Abstract
High sensation seeking has been linked to increased risk for drug abuse and other negative behavioral outcomes. This study explored the neurobiological basis of this personality trait using functional magnetic resonance imaging (fMRI). High sensation seekers (HSSs) and low sensation seekers (LSSs) viewed high- and low-arousal pictures. Comparison of the groups revealed that HSSs showed stronger fMRI responses to high-arousal stimuli in brain regions associated with arousal and reinforcement (right insula, posterior medial orbitofrontal cortex), whereas LSSs showed greater activation and earlier onset of fMRI responses to high-arousal stimuli in regions involved in emotional regulation (anterior medial orbitofrontal cortex, anterior cingulate). Furthermore, fMRI response in anterior medial orbitofrontal cortex and anterior cingulate was negatively correlated with urgency. Finally, LSSs showed greater sensitivity to the valence of the stimuli than did HSSs. These distinct neurobiological profiles suggest that HSSs exhibit neural responses consistent with an overactive approach system, whereas LSSs exhibit responses consistent with a stronger inhibitory system.
Sensation seeking is a multifaceted personality trait with components that include experience seeking, thrill and adventure seeking, disinhibition, and susceptibility to boredom (Zuckerman, 1994, 2005). High sensation seekers (HSSs) have a tendency to seek out and engage in novel and varied experiences—even if those experiences involve significant risk. Consequently, compared with low sensation seekers (LSSs), HSSs are more vulnerable to drug abuse and are more likely to engage in risky sexual behaviors or excessive gambling (Bardo, Donohew, & Harrington, 1996; Roberti, 2004).
The neurobiological basis of sensation seeking has been explored in both humans and nonhumans (Bardo et al., 1996; Zuckerman, 2005). Compared with LSSs, HSSs show enhanced neurobiological responses to intense and novel stimuli. For example, in humans, HSSs show a stronger orienting response to the presentation of a new stimulus, as well as greater cortical arousal in response to intense visual or auditory stimuli, than LSSs do, whereas LSSs show cortical inhibition in response to intense stimuli, especially at the highest levels of stimulus intensity (Zuckerman, 2005). HSSs also show a preference for and stronger skin conductance responses to sexually explicit and violent stimuli (Smith, Davidson, Perlstein, & Gonzalez, 1990). In addition, HSSs show a blunted cortisol response to stressors, relative to LSSs (Roberti, 2004). Taken together, the evidence suggests that HSSs show hypersensitivity to intense stimuli, but reduced sensitivity to stressors; this pattern, in turn, suggests that HSSs have a stronger appetitive-approach system and a weaker avoidance-withdrawal system than do LSSs (Depue & Collins, 1999; A. Lang, Shin, & Lee, 2005).
However, there is virtually no research examining the specific brain systems supporting the observed behavioral and peripheral physiological differences related to sensation-seeking status. Whether these differences can be attributed to brain systems regulating approach and avoidance remains an open question. Thus far, only a single functional magnetic resonance imaging (fMRI) study has explored the neurobiological basis of sensation seeking (Abler, Walter, Erk, Kammerer, & Spitzer, 2006). This particular study employed a monetary incentive task, which, although potentially useful for examining reward sensitivity, may be less well suited to examining preference for arousal (Zuckerman & Como, 1983) and strong reactivity to intense stimuli (Zuckerman, 1994). Emotional-induction tasks that use high- and low-arousal pictorial stimuli seem better suited to tap into these facets of sensation seeking. We used emotional induction and fMRI to explore the brain basis of emotional reactivity in HSSs and LSSs, because the emotion-induction task is widely used to study neurobiological and physiological arousal and emotional responses (Bradley, Codispoti, Cuthbert, & Lang, 2001; P.J. Lang et al., 1998).
HSSs and LSSs viewed photographs from the International Affective Picture System (IAPS; P.J. Lang, Bradley, & Cuthbert, that were characterized as high- or low-arousal pictures. Given prior evidence that HSSs, compared with LSSs, show greater reactivity to intense stimuli (Zuckerman, 2005), we expected that when viewing highly arousing and intense emotional stimuli, HSSs would exhibit stronger fMRI responses than LSSs in brain regions associated with autonomic arousal (e.g., the insula); such a pattern of results would be consistent with an overactive approach system in HSSs. We also expected that brain regions associated with emotional regulation and cognitive control, such as the anterior cingulate cortex, would be less strongly activated in HSSs than in LSSs, which would be consistent with a weaker avoidance-inhibitory system in HSSs.
METHOD
Participants
Participants were healthy adults, ages 18 through 25, whose scores on the Brief Sensation-Seeking Scale (Hoyle, Stephenson, Palmgreen, Lorch, & Donohew, 2002) placed them in the top (HSSs; n = 20; 10 males, 10 females) and bottom (LSSs; n = 20; 10 males, 10 females) quartiles of population-based norms. Exclusion criteria included the presence of metal in or on the body, pregnancy, a prior neurological or psychiatric diagnosis, learning disability, a history of substance abuse or smoking, current use of central nervous system medications, left-handedness (Oldfield, 1971), and poor vision that could not be corrected. Pregnancy and drug use were assessed prior to sessions via urinalysis. Participants also completed the Big-Five Inventory (BFI; John & Srivastava, 1999), the UPPS (Urgency, Premeditation, Perseverance, and Sensation Seeking) Impulsivity Scale (Whiteside & Lynam, 2001), the Eysenck Personality Inventory (EPI; Eysenck & Eysenck, 1975), the Zuckerman Sensation-Seeking Scale (Form V; Zuckerman, 1994), and the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ; Zuckerman, Kuhlman, Joireman, Teta, & Kraft, 1993).
Prior to making a final decision regarding participation, participants were given the option of viewing sample pictures that were not used during testing. All individuals provided informed consent in accord with the University of Kentucky Medical Institutional Review Board and received financial compensation.
Design and Stimuli
Two hundred images from the IAPS (P.J. Lang et al., 1999) were used—100 images from the upper 40% of arousal ratings (high- arousal images) and 100 images from the lower 40% (low- arousal images). Within each arousal category, pictures were divided equally into positive and negative valence groups (on the basis of the median valence rating within each arousal level). High-arousal stimuli depicted nudity, erotica, extreme sports, violence, bodily mutilation, insects, and snakes. Low-arousal stimuli included pictures of ordinary scenes, objects, people, and food. The high-arousal pictures had higher arousal ratings (M = 6.04, SE = 0.06) than the low-arousal pictures (M = 3.52, SE = 0.06), as confirmed with analysis of variance (ANOVA), F(1, 196) = 804.6, p < .0001. The positive pictures had higher valence ratings (M = 6.47, SE = 0.09) than the negative pictures (M = 3.5, SE = 0.09), F(1, 196) = 591.7, p < .0001. However, the Valence × Arousal interaction was significant for the valence ratings, F(1, 196) = 50.8, p < .0001; among high-arousal stimuli, positive pictures had higher valence ratings (M = 6.31, SE = 0.12) than negative pictures (M = 2.46, SE = 0.12), but this difference was not as pronounced for low-arousal stimuli (positive: M = 6.63, SE = 0.12; negative: M = 4.53, SE = 0.12). This outcome reflects an inherent property of the IAPS (P.J. Lang et al., 1999) and does not represent anything unusual about the stimuli selected for the present study. Because the Arousal × Valence interaction for valence ratings indicated that the valence manipulation was stronger for high-arousal pictures, we confined fMRI analyses of valence to the high-arousal condition only.
Procedure
Prior to the fMRI session, participants practiced the tasks outside of the MRI scanner for about 30 min. Once in the scanner, subjects were instructed to refrain from moving even if they felt startled by the pictures. Compliance with this request was high, and no data were excluded because of excessive head motion (defined as maximal absolute displacement larger than 1.7 mm). During scanning, participants viewed a series of the IAPS pictures and pressed a button using their right index finger when an image was displayed. Participants were instructed to view each picture even if the picture was aversive. Each picture appeared for 1 s and was followed by a fixation cross; the intertrial interval was 2.5 s. The onset of each picture was randomly sampled from one of three jitters (100, 300, or 500 ms), to minimize anticipation of the stimuli. The 250 trials (200 images and 50 visual-fixation trials that did not require a response) were randomly mixed according to the scripts for stimulus timing simulation using programs of the AFNI (Analysis of Functional Neuro-Images) software package (http://afni.nimh.nih.gov).
Data Acquisition
A 3-T Siemens Trio MRI system equipped for echoplanar imaging (repetition time = 2,500 ms, echo time = 30 ms, flip angle = 80°; 40 axial slices: matrix = 64 × 64, 3.5-mm3 resolution) was used. A high-resolution T1-weighted MP-RAGE (magnetization-prepared rapid gradient echo) anatomical volume (192 sagittal slices, matrix = 224 × 256, field of view = 224 × 256 mm2, slice thickness = 1 mm, no gap) was also collected for each participant. Stimuli were presented using a high-resolution rear-projection system (Avotec, Stuart, FL), and responses were recorded using a fiber-optic response pad (MRA Inc., Washington, PA). A computer running E-Prime (Version 1.1 SP3, Psychology Software Tools, Pittsburgh, PA) controlled stimulus presentation, which was synchronized with trigger pulses from the magnet.
Data Analysis
Prior to statistical analysis, the first four volumes of each run were discarded to allow the MR signal to reach steady state. The remaining images in each participant’s time series were motion-corrected, spatially smoothed with a 3-D Gaussian kernel (full width at half maximum = 7 × 7 × 7 mm3), and high-pass filtered using FSL, the Software Library of the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB; Version 3.1; http://www.fmrib.ox.ac.uk/fsl).
Customized square waveforms for each participant were generated for the specific counterbalanced order of experimental conditions and then convolved with a double-gamma hemodynamic response function. For each participant, hemodynamic parameters for the different explanatory variables (e.g., high arousal and low arousal) were estimated, and statistical maps of interest were generated for the contrasts across these explanatory variables. The contrast maps from individual subjects were warped into common stereotaxic space before mixed-effects group analyses were performed using the FMRIB’s Local Analysis of Mixed Effects module to obtain the group mean of brain activation.
Region-of-Interest Analysis
For nearly all contrasts, clusters were defined as 10 or more contiguous voxels (Xiong, Gao, Lancaster, & Fox, 1995) with z scores above 3.29 (p < .001, two-tailed); the one exception was that in the contrast of negative versus positive high-arousal items, the z-score cutoff was 2.81 (p < .005, two-tailed). Local peaks within each cluster were identified (Mintun, Fox, & Raichle, 1989). For larger clusters that covered adjacent but distinct anatomical regions (e.g., hippocampus, amygdala, and insula), anatomical masks were derived from the anatomical automatic label image packaged with MRIcro (Rorden & Brett, 2000). Customized MATLAB (Mathworks Inc., Natick, MA) codes were used to selectively average raw image intensity values elicited in each of the arousal conditions with regard to the fixation baseline. Raw intensity values were computed over the range of the hemodynamic response function (20 s) within the clusters (one average for each 2.5-s time window).
Three-way ANOVAs were conducted for each cluster to examine the effects of arousal (high, low), valence (positive, negative), and sensation seeking (HSS, LSS) on fMRI signal relative to the fixation baseline. The effects of arousal and valence were included to isolate any potential interactions between arousal and valence and between either arousal or valence and sensation seeking because the voxel-wise contrast did not test for such interactions. These initial ANOVAs were based on the average hemodynamic response in the 2.5-s to 7.5-s time window because ANOVAs conducted separately at each time point (from 2.5 to 15 s) did not reveal effects of sensation seeking or interactions outside this window. Clusters that showed a main effect of sensation seeking or an interactive effect involving sensation seeking (Table 1) served as regions of interest (ROIs) in additional analyses.
TABLE 1.
Results From the Voxel-Wise Analyses: Brain Regions Showing Effects of Arousal and Valence
| Region | No. of voxels | Coordinates
|
BA | Significant effects and interactions | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Greater response to HA than LA stimuli in HSSs (p < .001, two-tailed) | ||||||
| Right hippocampus | 454 | 20 | −6 | −14 | 34 | |
| Right insula | 36 | 20 | −16 | SS × Arousal* | ||
| Left hippocampus | 962 | −22 | −28 | −8 | ||
| Left insula | −34 | 12 | −16 | SS × Arousal* | ||
| Left posterior medial OFC | 33 | −4 | 34 | −14 | 11 | SS × Arousal† |
|
| ||||||
| Greater response to HA than LA stimuli in LSSs (p < .001, two-tailed) | ||||||
| Anterior medial OFC | 18 | 0 | 58 | −12 | 11 | SS × Arousal* |
| Left anterior cingulate | 28 | −10 | 44 | 10 | 32 | SS† |
|
| ||||||
| Difference between HA and LA response greater for HSSs than for LSSs (p < .001, two-tailed) | ||||||
| Right insula/IFG | 24 | 40 | 16 | 10 | SS × Arousal* | |
|
| ||||||
| Greater response to negative than positive HA stimuli in LSSs (p < .005, two-tailed) | ||||||
| Right putamen | 41 | 30 | −12 | 0 | SS × Valence* | |
|
| ||||||
| Greater response to positive than negative HA stimuli in LSSs (p < .001, two-tailed) | ||||||
| Left putamen | 49 | −22 | 20 | 4 | SS × Valence × Arousal* | |
Note. The first two panels present results comparing responses to high-arousal (HA) and low-arousal (LA) stimuli in high sensation seekers (HSSs) and low sensation seekers (LSSs), the third panel presents results comparing differential response to HA and LA stimuli in HSSs and LSSs, and the last two panels present results comparing responses to positive and negative HA stimuli in LSSs. BA = Brodmann’s area; IFG = inferior frontal gyrus; OFC = orbitofrontal cortex; SS = sensation seeking.
p = .077.
p < .05.
These ANOVAs were conducted for two main reasons. First, the voxel-wise analyses only isolated regions that were differentially sensitive to arousal level (i.e., high-arousal vs. low-arousal conditions) or valence within each sensation-seeking group or in the group comparison, but did not consider the fMRI response relative to the fixation baseline. The ROI analyses used fMRI signal for each arousal condition relative to the fixation baseline, rather than fMRI signal in the high-arousal condition relative to the low-arousal condition. The assumption was that fixation-baseline response would be less influenced by sensation-seeking status than the response to low-arousal stimuli would be (see Zuckerman, 2005). Second, a differential response to arousal or valence level in the voxel-wise analysis of a region would not necessarily imply that the region was activated more than during the baseline. A region could show a negative signal change relative to the fixation baseline even if it responded differentially to high-arousal versus low-arousal stimuli or to positive versus negative high-arousal stimuli. ROIs that showed a negative signal change for all conditions were not interpreted further.1
In addition to conducting these ANOVAs, within each ROI, we conducted one multiple regression analysis for each personality variable that was normally distributed within our sample (i.e., EPI extraversion score, all BFI and UPPS sub-scales, Form V Total and all Form V subscales except Thrill and Adventure Seeking from the Zuckerman Sensation-Seeking Scale). Normality was defined as a skewness value less than 2 times the standard error. Percentage signal change (e.g., between the high- and low-arousal conditions) was the outcome variable, and age, sex, education, sensation-seeking group (dummy-coded), and the personality-measure score were the predictor variables. By using sensation-seeking group as a control variable, these regression analyses revealed what influence these other personality variables had on fMRI signal, beyond the influence of sensation seeking. Finally, we used scores on the ZKPQ Sensation Seeking scale (which was also normally distributed) as a convergent measure of sensation seeking and calculated its correlation with differential activation for high-versus low-arousal stimuli in each ROI.
RESULTS
Arousal Analyses
Voxel-wise analyses isolated brain regions associated with differential sensitivity to high-arousal stimuli (see Table 1). The first analysis compared brain activation for high- versus low- arousal stimuli separately within each sensation-seeking group to assess sensitivity to high-arousal stimuli. The baseline level of cortical arousal is not expected to vary with sensation seeking (Zuckerman, 2005). However, should differences in baseline level of arousal exist, this within-groups analysis would not confound differential sensitivity to high-arousal stimuli with baseline differences in response to low-arousal stimuli. In this first analysis, both HSSs and LSSs showed strong fMRI responses to high-arousal stimuli. In addition to the regions highlighted in Table 1, this analysis revealed activation in the bilateral temporal pole and thalamus for HSSs. The reverse contrast of low-arousal pictures versus high-arousal pictures did not yield any clusters of activation when the same statistical threshold was used. This outcome was expected, given previous findings that the low-arousal stimuli from the IAPS produce less potent autonomic and electroencephalographic responses than the high-arousal stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000).
A second voxel-wise between-groups analysis compared the two sensation-seeking groups directly to examine differences in sensitivity to high-arousal versus low-arousal stimuli. This analysis revealed that the right anterior insula/inferior frontal gyrus (IFG) was more strongly activated in HSSs than in LSSs.
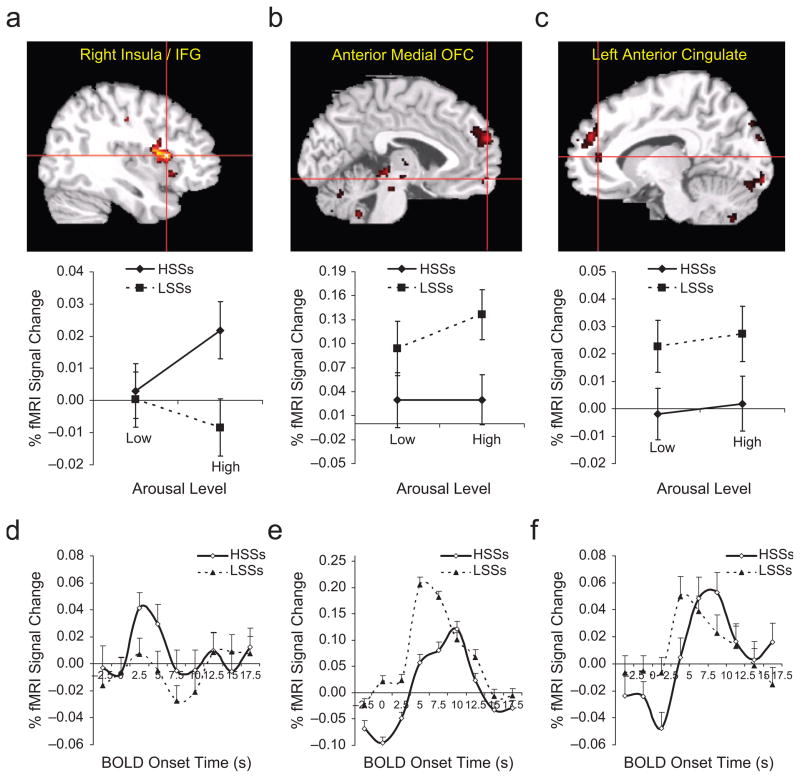
Figure 1 illustrates three of the brain regions showing significant differential effects of, or interactions with, sensation seeking, as confirmed by the three-way ANOVAs described earlier. These regions are highlighted because they have been consistently implicated in emotional processing and individual differences in other brain-imaging studies (Cunningham, Raye, & Johnson, 2005; Eisenberger, Lieberman, & Satpute, 2005; Kringelbach & Rolls, 2004; Paulus, Rogalsky, Simmons, Feinstein, & Stein, 2003; Suhara et al., 2001). As expected, the ANOVAs revealed main effects for arousal in all of the regions listed in Table 1; most of these regions showed a Sensation Seeking × Arousal interaction, and one region showed a main effect of sensation seeking. None of these regions showed interactions between sensation seeking and valence. Some of these regions also showed correlations with the personality measure of urgency (see Table 2).
Fig. 1.
Brain regions isolated from the contrast of high-arousal versus low-arousal stimuli and the time course of functional magnetic resonance imaging (fMRI) signal change in those regions. In the case of the region on the border of the right insula and inferior frontal gyrus (IFG; a), high sensation seekers (HSSs) showed greater activation than low sensation seekers (LSSs), whereas in the case of the anterior medial orbitofrontal cortex (OFC; b) and left anterior cingulate (c), LSSs showed greater activation than HSSs. In the top row, the red crosshair in each brain map indicates the peak of the corresponding cluster in Table 1. The graph below each map shows the percentage fMRI signal change from the resting baseline condition for each sensation-seeking group as a function of arousal condition. Each graph in the bottom row (d–f) shows hemodynamic response functions over a 20-s time window in the brain region in the illustration above. The fMRI response for high-arousal stimuli relative to the resting baseline is plotted separately for HSSs and LSSs. Error bars represent standard errors of the means. BOLD = blood-oxygenation-level-dependent.
TABLE 2.
Results From Multiple Regression Analyses: Brain Regions Showing an Association Between Personality Measures and Functional Magnetic Resonance Imaging Signal
| Region | Coordinates
|
Regressor | b | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Greater response to HA than LA stimuli in HSSs | |||||
| Right insula | 36 | 20 | −16 | Urgency | −0.364* |
|
| |||||
| Greater response to HA than LA stimuli in LSSs | |||||
| Anterior medial OFC | 0 | 58 | −12 | Urgency | −0.413* |
| Left anterior cingulate | −10 | 44 | 10 | Urgency | −0.566* |
|
| |||||
| Greater response to positive than negative HA stimuli in LSSs | |||||
| Left putamen | −22 | 20 | 4 | Disinhibition | −0.328* |
Note. Results are shown for regions showing greater response to high-arousal (HA) than to low-arousal (LA) stimuli in high sensation seekers (HSSs) and low sensation seekers (LSSs) and for a region showing greater response to positive than to negative HA stimuli in LSSs. OFC = orbitofrontal cortex.
p < .05.
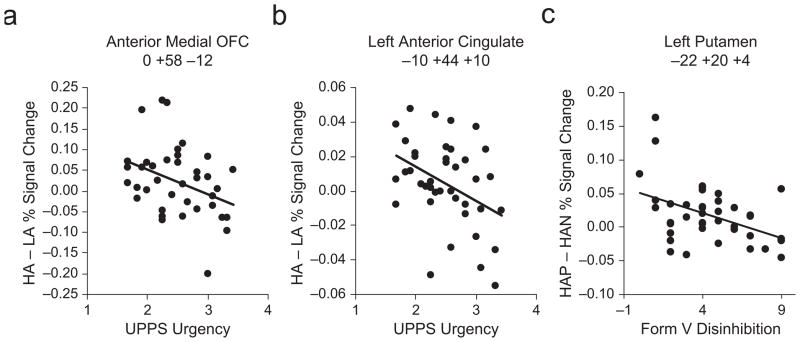
In the right insula/IFG, HSSs showed a greater response to high-arousal than to low-arousal pictures, whereas LSSs showed minimal differential response to high-arousal and low-arousal pictures (Fig. 1a). However, no personality variables (beyond sensation seeking) were associated with fMRI signal in this region. The correlation with the ZKPQ Sensation Seeking scale was positive and significant (r = +.56, p < .0001). In the anterior medial orbitofrontal cortex (OFC), LSSs showed more pronounced activation in the high-arousal condition than in the low-arousal condition, whereas HSSs did not (Fig. 1b). In this region, lower urgency scores (UPPS) predicted greater fMRI signal change (Fig. 2a, Table 2). In the left anterior cingulate cortex (Fig. 1c), LSSs showed marginally stronger activation than did HSSs, regardless of arousal level, and lower urgency scores also predicted greater fMRI signal change (Fig. 2b, Table 2). The correlation with the ZKPQ Sensation Seeking scale was not significant in either of these two regions (p > .17). The posterior medial orbitofrontal cortex region (not included in Fig. 1) showed a response similar to that of the right insula/IFG region in Figure 1a, with greater activation in response to high-arousal stimuli among HSSs than among LSSs, but the interaction of arousal and sensation seeking was only marginally significant.
Fig. 2.
Scatter plots illustrating the association between brain activation and personality traits. For the anterior medial orbitofrontal cortex (OFC; a) and left anterior cingulate (b) regions activated more strongly for high-arousal than for low-arousal stimuli in low sensation seekers, functional magnetic resonance (fMRI) signal change for high-arousal (HA) versus low-arousal (LA) pictures is plotted as a function of urgency (score on the UPPS Impulsivity Scale; Whiteside & Lynam, 2001). For the left putamen region activated more strongly for positive than for negative high-arousal stimuli in low sensation seekers (c), fMRI signal change for positive high-arousal pictures (HAP) versus negative high-arousal pictures (HAN) is plotted as a function of disinhibition (measured by Form V of the Zuckerman Sensation-Seeking Scale; Zuckerman, 1994). Results for both high and low sensation seekers are plotted in all three graphs. The Montreal Neurological Institute coordinates for each region are shown at the top of the figure. See Table 2 for details on the corresponding regression coefficients.
The time course of the fMRI signal in these three regions (as well as the posterior medial OFC) was examined for group differences in latency and amplitude of the peak fMRI response to high-arousal stimuli relative to baseline. In the right insula/IFG, the peak response was higher in magnitude for HSSs than for LSSs (Fig. 1d). In the left anterior cingulate cortex and anterior medial OFC, the peak response for high-arousal stimuli occurred earlier in LSSs than in HSSs (Figs. 1e and 1f). In addition, the response in the anterior medial OFC was more robust in LSSs.
Valence Analyses
Voxel-wise analyses were conducted to isolate brain regions associated with differential sensitivity to positive versus negative high-arousal stimuli. In LSSs, the right putamen showed greater sensitivity to negative than to positive high-arousal stimuli, whereas the left putamen showed greater sensitivity to positive than to negative high-arousal stimuli (Table 1). HSSs did not show significant differential activation to positive versus negative high-arousal stimuli. In the left putamen, lower Disinhibition scores on the Zuckerman Sensation-Seeking Scale Form V predicted greater fMRI sensitivity to valence (Fig. 2c, Table 2), and the correlation with the ZKPQ Sensation Seeking scale was significant and negative (r = −.437, p < .005).
DISCUSSION
This is the first fMRI study to characterize the neurobiological profile of emotional arousal and reactivity in sensation seeking. Previous research has shown that HSSs, compared with LSSs, show greater reactivity to intense stimuli (Smith et al., 1990; Zuckerman, 2005) and weaker avoidance responses to stressors (Lissek et al., 2005; Roberti, 2004). Such outcomes may be understood within a framework in which HSSs tend to have overactive approach systems and dampened avoidance systems (A. Lang et al., 2005). The present findings are consistent with this framework in that fMRI responses to high-arousal stimuli were stronger in HSSs than in LSSs in brain regions associated with arousal and reinforcement, including the insula and posterior medial OFC. In contrast, HSSs did not activate regions involved in emotional regulation, behavioral monitoring, and decision making (e.g., anterior cingulate and anterior medial OFC) as strongly or as early as LSSs did. Moreover, HSSs were not as sensitive to the valence of the stimuli as were LSSs. The propensity for arousal in HSSs may override the valence or content of the arousing material. These differences between the sensation-seeking groups may reflect a stronger arousal focus in HSSs and a stronger valence focus in LSSs (Feldman, 1995). The present study not only has outlined a core network of brain regions involved in emotional reactivity and regulation, but also has demonstrated that these components are differentially modulated by sensation seeking and related personality dimensions.
HSSs strongly activated brain regions involved in reinforcement (posterior medial OFC) and arousal (right anterior insula). In response to high-arousal stimuli, HSSs activated the posterior medial OFC more strongly than did LSSs, but the Arousal × Sensation Seeking interaction was only marginally significant. This region is often associated with the presentation of primary reinforcers, such as taste and pain, regardless of their reward value (Kringelbach & Rolls, 2004). The right anterior insula was also more strongly activated in HSSs than in LSSs for high-arousal stimuli. Strong neurobiological and neuroanatomical evidence supports the view that the right anterior insula may be a somatic marker for arousal and sympathetic stress responses (Craig, 2005; Critchley, Melmed, Featherstone, Mathias, & Dolan, 2002; Paulus et al., 2003; Zuckerman, 2005). In addition, the insula responds to pictures of appetizing food (Simmons, Martin, & Barsalou, 2005), is activated during sexual arousal (Stoleru et al., 1999), and may be involved in addictive cravings and urges (Naqvi, Rudrauf, Damasio, & Bechara, 2007); these findings suggest an association with appetitive behavior. In the present study, peak activation in the right insula occurred fairly early (around 2.5 s after image presentation; see Fig. 1d), and this early latency is consistent with the proposal that the insula may provide a direct and somewhat immediate representation of autonomic states.
Other neuroimaging studies have implicated right insula activity as related to the personality traits of reward or novelty seeking (Suhara et al., 2001) and neuroticism (Paulus et al., 2003). Activation in this region may be related to individual differences in part by reflecting sympathetic states that differ as a function of personality (e.g., Paulus et al., 2003). The present findings indicate that sensation seeking is the primary personality dimension predicting activation in this region because correlations with the convergent measure of sensation seeking were very strong, and no other personality variables predicted activation in this region when level of sensation seeking was controlled.
Compared with HSSs, LSSs more strongly activated brain regions associated with emotional regulation—the anterior cingulate and anterior medial OFC. The anterior cingulate may be involved in top-down, or intentional, modulation of intense emotional states (Matthews, Paulus, Simmons, Nelesen, & Dimsdale, 2004; Ochsner et al., 2004). In addition, the regulatory role of the rostral anterior cingulate cortex in affective behavior may depend on an interaction between incentive conditions and personality traits (Cunningham et al., 2005). Interestingly, in the present study, the fMRI response of the left anterior cingulate to high-arousal images was earlier in LSSs than in HSSs. This result suggests that this particular brain region may be engaged more readily in LSSs in the face of intense arousal. Because activation in the anterior cingulate was also negatively correlated with urgency, this region was engaged less strongly in impulsive sensation-seeking individuals than in individuals with low urgency scores. In contrast, HSSs engaged the right anterior insula more strongly in the face of intense arousal. Consequently, the anterior cingulate may be an important part of emotional regulation of somatic responses that are processed in the insula (Craig, 2005). This interpretation is also consistent with the negative correlation found between activation in the left anterior cingulate cortex and scores on urgency, a personality dimension believed to relate to emotional dyscontrol (Whiteside & Lynam, 2003).
Recall that LSSs also activated the anterior medial OFC more strongly than did HSSs. The peak response for LSSs occurred earlier and was more robust than the peak response for HSSs. Damage to the medial OFC leads to profound deficits in emotional decision making (Bechara, Damasio, & Damasio, 2000). In addition, Kringelbach and Rolls (2004) suggested that activation in anterior medial OFC could represent the availability of information on reinforcers to conscious processing. Other recent neuroimaging studies have reported that the anterior medial OFC region is activated in conscious processing of emotions (Takahishi et al., 2004) and emotional suppression (Ohira et al., 2006), and is related to self-consciousness as a personality trait (Eisenberger et al., 2005). The role of the anterior medial OFC in conscious processing of emotions and in emotional decision making suggests that it may play a role in emotional regulation. In the present study, activation in this region, as in the anterior cingulate, was negatively associated with urgency. Again, this association may implicate this region in emotional regulation and control.
In conclusion, HSSs and LSSs showed clear differences in brain response to high-arousal stimuli. The brain regions activated more strongly by HSSs are often associated with autonomic arousal. In contrast, the brain regions activated more strongly and more readily by LSSs are associated with emotional regulation and showed negative correlations with urgency, a personality dimension associated with emotional dysregulation. LSSs were also more sensitive to valence of the stimuli than were HSSs. Given that sensation seeking, as originally described by Zuckerman (1994), is composed of two distinct dimensions—one involving the degree of activation-approach and the other involving a lack of inhibition—future research should attempt to separate these aspects. Nevertheless, the present results may help to elucidate the processes by which sensation seeking leads to various negative behavioral outcomes, including substance use, risky sex, and antisocial behavior. Individuals high in sensation seeking not only are strongly activated by exciting, thrilling, and potentially dangerous activities, but also may be less likely than other people to inhibit or appropriately regulate that activation.
Acknowledgments
This research was sponsored by the National Institutes of Health (P50 DA005312, R01 HD052724, P20 RR015592) and the National Science Foundation (BCS-0224240). We thank Kathryn Bylica, Christine Corbly, Adam Lawson, David Powell, and Tory Vagnini for their technical assistance; Mike Bardo for his helpful input on study design; and the individuals who volunteered for this study.
Footnotes
Three regions isolated by the contrast of positive versus negative high-arousal stimuli in HSSs (right mid-cingulate gyrus, right inferior parietal cortex, and right middle frontal cortex) showed negative signal change relative to baseline for all combinations of valence and sensation seeking, but these regions were not interpreted further.
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. NeuroImage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behavioural Brain Research. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions to picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: A neuroanatomical basis? Trends in Cognitive Sciences. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: A functional magnetic resonance study. NeuroImage. 2002;16:909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:202–211. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychiatry. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: An fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:169–181. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder & Stoughton; 1975. [Google Scholar]
- Feldman LA. Valence focus and arousal focus: Individual differences in the structure of affective experience. Journal of Personality and Social Psychology. 1995;69:153–166. [Google Scholar]
- Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, Donohew RL. Reliability and validity of a brief measure of sensation seeking. Personality and Individual Differences. 2002;32:401–414. [Google Scholar]
- John OP, Srivastava S. The big-five trait taxonomy: History, measurement, and theoretical perspectives. 2. New York: Guilford; 1999. [Google Scholar]
- Kringelbach ML, Rolls E. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lang A, Shin M, Lee S. Sensation seeking, motivation and substance use: A dual system approach. Media Psychology. 2005;7:1–29. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-4. Gainesville, FL: Center for Research in Psychophysiology; 1999. International Affective Picture System (IAPS): Instruction manual and affective ratings. [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Lissek S, Bass JMP, Pine DS, Orme K, Dvir S, Rosenberger E, Grillon C. Sensation seeking and the aversive motivational system. Emotion. 2005;5:396–407. doi: 10.1037/1528-3542.5.4.396. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons A, Nelesen RA, Dims- dale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage. 2004;22:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Fox PT, Raichle ME. A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. Journal of Cerebral Blood Flow and Metabolism. 1989;9:96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura N, Ichikawa N, Isowa T, Iidaka T, Sato A, et al. Association of neural and physiological responses during voluntary emotional suppression. NeuroImage. 2006;29:721733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Roberti JW. A review of behavioral and biological correlates of sensation seeking. Journal of Research in Personality. 2004;38:256279. [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Smith BD, Davidson RA, Perlstein WM, Gonzalez F. Sensation-seeking: Electrodermal and behavioral effects of stimulus content and intensity. International Journal of Psychophysiology. 1990;9:179–188. doi: 10.1016/0167-8760(90)90072-l. [DOI] [PubMed] [Google Scholar]
- Stoleru S, Gregoire MC, Gerard D, Decety J, Lafarge E, Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Archives of Sexual Behavior. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. NeuroImage. 2001;13:891–895. doi: 10.1006/nimg.2001.0761. [DOI] [PubMed] [Google Scholar]
- Takahishi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: An fMRI study. NeuroImage. 2004;23:967–974. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: Application of the UPPS Impulsive Behavior Scale. Experimental and Clinical Psychopharmacology. 2003;11:210–217. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping. 1995;3:287–301. [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge, England: Cambridge University Press; 1994. [Google Scholar]
- Zuckerman M. Psychobiology of personality. Cambridge, England: Cambridge University Press; 2005. [Google Scholar]
- Zuckerman M, Como P. Sensation seeking and arousal systems. Personality and Individual Differences. 1983;4:381–386. [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models of personality: The big three, the big five, and the alternative five. Journal of Personality and Social Psychology. 1993;65:757–768. [Google Scholar]