Abstract
Background & Aims
Corticotropin-releasing factor receptor-1 (CRF1) mediates the stress-induced colonic motor activity. Less is known about the role of CRF2 in the colonic response to stress.
Methods
We studied colonic contractile activity (CCA) in rats and CRF2-/-, CRF-overexpressing, and wild-type mice using still manometry; we analyzed defecation induced by acute, partial-restraint stress (PRS), and/or intraperitoneal (IP) injection of CRF ligands. In rats, we monitored activation of the colonic longitudinal muscle myenteric plexus (LMMP) neurons and localization of CRF1 and CRF2 using immunohistochemical and immunoblot analyses. We measured phosphorylation of ERK1/2 by CRF ligands in primary cultures of LMMP-neurons (PC-LMMPn) and cAMP production in HEK-293 cells transfected with CRF1 and/or CRF2.
Results
In rats, a selective agonist of CRF2 (urocortin 2) reduced CRF-induced defecation (>50%), CCA, and Fos expression in the colonic LMMP. A selective antagonist of CRF2 (astressin2-B) increased these responses. Urocortin 2 reduced PRS-induced CCA in wild-type and CRF-overexpressing mice, whereas disruption of CRF2 increased PRS-induced CCA and CRF-induced defecation. CRF2 co-localized with CRF1 and neuronal nitric oxide synthase in the rat colon, LMMP, and PC-LMMPn. CRF-induced phosphorylation of ERK in PC-LMMPn; this was inhibited or increased by a selective antagonist of CRF1 (NBI35965) or astressin2-B, respectively. The EC50 for the CRF-induced cAMP response was 8.6 nM in HEK-293 cells that express only CRF1; this response was suppressed 10-fold in cells that express CRF1 and CRF2.
Conclusions
In colon tissues of rodents, CRF2 activation inhibits CRF1 signaling in myenteric neurons and the stress-induced colonic motor responses. Disruption of CRF2 function impairs colonic coping responses to stress.
Keywords: colonic contraction, myenteric neurons, nNOS, stress response
Introduction
Clinical and experimental studies show that chronic or uncontrolled stress triggers or exacerbates a number of pathologies including gastrointestinal diseases (1-4). Corticotropin-releasing factor (CRF) is the primary hypothalamic mediator of the mammalian neuroendocrine and behavioral responses to stress (5). The CRF signaling system, in addition to CRF, encompasses three CRF related peptides, urocortins (Ucns), Ucn 1, Ucn 2 and Ucn 3 and two receptor subtypes, CRF1 and CRF2 (6; 7). CRF and Ucn 1 bind to both CRF1 and CRF2 receptors, although with different affinities (5-7). On the other hand, Ucn 2 and Ucn 3 bind selectively to CRF2 receptors (7). CRF1 is found abundantly in the central nervous system with limited expression in peripheral tissues, whereas CRF2 is widespread in the periphery and confined in discrete brain nuclei (8-12). Multiple alternatively spliced transcripts of CRF1 have been identified (CRF1a-CRF1i), with only a few of them being functional. CRF2 is expressed in three major functional isoforms in humans (CRF2a, CRF2b, CRF2c), two in rodents (CRF2a, CRF2b) and 5 additional non-coding CRF2a variants (7; 11; 13-15). Rodent CRF2a is primarily expressed in the brain, whereas CRF2b is mainly found in the periphery, including the gastrointestinal tract (11; 14; 16; 17). CRF2a and CRF2b isoforms display similar pharmacological profile (18). The dominant mode of signaling for both CRF1 and CRF2 is the Gs-coupled adenylate cyclase-phosphokinase A cascade, although PLC-PKC and ERK-MAPK cascades are reported in different cell types (13; 19).
In rodents stress or CRF injected into the brain or periphery induces colonic secretomotor and pain sensation alterations that are blocked by CRF1 antagonists (22-26). These findings have led to the consensus that CRF1 receptor is the primary receptor involved in the stress-induced alteration of lower gut secretomotor and pain sensation. However except the preliminary data on the inhibitory actions of Ucn 2 in mice defecation (27) and rat visceral pain (48) responses, the mechanisms of action and role of peripheral CRF2 in the colonic response to CRF or stress are largely unknown.
In the present study, we investigated whether peripheral CRF2 activation or blockade modulates the colonic motor activity to peripheral injection of CRF or stress in rats and mice and assessed the underlying mechanisms involved in the CRF2-mediated inhibitory actions. We show that CRF2 activation plays a critical role in harnessing the CRF1-mediated stimulation of colonic motor function induced by acute partial restraint stress (PRS) or peripheral injection of CRF by modulating CRF1 signaling and/or recruiting inhibitory pathways such as nitric oxide (NO). Such modulation is essential to establish homeostasis and it is likely that alteration of CRF2-signaling impairs the normal stress-coping mechanisms and may contribute to the development of stress-related gut diseases.
Methods
Animals
Adult male Sprague-Dawley rats (SD, 280-300g, Harlan, Indianapolis, ID), male and female CRF2-/- (32.7±3.8g) and their wild-type littermates (WTL, C57BL/6J, 31.7 ± 0.3g), CRF-overexpressing (CRF-OE, 30.9±2.1g) and their WTL (C57BL/6, 28.8±0.6g), from the Oregon Health and Science University, Portland, OR (28), and the University of California Los Angeles, CA were used. Animals were maintained under temperature (20–24°C) and light-(12-h light-12−h dark) controlled environment and fed ad-libitum with standard rodent chow (Prolab RMH 2500-5P14; Purina LabDiet, St. Louis, MO) and tap water. Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Protocols (9906-820; 08047-05 and 06014-08) were approved by the VA Institutional Animal Care and Use Committee.
Substances
Human/rat/mice urocortin 1 (Ucn 1), human Ucn 2 (hUcn 2), mouse Ucn 2 (mUcn 2), h/rCRF and astressin2-B (Clayton Foundation Laboratories, The Salk Institute, La Jolla, CA) were synthesized and purified as previously described (29). NBI-35965 was obtained from Neurocrine Bisosciences Inc. (San Diego, CA).
Stress models
Water avoidance stress (WAS) in rats and partial-restraint stress (PRS) in rats and mice for 1h were used as acute-stressors (30; 31) and CRF-OE mice as a genetic model of chronic stress (27; 28; 31).
Measurements of colonic motor function in rats and mice
Colonic contractions
Contraction was recorded in conscious non-fasted rats and mice using newly developed minimally-invasive solid-state manometry catheter (23; 31). Pressure sensors were positioned at 8 and 4-cm (rats) and at 2-cm (mice) past the anus. The 8-cm site (rats) corresponds to proximal-transverse whereas the 4-cm (rats) and 2-cm (mice) correspond to the distal colon. Colonic contractions were quantified by measuring for every minute the area under the curve (AUC) of the phasic component of the intraluminal pressure change that was extracted from the original trace (32). Because acute PRS-induced activation of colonic contractions primarily occur during the first 20-min (31), the frequency, amplitude, duration and propagation of contractions were determined for the 0-20-min and 20-60 min time periods. See supplement for additional information.
Fecal pellet output (defecation) and diarrhea
In non-fasted conscious rats and mice, defecation was monitored as number of fecal pellets output (FPO) for 1 or 2-h (30; 31). The % of rats with diarrhea was calculated.
Immunohistochemistry: rat colon longitudinal muscle myenteric plexus (LMMP)-wholemount preparation
Neuronal Fos
Proximal and distal colonic LMMP wholemount preparations were dissected and Fos-immunohistochemistry assessed as in our previous studies (33; 34). The mean number of Fos-IR nuclei/myenteric-ganglion from each rat was used to generate a mean number.. See supplement for additional information.
Double labeling of CRF2 with CRF1 or neuronal nitric oxide synthase (nNOS)
The proximal and distal segments of colon collected from 2 naïve adult male SD rats were processed for LMMP wholemount preparations as above (34) and processed for CRF1, CRF2 and nNOS immunostaining as described (11; 34). See supplement for additional information.
Immunohistochemistry: rat colon primary culture LMMP neurons (PC-LMMPn)
LMMP neurons were cultured using slightly modified method (35). CRF1 and CRF2 receptors expression was determined using RT-PCR on 0.1 μg of poly A+ RNA isolated from non-fixed 5 days old primary culture cells by oligo-dT cellulose spin column (FastTrack 2.0 Kit, Invitrogen). In separate preparation, 4′,6-diamidino-2-phenylindole, anti-Hu, CRF1 and CRF2 receptor immunoreactivity (IR) was performed in 5 days old fixed cultured neurons. CRF1 and CRF2 presence in the PC-LMMPn was further confirmed by Western blot. See supplement for additional information.
pERK1/2 in rat colon PC-LMMPn
Phosphorylation of ERK1/2 in response to CRF, Ucn 1 or Ucn 2 and the CRF or Ucn 1 effects in the presence or absence of Ucn 2 or selective CRF1 or CRF2 antagonists, NBI35965 or astressin2-B respectively, was determined in 5-day cultured PC-LMMPn of rats by Western blot. Ucn 1 is used in this experiment because of its higher affinity to CRF1 and CRF2 than CRF (5-7). Selective inhibition of CRF1 or CRF2 receptors, in the presence of Ucn 1, would allow better detection of the respective role of CRF1 or CRF2 in the neuronal response. Band intensities were normalized to control (basal) for comparison. See supplement for additional information.
DNA transfection in HEK-293 cell lines and cAMP measurement
Stable CRF1 and CRF2-expression
Rat CRF1 and CRF2b cDNA encoding full-length CRF1 and CRF2b protein, respectively, was cloned into pcDNA3.1 expression vector (Invitrogen) as in our previous study (11). Confirmed plasmid DNA was then transfected into human embryonic kidney (HEK)-293 cells (1 μg/106 cells) using Lipofectamine 2000 as a carrier. Representative HEK-293 cell lines from at least three positive clones were used as controls to characterize CRF1-CRF2 interaction in subsequent functional experiments.
cAMP measurement
Similar protocol as in our previous study was used (11). See supplement for detailed information.
Data analysis
Values are presented as means±SEM. Colonic contractile or defecation differences between groups were analyzed by One Way ANOVA whereas time course data were compared using One Way ANOVA for repeated measures followed by a Student-Newman-Keuls post hoc test. Fos IR nuclei in the colon myenteric-ganglia and cAMP production difference between groups were tested by student’s t test. P < 0.05 was considered statistically significant.
Results
Urocortin 2 injected IP blunts IP CRF-induced defecation, diarrhea and myenteric neuron activation whereas IP astressin2-B enhances the responses
Defecation and diarrhea
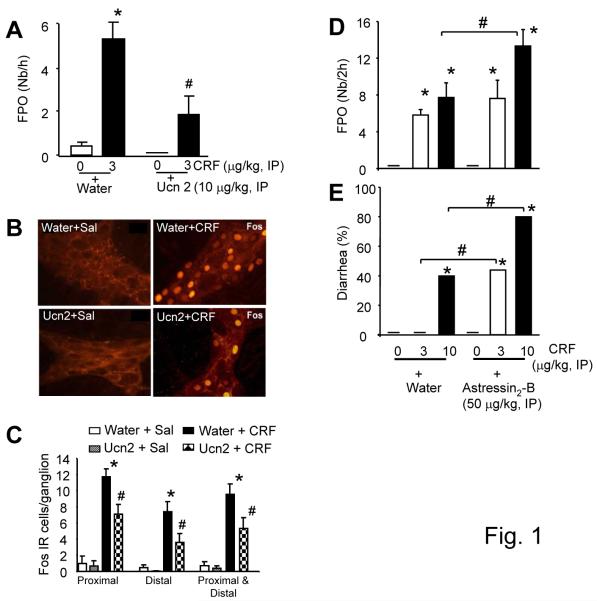
Control rats injected IP with vehicle (water) followed 5-min later by saline or rats injected with hUcn 2 (10 μg/kg)+saline exhibited similar defecation over the 60 min period (0.4±0.2 vs 0±0/h) (Fig 1A). CRF (3 μg/kg, IP) in vehicle pretreated rats, compared with vehicle+saline group, increased significantly the 60-min defecation (5.3±0.8 vs 0.4±0.2/h) (Fig 1A). Pretreatment with hUcn 2 (10 μg/kg, IP) inhibited the CRF-induced defecation by 64% (Fig. 1A).
Figure1.
Peripheral pretreatment (−5 min) with hUcn 2 blunts IP CRF-induced defecation and colonic myenteric Fos-IR whereas astressin2B enhances the response in rats. (A): Defecation response, expressed as fecal pellet output (FPO) to IP CRF in the presence or not of IP hUcn 2, *p<0.05 vs controls; #p<0.05 vs CRF; n=8-11/group. (B): Confocal photomicrograph of proximal colon LMMP neurons Fos expression. Colons are from rats in A above (8/group) that received IP water+saline (top-left), water+CRF (top-right), hUcn 2+saline (bottom-left) and hUcn 2+CRF (bottom-right). (C): Fos-IR (cell/ganglion) in the proximal, distal and proximal-distal colon in response to CRF (3 μg/kg, IP) in the presence or not of hUcn 2 (10 μg/kg, IP), *p<0.05 vs all others; #p<0.05 vs their respective water+sal or Ucn 2+sal; n=8/group (D-E): Selective blockade of CRF2 receptor by IP astressin2-B exacerbated IP CRF-induced defecation (D) and diarrhea (E). Bar, mean±SEM. n=8/group, *p<0.05 vs water+saline or astressin2-B+saline; #p<0.05 vs the corresponding water+CRF 10 μg/kg (D,E) or water+CRF 3 or 10 μg/kg (E).
In a separate experiment, IP vehicle+CRF at 3 and 10 μg/kg, increased defecation to 5.7±0.3 and 8.0±1.2 pellets/h and induced diarrhea in 0% and 40% of rats, respectively compared to vehicle+saline (0±0 pellets/h and 0% diarrhea). Astressin2-B (50 μg/kg, IP), compared to vehicle, enhanced the CRF-induced defecation at 10 μg/kg (13.5±1.3 vs 8.0±1.2 pellets/2h, p<0.05) and showed a trend to increase at 3 μg/kg (7.8±1.3 vs 5.7±0.3 pellets/2h, p>0.05, NS) (Fig. 1D). Likewise IP astressin2-B (50 μg/kg) enhanced significantly the IP CRF-induced diarrhea both at 3 μg/kg (0% to 44%) and 10 μg/kg (40% to 80%) (Fig. 1E).
LMMP neuronal activation
CRF (3 μg/kg, IP), compared to saline, increased the number of Fos-positive myenteric neurons in the proximal (11.7±1.9 vs 1.0±0.9 cells/ganglion), distal (7.4±1.5 vs 0.5±0.3 cells/ganglion) and the combined proximal-distal (9.6±1.1 vs 0.7±0.4 cells/ganglion) LMMP (Fig. 1B-C), simultaneous with increased defecation (Fig. 1A). Pretreatment with hUcn 2 (10 μg/kg, IP) decreased the CRF-induced neuronal activation in both segments (Fig. 1B-C). In a separate experiment, astressin2-B, compared to vehicle, enhanced the CRF-induced Fos-expression in the proximal colon LMMP neurons (13.2±0.5 vs 10.7±1.0 cells/ganglion, p<0.05) but not in the distal colon (11.2±0.4 vs 10.0±0.6 cells/ganglion, p>0.05).
Urocortin 2 injected IP prevents acute stress-induced stimulation of colonic motor function in rats
Partial-restrain stress
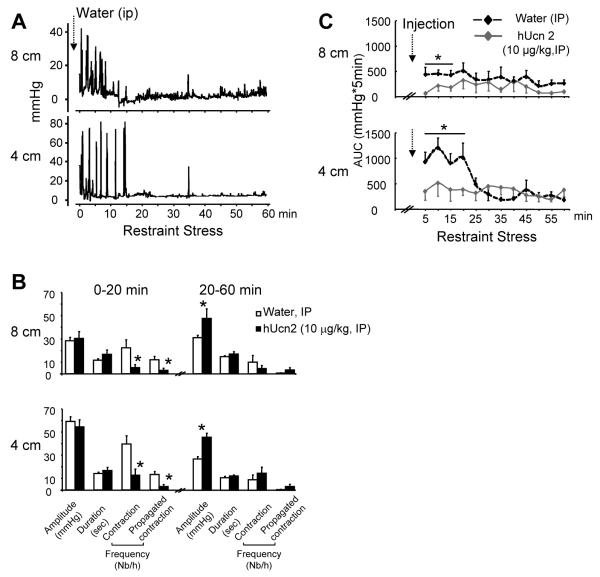
Rats injected IP with saline and exposed to 1h PRS exhibited robust contractile activities in the proximal-transverse and distal colon for the first 20-min followed by a period of relative quiescence (20-60 min) (Fig. 2A-C). High amplitude phasic contractions (>15 mmHg) occurred with a frequency of 22±7.1 and 40± 7.2/h in the proximal-transverse and distal colon respectively, with 33% propagating from proximal to the distal site (Fig. 2A-B). hUcn 2 (10 μg/kg, IP) prevented the enhanced initial 20-min colonic response as shown by the reduced AUC (Fig. 2C), frequency and propagation of contractions (Fig. 2B). However hUcn 2 did not affect the duration and amplitude (Fig 2B) of contractions during this period. During the 20-60 min period, Ucn 2 had no effect on frequency, duration and propagation while increasing amplitude at both 8 and 4-cm sites (Fig 2B).
Figure 2.
Acute partial-restraint stress-induced colonic contractions in rats is prevented by hUcn 2 (10 μg/kg, IP). (A): Representative solid state manometry trace with pressure sensor catheter probes placed in the colon at 8 and 4 cm past the anus. (B): Graphs showing amplitude, duration, total frequency (>15 mmHg) and propagated contraction frequency. Bar, mean±SEM of n=5/group*: p<0.05 versus water (C): Time course of AUC pressure changes. Dotted arrows show IP injection just before the onset of restraint stress. Values are rolling averages (mean±SEM) of AUC computed for every 5 min, *p<0.05 vs contractile responses on all other time points. n=8/group.
Water avoidance stress
Rats injected IP with saline and exposed to WAS (1h) had higher defecation than non-stressed rats (3.5±0.3 vs 0.4±0.1 pellets/h). hUcn 2 (10 μg/kg, IP)-reduced significantly the WAS-induced defecation by 49% (3.5±0.3 vs 1.8±0.2 pellets/h).
Urocortin 2 injected IP prevents stress-induced colonic motor response in mice
WTL mice
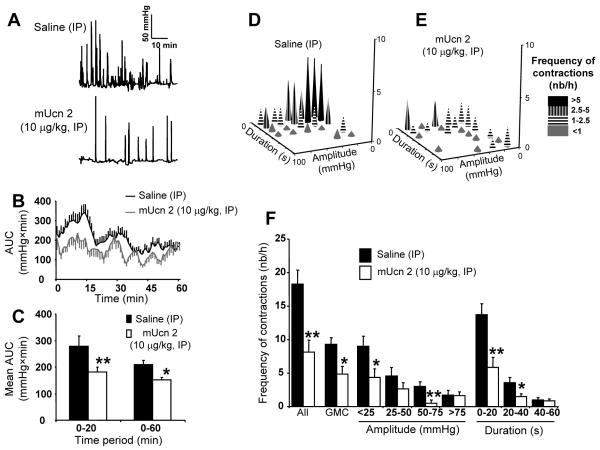
mUcn 2 (10 μg/kg, IP) prevented acute PRS-induced initial increase in distal colonic high amplitude contractions termed as giant migrating contractions (GMCs) compared to IP saline (0.1 ml) (Fig. 3A-C). mUcn 2 decreased the mean AUC of the 0-20-min period by 34.7% (Fig. 3B-C). This was mainly due to decreased frequency of all contractions (24.5±5.2 vs 54.9±6.1 contractions/h; p<0.05), and specifically in the short duration contractions (<40 sec) and GMCs (14.5±3.6 vs 27.9±3.0 contractions/h; p<0.05) (Fig. 3D-F). mUcn 2 decreased also the acute PRS-induced defecation compared with saline (5.3±0.6 vs 13.7±1.2; p<0.05), concomitant to the decrease in colonic contractions.
Figure 3.
mUcn 2 (10μg/kg) injected IP reduced distal colonic contraction to acute partial-restraint stress in wild-type mice. Representative distal CCA traces following saline or mUcn 2 treatment (A). Time course of AUC pressure changes over 1 h (B) and in blocks of 0-20 and 20-60 min period (C). Bar, mean ± SEM of n = 8-10/group. *p<0.05; **: p<0.01 vs saline. (D-E): Plots show distal colonic contractions pattern of WTL mice during the first 20-min of PRS following injection with saline (D) or mUcn 2 (E). Plots show the frequency of contractions as a function of amplitude or duration of contractions. (F): Distal colonic contractions profile (frequency, amplitude and duration of contractions), including giant migrating contractions (GMCs) over 1-h period. Bar, mean±SEM of n=6 mice/group*: p<0.05 versus saline; **: p<0.01 vs saline.
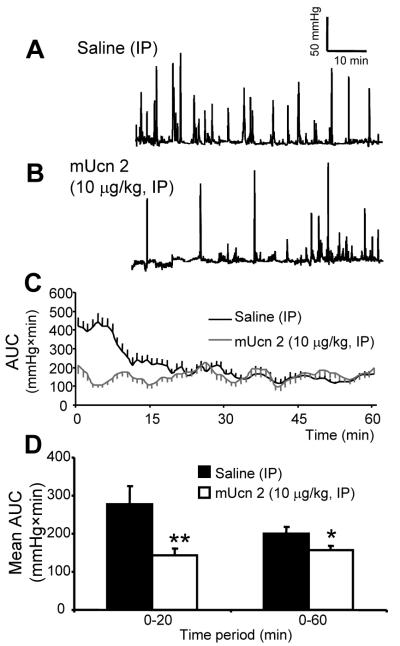
CRF-OE mice
The initial increase in distal colonic contractile activity in response to PRS was shortened to 10-min as reported before (31) but was significantly higher activity than in the remaining recording period (Fig. 4A-D). mUcn 2 (10 μg/kg, IP), compared to vehicle, reduced PRS-induced activation of colonic contractility, resulting in a significant 54.2% decrease of AUC in the first 20-min (Fig. 4A-D) together with a decrease in PRS-induced defecation (4.6±1.5 vs 11.0±2.3 pellets/h, p<0.05).
Figure 4.
mUcn 2 (10 μg/kg, IP) reduces distal colonic contractions to impartial-restraint stress in CRF overexpressing (CRF-OE) mice. (A-B): Representative distal CCA traces of CRF-OE mice injected with saline (A) or mUcn 2 (B). (C-D): Time course of the AUC of intracolonic pressure changes over 1-h (C) and in blocks of 0-20, 0-60-min (D). Bar, mean±SEM of n=6-7/group. *: p<0.05 **: p<0.01 vs saline.
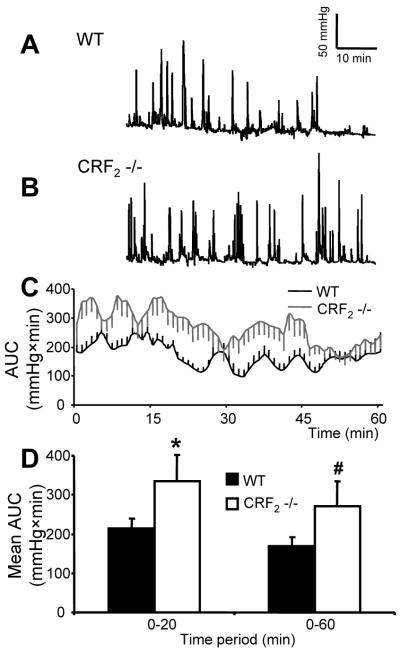
CRF2 receptor deletion (CRF2-/- mice) exaggerates colonic contraction and defecation response to acute PRS or IP CRF
CRF2-/- mice, compared to WTL, displayed increased frequency of GMCs, AUC for the 0-60 min period (p<0.05) (Fig. 5A-D) and defecation (11.5±0.8 vs 4.2±1.1 pellets/h; p<0.05) when exposed to PRS. Injection of CRF (10 μg/kg,IP), compared to saline, in female WTL mice, placed in novel individual cages, had no effect on defecation (4.0±1.0 vs 4.6±0.9 pellets/h) but significantly increased defecation in female CRF2-/- mice (8.5±0.7 vs 5.8±0.9 pellets/h).
Figure 5.
Distal colonic contractions to partial-restraint are enhanced in CRF2-/- mice compared to wild-type. (A-B): Representative trace in WTL mouse (A) and CRF2-/- mouse (B). (C): Time course of AUC of intracolonic pressure changes over 1h (D) and in blocks of 0-20 and 0-60-min period (D). Bar, mean±SEM of n=7/group.*: p<0.05 vs WTL 0-20-min time period; #: p<0.05 vs WTL 0-60 min.
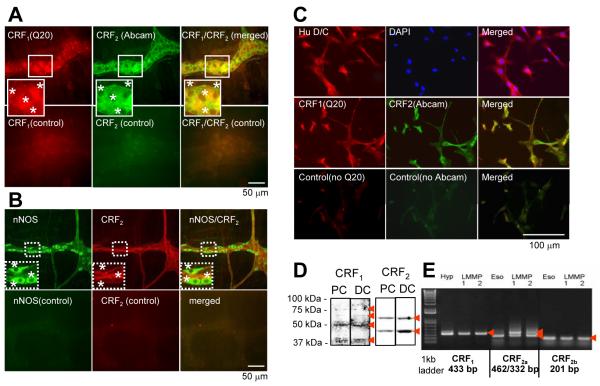
Double-immunostaining of CRF2/CRF1 in the rat colon LMMP and PC-LMMPn
LMMP
Rat proximal and distal colon LMMP neurons exhibited CRF2 (cell bodies and fibers) and CRF1 (mainly cell membrane)-receptor antibody IR (Fig. 6A, supplement Fig. 1). The two receptors are co-localized in the majority of cells. Similarly CRF2 are co-localized with nNOS (Fig. 6B, supplement Fig 1).
Figure 6.
CRF1 and CRF2 are coexpressed in rat colonic myenteric neurons. (A-B ): Confocal microscope images showing double labeling of CRF2 with CRF1 (A) and CRF2 with nNOS (B) in rat distal colon wholemount LMMP preparation. (C): double labeling of nuclear marker, 4′,6-diamidino-2-phenylindole (DAPI) and anti-Hu IR in rat colon myenteric ganglion primary culture neurons (top panel) and double labeling of CRF2 with CRF1 (middle panel). Lower panels in A,B,C show negative control, stained with normal goat IgG. (D): Western blot analysis of CRF1 (bands at 39, 50, 60, 75 kDa, arrows) and CRF2 (at 42, 55 kDa, arrows) in proximal colon (PC) and distal colon (DC) PC-LMMPn. (E): RT-PCR analysis for expression of CRF1, CRF2b and CRF2a splice variants in the rat proximal colon PC-LMMPn. Hypothalamus (Hyp) was used as positive control for CRF1 and Esophagus (Eso) for CRF2b and CRF2a to generate predicted PCR products (arrows).
PC-LMMPn
5-day cultured primary neurons uniformly displayed IR to Anti-Hu D/C demonstrating the neuronal nature of these cells (Fig. 6C). CRF1 and CRF2 are co-localized in the neuronal cells (Fig 6C). The presence of CRF1 and CRF2 in the primary neurons was further confirmed by Western blot (Fig 6D) and RT-PCR (Fig. 6E).
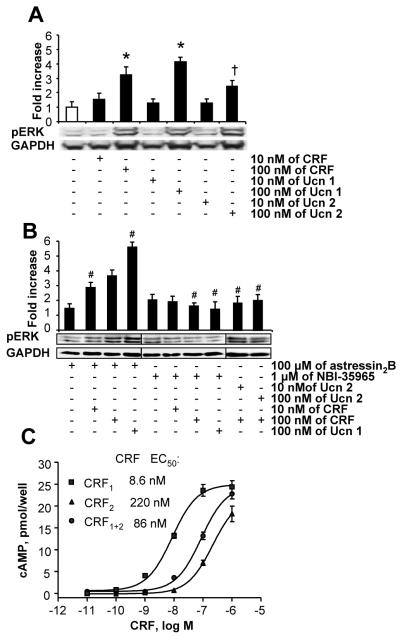
CRF-induced ERK phosphorylation in PC-LMMPn is enhanced by CRF2 blockade
CRF and Ucn 1 (10 and 100 nM)-induced phosphorylation of ERK1/2 in neuronal cells in a dose-dependent manner (Fig. 7A). Pre-incubation with the selective CRF1 antagonist, NBI-35965 (1 μM), prevented CRF or Ucn 1 (100 nM)-induced phosphorylation (Fig. 7B) while astressin2-B further enhanced phosphorylation stimulated by CRF and Ucn 1 (Fig. 7B). Ucn 2 by itself had no (10 nM) or moderate (100 nM) effect on pERK (Fig. 7A). However pre-incubation with Ucn 2 (10 or 100 nM) prevented the CRF (100 nM)-induced pERK (Fig. 7B).
Figure 7.
Activation of CRF2 suppresses phosphorylation of pERK in PC-LMMPn and cAMP production in HEK-293 cells. (A-B) Western blot analysis of pERK1/2 in response to CRF, Ucn 1 or Ucn 2 (A). Blockade of the CRF-induced pERK by Ucn 2 or by a selective CRF1 antagonist, NBI-3965 and enhancement of the response by selective CRF2 antagonist astressin2-B (B). Bar, mean±SEM, n=3/group, * p<0.05 vs no-treatment and the respective10 nM dose in A, †p<0.05 vs all other groups in A, #p<0.05 vs the no-treatment and the respective CRF or Ucn 1 alone dose in A. (C): Dose-dependent increases in intracellular cAMP production in CRF1α, CRF2β and CRF1α+CRF2β transfected HEK-293 cells stimulated with CRF for 30 min. Note the 10-fold decrease in potency of CRF in CRF1 and CRF2 coexpressing cells vs. in CRF1 only expressing cells.
CRF-induced cAMP production in CRF1-only transfected cells is blunted in CRF1/CRF2b double-transfected cells
CRF (10−10-10−6 M) induced a concentration dependent cAMP response (EC50:8.6 nM) through CRF1 in HEK-cells that expressed only CRF1-receptors. CRF induced also cAMP production in CRF2-only transfected cells with an EC50 value of 220 nM. However compared to the effect of CRF on CRF1-only cells, the potency of CRF was decreased by 10-fold (EC50: 8.6 nM→ 86 nM) in cells that co-expressed both CRF1 and CRF2-receptors (Fig. 7C).
Discussion
The bodily response to stress, including that of the colon, is primarily initiated by the activation of CRF1 (5). However little is known about the role of CRF2.in the colonic responses to stress. The present studies provide convergent evidence that activation of peripheral CRF2 plays a physiological role to counteract CRF1-mediated stimulation of colonic motor response to acute-stress and IP CRF in rodents. In addition, we show that CRF1-CRF2 receptors are colocalized and interact in the rat colon wholemount as well as in primary myenteric neurons and in CRF receptor transfected cell line where CRF2 activation curtails a CRF1-mediated phosphorylation of ERK1/2 and cAMP production.
Peripheral activation of CRF2 by IP Ucn 2 consistently blunted acute-stress or CRF-induced defecation and colonic contractile responses in rodents. Colonic contraction in rats and mice submitted to PRS and monitored by still manometry is characterized by the occurrence of an immediate (first 20-min) high amplitude (>15 mmHg) and propagative contractile activity. Comparable propagative contractions termed giant migrating contractions (GMC) in the colon of non-fasted freely moving (36; 37), or fasted and anesthetized rats (37; 38) are reported, albeit with different frequencies. The frequency differences are probably due to the acute-stress (present) versus the freely moving (36) and anesthetized state (37; 38) as well as the use of still manometry (present) versus strain-gauge (36; 37) or perfused manometry (38). Increased and coordinated colonic high amplitude contractions or GMCs are associated with defecation in several species including humans (36; 37; 39). We previously established in mice that the initial 20-min colonic activity in response to acute-PRS is strongly correlated with defecation (31). The present CRF2-mediated reduction of defecation thus is attributable to the suppression of the overall AUC and specifically the frequency and propagation of the high amplitude contractions.
Given that acute-stress and IP CRF-induced colonic stimulation is primarily mediated by CRF1-activation (22; 33) and that CRF also binds to CRF2, although with less affinity compared to CRF1 (5), we set out to determine whether simultaneous activation of both receptors, leads to the modulation of CRF1 mediated colonic propulsive motor function. The data indicate that blockade of CRF2, in rats, exacerbates IP CRF-induced defecation and diarrhea. Similarly, the defecation response to a sub-threshold dose of CRF and the colonic contractile response to acute-PRS are enhanced by CRF2 deletion in mice. These data clearly show that CRF2 indeed has a physiological role in the colonic secretomotor-response to acute-stress or IP CRF and point to the existence of a possible CRF1-CRF2 receptor interaction in the responses. In addition, the data that mUcn 2 not only blunts the acute stress-induced colonic contractions in WTL but also does so in mice under chronic stress setting, i.e. the CRF-OE mice, is of significance because chronic stress has more relevance in several diseases including colonic sensorimotor-responses in humans and animals (1-4; 40).
A potential target for the CRF1-CRF2 interaction in the colonic responses includes colonic myenteric neurons. This is supported first by the dense colocalization of CRF1-CRF2 and CRF2-nNOS in the rat colonic myenteric wholemount and primary neurons. Second CRF2 activation blunted IP CRF-induced myeneteric Fos-expression, a marker for neuronal activation (41), while blockade of CRF2 receptor enhanced the Fos-response. We previously established that IP CRF activates colonic myenteric neuron through peripheral CRF1 (33; 34) and that nearly all (96-98%) Fos-expressing cells are CRF1-IR (34). The study showed also that Fos-activation by IP CRF is correlated with increased defecation (34). Although Fos-expression is a general marker for neuronal activation (41), the fact that CRF1-CRF2 are colocalized on myenteric neurons and that CRF2 inhibits in tandem the CRF-induced myenteric neuron Fos-expression and defecation suggest that enteric neurons are direct or indirect targets of CRF ligands. In support for a direct action on enteric neurons, studies in guinea pigs have shown that CRF or Ucn 1 increases colon myenteric neurons firing through a direct action on neuronal CRF1 (25; 42). Collectively these findings point to a possible direct inhibitory action of CRF2 on CRF1-containing neurons and/or indirectly through the release of inhibitory neurotransmitters such as NO
Further evidence in support of a CRF1-CRF2 interaction in the observed CRF2 mediated inhibition of the colon to CRF and acute-stress come from data in the primary myenteric neuron and HEK-293 cell. CRF- and Ucn 1-activated myenteric neuron ERK1/2 is prevented by selective CRF2 agonist and selective CRF1 antagonist but enhanced by selective CRF2 antagonist. Since, CRF and Ucn 1 bind to both receptors, although with different affinities (5), the data clearly show that when both receptors are simultaneously activated, CRF2 modulates a CRF1 mediated event. The physiological significance of CRF2 dependent inhibition of a CRF1 mediated ERK activation in primary LMMP neurons can not be fully explained in the present study because we only assessed pERK levels at one time point and that events that precede or post ERK1/2 phosphorylation following CRF1 and/or CRF2 activation are not yet characterized in this system. However, it is shown that Fos-expression in enteric neuron is associated with defecation (34) and that c-fos transactivate transcription of several neurotransmitter biosynthetic enzymes that have AP-1 responsive elements including choline acetyltransferase (ChAT), a key enzyme in the synthesis of acetylcholine (43). Blockade of this cascade thus could interfere with the CRF1 mediated, at least neuronal activation, and possibly release and/or synthesis of neurotansmitters.
Similarly, CRF’s potency to induce cAMP production in CRF1/CRF2b transfected cells was 10-fold lower than that in cells transfected with CRF1 only. This potency loss in the double-transfected cells is unlikely to be due to differential availability of CRF. First HEK-293 cells do not constitutively express the CRF1 and CRF2 (11) and the density of transfected receptors is about the same. Second, the concentration of CRF used in the current study is 100 times in excess to the EC50 (8.6 nM) required to activate CRF1 receptors in the transfected cells. Third, although CRF1 has higher affinity (>4-20X) to CRF than CRF2 (5), CRF in low (nM) concentrations can induce a CRF2 mediated cAMP production in HEK-293 cells (11; 44). Thus, the blunted CRF-effect on cAMP production is likely due to a receptor-to-receptor interaction at coupling and/or signaling level. Such opposite effects of CRF may be explained through a dual mechanism where CRF-peptides, which bind to both receptors, activate cAMP primarily through Gs-coupled CRF1 and inhibit it through Gi-coupled CRF2 variant, as has been recently reported (44). It is conceivable also that CRF or Ucn 1 would activate both receptors, but that CRF1 remains desensitized longer than CRF2 as shown for NK1 and NK3 receptors (45) and/or that CRF2 activation may share intracellular signaling targets of CRF1.
Acute-stress triggers integrated responses to maintain homeostasis and ensure survival of an organism. In the absence of proper counter-regulation, the stress-response runs in an overdrive state that can become maladaptive and could predispose to diseases (46) The altered colonic motor response to acute or chronic stress following blockade or deletion of CRF2 is consistent with an adaptive counter regulatory role of CRF2 in the stress-response. Interestingly, in mice heart CRF2b confers cardioprotection that is lost following chronic stress-induced down-regulation of the CRF2b variant (47). Protective effects mediated by CRF2 are also reported in experimental visceral pain (48) and colitis (49). In line with this, the present data provide a basis for the concept that stress-related gut functional alterations should not be solely viewed through a CRF1-mediated pathway but also through a dampened CRF2 signaling that overstrains the colonic motor response to stress The notion is particularly relevant in view of the recent data on the lack of effect of a CRF1 antagonist to improve intestinal transit symptoms in IBS patients (50). Understanding the role of CRF2 and its regulation in the gut response to stress may open new pharmacological targets in the treatment of stress related disorders of the gut, such as IBS.
Supplementary Material
Acknowledgements
Work is supported by NIDDK Grants R21 DK-068155 and RO1 DK078676 (MM), DK-57238, (YT) and DK-41301 (Center Grant, Animal core, YT) and VA Career Scientist Award (YT); the French Foreign Office (Egide program) and the French Society of Gastroenterology (S.N.F.G.E.) (GG); DK PO1-26741 (JR); P01 DK 33506 (CP); KO1 DK083336 (EI). Authors thank Dr Stenzel-Poore M for the CRF-OE and CRF2-/- mice, Dr Dimitri Grigoriadis for supplying NBI-39564 and Ms HongHui Liang for technical support.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- CCA
colonic contractile response
- CRF
corticotropin releasing factor
- CRF1
corticotropin releasing factor receptor 1
- CRF2
corticotropin releasing factor receptor 2
- CRF2 -/- or CRF2-KO
CRF2 deficient mice
- CRF-OE
corticotropin releasing factor overexpressing
- EIA
Enzyme Immuno Assay
- ERK1/2
extracellular signal-regulated kinase1/2
- FPO
Fecal pellet output
- GMCs
Giant migrating contractions
- HEK
human embryonic kidney cells
- hUcn 2
human urocortin 2
- IR
immunoreactivity
- LMMP
longitudinal muscle myenteric plexus
- mUcn 2
mouse urocortin 2
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- PC-LMMPn
primary culture longitudinal muscle myenteric plexus neurons
- PRS
partial-restraint stress
- Ucn 1
urocortin 1
- WAS
water avoidance stress
- WLT
wild type littermate
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Santos J, Alonso C, Vicario M, Ramos L, Lobo B, Malagelada JR. Neuropharmacology of stress-induced mucosal inflammation: implications for inflammatory bowel disease and irritable bowel syndrome. Curr Mol Med. 2008;8:258–273. doi: 10.2174/156652408784533788. [DOI] [PubMed] [Google Scholar]
- 2.Maunder RG, Levenstein S. The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Curr Mol Med. 2008;8:247–252. doi: 10.2174/156652408784533832. [DOI] [PubMed] [Google Scholar]
- 3.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bale TL, Vale WW. CRF and CRF receptor: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 6.Grace CR, Perrin MH, Cantle JP, Vale WW, Rivier JE, Riek R. Common and divergent structural features of a series of corticotropin releasing factor-related peptides. J Am Chem Soc. 2007;129:16102–16114. doi: 10.1021/ja0760933. [DOI] [PubMed] [Google Scholar]
- 7.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 8.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 10.Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis D. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu SV, Yuan PQ, Wang L, Peng YL, Chen CY, Tache Y. Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology. 2007;148:1675–1687. doi: 10.1210/en.2006-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcher C, Juhem A, Peinnequin A, Sinniger V, Bonaz B. Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1091–G1103. doi: 10.1152/ajpgi.00302.2004. [DOI] [PubMed] [Google Scholar]
- 13.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 14.Chen A, Perrin M, Brar B, Li C, Jamieson P, DiGruccio M, Lewis K, Vale W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol. 2005;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- 15.Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2ÿ receptor. Mol Endocrinol. 1998;12:1077–1085. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- 16.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2α and CRF2β receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 17.Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardati A, Goetschy V, Gottowick J, Henriot S, Valdenaire O, Deuschle U, Kilpatrick GJ. Human CRF2 α and β splice variants: pharmacological characterization using radioligand binding and a luciferase gene expression assay. Neuropharmacology. 1999;38:441–448. doi: 10.1016/s0028-3908(98)00201-9. [DOI] [PubMed] [Google Scholar]
- 19.Refojo D, Holsboer F. CRH signaling. Ann N Y Acad Sci. 2009;1179:106–119. doi: 10.1111/j.1749-6632.2009.04983.x. [DOI] [PubMed] [Google Scholar]
- 20.Ising M, Holsboer F. CRH-sub-1 receptor antagonists for the treatment of depression and anxiety. Exp Clin Psychopharmacol. 2007;15:519–528. doi: 10.1037/1064-1297.15.6.519. [DOI] [PubMed] [Google Scholar]
- 21.Stengel A, Tache Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maillot C, Million M, Wei JY, Gauthier A, Tache Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–1579. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- 23.Larauche M, Gourcerol G, Wang L, Pambukchian K, Brunnhuber S, Adelson DW, Rivier J, Million M, Tache YF. Cortagine, a CRF1 agonist, induces stress-like alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Ren W, Qu MH, Bishop GA, Wang GD, Wang XY, Xia Y, Wood JD. Differential actions of urocortins on neurons of the myenteric division of the enteric nervous system in guinea pig distal colon. Br J Pharmacol. 2010;159:222–236. doi: 10.1111/j.1476-5381.2009.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trimble N, Johnson AC, Foster A, Greenwood-Van Meerveld B. Corticotropin-releasing factor receptor 1-deficient mice show decreased anxiety and colonic sensitivity. Neurogastroenterol Motil. 2007;19:754–760. doi: 10.1111/j.1365-2982.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 27.Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Tache Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1429–R1438. doi: 10.1152/ajpregu.00626.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- 29.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Tache Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 30.Bonaz B, Tache Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- 31.Gourcerol G, Wang L, Adelson DW, Larauche M, Tache Y, Million M. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol Gastrointest Liver Physiol. 2009;296:G992–G1002. doi: 10.1152/ajpgi.90436.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gourcerol G, Coskun T, Craft LS, Mayer JP, Heiman ML, Wang L, Million M, St Pierre DH, Tache Y. Preproghrelin-derived peptide, obestatin, fails to influence food intake in lean or obese rodents. Obesity (Silver Spring) 2007;15:2643–2652. doi: 10.1038/oby.2007.316. [DOI] [PubMed] [Google Scholar]
- 33.Miampamba M, Maillot C, Million M, Tache Y. Peripheral CRF activates myenteric neurons in the proximal colon {Yuan, Million, et al. 2007 8318 /id}through CRF1 receptor in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G857–G865. doi: 10.1152/ajpgi.00434.2001. [DOI] [PubMed] [Google Scholar]
- 34.Yuan PQ, Million M, Wu SV, Rivier J, Tache Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol Motil. 2007;19:923–936. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristensson E, Themner-Persson A, Ekblad E. Survival and neurotransmitter plasticity in cultured rat colonic myenteric neurons. Regul Pept. 2007;140:109–116. doi: 10.1016/j.regpep.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G544–G552. doi: 10.1152/ajpgi.00114.2001. [DOI] [PubMed] [Google Scholar]
- 37.Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am J Physiol. 1999;277:G275–G279. doi: 10.1152/ajpgi.1999.277.2.G275. [DOI] [PubMed] [Google Scholar]
- 38.Tomaru A, Ishii A, Kishibayashi N, Karasawa A. Colonic giant migrating contractions induced by glycerol enema in anesthetized rats. Jpn J Pharmacol. 1993;63:525–528. doi: 10.1254/jjp.63.525. [DOI] [PubMed] [Google Scholar]
- 39.Sarna SK. Myoelectrical and contractile activities of the gastrointestinal tract. In: Schuster MM, Crowell MD, Koch KL, editors. Schuster Atlas of GASTROINTESTINAL MOTILITY in Health and Disease. Second ed BC Decker Inc; Hamilton. London: 2002. pp. 1–18. [Google Scholar]
- 40.Choudhury BK, Shi XZ, Sarna SK. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1238–G1247. doi: 10.1152/ajpgi.90712.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krukoff TL. Expression of c-fos in studies of central autonomic and sensory systems. Mol Neurobiol. 1993;7:247–263. doi: 10.1007/BF02769178. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Gao X, Gao N, Wang X, Fang X, Hu HZ, Wang GD, Xia Y, Wood JD. Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J Comp Neurol. 2005;481:284–298. doi: 10.1002/cne.20370. [DOI] [PubMed] [Google Scholar]
- 43.Pongrac JL, Rylett RJ. Molecular mechanisms regulating NGF-mediated enhancement of cholinergic neuronal phenotype: c-fos trans-activation of the choline acetyltransferase gene. J Mol Neurosci. 1998;11:79–93. doi: 10.1385/jmn:11:1:79. [DOI] [PubMed] [Google Scholar]
- 44.Schulz K, Rutz C, Westendorf C, Ridelis I, Vogelbein S, Furkert J, Schmidt A, Wiesner B, Schuelein R. The pseudo signal peptide of the corticotropin-releasing factor receptor type 2a decreases receptor expression and prevents Gi-mediated inhibition of adenyly cyclase activity. J Biol Chem. 2010 doi: 10.1074/jbc.M110.129627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidlin F, Dery O, Bunnett NW, Grady EF. Heterologous regulation of trafficking and signaling of G protein-coupled receptors: beta-arrestin-dependent interactions between neurokinin receptors. Proc Natl Acad Sci U S A. 2002;99:3324–3329. doi: 10.1073/pnas.052161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 47.Sztainberg Y, Kuperman Y, Issler O, Gil S, Vaughan J, Rivier J, Vale W, Chen A. A novel corticotropin-releasing factor receptor splice variant exhibits dominant negative activity: a putative link to stress-induced heart disease. FASEB J. 2009;23:2186–2196. doi: 10.1096/fj.08-128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu VS, Wei JY, Rivier J, Vale W, Mayer EA, Tache Y. CRF2 receptor activation prevents colorectal distension-induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Im E, Rhee SH, Park YS, Fiocchi C, Tache Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology. 2010;138:2457–67. 2467. doi: 10.1053/j.gastro.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sweetser S, Camilleri M, Nord SJ Linker, Burton DD, Castenada L, Croop R, Tong G, Dockens R, Zinsmeister AR. Do Corticotropin Releasing Factor-1 Receptors Influence Colonic Transit and Bowel Function in Females with Irritable Bowel Syndrome? Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.