Abstract
Objectives
The objective of this study was to test the hypothesis that physical frailty is associated with an increased risk of MCI.
Design
Prospective, observational cohort study.
Setting
Approximately 40 retirement communities across the Chicago metropolitan area.
Participants
More than 700 older persons without cognitive impairment at baseline.
Measures
Physical frailty, based on four components (i.e., grip strength, timed walk, body composition and fatigue), was assessed at baseline and cognitive function was assessed annually. Proportional hazards models adjusted for age, sex, and education were used to examine the association of physical frailty with the risk of incident MCI, and mixed effect models were used to examine the association of frailty with the rate of change in cognition.
Results
During up to 12 years of annual follow-up, 305 of 761 (40%) persons developed MCI. In a proportional hazards model adjusted for age, sex, and education, physical frailty was associated with a substantially increased risk of incident MCI, such that each one unit increase in physical frailty was associated with a 63% increase in the risk of MCI (hazard ratio: 1.63; 95% CI: 1.27, 2.08). This association persisted in analyses that required MCI to persist for at least one year and after controlling for depressive symptoms, disability, vascular risk factors, and vascular diseases. Further, a higher level of physical frailty was associated with an increased rate of decline in global cognition and 5 cognitive systems (i.e., episodic memory, semantic memory, working memory, perceptual speed, and visuospatial abilities).
Conclusion
Physical frailty is associated with an increased risk of MCI and a more rapid rate of cognitive decline in aging.
Keywords: frailty, mild cognitive impairment, cognitive decline
INTRODUCTION
Mild cognitive impairment (MCI) is a heterogeneous condition that is increasingly recognized as a precursor to dementia, particularly Alzheimer's disease (AD)1,2. Persons with MCI have a substantially increased risk of developing AD and a more rapid rate of cognitive decline compared to those without cognitive impairment3–7. Although recognition of MCI as the earliest manifestation of AD has generated interest in identifying the factors associated with its development, to date, relatively few such factors have been identified8–10. This is in part because MCI develops gradually over a number of years and the identification of risk factors requires longitudinal observation of large groups of older persons initially free of cognitive impairment. The identification of risk factors for MCI is crucial for the development of disease-modifying therapies for AD and interventions to prevent age-related cognitive decline.
An emerging body of work raises the possibility that physical frailty may be a risk factor for the development of MCI12. Physical frailty is common among older persons and thought to represent an age-related decrease in physiologic reserve that leads to an increased susceptibility to adverse health outcomes11–14. Cross-sectional studies have reported associations between physical frailty and cognitive function11,15–17. In addition, in this cohort, we recently documented that a higher level of physical frailty is associated with an increased risk of incident AD18. We are not aware, however, of prior studies that have examined whether physical frailty is a risk factor for the development of MCI among persons initially free of cognitive impairment.
We tested the hypothesis that a higher level of physical frailty is associated with an increased risk of MCI using data from more than 750 community-based older persons without cognitive impairment (no dementia or MCI) from the Rush Memory and Aging Project, an ongoing longitudinal epidemiologic study of aging19,20. Participants underwent baseline assessments of frailty and detailed annual structured clinical evaluations to document the level of cognitive function and determine the presence of MCI, AD and other causes of dementia. Proportional hazards models were used to examine the association of baseline physical frailty with the risk of developing MCI. In subsequent models, we examined whether the association of frailty with MCI was influenced by other factors including depressive symptoms, disability, vascular risk factors and diseases. Finally, we examined the association of physical frailty with the rate of cognitive decline.
METHOD
Participants
Participants were from the Rush Memory and Aging Project, a longitudinal clinical-pathologic study of common chronic conditions of old age20. Participants come from more than 40 residential facilities across the metropolitan Chicago area, including subsidized senior housing facilities, retirement communities, and retirement homes, in addition to social service agencies and Church groups. As a condition of entry, all participants agreed to annual detailed clinical evaluations and donation of brain, spinal cord, nerve, and muscle at the time of death. The study was in accordance with the latest version of the Declaration of Helsinki and was approved by the Institutional Review Board of Rush University Medical Center.
Each participant of the Rush Memory and Aging Project undergoes a uniform structured clinical evaluation at baseline. This evaluation includes a medical history, neurological and physical examination, and assessment of cognitive function. Follow-up clinical evaluations are identical in all essential details to the baseline examination and are performed at one-year intervals by examiners blinded to previously collected data. At the time of these analyses, 1222 participants had completed the baseline evaluation. Eligibility for these analyses required the absence of clinical dementia or mild cognitive impairment based on the detailed cognitive function testing (see below), as well as a valid frailty score from the baseline evaluation and at least one valid follow-up evaluation. Thus, 72 persons who met criteria for dementia at the baseline evaluation, 327 persons who met criteria for MCI at the baseline evaluation, 14 without a valid frailty score at baseline, 18 who died before the first follow-up, and 30 who had only one evaluation were excluded from these analyses. This resulted in a final group of 761 persons included in these analyses.
Assessment of Cognitive Function
Cognitive function was assessed via a battery of 21 tests, as previously described5–7,20,22. This battery included the MMSE21, but MMSE scores are used only to describe the cohort. Scores on 19 tests are used to create summary indices of global cognitive function and five specific cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. Episodic memory was assessed via seven tests: immediate and delayed recall of story A from Logical Memory, immediate and delayed recall of the East Boston Story, Word List Memory, Word List Recall, and Word List Recognition; semantic memory was assessed via three tests: a 15-item version of the Boston Naming Test, Verbal Fluency, and a 15-item reading test; working memory was assessed via three tests: Digit Span Forward, Digit Span Backward and Digit Ordering; perceptual speed was assessed via four tests: Symbol Digit Modalities Test, Number Comparison, and two indices from a modified version of the Stroop Neuropsychological Screening Test; and visuospatial abilities were assessed via two tests: a 15-item version of Judgment of Line Orientation and a 16-item version of Standard Progressive Matrices. One additional test, Complex Ideational Material, was used for diagnostic classification purposes only.
To compute the composite measure of global cognitive function, raw scores on each of the individual tests are converted to z-scores using the baseline mean and standard deviation of the entire cohort, and the z-scores of all 19 tests are averaged5–7,20,22. In addition, summary scores for five cognitive domains (i.e. episodic memory, semantic memory, working memory, perceptual speed, and visuospatial abilities), are derived by converting raw scores on each of the individual tests to z-scores using the mean and standard deviation of the entire cohort and then averaging the z-scores from tests within a specific domain. Psychometric information on these summary scores, including factor analytic support for the five domains, is contained in previous publications5–7,20,22.
Clinical Diagnoses
All participants underwent a uniform structured clinical evaluation including a medical history, neurologic examination, and cognitive performance testing, as previously described (9). Cognitive tests were reviewed by an experienced neuropsychologist. Participants were evaluated in person by a physician, who used all available cognitive and clinical testing results, to diagnose dementia and other common neurologic conditions affecting cognitive function. The diagnosis of dementia followed the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association23. These require a history of cognitive decline and evidence of impairment in two or more domains of cognition, one of which must be memory, for classification as Alzheimer's disease. Persons were diagnosed with MCI if they were determined to have cognitive impairment by the neuropsychologist but did not meet criteria for dementia. The criteria employed for the diagnosis of MCI in this cohort are equivalent to those used for a diagnosis of cognitive impairment, no dementia (i.e., CIND) in some other cohorts24. Importantly, however, there are no universally agreed upon criteria for MCI, and these criteria have been validated in several prior publications using data from this and other cohorts and this definition of MCI is related to the subsequent development of dementia and further cognitive decline4–7.
Composite Frailty Measure
A continuous composite measure of frailty based on four components (i.e., grip strength, timed walk, body composition and fatigue) was used in the present study, as previously described18,19,26. Strength was based on grip strength measured with the Jamar hydraulic hand dynamometer (Lafayette Instruments, Lafayette, IN). Gait was based on the time to walk eight feet. Body composition was based on body mass index (BMI) = weight/height2. As done in previous studies of frailty1–4, we used two questions derived from a modified version of the Center for Epidemiologic Studies-Depression (CES-D) Scale to assess fatigue. The four components used to construct the composite measure of frailty were structured so that higher values would indicate poorer performance (more frailty) and lower values would indicate better performance (less frailty), to be consistent with prior literature. The composite measure of frailty was constructed by converting the raw score from each of the four component measures to z scores using the mean and standard deviation from all participants at baseline, as previously described17,18. The composite measure of frailty has been shown to predict disability, AD and mortality18,19.
Comorbidities and Other Covariates
Gender, race, and age are recorded at the baseline interview. Gender was coded as “1” for men, “0” for women. Race questions and categories were those used by the 1990 U.S. Census. Age in years was computed from self-reported date of birth and date of the clinical examination.
Education (reported highest grade or years of education) was obtained at the time of the baseline cognitive testing.
Depressive symptoms were assessed with a 8-item version of the Center for Epidemiologic Studies Depression (CES-D) scale27,28. Persons were asked whether they had experienced each of 8 symptoms in the past week, and the score was the number of symptoms reported. The mean score on the CESD was 0.9 (SD= 1.4, range: 0–8).
Disability was assessed via two measures. The Katz includes six items that address basic activities of daily living (ADLs): walking across a small room, bathing, dressing, eating, transferring from a bed to a chair, and toileting29. A composite measure was created by summing the number of items on which participants reported the need for assistance; thus, higher scores indicate greater disability. The mean score on the Katz scale was 0.14 (SD=0.5, range: 0–5). Instrumental activities of daily living were assessed using items adapted from the Duke Older Americans Resources and Services project20. Items assessed include eight activities: telephone use, meal preparation, money management, medication management, light and heavy housekeeping, shopping, and local travel. For these analyses, participants who reported an inability to perform one or more tasks were classified as having disability on the measure of instrumental activities of daily living.
To assess the influence of cumulative vascular risk factors and vascular diseases, we computed summary scores indicating each individual's vascular risk factor (i.e,. the sum of hypertension, diabetes mellitus, and smoking as determined by medication use or self-report, resulting in a score from 0–3 for each individual (mean 1.1 SD=0.8) and vascular disease burden (i.e. the sum of heart attack, congestive heart failure, claudication, and stroke by self-report or clinician diagnosis of stroke, resulting in a score from 0–4 for each individual (mean 0.3 SD= 0.6)5. These summary scores were used as covariates in the analyses.
Current income was measured at baseline via a single question. Persons were asked to select one of 10 levels of total family income using the “show-card” method20.
Statistical Analyses
Pearson correlations were used to examine the associations of frailty with age and education. Student's t-tests were used to compare measures among men and women as well as those who did and did not develop MCI. We examined the association of frailty with incident MCI via a series of proportional hazards models for discrete (tied) data30,45; that is, because examinations are scheduled in an annual cycle, differences of a few months in the date of the first MCI diagnosis correspond to the sequence in which participants were studied, not the sequence in which the participants developed cognitive impairment; thus, the time from baseline examination to the examination at which MCI was first diagnosed was rounded to the nearest year. All models were adjusted for age, sex and education. The first occurrence of MCI was used as the outcome in the initial series of models, and we then repeated these analyses in models requiring MCI to have persisted for at least 1 year (i.e., MCI followed by MCI, dementia, or death). In subsequent models, we tested for interactions of frailty with age, sex, and education and examined several potential confounders of the association of frailty with MCI. Finally, we examined the association of the components of frailty with the risk of MCI and conducted mixed models31 to examine whether the association of physical frailty with cognitive decline varied across cognitive abilities. Models were examined graphically and analytically and assumptions were judged to be adequately met. Programming was done in SAS®32.
RESULTS
Metric Properties of Physical Frailty
The 761 persons included in this study had an average of 6 clinical evaluations (SD=2.3, range: 2–12). Their mean age was 79 years (SD = 7.1, range: 54–100), mean education was 14.5 years (SD = 3.2, range: 0–28), mean Mini-Mental State Examination21 score was 28.4 (SD = 1.7, range: 18–30), and mean global cognitive function score was 0.29 (SD = 0.4, range: −1.2 to 1.4), 76% were women and 89% were white and non-Hispanic. Of the 11% (81) who were racial and ethnic minorities, 28 were Hispanic, 46 were African American, 3 were Native American, and 4 were Asian.
The mean score on the composite measure of physical frailty was −0.09 (SD=0.56, range: −1.71 to 1.89) with higher scores indicating more frailty (poorer physical performance). Physical frailty was positively related to age (r = 0.33, p < 0.0001), negatively related to education (r = −0.23, p < 0.0001) and the global cognitive function score (r = −0.25, p < 0.0001), and men were less frail than women (t [759] = 12.22, p < 0.0001).
Physical Frailty and the Risk of Incident MCI
Over the course of the study, 305 persons (40% of 761) developed MCI. Those who developed MCI were older (t(700)=−6.11, p<0.001), had lower global cognitive function scores (t(727)=9.43, p<0.001), greater disability (t(727)=3.21, p=0.005), and higher levels of physical frailty at baseline (t(727)=−4.71, p<0.001) than those who did not develop MCI (Table 1).
Table 1.
Baseline characteristics of participants who did not versus those who developed MCI.
| Characteristic* | Did not develop MCI N=456 | Developed MCI N=305 | P Value |
|---|---|---|---|
| Age | 77.9, 7.21 | 81.0, 6.30 | <0.001 |
|
| |||
| Gender (% Female) | 76.7% | 74.4% | 0.489 |
|
| |||
| Race (% White, non-Hispanic) | 89.6% | 92.8% | 0.142 |
|
| |||
| Education | 14.5, 2.91 | 14.8, 3.25 | 0.247 |
|
| |||
| Global cognitive function | 0.42, 0.38 | 0.14, 0.40 | <0.001 |
|
| |||
| Physical frailty | −0.19, 0.54 | 0.00, 0.55 | >0.001 |
| Grip strength | −51.43, 19.05 | −47.99, 18.43 | 0.007 |
| Timed walk | 2.59, 1.37 | 3.07, 1.46 | <0.001 |
| BMI | −27.87, 5,43 | −27.27, 4.84 | 0.165 |
| Fatigue | 1.14, 0.28 | 1.18, 0.33 | 0.153 |
|
| |||
| Depressive symptoms | 0.85, 1.39 | 0.92, 1.36 | 0.319 |
|
| |||
| Vascular risk factors | 1.16, 0.85 | 1.10, 0.79 | 0.430 |
| Hypertension (% yes) | 59% | 42% | 0.878 |
| Smoking (% yes) | 41% | 39% | 0.692 |
| Diabetes (% yes) | 15% | 11% | 0.104 |
|
| |||
| Vascular diseases | 0.32, 0.62 | 0.26, 0.51 | 0.539 |
| Claudication (% yes) | 5% | 6% | 0.613 |
| Congestive heart failure (% yes) | 5% | 5% | 0.920 |
| Heart attack (% yes) | 12% | 36% | 0.290 |
| Stroke (% yes) | 9% | 7% | 0.400 |
|
| |||
| Katz Disability | 0.10, 0.48 | 0.19, 0.63 | 0.005 |
| Katz=1 or more (%) | 6% | 11% | 0.005 |
| IADL Disability | 0.60, 0.96 | 0.99, 1.30 | <0.001 |
| IADL=1 or more (%) | 12% | 25% | <0.001 |
|
| |||
| Income | 6.80 (2.52) | 6.40 (2.57) | 0.061 |
Mean values and standard deviations are presented unless otherwise noted and statistical significance is based on t-tests, Wilcoxon rank sum test or Chi-Square tests, as appropriate.
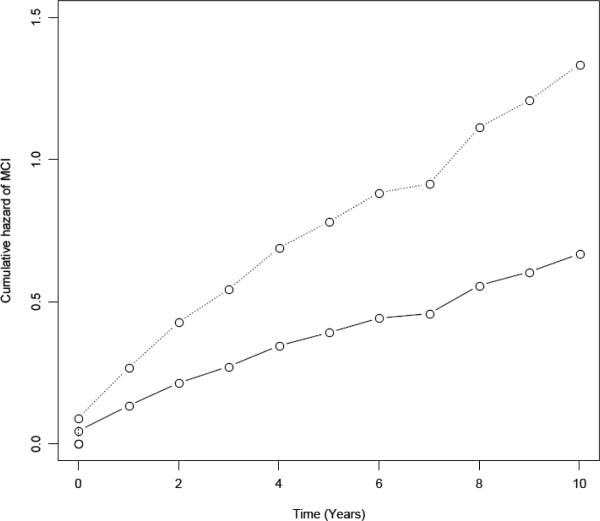
We examined the relation of the baseline level of physical frailty with the risk of developing MCI in a proportional hazards model for discrete (tied) data45,46 adjusted for age, sex, and education. In this core model, physical frailty was associated with a substantial increase in the risk of MCI; that is, each one unit increase in frailty at baseline was associated with more than a 60% increase in the risk of MCI (hazard ratio: 1.63; 95% 95% CI 1.27, 2.08). This is illustrated in Figure 1, which shows that a person with a higher level of frailty at baseline (90th percentile, solid line, score=0.61) had about a 1.1-fold greater risk of developing MCI compared to a person with a lower level of frailty (10th percentile, dotted line, score=−0.81).
Figure 1.
Association of physical frailty with the risk of MCI for a typical participant with a low level of physical frailty (solid line, 10th percentile, score=−0.81) vs. a high level of physical frailty (dotted line, 90th percentile, score=0.61).
Next, because MCI does not uniformly progress to dementia or even persist1–3,5, we constructed a proportional hazards model to examine the association of frailty with persistent MCI (persistent meaning that MCI was present on consecutive examinations or followed by dementia or death). Of 305 persons with incident MCI in the analyses described above, 133 had persistent MCI; in this model, the 172 persons without persistent MCI were included in the reference group. In this analysis, the risk of MCI increased by about 65% for every unit increase in physical frailty at baseline (HR=1.65, 95% CI 1.15, 2.36). Thus, these findings were similar to the results above and suggest that they were not influenced by diagnostic misclassification.
In subsequent models, we repeated the models described above with additional terms to test for interactions of frailty with age, sex, and education and the potential influence of depressive symptoms, disability, vascular risk factors, and vascular diseases on the association of frailty with MCI. The association of frailty with MCI was not influenced by demographic characteristics (data not shown). Further, even in models controlling for all covariates simultaneously, the association of physical frailty with MCI persisted (HR=1.59, 95% CI 1.21, 2.10 for first occurrence of MCI and HR=1.63, 95% CI 1.08, 2.44 for persistent MCI).
Sensitivity analyses
Our definition of MCI differs from that used in some other cohorts in that disability is not taken into account for the diagnosis of MCI. Thus, we conducted a series of sensitivity analyses to examine the potential effect of disability on the association of frailty with MCI (Table 2). First, we repeated the core model controlling for disability on the Katz and IADL measures at baseline (Part 1, Models A and B). Second, we repeated the core model after excluding all persons with disability (defined as scores of 1 or more or 2 or more on the disability measures, respectively; Part 2, Models A–D). Finally, we constructed a proportional hazards model in which we excluded persons who developed disability coincident with the development of MCI; this is essentially the same as requiring the absence of disability for the diagnosis of MCI (Part 3, Models A and B). In these analyses, the association of frailty with MCI persisted and was not substantially reduced, suggesting that it is robust and not strongly influenced by concomitant disability.
Table 2.
Sensitivity analyses examining the potential influence of disability on the association of frailty with MCI
| Analysis (n=# persons included in the analysis) | MCI, first occurrence Hazard ratio and 95% CI | MCI, persistent Hazard ratio and 95% CI |
|---|---|---|
| 1) Controlling for disability at baseline | ||
| A: Katz (n=729) | 1.59 (1.23, 1.28) | 1.65 (1.13,2.42) |
| B: IADL (n=729) | 1.46 (1.04, 1.29) | 1.53 (1.04, 2.24) |
|
| ||
| 2) Excluding persons with disability at baseline | ||
| A: Katz=1 or more (n=670) | 1.58 (1.17, 2.06) | 1.94 (1.29, 2.92) |
| B: Katz=2 or more (n=719) | 1.60 (1.24, 2.06) | 1.66 (1.14, 2.41) |
| C: IADL=1 or more (n=402) | 1.66 (1.08,2.54) | 2.37 (1.25, 4.48) |
| D: IADL=2 or more (n=666) | 1.60 (1.22, 2.11) | 1.88 (1.25, 2.80) |
|
| ||
| 3) Excluding persons who developed disability coincident with MCI | ||
| A: Katz=1 or more (n=729) | 1.87 (1.32, 2.65) | 1.64 (0.98, 2.75) |
| B: IADL=1 or more (n=729) | 2.07 (1.55, 2.77) | 1.98 (1.30, 3.02) |
Components of physical frailty and risk of MCI
Because physical frailty is multidimensional and the associations of its components with MCI may differ1–4, we conducted a series of discrete proportional hazards models adjusted for age, sex, and education to examine the association of the four components used to construct the composite of frailty with the risk of MCI. In these analyses, grip strength and timed walk were associated with the risk of first occurrence of MCI (HR for grip=1.28, 95% CI 1.07, 1.54; HR for timed walk=1.27, 95% CI 1.11, 1.45); BMI and fatigue were not (HR for BMI=1.01, 95% CI 0.89, 1.16; HR for fatigue=1.10, 95% CI 0.98, 1.24). Only grip strength was associated with persistent MCI (HR=1.34, 95% CI 1.02, 1.75).
Physical frailty and the rate of change in cognitive function
Because the principal manifestation of MCI is cognitive impairment that develops slowly over time, to examine further the robustness of the association of physical frailty with MCI we examined the relation of physical frailty with the rate of change in cognitive function in a series of mixed-effect models controlled for age, sex, and education. In these models, a higher level of physical frailty at baseline was associated with a lower level of function in global cognition, as indicated by the term for physical frailty (Table 3). In addition, with this baseline effect controlled for, a higher level of physical frailty was associated with a more rapid rate of decline in global cognition, as indicated by the term for physical frailty × time (Table 3). Additional models examined five separate cognitive abilities starting with episodic memory, the hallmark of AD, and four other measures including semantic memory, working memory, perceptual speed, and visuospatial ability (Table 2). Physical frailty was associated with a more rapid rate of decline in all five systems.
Table 3.
Relation of physical frailty with change in cognitive function
| Cognitive Measure | Model Term | Estimate (SE) | P-Value |
|---|---|---|---|
| Global cognition | Physical frailty | −0.085 (0.03) | 0.003 |
| Physical frailty × time | −0.038 (0.01) | <0.001 | |
|
| |||
| Episodic memory | Physical frailty | −0.054 (0.03) | 0.092 |
| Physical frailty × time | −0.039 (0.01) | 0.001 | |
|
| |||
| Semantic memory | Physical frailty | −0.061 (0.04) | 0.125 |
| Physical frailty × time | −0.026 (0.01) | 0.004 | |
|
| |||
| Working memory | Physical frailty | −0.065 (0.05) | 0.190 |
| Physical frailty × time | −0.033 (0.01) | 0.003 | |
|
| |||
| Perceptual speed | Physical frailty | −0.175 (0.05) | <0.001 |
| Physical frailty × time | −0.034 (0.01) | 0.002 | |
|
| |||
| Visuospatial ability | Physical frailty | −0.076 (0.05) | 0.091 |
| Physical frailty × time | −0.038 (0.01) | 0.003 | |
Derived from models that included terms for age, sex, and education, time, time-squared, and the interactions of the demographic variables with time.
DISCUSSION
In a cohort of more than 750 well-characterized older persons free of cognitive impairment at baseline, we found that physical frailty was associated with an increased risk of developing MCI, whether defined as the first occurrence of or persistent MCI. This association remained in analyses that controlled for depressive symptoms, disability, vascular risk factors and diseases. Further, physical frailty was associated with an increased rate of decline in global cognition and five specific cognitive systems. These findings demonstrate that a higher level of physical frailty predicts the development of MCI and is associated with an accelerated rate of cognitive decline in older persons. Together with prior studies showing an association between frailty, clinical AD and AD pathology, these data may suggest that physical frailty and cognitive impairment share a common underlying pathogenesis.
Importantly, little is known regarding the factors associated with the development of MCI. Research examining the determinants of MCI is challenging because MCI develops slowly over many years and incidence studies require large samples of persons without cognitive impairment in whom data from multiple years of observation are available. The finding that frailty predicts MCI and cognitive decline has important public health implications. Public policy and aging research are increasingly focusing on the development of strategies to maintain cognitive health and vitality in aging12. MCI represents the transition state between normality and dementia and may in fact be much more common than AD47. Moreover, by the time older persons meet criteria for MCI, they are already experiencing cognitive decline, the core feature of AD, and often are accumulating the neuropathologic hallmarks of AD3,5–7. As new and more effective treatments to prevent or delay the onset of dementia are developed, it will be essential to be able to identify persons who are not yet exhibiting cognitive impairment1–4,33 but who are at increased risk. The current study extends the limited prior work on frailty and cognitive decline33 and suggests that measures of physical frailty may help identify those persons likely to develop cognitive impairment and who are most likely to benefit from interventions to maintain cognitive function.
In this study, the frailty components of grip strength and timed walk were most strongly associated with MCI. Idiopathic decline in motor function is a familiar consequence of aging, with older persons displaying a wide spectrum of loss of muscle strength, muscle bulk, and walking speed34. These deficits are subsumed under several constructs including physical frailty, sarcopenia, and parkinsonism and there is now considerable evidence showing that idiopathic decline in motor function is common in old age and precedes and predicts a wide range of important health and cognitive outcomes, including death, disability, MCI and AD35–38. It is possible that specific aspects of motor function have particular prognostic implications.
Although physical frailty may represent a true risk factor for cognitive impairment, it is noteworthy that the biologic basis of the association remains unknown. We suspect that physical frailty, MCI and AD may share an underlying pathogenesis. For example, several factors that are related to physical frailty also are related to cognitive impairment, including inflammatory markers, diabetes, congestive heart failure, and stroke39–42. Thus, physical frailty may result in part from disorders of the central nervous system (e.g., stroke, neurodegenerative diseases), some of which may also unmask subclinical AD. In this study, the association between frailty and MCI persisted in analyses controlling for vascular risk factors and diseases; however, many of our vascular measures were determined by self-report. Given that vascular findings are common and have functional consequences in older persons, further investigation of the influence of vascular factors on cognitive-physical relationships is warranted. Further, although we did not examine this issue directly in this study, it is possible that AD pathology underlies (to some degree) the association of frailty with cognitive impairment. AD pathology is widespread in persons with MCI and even some without cognitive impairment, and this pathology is associated with motor dysfunction as well as cognitive impairment6,7,19,43,44. We previously reported that AD pathology was associated with physical frailty in older persons with and without dementia from the same cohort; notably, however, AD pathology accounted for only a small percentage of the variance in frailty even in analyses that included persons with clinical dementia19. Given that it is very likely that non-frail and non-demented persons have AD pathology and that persons with clinically diagnosed cognitive impairment have co-morbid non-AD pathologies46, other mechanisms must also be important. Some other less well-studied but potential mechanisms may include decreased energy production or metabolic issues and stress. Future studies are needed to explicate the biologic basis of the association between physical frailty and cognitive impairment in old age.
Notably, there is ongoing debate regarding the optimal approach to the classification of MCI and the potential role of disability and/or executive impairment with associated functional limitations48,49. In this study, persons were diagnosed with MCI if they were determined to have cognitive impairment but did not meet criteria for dementia. The absence of disability was not required, although the absence of disability frequently is cited as a pre-requisite for MCI case finding49. Thus, the criteria employed for the diagnosis of MCI in this cohort are equivalent to those used for a diagnosis of cognitive impairment, no dementia (i.e., CIND) in some other cohorts24. It is likely that the use of more restrictive criteria would have captured fewer persons than included in this study; for example, in a study that examined the prevalence and course of amnestic MCI in community-based persons using criteria that required intact instrumental activities of daily living, only about 3–4% of those studied met criteria for MCI48. It is also possible that more restrictive criteria would have yielded different results. The criteria used here and have been validated in several prior publications using data from this and other cohorts and this definition of MCI is associated with the subsequent development of dementia, particularly AD, and an increased rate of cognitive decline4–7, 24,25. Further, in this study, we examined the association of frailty with both the first occurrence and persistence of MCI and conducted a series of sensitivity analyses that showed that the association of MCI with frailty was robust and persisted even after controlling for the baseline level of disability or excluding persons with disability either at baseline or at the time of MCI diagnosis.
This study has some limitations, including the selected nature of the cohort, the relatively short duration of follow-up and the need to exclude persons with cognitive impairment (i.e., dementia and MCI) at baseline, as well as those who were not yet eligible for follow-up from analyses, which may have affected the results. Further, participants were from a study that requires older persons to agree to organ donation at death, thereby introducing selection bias. Finally, some of the individual components of frailty, such as fatigue, were assessed fairly crudely and this may have resulted in an underestimation of the association of physical frailty with MCI. However, several factors lend confidence in the findings from this study, including the use of a composite measure of frailty and examination of frailty and incident MCI (first occurrence and persistent) using uniform structured procedures in a large number of well-characterized older persons free of dementia and MCI at baseline and with high rates of annual follow-up.
ACKNOWLEDGMENTS
We are indebted to the participants and the staff of the Rush Memory and Aging Project and the Rush Alzheimer's Disease Center for this work. The study was supported by NIA grants R01AG17917, R01AG024480, and K23AG023040.
Sponsor's Role: None
Funding: This research was supported by National Institute on Aging grants K23AG23040, R01AG17917, R01AG24480, the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund.
Footnotes
Author Contributions: Drs. Boyle and Buchman participated in the study conceptualization, data analysis, interpretation, and drafting of this manuscript. Drs. Bennett and Wilson participated in study conceptualization, study design, data acquisition and interpretation of findings, and critical review of this manuscript. Dr. Leurgans participated in the data analysis and interpretation and critical review of the manuscript.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
REFERENCES
- 1.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 2.Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. doi: 10.1212/01.wnl.0000089238.07771.c7. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease. Arch Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 5.Boyle PA, Wilson RS, Aggarwal NT, et al. Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 8.Reitz C, Tang MX, Manly J, et al. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64:1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RS, Schneider JA, Arnold SE, et al. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 10.Solfrizzi V, Panza F, Colacicco AM, et al. Italian Longitudinal Study on Aging Working Group. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Kuh D. A life course approach to healthy aging, frailty, and capability. J Gerontol A Biol Sci Med Sci. 2007;62A:717–721. doi: 10.1093/gerona/62.7.717. [DOI] [PubMed] [Google Scholar]
- 13.Montero-Odasso M, Bergman H, Béland F, et al. Identifying mobility heterogeneity in very frail older adults. Are frail people all the same? Arch Gerontol Geriatr. 2009 Sep–Oct;49(2):272–7. doi: 10.1016/j.archger.2008.09.010. Epub 2008 Nov 4. [DOI] [PubMed] [Google Scholar]
- 14.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 17.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59:M627–632. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 18.Buchman AS, Boyle PA, Wilson RS, et al. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med. 2007;69:483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 19.Buchman AS, Schneider JA, Leurgans S, et al. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71:499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 21.Folstein M, Folstein S, McHugh P. Mini-Mental State: a practical method for grading the mental state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Tuokko H, Frerichs R, Graham J, et al. Five-year follow-up of cognitive impairment with no dementia. Arch Neurol. 2003;60:577–582. doi: 10.1001/archneur.60.4.577. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal NT, Wilson RS, Beck TL, et al. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63:1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 26.Buchman AS, Wilson RS, Bienias JL, et al. Change in frailty and risk of death in older persons. Exp Aging Res. 2009;35:61–82. doi: 10.1080/03610730802545051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 30.Cox DR. Regression models and life tables (with discussion) J Soc Stat Soc B. 1972;74:187–220. [Google Scholar]
- 31.Laird N, Waire J. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 32.SAS Institute Inc . SAS 9.1.3 Help and Documentation. SAS Institute Inc; Cary, NC: 2000. [Google Scholar]
- 33.Samper-Ternent R, Al Snih S, Raji MA, et al. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc. 2008;56:1845–1852. doi: 10.1111/j.1532-5415.2008.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camicioli R, Wang Y, Powell C, et al. Gait and posture impairment, parkinsonism and cognitive decline in older people. J Neural Transm. 2007;114:1355–1361. doi: 10.1007/s00702-007-0778-5. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell SL, Rockwood K. The association between parkinsonism, Alzheimer's disease, and mortality: A comprehensive approach. J Am Geriatr Soc. 2000;48:422–425. doi: 10.1111/j.1532-5415.2000.tb04701.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Larson EB, Bowen JD, et al. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 37.Buchman AS, Wilson RS, Bienias JL, et al. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 38.Waite LM, Grayson DA, Piguet O, et al. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J Neurol Sci. 2005;229–230:89–93. doi: 10.1016/j.jns.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 40.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 41.Puts MT, Visser M, Twisk JW, et al. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63:403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 42.Weaver JD, Huang MH, Albert M, et al. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 43.Schneider JA, Li JL, Li Y, et al. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 44.Buchman AS, Schneider JA, Wilson RS, et al. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 45.Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 46.Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;11(69):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 47.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganguli M, Dodge HH, Shen C, et al. Mild cognitive impairment, amnestic type: An epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 49.Royall DR. Mild cognitive impairment and functional status. J Am Geriatr Soc. 2006;54:163–165. doi: 10.1111/j.1532-5415.2005.00539.x. [DOI] [PubMed] [Google Scholar]