Abstract
A Brain-Computer Interface (BCI) is a device that enables severely disabled people to communicate and interact with their environments using their brain waves. Most research investigating BCI in humans has used scalp-recorded electroencephalography (EEG) or intracranial electrocorticography (ECoG). The use of brain signals obtained directly from stereotactic depth electrodes to control a BCI has not been previously explored. In this study, event related potentials (ERPs) recorded from bilateral stereotactic depth electrodes implanted in and adjacent to hippocampus were used to control a P300 Speller paradigm. The ERPs were preprocessed and used to train a linear classifier to subsequently predict the intended target letters. The classifier was able to predict the intended target character at or near 100% accuracy using fewer than 15 stimulation sequences in the two subjects tested. Our results demonstrate that event related potentials from hippocampal and hippocampal adjacent depth electrodes can be used to reliably control the P300 Speller BCI paradigm.
1. Introduction
A brain-computer interface (BCI) is a device that uses brain signals to provide a non-muscular communication channel [1], particularly for individuals with severe neuromuscular disabilities. One of the most promising signals for controlling a BCI are event-related potentials (ERPs) such as the P300. The P300 event related potential is an evoked response to an external stimulus that has been traditionally observed in scalp-recorded electroencephalography (EEG). The scalp-recorded P300 response has proven to be a reliable signal for controlling a BCI using the P300 Speller paradigm [2]. Based on multiple studies in healthy volunteers [3-5], and initial results in persons with physical disabilities [6-7], the P300 Speller has the potential to serve as an effective communication device for persons who have lost or are losing the ability to write and speak.
It is hypothesized that electrodes positioned closer to the source of the brain’s electrical activity will improve the signal-to-noise ratio and hence the communication rate. Intracranial surface grid arrays and depth electrodes are routinely implanted in humans for localizing epileptic seizure foci. Both styles of electrodes record local field potentials (LFPs), with the surface grid array recordings referred to as the electrocorticogram (ECoG). It has recently been shown that the P300 Speller can be effectively controlled using ECoG [8]. It has also been shown that P300 ERPs can be recorded from hippocampal depth electrodes in humans [9]. The present study characterizes the ERPs recorded from bilateral stereotactic depth electrodes (SDEs) implanted in and adjacent to hippocampus and shows that they can be used to control the P300 Speller.
2. Methods
2.1 Patients
Two subjects with medically intractable epilepsy were tested for the ability to control a visual keyboard using ERPs. Both subjects underwent Phase 2 evaluation for epilepsy surgery with temporary placement of bilateral SDEs to localize seizure foci prior to surgical resection. Both subjects were presented at Mayo Clinic Florida’s multidisciplinary Surgical Epilepsy Conference where the consensus clinical recommendation was for the subject to undergo invasive monitoring primarily to localize the epileptogenic zone. The study was approved by the Institutional Review Board of both Mayo Clinic and the University of North Florida. Both subjects gave their informed consent. Clinical data on each subject are provided in Table 1.
Table 1.
Subject Clinical Information
| Age/Sex | AED | MRI | Neuropsychological Language Testing | Language (Wada) | Memory (Wada) | |
|---|---|---|---|---|---|---|
| Subject A | 48/F | CBZ, ZSM | (R) MTS, ? left hippocampal atrophy | Mild learning inefficiency for nonverbal material; mild nonspecific cognitive dysfunction | Left | Left |
| Subject B | 33/F | LVT | Normal | Variability in attention and processing speed | Right | Bilateral R > L |
Anti-epileptic drug (AED); carbamazepine (CBZ); zonisamide (ZSM); levetiracetam (LVT); mesial temporal sclerosis (MTS); intracarotid sodium amytal study (Wada); (?) denotes “questionable” or “possible”
2.2 Electrode Locations and Clinical Recordings
Electrode (AD-Tech Medical Instrument Corporation, WI, USA) placements and duration of intracranial monitoring were based solely on the requirements of the clinical evaluation, without any consideration of this study. All electrode placements were stereotactically guided intra-operatively by Stealth MRI neuronavigational system (Medtronics, Inc., MN, USA). Each subject had post-operative anterior–posterior and lateral radiographs to verify electrode locations. The inter-electrode spacing along the arrays was 10 mm for Subject A and 5 mm for Subject B. Electrode locations are illustrated in Figure 1. By capturing seizures with an electrographic pattern typical for hippocampal onset seizures, the three most anterior right temporal contacts for Subject A, and the five most anterior left temporal contacts for Subject B were confirmed to be in or adjacent to hippocampal tissue. Aside from the most posterior electrodes for Subject A, it is highly probable that all electrodes were in or adjacent to hippocampal tissue.
Figure 1.
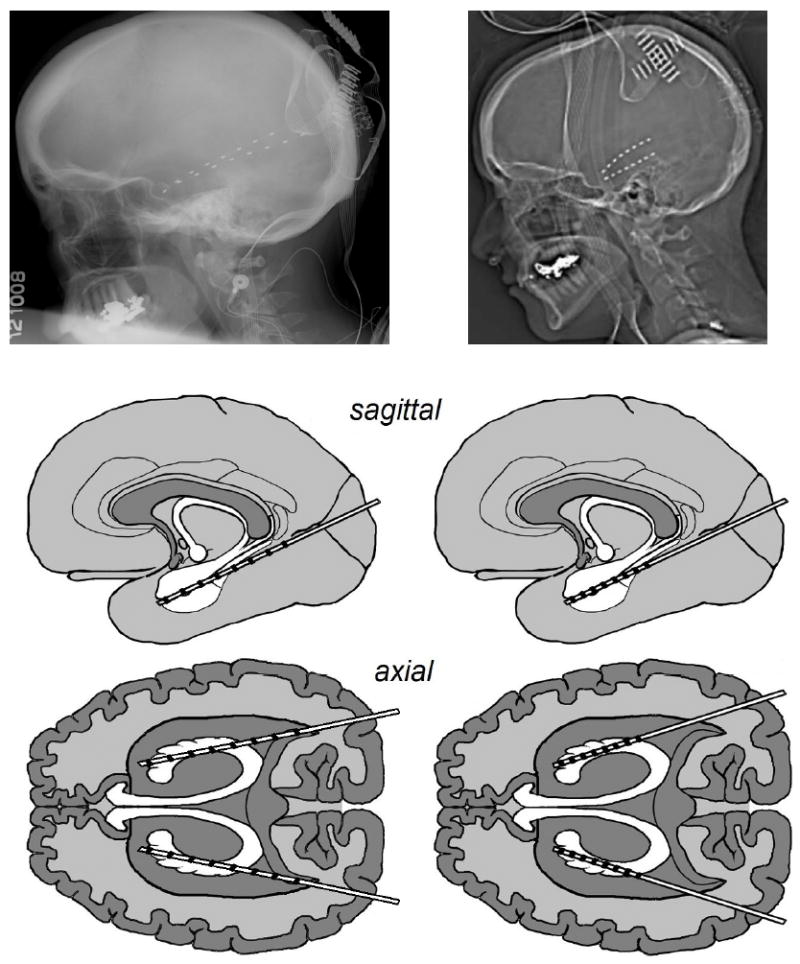
The lateral radiographs and approximate sagittal and axial electrode locations. Left column: Subject A, Right column: Subject B.
After electrode implantation, all subjects were admitted to an ICU room with epilepsy monitoring capability. Clinical data were gathered with a 64-channel clinical video-EEG acquisition system (Natus Medical, Inc.; CA, USA).
2.3 BCI Data Acquisition
Both subjects performed BCI testing between 24-48 hours after electrode implantation. Testing was performed only when the subject was clinically judged to be at cognitive baseline and free of physical discomfort that would affect attention and concentration. Testing was performed at least six hours after a clinical seizure. Stimuli were presented and the data were recorded using the general-purpose BCI system BCI2000 [10]. All electrodes were referenced to a scalp vertex electrode, amplified, band pass filtered (0.5–500 Hz), digitized at 1200 Hz using 16-channel g.USB amplifiers (Guger Technologies, Graz, Austria), and stored. A laptop with a 2.66 GHz Intel Core 2 Duo CPU, 3.5 GB of RAM, and Windows XP was used to execute BCI2000. The signals for the BCI experiments were acquired concurrent with the clinical monitoring via a 32-channel electrode splitter box (AD-Tech Medical Instrument Corporation, WI, USA).
2.4 Task, Procedure, & Design
The experimental protocol was based on the protocol used in an EEG-based P300 Speller study [3]. Each subject sat in a hospital bed about 75 cm from a video monitor and viewed the matrix display. The monitor was centered in the subject’s visual field. The task was to focus attention on a specified letter of the matrix and silently count the number of times the target character flashed, until a new character was specified for selection. All data were collected in the copy speller mode: words were presented on the top left of the video monitor and the character currently specified for selection was listed in parentheses at the end of the letter string as shown in Figure 2. Each session consisted of 8-11 experimental runs of the P300 Speller paradigm; each run was composed of a word or series of characters chosen by the investigator. This set of characters spanned the set of characters contained in the matrix and was consistent for each subject and session. Each session consisted of between 32-39 character epochs. A single session lasted approximately one hour. One to two sessions were collected for each subject, depending on the subject’s physical state and willingness to continue.
Figure 2.

The 6×6 matrix used in the current study. A row or column flashes for 100 ms every 175 ms. The letter in parentheses at the top of the window is the current target character “D.” A P300 should be elicited when the fourth column or first row is flashed. After 15 flash sequences (i.e., each row and each column has been flashed 15 times), the collected brain responses are processed, classified, and online feedback is provided directly below the character to be copied. The process is then repeated for the next target character “I” and so forth until all characters in the word "DICE" have been presented as targets.
2.5 Online Response Classification
For each of the 16 channels used in the analysis, 800-ms segments of data following each flash were extracted for the offline analysis. The data segments were lowpass filtered and decimated to 20 Hz and concatenated by channel for each flash, creating a single feature vector corresponding to each stimulus. The features from the first four runs (16 characters) from the first uncorrupted session were used to generate a linear classifier for each subject using stepwise linear regression (SWLDA) [11]. The response processing and classification are detailed in [3]. The performance of the classifier for selecting the attended character was tested on the four subsequent runs (16 characters) from the same session.
2.6 Response Visualization
Nearly all electrodes were included in the linear regression model for each subject, although it is evident that only select electrodes contribute the bulk of the discriminable information for the task while the others merely serve as suppressor variables [12], which are not correlated with the task but are correlated with one or more of the independent variables of a regression model. The ERPs from all electrodes and their r2 correlations (i.e. the proportion of the variance of the instantaneous signal amplitude accounted for by the stimulus type, i.e., target or standard) with the task are presented in Figure 3. The waveforms were generated using the average of all training and test data used for classification for each subject. The averaged waveforms were smoothed for visualization using a 0-30 Hz lowpass filter. The color scale in Figure 3 corresponds to the electrode coloring and indicates the classification accuracy after 15 flash sequences obtained by constructing a least-squares linear classifier from the individual electrode’s ERPs in isolation (evaluated using the same training and test sessions as the previous classification). This provides some indication of the relative discriminative power of the ERPs at each electrode for the task. Separate SWLDA classifiers were also trained using the responses from the 8 left hemisphere electrodes and 8 right hemisphere electrodes, respectively. Likewise, this was done to evaluate the relative discriminative power of the ERPs from each hemisphere.
Figure 3.
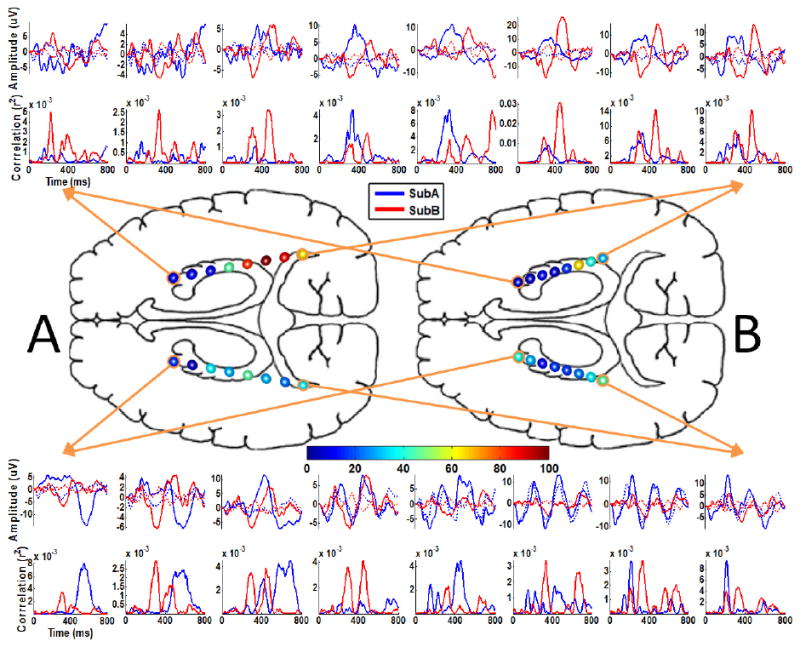
The ERPs and individual electrode classification. The ERPs and the respective r2 correlations with the task are plotted in the periphery. These plots have been arranged according to the electrode position on the respective arrays and do not indicate an anatomic correspondence of the electrode positions between subjects. These waveforms represent the average responses to the target (solid) and standard (dotted) stimuli. The waveforms were generated using the average of all training and test data used for classification for each subject. The color scale corresponds to the electrode coloring and indicates the classification accuracy after 15 flash sequences obtained by constructing a least squares linear classifier with the individual electrode’s ERPs in isolation (chance accuracy is 2.8%). Note that this figure represents an approximate axial view of depth electrodes and that the relative electrode size is enlarged for visualization purposes; refer to Figure 1 for details on the electrode positioning.
3. Results
Both subjects were able to accurately spell words via the P300 Speller using depth electrode signals. As shown in Figure 4, both subjects achieved over 90% after 15 flash sequences online, with Subject A reaching 100% after 6 flash sequences. Figure 4 also indicates that higher bitrates [13] can be achieved for both subjects using fewer than 15 flash sequences. Offline analysis revealed that classifiers constructed from right hemisphere signals performed significantly better that the left hemisphere for Subject A and marginally better for Subject B. Interestingly, for Subject A, the right hemisphere classifier is comparable to the classifier that spans the hemispheres. Based on the offline analysis of individual electrode classification, Subject A achieved 100% accuracy and Subject B achieved 63% after 15 flash sequences using an individual electrode, with 2.8% being chance accuracy. Interestingly, the individual electrode that achieved the highest accuracy was located in the right posterior hippocampal region for both subjects.
Figure 4.
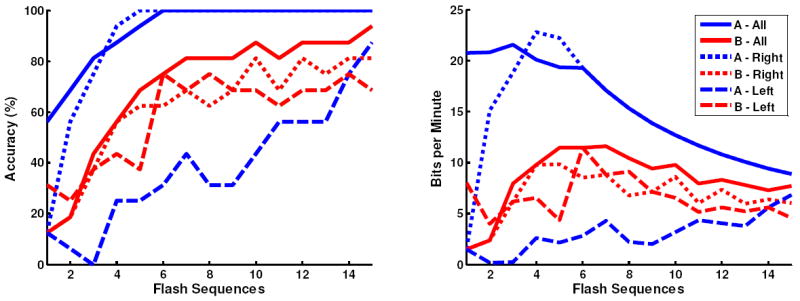
The offline classification accuracy (left figure) and bitrate (right figure) with respect to the number of flash sequences for subjects A and B. The individual traces indicate the performance based on training the classifier using all 16 electrodes, the 8 right hemisphere electrodes, and the 8 left hemisphere electrodes. The online accuracy corresponds to the accuracy after 15 flash sequences using all electrodes.
4. Discussion
Multiple studies have shown that BCI-based methods using scalp EEG and ECoG in humans can be used to control a prosthetic device [14] or a cursor on a computer monitor [15-22]. However, previous BCI-related language research has mainly utilized scalp EEG [23-26]. Several EEG-based BCI systems have been developed with sophisticated paradigms to translate neuroelectric signals for the purpose of communication. The performance of these scalp-based translational systems has been hampered by the fact that electrical signals are degraded and attenuated while traveling through skull and scalp layers, in addition to muscle-related and electrode interface artifacts common to scalp recordings. These factors result in suboptimal signal-to-noise characteristics and lower information transfer rates and likely contribute to slower speed and decreased accuracy in performing language tasks.
Our previous study shows that electrical recordings from human cortex can be translated by P300-based BCI systems to produce accurate and reliable language output at least equal to and probably superior to recordings obtained from scalp EEG [8]. Our previous study results attest to the possible superiority of the ECoG signal over the EEG signal in controlling a BCI-based language communication system. Further improvements in ECoG-based bitrate could potentially be achieved with optimization of classification parameters for each individual. In addition, our results suggest that control of a visual keyboard can be achieved by directly monitoring a small area of brain as opposed to monitoring the entire scalp. These findings open a new avenue for research on improving communication devices for patients with ALS, spinal cord injuries, stroke, and severe inflammatory polyradiculopathies. As the risks associated with implantation of chronic intracranial electrodes continue to decrease with advances in electrode design and surgical techniques, the ECoG-based P300 Speller may become a viable option for severely disabled individuals with no reliable means of communication.
Although the P300 signal has been recorded with intracranial electrodes in the hippocampus [27-28], this study is the first to demonstrate the use of human hippocampal and adjacent signals to control a brain-computer interface. The ability to utilize a SDE-based P300 Speller for communication may improve the risk/benefit ratio for chronic intracranial implantation compared to ECoG with grid electrodes. SDEs are often implanted through occipital burr holes with stereotactic guidance. In contrast, a craniotomy procedure is most commonly used to place grid or strip electrodes. Postoperative steroids to reduce brain swelling is often used after grid/strip implantation, but not after SDE inserted through the occipital approach1. Surgical case series [29-32] suggest epilepsy patients undergoing SDE as opposed to subdural grids/extended strips have less morbidity.
An interesting finding is that an individual electrode, similarly located in the posterior right hippocampal region for both subjects, recorded data which achieved the highest accuracy for our classifier. While Subject A may have had left hippocampal atrophy (the radiologist could not be definitive), she clearly had right mesial temporal sclerosis by MRI criteria. Therefore, she was expected to exhibit more right temporal lobe dysfunction. This was confirmed by her neuropsychometric test which showed mild inefficiency in learning nonverbal material (typically a right hemisphere function) and on Wada testing, in which Subject A was left hemisphere dominant for both language and memory. Because Subject A’s structural and neurocognitive abnormalities were lateralized to the right temporal region, the finding that classifiers constructed from right hemisphere signals performed significantly better than that from the left hemisphere was unexpected. Subject B was dominant for language in the right hemisphere, and had bilateral memory functioning albeit with greater representation in the right hippocampus. We recognize the N is small, but based on these two subjects, the presence of discriminable P300 responses appears independent of memory or language lateralization. Ludowig et al. [9] studied the topography of the medial temporal P300 and found the highest signal amplitude in the anterior subiculum and posterior hippocampal body. Our findings are consistent with their results. Further studies are needed to determine if the posterior right hippocampal region preferentially produces the best P300 signals for BCI classification.
A limitation of this study was the lack of millimeter spatial localization of the individual electrodes of the SDE array to the anatomic location region in the brain. Our IRB approval letter was very specific in prohibiting any testing outside of what the clinical team felt was appropriate to treat the patient’s medical problem. Routine post-operative CT or MRI for anatomic localization of stereotatic depth electrodes is not standard of care for these patients’ treatment teams. Future studies with SDE in this type of research may benefit from involvement of treatment teams with different practice patterns.
These preliminary results indicate that adequate performance can be achieved using a unilateral array or possibly even a single SDE. We believe a SDE-based P300 Speller with the same efficiency and accuracy as an ECoG-based P300 Speller may represent a better long-term option for patients needing chronic brain-computer interface devices for communication control. Further studies will be needed to compare the overall feasibility of the SDE-based P300 Speller compared to scalp EEG and ECoG.
Acknowledgments
This work was funded in part by the National Science Foundation (0905468) and the National Institutes of Health (NIBIB/NINDS EB00856).
Footnotes
Personal communication, Robert Wharen, MD, Chief of Neurosurgery, Mayo Clinic Florida
References
- 1.Wolpaw JR, Birbaumer N, McFarland DJ, et al. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002 Jun;113(6):767–91. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 2.Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988 Dec;70(6):510–23. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- 3.Krusienski DJ, Sellers EW, McFarland DJ, et al. Toward enhanced P300 speller performance. J Neurosci Methods. 2008 Jan 15;167(1):15–21. doi: 10.1016/j.jneumeth.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenhardt A, Kaper M, Ritter HJ. An adaptive P300-based online brain-computer interface. IEEE Trans Neural Syst Rehabil Eng. 2008 Apr;16(2):121–30. doi: 10.1109/TNSRE.2007.912816. [DOI] [PubMed] [Google Scholar]
- 5.Serby H, Yom-Tov E, Inbar GF. An improved P300-based brain-computer interface. IEEE Trans Neural Syst Rehabil Eng. 2005 Mar;13(1):89–98. doi: 10.1109/TNSRE.2004.841878. [DOI] [PubMed] [Google Scholar]
- 6.Nijboer F, Sellers EW, Mellinger J, et al. A P300-based brain-computer interface for people with amyotrophic lateral sclerosis. Clin Neurophysiol. 2008 Aug;119(8):1909–16. doi: 10.1016/j.clinph.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan TM, McFarland DJ, Schalk G, et al. The Wadsworth BCI Research and Development Program: at home with BCI. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):229–33. doi: 10.1109/TNSRE.2006.875577. [DOI] [PubMed] [Google Scholar]
- 8.Krusienski DJ, Shih JJ. Control of a Visual Keyboard using an Electrocorticographic Brain-Computer Interface. Neurorehabilitation and Neural Repair. doi: 10.1177/1545968310382425. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludowig E, Bien CG, Elger CE, et al. Two P300 generators in the hippocampal formation. Hippocampus. 2010 Jan;20(1):186–95. doi: 10.1002/hipo.20603. [DOI] [PubMed] [Google Scholar]
- 10.Schalk G, McFarland DJ, Hinterberger T, et al. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004 Jun;51(6):1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 11.Draper NR, Smith H. Applied regression analysis. 2. New York, New York: John Wiley and Sons; 1981. [Google Scholar]
- 12.Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2. Hillsdale, N.J.: Erlbaum; 1983. [Google Scholar]
- 13.McFarland DJ, Sarnacki WA, Wolpaw JR. Brain-computer interface (BCI) operation: optimizing information transfer rates. Biol Psychol. 2003 Jul;63(3):237–51. doi: 10.1016/s0301-0511(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006 Jul 13;442(7099):164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 15.McFarland DJ, Sarnacki WA, Vaughan TM, et al. Brain-computer interface (BCI) operation: signal and noise during early training sessions. Clin Neurophysiol. 2005 Jan;116(1):56–62. doi: 10.1016/j.clinph.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004 Dec 21;101(51):17849–54. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leuthardt EC, Schalk G, Wolpaw JR, et al. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004 Jun;1(2):63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 18.Schalk G, Miller KJ, Anderson NR, et al. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008 Mar;5(1):75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felton EA, Wilson JA, Williams JC, et al. Electrocorticographically controlled brain-computer interfaces using motor and sensory imagery in patients with temporary subdural electrode implants. Report of four cases. J Neurosurg. 2007 Mar;106(3):495–500. doi: 10.3171/jns.2007.106.3.495. [DOI] [PubMed] [Google Scholar]
- 20.Santhanam G, Ryu SI, Yu BM, et al. A high-performance brain-computer interface. Nature. 2006 Jul 13;442(7099):195–8. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- 21.Blakely T, Miller KJ, Zanos SP, et al. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurg Focus. 2009 Jul;27(1):E13. doi: 10.3171/2009.4.FOCUS0977. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JA, Schalk G, Walton LM, et al. Using an EEG-based brain-computer interface for virtual cursor movement with BCI2000. J Vis Exp. 2009;(29) doi: 10.3791/1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allison BZ, Pineda JA. ERPs evoked by different matrix sizes: implications for a brain computer interface (BCI) system. IEEE Trans Neural Syst Rehabil Eng. 2003 Jun;11(2):110–3. doi: 10.1109/TNSRE.2003.814448. [DOI] [PubMed] [Google Scholar]
- 24.Lee PL, Hsieh JC, Wu CH, et al. The brain computer interface using flash visual evoked potential and independent component analysis. Ann Biomed Eng. 2006 Oct;34(10):1641–54. doi: 10.1007/s10439-006-9175-8. [DOI] [PubMed] [Google Scholar]
- 25.Lee PL, Hsieh JC, Wu CH, et al. Brain computer interface using flash onset and offset visual evoked potentials. Clin Neurophysiol. 2008 Mar;119(3):605–16. doi: 10.1016/j.clinph.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Kubler A, Kotchoubey B, Kaiser J, et al. Brain-computer communication: unlocking the locked in. Psychol Bull. 2001 May;127(3):358–75. doi: 10.1037/0033-2909.127.3.358. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy G, Wood CC, Williamson PD, et al. Task-dependent field potentials in human hippocampal formation. J Neurosci. 1989 Dec;9(12):4253–68. doi: 10.1523/JNEUROSCI.09-12-04253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007 Oct;118(10):2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong CH, Birkett J, Byth K, et al. Risk factors for complications during intracranial electrode recording in presurgical evaluation of drug resistant partial epilepsy. Acta Neurochir (Wien) 2009 Jan;151(1):37–50. doi: 10.1007/s00701-008-0171-7. [DOI] [PubMed] [Google Scholar]
- 30.Burneo JG, Steven DA, McLachlan RS, et al. Morbidity associated with the use of intracranial electrodes for epilepsy surgery. Can J Neurol Sci. 2006 May;33(2):223–7. doi: 10.1017/s0317167100005023. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Hwang YS, Shin JJ, et al. Surgical complications of epilepsy surgery procedures : experience of 179 procedures in a single institute. J Korean Neurosurg Soc. 2008 Oct;44(4):234–9. doi: 10.3340/jkns.2008.44.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behrens E, Schramm J, Zentner J, et al. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997 Jul;41(1):1–9. doi: 10.1097/00006123-199707000-00004. discussion 9-10. [DOI] [PubMed] [Google Scholar]


