Abstract
The pseudouridine synthase TruB handles 5-flurouridine in RNA as a substrate, converting it to two isomeric, hydrated products. Unexpectedly, the two products differ not in the hydrated pyrimidine ring but in the pentose ring, which has been epimerized to arabinose in the minor product. This inversion of stereochemistry at C2′ suggests that pseudouridine generation may proceed by a mechanism involving a glycal intermediate or that the previously proposed mechanism involving an acylal intermediate operates but with an added reaction manifold for 5-fluorouridine versus uridine. The arabino product strongly disfavors a mechanism with a Michael addition to the pyrimidine ring.
The pseudouridine synthases (Ψ synthases) isomerize uridine to pseudouridine (Ψ) in RNA and fall into six families. Despite their statistically insignificant global sequence similarity, the families share a common protein fold and constellation of active site residues, so they also likely share a common mechanism.1 The only absolutely conserved amino acid residue is an esssential Asp that has been proposed as a nucleophile that forms either a Michael adduct by attacking C6 of the uracil ring or an acylal intermediate by attacking C1′ of the ribose ring.2,3 Pioneering mechanistic studies using RNA containing 5-fluorouridine, [F5U]RNA, and the Ψ synthase TruA demonstrated the formation of a protein-RNA adduct that was stable in denaturing gels unless the sample was heated, in which case a hydrated product of F5U was isolated. These results were reasonably interpreted in favor of the mechanism involving a Michael adduct, with hydration resulting from the hydrolysis of the ester linkage between the active site Asp and pyrimidine ring.2,3 Subsequent 18O-labeling studies, however, showed that the direct hydration of F5U rather than ester hydrolysis gives rise to the products.4 Similar studies had already shown the same result for the Ψ synthases TruB5 and RluA6, and the cocrystal structures7,8 of both enzymes with [F5U]RNA showed that a covalent adduct was not present and that the F5U was rearranged to a C-glycoside (like Ψ) as well as becoming hydrated. Furthermore, analysis of the products after digestion of the RNA with S1 nuclease and alkaline phosphatase revealed that both TruB and RluA generate two hydrated products of F5U (at a ratio of ~3:1), which are both C-glycosides and recovered as dinucleotides with the residue following F5U in the RNA.4 To seek any mechanistic insight that the products might offer, their full structural elucidation was undertaken by NMR methods. TruB was examined first, for it does not make a tight adduct with [F5U]RNA (as TruA and RluA do) but instead handles it as a simple substrate,5 which eases the technical demands of generating appropriately large quantities of the products, the characterization of which is reported here.
The isolation of a mixture of the major and minor dinucleotide products after incubation of [F5U]RNA with TruB was reported earlier.4 Overlap of the ribose protons in the 1D 1H NMR spectrum necessitated their assignment by a suite of 2D methods. For both dinucleotide products, all protons and carbon atoms in the product of F5U (for convenience, F5U*) and the cytidine (Cyd) that follows it in the RNA sequence were unequivocally assigned (supplementary material). The major product is, unsurprisingly, the one observed in the cocrystal of TruB and [F5U*]RNA, but what is the minor product?
Both products share the same skeletal structure (supplementary material), leaving conformational or stereochemical isomerism as the difference between them. The dogged maintenance of the ratio of the two products through the digestion, isolation, and storage argued against conformational isomerism unless the conformers were remarkably strongly locked. Degradation of the 3′-Cyd by treatment with periodate and base yielded a mononucleotide F5U*, the spectrum of which matched that of the major product. The yield, however, was less than the major product originally present and a commensurate loss of the minor product would take it below the threshold of NMR detection, so no definitive conclusion can be drawn concerning the conversion of the minor to the major product by this treatment.
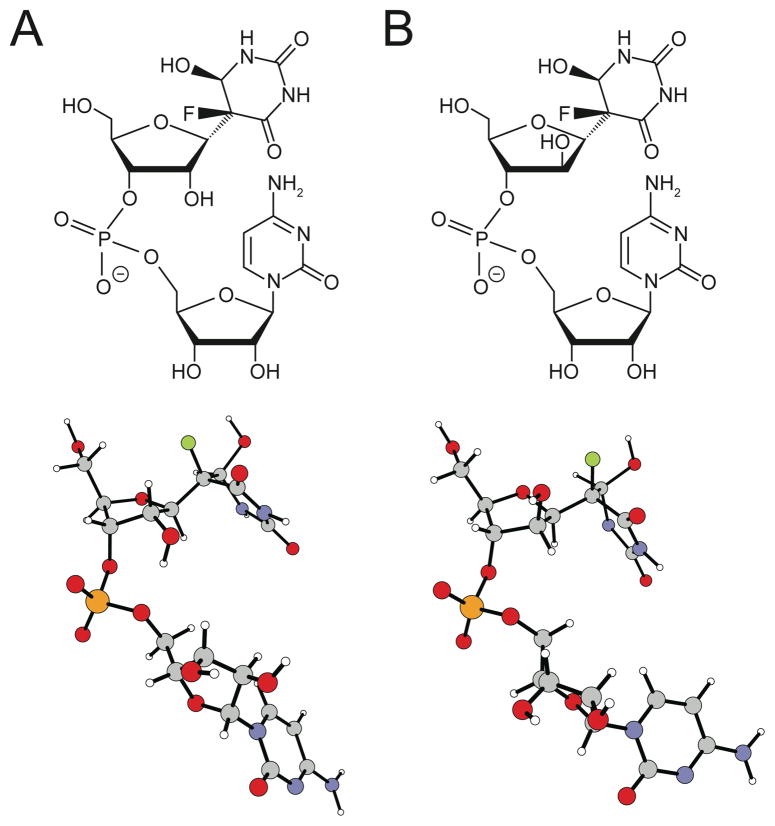
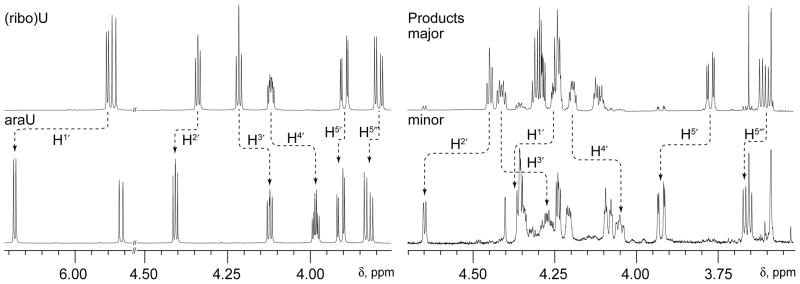
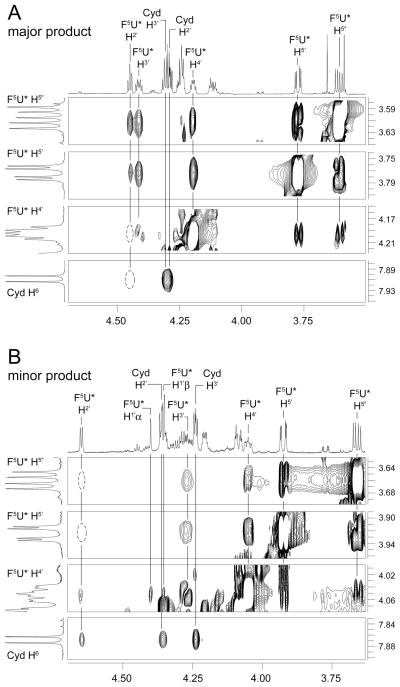
The evidence for stereochemical isomerism, on the other hand, is overwhelming. Surprisingly, the difference between the major and minor products is not at C6 (the site of hydration in the pyrimidine ring) of F5U* as previously proposed5,9 but instead at C2′ of its pentose ring so that the minor product is the arabino isomer of the product seen in the cocrystal (Figure 1). This outcome was first indicated by a comparison of the chemical shifts of the pentose protons in F5U versus F5U*, which match the differences between (ribo)uridine and arabinouridine with a diagnostic deshielding of H2′ and C2′ (Figure 2; also supplementary material Figure S25). Confirming a difference in configuration at C2′ between the major and minor products, both 1H-1H and 1H-19F NOE experiments (Figure 2; supplementary material, Figs S19-S21) showed the expected differences for the approach of H2′ to other pentose protons and F5 in a ribose ring for the major product and an arabinose ring for the minor product. Saliently, the major product evinces strong NOEs between H2′ and H5′/H5″, but these interactions are absent in the minor product, the H2′ of which lies on the opposite side of the pentose ring as H5′/H5″ in arabinose. Even though strong NOEs between F5 and H3′ in both the major and minor products indicate that the fluoro group lies over the pentose ring, only the major product shows a strong interaction between F5 and H2′. Similarly, the minor product lacks a detectable NOE between H2′ and H6, an interaction evident in the major product, but does show an NOE between H2′ and H4′ (Figure 3), albeit a somewhat weak one as expected since the energy-minimized structures reveal that these two protons are splayed out away from each other (Figure 1B) and lie 3.99 Å apart; the intensity is also approximately equal to that of the NOE between H1′ and H4′, which clearly lie on the same side of the pentose ring. Together, these data show the opposite disposition of H2′ on the pentose ring in the two products. That conclusion is bolstered (Figure 3) by the detectable NOE between H2′ of F5U* and H6 of C in the minor product (with reference to the standard Haworth projection, H2′ points “down” towards Cyd and H6 points “up” towards F5U*) but not in the major product (in which H2′ points “up” and away from Cyd).
Figure 1.
The structures of the (A) major and (B) minor products, which are isolated as dinucleotides. The models were energy-minimized using density functional theory (supplementary material).
Figure 2.
The differences in chemical shifts of the F5U* pentose protons in the major and minor products qualitatively match those between uridine (riboU) and arabinouridine (araU).
Figure 3.
1H-1H NOE spectra showing the correlations with H5′, H5″, and H4′ of F5U* and H6 of Cyd in the major (A) and minor (B) products. Dotted ovals indicate NOEs the absence of which are stereochemically informative.
Residual dipolar coupling methods can determine the relative orientation of bond vectors within a molecule10, and the data for the F5U* moieties fit an arabino minor product (Q = 0.251) better than a ribo one (Q= 0.571) and also indicate a ribo (Q = 0.290) rather than an arabino (Q = 0.442) major product (Q values represent the extent of deviation of the data from that calculated for a candidate structure so that a lower value indicates a better fit). The inter-atomic distances from the energy-minimized proposed structures and the density functional theory chemical shift predictions for F5 match the NMR data very well (supplementary material). For the major product, the conformations seen in molecular dynamics runs and the predicted ring pucker from the coupling constants of the ribose protons match well, but they diverge notably for the minor product. The correlation between pucker and coupling constants was, however, derived for ribo- and deoxyribo-nucleotides, and the inadequacy of the correlation for α-arabinofuranosides has been reported11, and a β-arabinofuranoside posed even greater difficulties for accurate theoretical treatment.12 Although the matter is under further examination, the unusual nature of this particular β-arabinonucleoside (a C-glycoside with both carbons fully saturated) make it a test case for method development rather than a candidate for description by “canned” protocols based on ribonucleoside parameters.
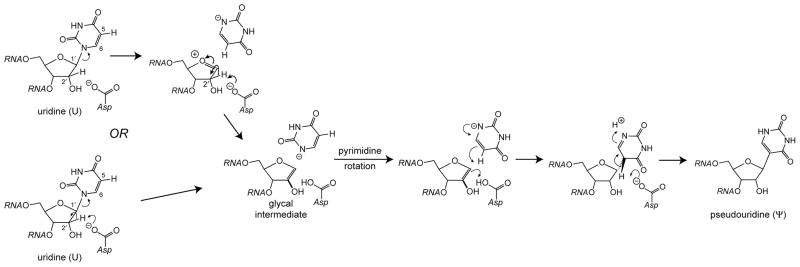
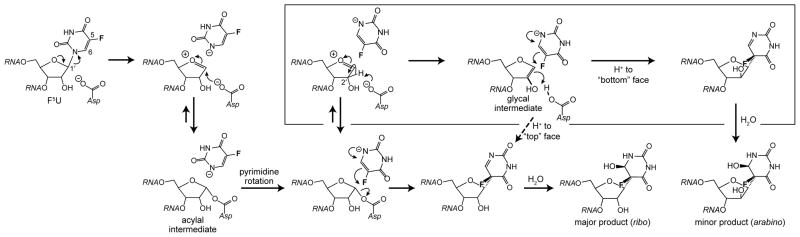
The arabino minor product indicated by the preponderance of the data offers mechanistic insight. Epimerization at C2′ requires deprotonation to form a glycal intermediate followed by reprotonation on the opposite face. Uridine phosphorylase offers a precedent for such an intermediate in an enzyme-catalyzed reaction13, and the Ψ synthase reaction with the natural substrate (U) may proceed by a similar mechanism (Scheme 1), with deprotonation effected either by the active site Asp (as shown) or O2 of uridine. The elimination may be stepwise with deprotonation of an oxocarbenium intermediate or a concerted syn elimination, a process generally disfavored but which is catalyzed by α-1,4-glucan lyase.14 No evidence for arabino Ψ has ever appeared in our numerous experiments with TruB, RluA, and TruA, so they must be rigidly stereospecific with their natural substrates, which is not surprising.
Scheme 1.
The “glycal mechanism” for Ψ generation. The elimination of uracil may be stepwise (upper path) or concerted (lower path).
A variation of the mechanism involving attack of the active site Asp at C1′ can also accommodate the arabino minor product of F5U (Scheme 2). The decreased nucleophilicity of the anion of 5-fluorouracil versus uracil increases the lifetime of the acylal intermediate, which is in equilibrium with an oxocarbenium species and free Asp, effectively giving both of these intermediates more time to access an alternate reaction pathway. The acidity of C2′ is accentuated in the oxocarbenium intermediate, which facilitates deprotonation to generate the glycal intermediate. The glycal intermediate can be reprotonated on either face during pyrimidine reattachment (as shown, Scheme 2) or beforehand to regenerate oxocarbenium intermediates of each configuration at C2′ that subsequently undergo pyrimidine reattachment.
Scheme 2.
The “acylal mechanism” for Ψ generation modified to account for epimerization at C2′ via the boxed reaction manifolda
aThe stereospecific hydration across C=N could occur at the active site or in solution with a directing hydrogen bond between the fluoro group and incoming water molecule. The reaction manifold made accessible by the longer lifetime of the acylal/oxocarbenium intermediates is boxed.
Whether or not O2 or O4 of the 5-fluorouracilate anion or the Asp effects the deprotonation (from the “top”), the proton must transfer to Asp to afford the arabino product since no other active site group in the cocrystal7 is positioned to protonate the glycal from the “bottom”, at least not without major structural reorganization. The alternative protonation of the gylcal directly from solution to generate the arabino product is ruled out by the lack of deuterium incorporation when the reaction (with either U or F5U in RNA) is run in buffered D2O with protein and RNA pre-equilibrated in the same buffer; this result also indicates that the protonated Asp is sequestered from exchange with solvent. The need for participation by the active site Asp as an acid precludes the mechanism involving it in a Michael addition to the pyrimidine ring, for the Asp would be tied up in the resulting ester linkage throughout the subsequent course of events and therefore be unable to protonate C2′ of the glycal intermediate.
A major caveat of experiments with substrate analogs is the chance that the alteration of the substrate significantly perturbs its chemistry. In this instance, however, the fluoro group appears not to alter the fundamental chemistry but merely enhance the lifetime of an intermediate (glycal or acylal/oxocarbenium) and thereby allow it to access a secondary—and mechanistically informative—reaction manifold. Hydrogen bonding to the fluoro group may also help to depress the pKa of C2′ in the intermediates, but this effect seems likely to be minor compared to the increased lifetime of the intermediate with a detached pyrimidine.
The arabino minor product of F5U from the action of TruB, then, suggests another plausible mechanism for the Ψ synthases and strongly disfavors one of the two mechanisms that have dominated discussion. Given the similarity of the NMR spectra of the products of F5U from the action of RluA to those reported here, the conclusions will likely prove general for all Ψ synthases, but the full characterization of the “RluA products” are underway to confirm that supposition, as are experiments to test for the formation of a glycal intermediate during the conversion of U to Ψ.
Supplementary Material
Acknowledgments
This work was supported by NIH grant GM059636 (to E.G.M.), the Commonwealth of Kentucky Research Challenge Trust Fund (“Bucks for Brains”), and the Charles L. Bloch, M.D. Professorship. We thank N. Stolowich, S. Aramgum, and S. Bai for technical assistance. We acknowledge the helpful discussions of R. Wittebort, A. Lane, R. Silverman, and T. Lowary.
Footnotes
Supporting Information Available: NMR spectra and assignments, detailed structural analysis, and experimental procedures are available. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Mueller EG, Ferré-D’Amaré AR. In: DNA and RNA Modification Enzymes: Structure, Mechanism, Function and Evolution. Grosjean H, editor. Landes Bioscience; Austin, TX: 2009. p. 363. [Google Scholar]
- 2.Gu XR, Liu YQ, Santi DV. Proc Natl Acad Sci U S A. 1999;96:14270. doi: 10.1073/pnas.96.25.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang LX, Pookanjanatavip M, Gu XG, Santi DV. Biochemistry. 1998;37:344. doi: 10.1021/bi971874+. [DOI] [PubMed] [Google Scholar]
- 4.McDonald MK, Miracco EJ, Chen J, Xie Y, Mueller EG. Biochemistry. 2011;50:426. doi: 10.1021/bi101737z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spedaliere CJ, Ginter JM, Johnston MV, Mueller EG. J Am Chem Soc. 2004;126:12758. doi: 10.1021/ja046375s. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton CS, Greco TM, Vizthum CA, Ginter JM, Johnston MV, Mueller EG. Biochemistry. 2006;45:12029. doi: 10.1021/bi061293x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoang C, Ferré-D’Amaré AR. Cell. 2001;107:929. doi: 10.1016/s0092-8674(01)00618-3. [DOI] [PubMed] [Google Scholar]
- 8.Hoang C, Chen JJ, Vizthum CA, Kandel JM, Hamilton CS, Mueller EG, Ferre-D’Amare AR. Mol Cell. 2006;24:535. doi: 10.1016/j.molcel.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Spedaliere CJ, Mueller EG. RNA. 2004;10:192. doi: 10.1261/rna.5100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kummerlöwe G, Luy B. Trends Anal Chem. 2009;28:483. [Google Scholar]
- 11.Taha HA, Castillo N, Sears DN, Wasylishen RE, Lowary TL, Roy PN. J Chem Theory Comp. 2010;6:212. doi: 10.1021/ct900477x. [DOI] [PubMed] [Google Scholar]
- 11.Taha HA, Roy PN, Lowary TL. J Chem Theory Comp. 2011 doi: 10.1021/ct100450s. [DOI] [PubMed]
- 13.Paul D, O’Leary SE, Rajashankar K, Bu W, Toms A, Settembre EC, Sanders JM, Begley TP, Ealick SE. Biochemistry. 2010;49:3499. doi: 10.1021/bi902073b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SS, Yu S, Withers SG. Biochemistry. 2003;42:13081. doi: 10.1021/bi035189g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.