responses. Kunii et al. [31] also showed that intra-arterial infusion of activated Valpha24 NKT cells and the sub-mucosal injection of α-GalCer -pulsed APC have been shown to induce significant anti-tumor immunity. Several studies show that α-GalCer has a potent adjuvant effect on generation of antigen-specific cytotoxic T lymphocytes through stimulation of DCs but offer little therapeutic effects against tumor by co-administration of DNA vaccine inducing CTLs responses with α-GalCer as adjuvant. Our studies show the new measure to overcome low immunogenicity of DNA vaccine with α-GalCer for inducing strong tumor antigen-specific CTLs responses through stimulating maturation of DCs.
α-GalCer previously displayed adjuvant effects on HIV DNA vaccines after administration at priming, leading to the enhancement of both antigen-specific cellular and humoral responses. The adjuvant activity of α-GalCer is not restricted to certain antigens and is more profound when administered with low-dose DNA vaccines [12,32]. Our results are consistent with previous reports which demonstrated that mice primed with CRT/E7 in the presence of α-GalCer (which has adjuvant role for DC maturation) generated a significant number of E7-specific CD8+ effector T-cells after CRT/E7 boosting as well as significant therapeutic anti-tumor effects against TC-1 tumors. Co-administration of E7-pulsed DC-1 (DC-1-E7) with α-GalCer at either the prime or boost phase generated greater E7-specific CD8+ T-cell precursors compared to mice vaccinated without α-GalCer.
However, mice boosted with CRT/E7 and α-GalCer did not enhance or suppress immune responses and mice co-administered CRT/E7 with α-GalCer both at prime and boost phase produced only 20% E7-specific CD8+ T-cells when compared with mice primed and boosted with CRT/E7 alone. Previous report also showed that the adjuvant activity of α-GalCer was most profound when co-administered at the priming, but not at the boosting phase and mice that received α-GalCer during both priming and boosting had reduced CD8+ IFN-γ T-cell responses. [19]. It appears that mice exposed to α-GalCer frequently resulted in a minimal production of cytokines by NKT cells, leading to a failed adjuvant effect in the boosting phase [33]. However, we observed that 80% of the mice depleted NK cells remained tumor-free conditions at day 60 after tumor challenge. We need further investigation to find out the specific mechanism of NKT cells or α-GalCer for DC maturation and generation of antigen-specific CD8+ T cell responses after DNA vaccination. The induction of an effective immune response against an established tumor via DNA vaccines may require repeated immunization. Heterologous prime-boost vaccination with a different agent (recombinant virus or protein vaccine) may eliminate the poor efficacy of boosting with the same viral vector or DNA vaccine [34–36]. After intramuscular injection of a naked plasmid DNA, myocytes are the predominant cells transfected; however, DCs and macrophages within the muscle tissue are also transfected [37,38]. DNA vaccines are more safe than live attenuated viral vector and relatively simple and inexpensive to design and create. Although the exact mechanism of cellular macromolecule entry is still unknown, larger molecules such as naked plasmid DNA are thought to enter through a multistep mechanism. Electroporation (EP) is a method whereby cellular membranes are transiently destabilized to facilitate the entry of foreign molecule into cells and tissues [39]. Recently, EP can boost cellular expression of DNA plasmids and immunogenicity across several species, different types of cells and tissues, even human clinical trials. For instance, it has been shown that EP can be used to enhance CRT/E7 DNA vaccine in the preclinical model [40]. Furthermore, the intramuscular administration of clinical grade CRT/E7 DNA vaccine followed by electroporation will be evaluated in patients with advanced head and neck cancer (Dr. Sara Pai, personal communications). However, the clinical application of EP technology may require appropriate devices that can provide a precise control of voltage and current to prevent excessive tissue damage. For this reason, it is necessary to consider the development of a more available and potent adjuvant system to enhance DNA vaccine immunogenicity.
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) and mediate effective in vivo and in vitro immune responses [41]. Administration of DCs with tumor-specific antigens may elicit anti-tumor cell-mediated immune responses and has utility as an anti-cancer therapy. However, the preparation of individualized DC-based vaccines is difficult to generate because it requires large scale culture, high costs, and prolonged time periods. For these reasons, appropriate combinations of DNA- and DC-based vaccines in prime-boost regimens might be more effective than either alone. Interestingly, we observed that priming with α-GalCer + CRT/E7 and boosting with E7-pulsed DC-1 further enhanced E7-specific CD8+ T-cell immune responses in vaccinated mice compared to mice primed with CRT/E7 and boosted with α-GalCer + DC-1-E7. We additionally observed that the α-GalCer + CRT/E7 prime and DC-1-E7 boost combination generated the best therapeutic anti-tumor effects and led to significant prolonged survival in vaccinated mice among all tested DNA-DCs vaccine combinations.
An important feature of a therapeutic anti-tumor vaccine is the ability to generate long-term memory immune responses. Mice primed with α-GalCer + CRT/E7 and boosted with DC-1-E7 demonstrated complete inhibition of tumor growth compared with mice vaccinated other vaccination regimens. We also demonstrated that priming with CRT/E7 followed by DC-1-E7 in the presence of α-GalCer also produced significant levels of E7-specific CD8+ memory T-cells in vaccinated mice. However, mice primed with CRT/E7 and boosted with DC-1-E7 in the presence of α-GalCer started to die on the 43rd day after tumor challenge. α-GalCer may influence the APC function or generation of E7-specific CD8+ memory T-cell, and may result in the enhancement of protection against TC-1 tumor cells. The specific mechanisms for the observed effects of α-GalCer on antigen-specific T-cells at prime phase warrant further investigation.
In summary, our data suggests that the enhancement of DNA vaccine potency by the co-administration of α-GalCer and DCs-based vaccine led to enhanced antigen-specific CD8+ T-cell activity and potent in vivo anti-tumor effect. These findings may have significant therapeutic utility. This innovative strategy may be a highly useful approach in the future.
Supplementary Material
Acknowledgments
This work supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-314-E00195). Dr. T.-C. Wu and Dr. Chien-Fu Hung are supported by the National Cancer Institute SPORE in Cervical Cancer P50 CA098252 and the 1 RO1 CA114425-01.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2010.08.079.
References
- 1.Liu MA. DNA vaccines: a review. J Intern Med. 2003;253:402–10. doi: 10.1046/j.1365-2796.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyd D, Hung CF, Wu TC. DNA vaccines for cancer. IDrugs. 2003;6:1155–64. [PubMed] [Google Scholar]
- 3.Nichols WW, Ledwith BJ, Manam SV, Troilo PJ. Potential DNA vaccine integration into host cell genome. Ann N Y Acad Sci. 2001;772:30–9. doi: 10.1111/j.1749-6632.1995.tb44729.x. [DOI] [PubMed] [Google Scholar]
- 4.Shan Lu. Immunogenicity of DNA vaccines in humans. Human Vaccines. 2008;4(6):449–52. doi: 10.4161/hv.4.6.6179. [DOI] [PubMed] [Google Scholar]
- 5.Tarte K, Klein B. Dendritic cell-based vaccine: a promising approach for cancer immunotherapy. Leukemia. 1999;13:653–63. doi: 10.1038/sj.leu.2401394. [DOI] [PubMed] [Google Scholar]
- 6.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–41. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 7.Tracy Doan, Herd Karen A, Lambert Paul F, Fernando Germain JP, Street Michael D, Tindle Robert W. Peripheral tolerance to human papillomavirus E7 oncoprotein occurs by cross-tolerization, is largely Th-2-independent, and is broken by dendritic cell immunization. Cancer Res. 2000;60:2810–5. [PubMed] [Google Scholar]
- 8.Jiang XB, Lu XL, Hu P, Liu RE. Improved therapeutic efficacy using vaccination with glioma lysate-pulsed dendritic cells combined with IP-10 in murine glioma. Vaccine. 2009;27:6210–6. doi: 10.1016/j.vaccine.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Hung CF, Wu TC, Monie A, Roden R. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol Rev. 2008;222:43–69. doi: 10.1111/j.1600-065X.2008.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TG, Kim CH, Won EH, Bae SM, Ahn WS, Park JB, et al. CpG-ODN-stimulated dendritic cells act as a potent adjuvant for E7 protein delivery to induce antigen-specific antitumour immunity in a HPV 16 E7-associated animal tumour model. Immunology. 2004;112:117–25. doi: 10.1111/j.1365-2567.2004.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helson R, Olszewska W, Singh M, Megede JZ, Melero JA, O’Hagan D, et al. Polylactide-co-glycolide (PLG) microparticles modify the immune response to DNA vaccination. Vaccine. 2008;26:753–61. doi: 10.1016/j.vaccine.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Chen A, Li X, Chen Z, Zhang W, Song Y, et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008;26:1807–16. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, et al. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol. 1998;161:3271–81. [PubMed] [Google Scholar]
- 14.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–34. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med. 2007;204:2641–53. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood. 2007;110:2013–9. doi: 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaraj S, Ziske C, Strehl J, Messmer D, Sauerbruch T, Schmidt-Wolf IG. Dendritic cells pulsed with alpha-galactosylceramide induce anti-tumor immunity against pancreatic cancer in vivo. Int Immunol. 2006;18:1279–83. doi: 10.1093/intimm/dxl059. [DOI] [PubMed] [Google Scholar]
- 19.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a co administered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 21.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–42. [PubMed] [Google Scholar]
- 23.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–9. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 24.Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;61:3698–703. [PubMed] [Google Scholar]
- 25.Donnelly JJ, Ulmer JB, Liu MA. DNA vaccines. Life Sci. 1997;60:163–72. doi: 10.1016/s0024-3205(96)00502-4. [DOI] [PubMed] [Google Scholar]
- 26.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 27.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. CD1d-restricted and TCR-mediated activation of Vα14NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 28.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-γ-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–74. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg SM, Bartido SM, Gardner JP, Guevara-Patiño JA, Montgomery SC, Perales MA, et al. Comparison of two cancer vaccines targeting tyrosinase: plasmid DNA and recombinant alphavirus replicon particles. Clin Cancer Res. 2005;11:8114–21. doi: 10.1158/1078-0432.CCR-05-1410. [DOI] [PubMed] [Google Scholar]
- 30.Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, et al. A phase I–II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 31.Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–8. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–24. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi H, Rong Y, Yankai Z, Wentao L, Hongxia Z, Jie W, et al. Improved efficacy of DNA vaccination against breast cancer by boosting with the repeat beta-hCG C-terminal peptide carried by mycobacterial heat-shock protein HSP65. Vaccine. 2006;24:2575–84. doi: 10.1016/j.vaccine.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Ferraz JC, Stavropoulos E, Yang M, Coade S, Espitia C, Lowrie DB, et al. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infect Immun. 2004;72:6945–50. doi: 10.1128/IAI.72.12.6945-6950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santra S, Barouch DH, Korioth-Schmitz B, Lord CI, Krivulka GR, Yu F, et al. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc Natl Acad Sci USA. 2004;101:11088–93. doi: 10.1073/pnas.0401954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupuis M, Denis-Mize K, Woo C, Goldbeck C, Selby MJ, Chen M, et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J Immunol. 2000;165:2850–8. doi: 10.4049/jimmunol.165.5.2850. [DOI] [PubMed] [Google Scholar]
- 38.Chattergoon MA, Kim JJ, Yang JS, Robinson TM, Lee DJ, Dentchev T, et al. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered Fas-mediated apoptosis. Nat Biotechnol. 2000;18:974–9. doi: 10.1038/79470. [DOI] [PubMed] [Google Scholar]
- 39.Rols MP. Mechanism by which electroporation mediates DNA migration and entry into cells and targeted tissues. Methods Mol Biol. 2008;423:19–33. doi: 10.1007/978-1-59745-194-9_2. [DOI] [PubMed] [Google Scholar]
- 40.Best SR, Peng S, Juang CM, Hung CF, Hannaman D, Saunders JR, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27:5450–9. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53:240–8. doi: 10.1007/s00262-003-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.