Abstract
The recent emergence of optical imaging has brought forth a unique challenge for chemists: development of new biocompatible dyes that fluoresce in the near-infrared (NIR) region for optimal use in biomedical applications. This review describes the synthesis of NIR dyes and the design of probes capable of noninvasively imaging molecular events in small animal models.
Keywords: Cyanine, fluorescence, FRET, imaging, optical, probe, rhodamine
1. INTRODUCTION
Fluorescent light is a natural phenomenon that has been known by humans for thousands of years. This fascinating event has been employed in many areas of research including the fields of chemistry and organic photochemistry for explaining reaction mechanisms and identifying other reactive intermediates such as carbenes, radical pairs, and singlet oxygen. It has been used in the areas of supramolecular chemistry, host-guest chemistry, and physics, especially for studying the electronic vibration of molecules. It has also found use in high technology research such as information processing, biophotonics, and optical data recording in the past decade. One of the latest applications of this phenomenon is in the biomedical imaging of disease. Optical imaging is an emerging technology, thanks to several research breakthroughs from a number of scientific areas including fluorescence technology, biology, and chemistry. Furthermore, the field is greatly indebted to the development of laser technology and physics, specifically for the invention of the CCD camera. Optical imaging has increasingly become a more favorable research tool as an alternative to other techniques.
In vivo optical imaging makes use of molecular probes to visualize the underlying mechanisms of biological processes at the cellular and molecular levels. In general, an optical probe is comprised of an active biological component tagged with one or many fluorophores. The mode of construction of the molecular imaging probes relies on the creativity of chemists. The attachment of fluorophores onto biological factors is diverse and can be exploited to exert the best reporting mechanism depending upon the study design. For example, using fluorescence resonance energy transfer (FRET) methods, researchers have created a conditionally activated fluorescent signal to gauge enzyme activity.[1] Other researchers have developed innovative methods to manipulate the intrinsic structures of dyes to provide logically activated modes that are indicated by enhanced quantum efficiencies, shifted spectrums, and/or altered fluorescence lifetimes.[2] Despite the large number of optical probes reported in the past few decades, they can be classified into three categories: targeted probes, topologically activated probes, and FRET probes.
Since scattering and auto fluorescence are prominent in living systems, it is imperative to tune the in vivo imaging parameters away from potential interference from endogenous fluorescence factors and into the near-infrared (NIR) spectral region (650-900 nm). NIR fluorescence imaging offers a considerable advantage over imaging in the visible range. Not only does it help to enhance the signal-to-noise ratio, but fluorescent labels associated with the NIR emission wavelengths also penetrate deeper into tissue than those associated with blue emission wavelengths. Owing to its excellent sensitivity and temporal resolution, optical imaging in the NIR window provides enormous potential for detecting diseases and monitoring therapy non-invasively. Furthermore, it allows for the assessment of treatment efficacy and thereby facilitates the potential to adjust and customize treatment in vivo.
While the importance of optical imaging in biomedical research is increasingly recognized, its usefulness is limited by the chemical synthesis of fluorescent probes. Unfortunately, there is very little reported literature that shows comprehensive information on probe development and subsequent application. In this review article, we hope to fill that gap by describing the fundamental design and synthesis of common fluorescent dyes that are currently used in molecular imaging. In the following sections, we will describe how probes are assembled onto biologically compatible scaffolds to create optical molecular probes. Finally, we will discuss the applications of these molecular probes for imaging a number of disease models in small animals.
2. FUNDAMENTALS OF FLUORESCENCE
When a small organic chromophore absorbs light, the molecule will excite from the ground state of the atom, S0, to a higher energy level excited state (mostly S1 and sometimes S2). Immediately, it will relax and return to the ground state via several mechanisms that depend on the structure of the molecule. In general, the cascade of electrons to the S0 state follows a radiation or radiationless pathway. In the latter mechanism, electrons move from the S1 energy level to S0 via internal conversion or intersystem crossing, thereby generating heat. The radiationless mechanism can be exploited in the field of photochemistry by creating highly strained organic molecules. The excited molecule relaxing to the ground state is associated with new bond formation due to changes in the molecule’s electronic configuration. In the radiation mechanism, the direct return of the excited electrons from the S1 to the S0 energy state emits fluorescent light at a wavelength corresponding to the energy difference of the two energy states. Because of this relationship, the atomic absorbance and emission spectra are usually mirror images of each other. The energy of emission is lower than that of excitation, which results in a longer wavelength than the excitation light. The peak difference between the excitation and emission spectra is called the Stokes shift. For cyanine dyes, the Stokes shift is very narrow and typically falls somewhere in the range of 30 nm. On the other hand, rhodamine dyes have much larger Stokes shift. In general, fluorescence intensity is proportional to the total number of fluorophores in the excited state. It is extremely sensitive to the surrounding environment, and therefore can be used to provide valuable information on biological processes. Environmental changes such as ion concentration, hydrophobicity, and pH can alter not only quantum efficiency, but also the actual spectra of the fluorophores.[3]
3. FLUORESCENT DYES
There are a large number of fluorescent dyes available for use in microscopy, immunohistology, and other high technology research. These dyes have extended conjugated carbon chains embedded in their chemical structures. The molecules are able to absorb light energy and emit light of a different color. According to molecular orbital theory, this phenomenon occurs due to electron transitions from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). The emission wavelengths of organic dyes can be fine tuned to emit light of longer wavelength by incorporation of an electron sink within the molecule that allows for delocalization of π electrons along the unsaturated chain.[4] Even though NIR dyes have been developed for many years for use in high technology fields, just a handful of them have found use for in vivo applications.
The development of NIR fluorescent dyes has played a critical role in the optical imaging field, allowing it to become an increasingly important contributor to imaging science. In contrast to the classical application of fluorescent dyes in other technologies; the design of fluorescent dyes for in vivo applications needs to incorporate several important criteria including (1) water solubility, (2) structural and chemical stability, (3) NIR fluorescence, (4) high quantum yield and last but, not least (5) a functional group for bioconjugation.
The chemical syntheses of fluorescent dyes have been well developed over the past few decades. A large part of the current knowledge base is a result of fundamentals discovered during the last half of the century.Novel reaction mechanisms have been developed that are paving the way for the rational design of more synthetically demanding dyes. In the narrow context of this review, we are going to highlight the fundamental design of two major families of dyes, namely the cyanines and rhodamines. The discussion focuses on the impact of their design for molecular imaging research and their potential capability for use in in vivo imaging. Efforts to improve their performance in biological applications will also be emphasized.
3.1. CYANINE DYES
Cyanine dyes are a large family of dyes that, in general, contain an unsaturated carbon chain linked by heterocyclic rings such as, but not limited to indole, quinoline, isoquinoline, benzothiazole, and benzooxazole. These ring systems play an important role in the final characteristics of the dyes. Additionally, these rings serve as a critical framework to fine-tune the photophysical properties of the dyes. Modification of the rings enhances the feasibility of the dyes for biological use by helping tune the emission wavelength of the dye into the NIR region. The color of the dye is generated by electron propagation along the polymethine moieties bridging the heterocyclic ring system. The quaternary amines on the rings act as electron sinks. As shown in Fig. (1), the wave function of polymethine cyanine dyes has equal contributions from two cationic resonance structures. What makes cyanine dyes unique compared to other dyes is their flexible chemistry that allows for modifications at many possible positions on the carbon backbone. These modifications allow for (1) the fine-tuning of the absorbance wavelengths as precisely as possible, (2) enhanced solubility, (3) chemical stability, and (4) functionalization.
Fig. (1).
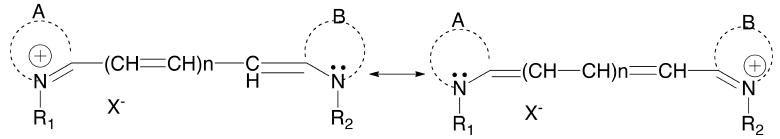
Representative chemical resonance structures of a cyanine dye.
Condensation reactions are a major synthesis pathway for the production of cyanines. For example, one of the early syntheses of hemicyanine dyes, Basic Yellow and its analogs were based on the condensation of Indole Fisher aldehydes with anilines in the presence of acetic acid.[5] Under these conditions, the rate-determining step of the reaction is the nucleophilic attack of the free amine on aniline with the carbonyl group of the Fisher base aldehyde.[6] Therefore, aniline was used in excess compared to the aldehyde in order to assure reaction completion via pseudo-first-order conditions. The concentration of acetic acid plays a crucial role in this condensation reaction. A high concentration of acetic acid decreases the free amine concentration and increases the amount of protonated amine. The protonated amine reduces the chance of nucleophilic attack toward the electrophilic carbon on the aldehyde by free amine. Such dyes were used in imaging cell membrane potentials and as intracellular pH sensors.[7]
A second condensation reaction used to make cyanines employs orthoester intermediates and reactive methyl groups on heterocyclic aromatic rings. One of the earliest reactions performed by Konig et al. at the beginning of the last century involved the condensation of benzothiazolium, a quaternary ammonium salt, with triethyl orthoformate in the presence of sodium tetrafluroborate (Fig. (2)). The blue-violet prism shaped product was collected by recrystalization resulting in an overall yield of 70%.[8] This approach is generally employed for synthesizing any trimethine cyanine dyes (Cy3), not only those with benzothiazole rings, but ones made with other ring systems such as N-alkylquinoline, N-alkylpyridine, and N-alkylbenzazole are also possible. The emission λmax of most trimethine cyanine dyes extends below 600 nm, and the molar extinction coefficient is high enough for use in microscopic or in vitro imaging. However, they are not suitable for in vivo studies given the limited tissue penetration of visible light and the high background fluorescence signal caused by intrinsic biological materials such as hemoglobin and others that can emit light in the visible spectrum (400-600 nm).
Fig. (2).
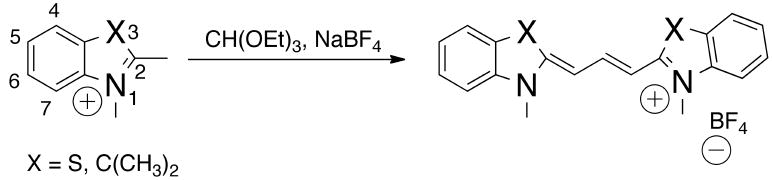
Synthesis of Cy3 dyes.
In an effort to develop polymethine cyanine dyes that absorb longer wavelengths for in vivo imaging applications, several studies have focused on polyenes since each double bond enhancement in this region would increase the bathochromic shift (sometimes called vinylene shift) by ~100 nm.[9] This feature shows the advantage of cyanines compared to other dyes where tuning is contingent upon the expansion of the aromatic rings. Several lines of research have demonstrated that addition of an aromatic 6-membered ring would shift the absorbance by approximately 20 nm.[9] The major drawback to this approach is the increased hydrophobicity of the resulting compound.
Various NIR polymethine dyes became available at the beginning of the 1930s and proved very useful in photographic sensitization up to 1300 nm.[4] It was not until a few decades later that biologically useful NIR dyes were developed with reasonable chemical accessibility. To generate dyes with a bathochromic shift, a number of innovative chemistries have been developed to generate extended and reactive unsaturated carbon chains. For example, Mujumdar et al. reported the synthesis of NIR polymethine cyanine dyes employing polyene intermediates with longer carbon chains.[10] The key intermediate underlying this pioneering approach for making dyes for in vivo imaging was the synthesis of indoleninium-5-sulfonate from p-hydrazinobezenesulfonic acid and methyl isopropyl ketone by Fisher indole condensation. Condensation of an indolenine intermediate with malonaldehyde dianil salt or glutaconaldehyde dianile salt resulted in NIR cyanine dyes. The sulfonate-associated cyanine dyes have SO3- groups at the 5 and 5’ positions to enhance their water solubility. Incorporation of multiple sulfonate groups in the dye structure not only increases water solubility, but also improves the brightness of labeled products in biological medium resulting from the minimization of dye aggregation and the reduction of nonspecific binding to cellular constituents. The dyes are pH insensitive, making them suitable for in vivo applications.
The choice of dye end groups can also contribute to the dye spectra. Dyadyusha and Kachkovskii et al. developed a clever set of rules based on topology to explain how to tune the color of dyes.[11] Using the molecular orbital perturbation theory, paying particular attention to π-electrons of aromatic end rings, the absorbance of a cyanine dye is not only affected by polymethine bridges, but also by the electron-donating power and the effective length (L) of the terminal groups. Altogether, it seems the combination of extending the polyenes, together with the electronic effect, play a key role in the design of compact NIR dyes for in vivo imaging. To illustrate the power of using the electronic effect on cyanines, Pham et al. developed two distinct azulene-based NIR dyes.[12] Even though the two dyes have an equal number of unsaturated carbons, the electron-donating group of guaiazulene at the aromatic end of the ring helped to red-shift the dye up to 50 nm. Thus, this rule is a powerful tool for tuning the wavelength of NIR dyes as shown in Fig. (3).
Fig. (3).
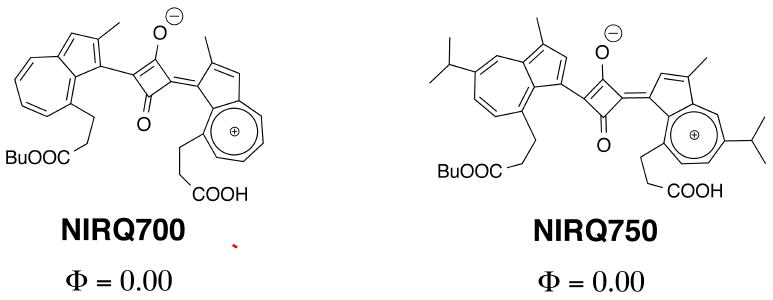
Azulene-based squaric NIR dyes.
For most biological applications, the stability of fluorescent dyes is crucial for sustaining their functions over the course of a study. This issue is worth mentioning, particularly for cyanine dyes, given that most of the polymethine bridges connecting the heterocycles of cyanines are linear carbon chains with the potential for rotation or flip-flop of the end rings thereby destroying the dye. The issue becomes more problematic when the dyes need to be tuned for NIR wavelengths. To overcome this drawback, Reynolds et al. proposed making the polymethine chain rigid by incorporating the methine groups into a cyclic frame, thus minimizing rotation.[13] This work demonstrated that five- and six-member rings in the methine groups make the dyes a hundred-fold more stable than their linear counterparts.
Polyene Modifications
Symmetrical synthesis of polymethine cyanine dyes are highly preferred because purification would be very complicated and time consuming otherwise. However, if the end groups have been incorporated with a handle for conjugation, symmetrical synthesis results in dyes with two activating groups, which may result in cross-linking of different amino groups on the same target or on different targets.[14] One solution to resolve this drawback is asymmetrical synthesis.
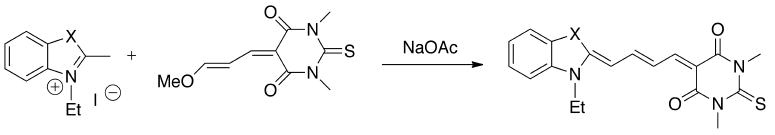
Nonetheless, experience from past studies shows that asymmetric synthesis usually results in low yields, and the by-product, specifically the symmetric dyes, mix with the desired product making purification a challenging task.[15] Altogether, efforts have focused on the modification of the polymethine for bioconjugation. This focus enables symmetric synthesis. Furthermore, it allows for incorporation of sulfonate groups to increase water solubility. Shan et al. reported a novel approach for making monofunctional pentamethines by incorporating functional groups into malonaldehyde dianil intermediates.[16] The malonaldehyde dianil derivatives were prepared via carboxylic acid intermediates, which were synthesized from methyl 5,5-dimethoxyvalerate by the Vilsmeier-Haack-Arnold mainoformylation followed by basic hydrolysis. This chemistry enables the incorporation of a number of common functional groups such as carboxylic acids, alkynes, or azides. The latter allows for coupling of dyes to biologically relevant molecules via “click” chemistry.
Modification of the polymethines can serve several different purposes. Pham et al. modified the polymethine aiming to develop large Stokes shift dyes for multiplex imaging.[17] In this work, the nucleophilic substitution (SNR1) at the central vinylogous halide carbon (C(sp2)-X) by an amine moiety from an alkylamine contributes to the intramolecular charge transfer (ICT) process.[18] As a result, the perturbation of the electronic effect causes an apparent hypsochromic shift. This shift causes a large Stokes shift of approximately 150 nm, which allows for the use of this dye along with other conventional NIR fluorophores for imaging multiple targets in one environment (Fig. (4)).
Fig. (4).
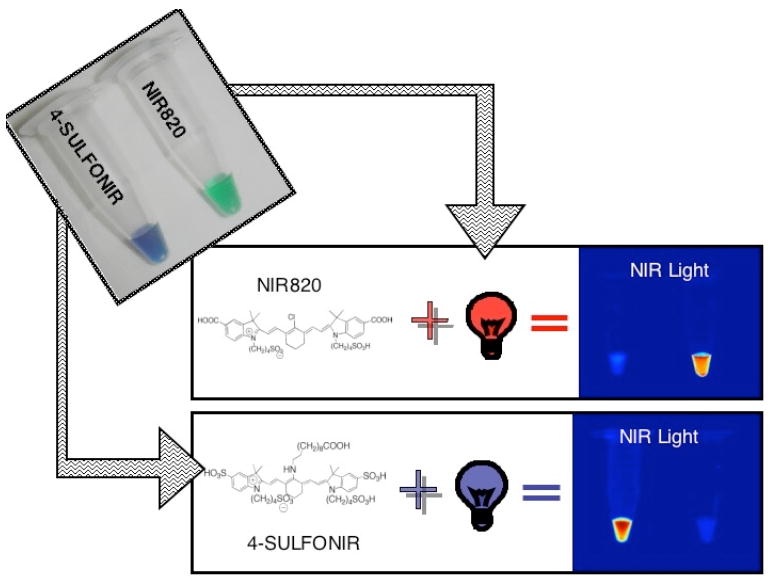
Multichannel-imaging using large Stokes shift dyes. NIR820 fluoresces when both samples are irradiated with light at an excitation wavelength of 750 nm. Switching the excitation wavelength to 600 nm causes only 4-SULFONIR to fluoresce.
So far, all of the condensation reactions discussed above provide cyanines with an odd number of methine carbons. It is possible to synthesize cyanines with an even number of methine carbons, also known as merocyanine dyes. For this particular chemistry, it is necessary to have an activated methylene group on a heterocycle, which acts as a nucleophile and reacts with another activated polymethine (Fig. (5)). As a result, merocyanines usually have asymmetric structures. There are three unique differences between these dyes compared to other cyanine dyes. First, merocyanines are uncharged colored molecules (sometimes referred to as neutrocyanines). Second, they contain electron-withdrawing and electron-donating substituents on their end rings, and third, the degree of aggregation of merocyanines in solvent is typically less profound when compared to the corresponding cationic polymethines. Although merocyanine dyes have not been used in molecular imaging, they have been used as fluorescent probes for the determination of polarity gradients in biological media, intracellular pH, microviscosity of membranes and biopolymers, and as markers for specific biological sites.[19] It is worth mentioning their potential for use as contrast agents for in vivo imaging since the tuning of the emission wavelengths of these dyes into the NIR region is simple and robust. For example, with a very compact structure, merocyanines emit at very long wavelengths extending into the NIR region when dissolved in polar solvents. Moreover, the photophysical properties of these dyes are very sensitive to changes in the electronic effects on the end groups. Therefore, merocyanines have wide applications in high technology. Some of the most well known examples of the merocyanines are the Spiropyrans (Fig. (6)). This family of compounds has found wide application in memory switches[20], logic devices[21], displays, non-linear optics, and other nanodevices given their ability to conditionally control switching from the neutral, closed form to the charge-separated merocyanine isomer.
Fig. (5).
Synthesis of cyanine dyes with an even number of methine carbons.
Fig. (6).
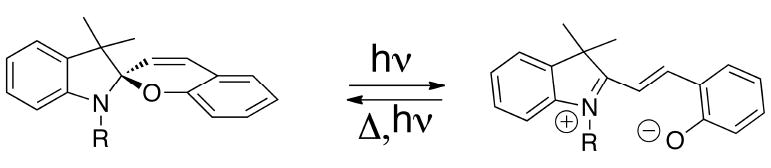
Closed (spiro) and open (merocyanine) structure of Spiropyran.
Because of the nature of an asymmetric molecule, the syntheses of merocyanine dyes that contain electron donors and electron acceptors on the ends of polyenes often encounter multiple challenges. The synthesis of these compounds requires careful design in order to minimize undesired products of symmetric cationic or anionic dyes. The problems become particularly more challenging when synthesizing merocyanines with long polyenes. Not only are there a large number of side reactions and difficulties with product isolation, but the scarcity of the starting materials is also a concern.[22] However, tetramethinemerocyanines and hexamethinemerocyanines can be synthesized using commercially available malonaldehyde and glutaconaldehyde derivatives, respectively.
3.2. RHODAMINE DYES
Rhodamines and oxazines have honeycomb-like structures since they are architectural clusters of hexagonal frameworks formed by conjugated π-systems. Because of their structure, this super family of dyes exhibits notable photostability and photophysical properties when compared to other families of dyes. Many rhodamines found use in a number of biological studies even before the arrival of molecular imaging such as in microcopy, histology, and as molecular switches[23]. Some well known versions have common names like rhodamine 6G, rhodamine B, Alexa dyes, TAMRA, and Texas Red.
Simple rhodamines can be synthesized using condensation reactions between phthalic anhydride and m-aminophenol in the presence of concentrated sulfuric acid (Fig. (7)). A few unique physical characteristics that make rhodamines different from cyanines include high absorption coefficients, larger fluorescence quantum yields, and broad fluorescence in both the visible and NIR regions.[24] Another interesting feature of rhodamines is the relationship of the structural amino end groups towards temperature, pH, or polarity. This phenomenon is explained by non-radiative deactivation by internal conversion. Internal conversion has both activated and non-activated components.[25] If the end amino groups on rhodamine carry none or only one alkyl substituent at each nitrogen, or when the amino groups are rigid, the activation process is absent and the quantum yield of these dyes is very high and independent of temperature. However, N-dialkyl substitution introduces a dominant, activated internal conversion. As a consequence, the quantum yield and fluorescence lifetime varies with temperature.[25]
Fig. (7).
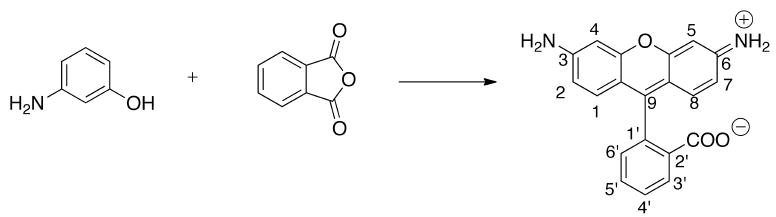
Common synthesis of rhodamine dyes using m-aminophenol and phthalic anhydride.
The capability to switch fluorescence on and off upon modification of the amino groups make rhodamines versatile probes for in vitro analysis by a number of important biological assays. In this construct, the free amino groups are acylated with specific substrates for proteases, caspases, and other kinds of enzymes such as esterases. The fundamental underlying design is that when the amino groups are acylated, rhodamine converts into a lactone due to the rearrangement of the π-conjugated system. The fluorescent signal intensity of rhodamine is reduced significantly when the dyes are locked in the lactone form. For example, Chandran et al.,[26] recently developed activated rhodamines by acylation of the 3- and 6- amino groups with a “trimethyl lock” to quench the fluorescence (Fig. (8)).[26] The “trimethyl lock” strategy employs the use of strain to enhance reactivity. The o-hydroxycinnamic acid introduces unfavorable steric interactions between the three methyl groups which induces facile lactonization to form a hydrocoumarin.[27] By treating these latent probes with Hela cells, the intra-cellular esterase removes the acetate moiety and triggers delactonization resulting in enhanced fluorescence.
Fig. (8).
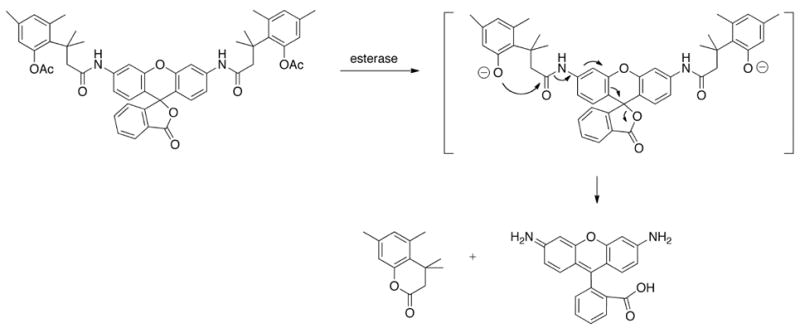
“Smart” activation of a rhodamine dye by hydrolysis of bis(acetylated trimethyl lock) Rhodamine 110 by an esterase.
For imaging applications, rhodamines are designed with functional groups to meet certain parameters. The functional groups shift the emission of the dyes into the NIR range and make the dyes more soluble. The usual method for making functionalized rhodamines is by the condensation reaction between m-aminophenol and trimellitic anhydride. This reaction produces isomeric products where the carboxylate group is derivatized at either the 4’ or 5’ positions. The dyes are activated as succinimidyl esters using dicyclohexyl carbodiamide and N-hydroxysuccinimide. However, it is apparently more difficult to develop rhodamines with isothiocyanates as an amine-reactive group. In this approach, Riggs et al.[28] demonstrated the synthesis starting with the condensation reaction between m-diethylaminophenol and p-nitrophthalic anhydride (Fig. (9)). The nitro group from the product was reduced to an amine that was further converted to isothiocyanate using thiophosgene.
Fig. (9).
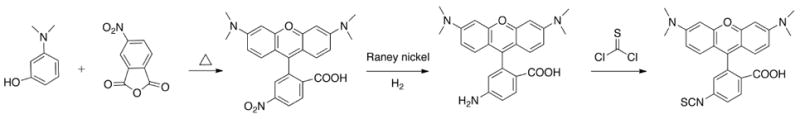
Classical synthesis of rhodamine dyes with an isothiocyanate activating group.
Perhaps one of the biggest breakthroughs in the development of rhodamines for in vivo applications stems from the work of Lefevre and Haugland et al. on the modification of Rhodamine Red-X© from Texas Red sulfonyl chloride and rhodamine B sulfonyl chloride.[29] The dyes have strong red fluorescence, chemical stability, and water solubility. These rhodamines are unique in that the carboxylic group at the 2’ position is removed so the dyes are always locked in the strongly fluorescent mode. In addition, attaching a linker for bioconjugation through the sulfonyl chloride helps reduce instability that the precursor dyes usually encounter. The authors demonstrated the versatility of the dyes by activating them as either a succinimidyl ester or a maleimide for labeling amine or sulfhydryl groups, respectively.[29]
Due to the intrinsic nature of their structure, it is more demanding to tune rhodamines to extend their emission wavelength into the NIR region compared to cyanines. One of the reasons researchers hesitate to expand the rings in the backbone of rhodamines is because they will become much more hydrophobic. To overcome many of the shortcomings associated with ring expansions, Sauer et al.[30] demonstrated that incorporation of CF2 in place of the aromatic ring at position 9 on the xanthene backbone helps shift the dye to longer wavelengths (Fig. (10)). It was also found that placement of two additional double bonds into the outside rings help to create a promising NIR feature and a greater than 30 nm bathochromic shift compared to comparable molecules that do not possess the extra olefins.
Fig. (10).
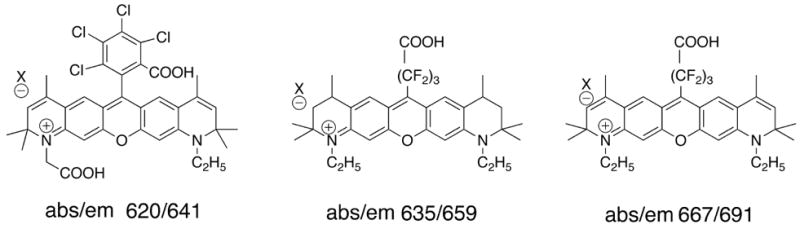
Rhodamine dyes with long wavelengths.
4. Optical Imaging Probes
Optical imaging is one of just a few imaging modalities at the forefront of in vivo molecular imaging research. Optical in vivo molecular imaging techniques refer to the imaging of light photons with charge-coupled device (CCD) cameras that allow for imaging of light both within and outside the visible range.[31] Compared to other imaging modalities, optical imaging techniques are cost effective, widely available, and do not involve any form of ionizing radiation. The most often used optical techniques for in vivo imaging are bioluminescence and fluorescence intensity imaging (FII).[31] Bioluminescence refers to the production and emission of light by a living organism. This naturally occurring, inherent chemiluminescent reaction is used in imaging to follow enzymatically mediated chemical reactions in vivo that produce light.[31] FII on the other hand, uses injected or applied fluorophores that are stimulated to fluoresce by an exogenous light source.[31] FII is more applicable to potential clinical use due to the fact that bioluminescence methods involve gene transfer techniques that result in cross species proteins.[31] Therefore, this section will focus on FII and the wide range of molecular probes that have been developed for this optical imaging technique.
FII is very sensitive, and as little as 10-9 to 10-12 molar concentration of a probe can be imaged.[31],[32] FII also has very good spatial resolution (2-10 mm).[32] FII can incorporate the use of a wide range of fluorophores that are available (including GFP, RFP, NIR fluorophores, and quantum dots), with emission spectra ranging from the visible spectrum (390-650 nm) to the near infrared (650-900).[32] Most fluorescent-based probes display only modest fluorescence changes, which leads to insufficient resolution. This insufficient resolution is a result of low fluorescent signal amplification and poor selectivity of the imaging probe for the molecules or events of interest.[33]
Progress in the field of optical imaging is becoming more and more dependent on the development of novel imaging probes.[33] Strategies that amplify the fluorescent signal and/or boost specific target recognition properties are driving the development of highly sensitive fluorescence-based imaging probes.[33] In recent years, fusing of fluorophores with materials such as peptides, polymers, and different metals has increased the pool of available imaging probes.[33] Optical imaging probes can be lumped into two generalized groups: probes that can be targeted and those that can be activated. Targeted probes accumulate at a region of interest (Fig. (11)). Therefore, the signal of the probe increases over time, but they are always “on”. Activated probes are those that can be turned “on” or “off”, usually through some type of quenching mechanism (Fig. (12) and (13)). Activated probes may also be referred to as “smart” imaging probes. The vast majority of in vivo optical imaging probes fall into the latter category.
Fig. (11).
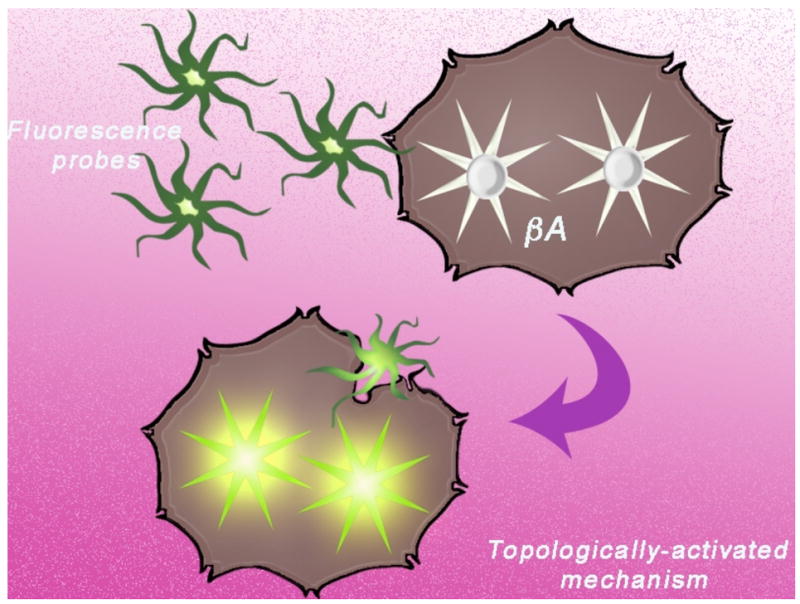
Mechanism of activation of topologically activated fluorescent dyes. Dyes change their conformation due to a change in environment leading to an increase in fluorescence. For example, conformational degrees of freedom quench the fluorescent signal when the dye is unbound, but upon binding to β-amyloid plaques, the dye becomes rigid and fluoresces.
Fig. (12).
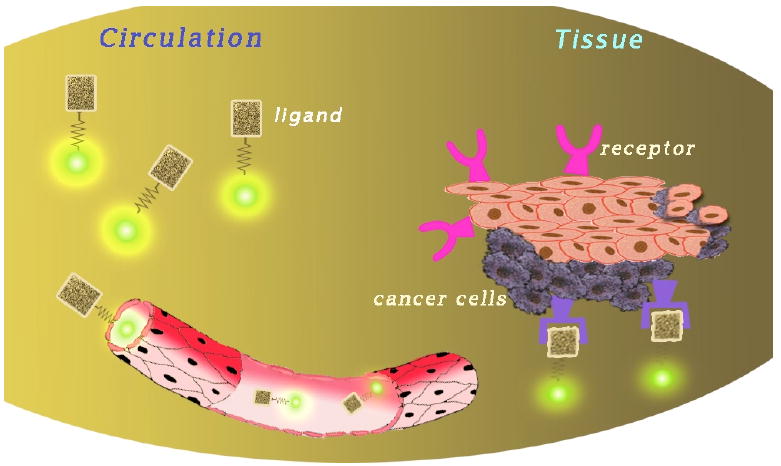
Mechanism of activation of targeted optical imaging probes. Dyes are conjugated to a ligand and circulate through the body until they reach the desired target and accumulate in tissues rich in the targeted receptor.
Fig. (13).
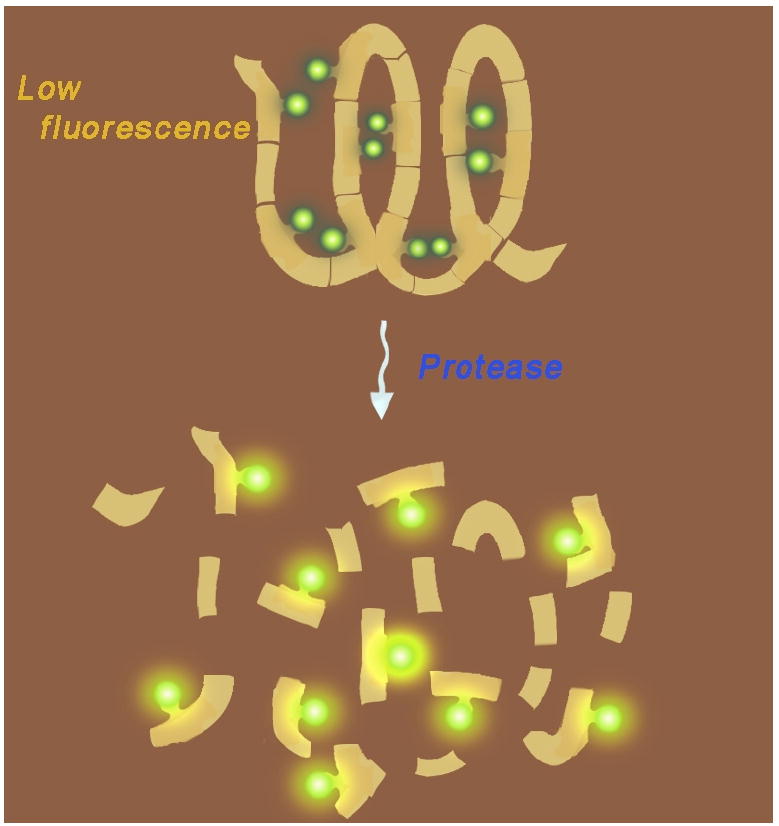
Mechanism of activation of FRET and self-quenched optical imaging probes. In the intact probe, donor and acceptor dyes are in close proximity to one another and fluorescence is quenched. Upon cleavage, fluorescence increases due to spatial separation of the dyes.
4.1. Targeted optical probes
There are broad arrays of exogenous fluorescent dyes that are available to make optical imaging probes. NIR cyanine-based dyes are the most commonly used for in vivo FII. Indocyanine green (ICG) is the only NIR fluorescent dye currently approved for use in human subjects.[34] ICG is a nonspecific contrast agent that does not have reactive functional groups that would allow it to be conjugated to biological carriers to enhance its target selectivity in vivo. However, it can be used to image the lymphatic system. Rasmussen et al. has shown that ICG, which can be excited between 760 and 785 nm and fluorescence imaged between 820 and 840 nm, can be used to passively image the lymphatic system in human subjects.[35] More selective imaging probes of the lymphatic system will be described later in this review.
Although ICG is nonspecific, analogs of ICG have been synthesized which allow them to be conjugated to biological carriers.[36] These functionalized fluorescent probes allow researchers to obtain tissue-specific or molecular information with an optical contrast agent.[37] Many carriers can be used to impart molecular selectivity to fluorescent optical dyes. Peptides, proteins, antibodies, and aptamers have all been used in the synthesis of targeted fluorescent optical probes.[38]
For example, early studies observed the behavior of synthesized ICG analogs conjugated to peptides that were targeted to somatostatin and bombesin receptors.[36] This approach resulted in the compounds being preferentially localized over the course of 24 hours in tumors known to over-express somatostatin and bombesin receptors.[36] Moon and Tung et al. decided to target folate receptors (FR) since they are overexpressed in many cancers.[39] Using the NIR fluorophore NIR2, the group showed that the FR targeted probe significantly increased tumor fluorescence intensity and contrast compared to normal tissue when compared to the nontargeted fluorescent dye.[39] Another group used this same method to develop a NIR dye-labeled hexapeptide, Cyp-GRD, that was formulated to target non-small cell lung cancer cells (A549) in a whole-body fluorescent lifetime imaging study in mice.[34]
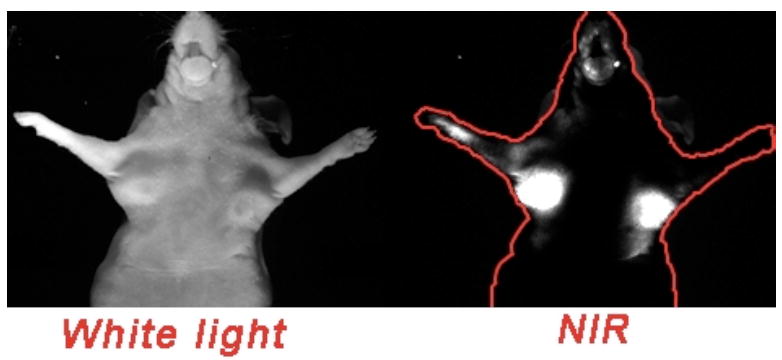
In a different approach, Pham et al. developed a novel water-soluble cyanine dye that incorporated a cyclic polymethine.[15] This dye was conjugated onto a peptide capable of targeting the uMUC-1 antigen. In vivo NIR optical imaging on tumor bearing athymic nude mice (tumors induced from injection of human pancreatic adenocarcinoma cells) was performed and the fluorescence signal was found to be 2.5 times greater in the tumors versus muscle (Fig. (14)).
Fig. (14).
Targeted imaging probe: imaging of uMUC-1 tumor antigen in a xenograft mouse model. Picture modified and used with permission from Ref. 15.
Lysosomes have also been targeted with fluorescent optical probes to potentially elucidate their role in cancer.[40] Recent studies have shown that NIR probes conjugated onto glucosamine have selectivity for lysosomes.[41] These NIR fluorescent glucosamine-bound probes demonstrated selective accumulation in lysosomes in human breast cancer tumor xenografts.[40]
Another tactic for making peptide-dye conjugates has been to use the dye as the scaffold and attach multiple proteins to it. A recent study made use of multimeric RGD peptide-dye conjugates to target αVβ3 integrin receptors.[42] Using the ICG analog cypate, they were able to conjugate one or two RGD peptide chains with each chain containing up to four linear RGD units. In vitro binding assay results showed that the binding affinity of linear RGD peptide-dye conjugates increased with a greater number of RGD units and was similar to control and cyclic RGD peptide conjugates.[42] In vivo results showed that probes with greater binding affinities localized in tumors faster, but after 24 hours, low binding compounds were also retained in the tumor.[42]
Tagging technology has also been used to make fluorescent peptides and proteins.[43], [44] Using a linear, random 7 amino acid phage display library, Kelley et al. were able to determine a peptide sequence (IQ-tag) that had subnanomolar binding affinity for a variety of (benz)indolium fluorophores.[43] They envision the IQ-tag system will be amenable to a number of different modalities and will find use in site-specific protein expression and cell tagging applications to name a few. Other studies have used the dehalogenase-based protein-Tag (HaloTag) system to image cancer in vivo.[44] Introduction of highly expressed HaloTag receptors in cancer cells followed by an external injection of fluorophore-conjugated dehalogenase-targeted linkers allowed for successful in vivo cancer imaging. In fact, the HaloTag system can be used for practically every step of cancer imaging studies: from in vitro validation and quantification of receptor expression to in vivo imaging and ex vivo histological analysis.[44] Since there are a wide range of fluorophores that can be attached to the reactive linker, an optimal color can be selected to meet the requirements of whatever experiment is being performed. The HaloTag system allows the tumor to be imaged at various wavelengths without changing the underlying tumor model.[44]
Targeted optical imaging probes have more uses than just detecting tumors. Many groups have shown fluorescent optical probes to be useful in visualizing antitumor treatments. Apoptosis is a programmed cell death process in multicellular organisms that plays a key role in the pathogenesis of many disorders.[33] Due to the fact that many effective antitumor/anticancer therapies initiate apoptosis, non-invasive methods to detect the progression of apoptosis could clinically assist in determining whether chemotherapy regimens are working appropriately.[33] Conjugation of fluorescent Cy5.5 dyes to annexin V allows for the imaging of cell death resulting from tumor response to chemotherapy.[45], [46] Labeling annexin V with NIR700 dye allowed for imaging tumor response to trastuzumab in mouse models of HER2-overexpressing breast cancer.[47]
Tracking of cells and mapping of the lymph node system can also be accomplished using targeted fluorescent optical imaging probes. Pham et al. recently showed that dendritic cells can be tracked in vivo using FITC conjugated to a myristoylated polyarginine peptide.[48] This peptide sequence has also been shown to cross the blood-brain barrier with a fluorescent dye (Cy5.5) payload and remain intact.[49]
As stated previously, the lymphatic system has been imaged with ICG.[35] However, in order to image multiple lymph nodes at one time, a multicolor nanoprobe has been developed. Building upon a generation-6 PAMAM dendrimer scaffold, five different NIR fluorophores were conjugated onto dendrimers resulting in fluorescent probes with nearly identical size and chemical characteristics.[50] Only one specific fluorescent dye was attached to each dendrimer with at most four dye molecules conjugated onto each scaffold in order to minimize dye self quenching. By using three different excitation filters, the five distinct fluorescent dyes used could be differentially excited simultaneously and the lymphatic drainage of the head and neck region of mice could be imaged in vivo.[50]
An interesting use of fluorescent optical imaging probes is their use in the optical imaging of bacterial infections. It was recently discovered that zinc (II) dipicolylamine (Zn-DPA) coordination complexes have a high affinity for the anionic surfaces of bacterial cells and apoptotic animal cells.[51] Studies have shown that a NIR fluorophore attached to Zn-DPA ligands can stain bacterial cells in vitro and selectively target them in vivo.[51], [52]
There are wide ranges of targeted fluorescent optical imaging probes being developed. These techniques allow for highly specific targeting using low amounts of fluorophores. However, these techniques may not have the best signal-to-background ratio since non-bound probes are also fluorescent and therefore contribute to background noise.[37], [32], [38]
4.2. Activated optical probes
Alternative strategies employed to impart molecular selectivity into optical imaging contrast agents is to design activatable probes. These probes interact with the intended target and undergo a chemical reaction or conformational change causing the probe to switch from a weakly fluorescent state to a strongly emitting form.[37-38] A number of mechanisms can impact the emission of a fluorophore: (1) photon-induced electron transfer (PeT), (2) fluorescence resonance energy transfer (FRET), and (3) self-quenching to name a few.[31]
The PeT mechanism involves the silencing of a fluorophore via electron transfer from a donor to acceptor fluorophore where the donor and acceptor are part of the same fluorophore.[53] While useful, PeT probes will not be discussed further in this review. A more common approach to developing activatable probes is the combining of a fluorophore and a quencher which fluoresces when activated by spatial separation.[31] This approach involves the FRET or self-quenching mechanisms. FRET results from the nonradiative transfer of energy between a donor (dye) and an acceptor (dye or quencher) to prevent the radiative transition of an electronically excited dye which usually results in fluorescent output.[33] Similarly, many fluorophores can self-quench if conjugated to the same targeting substrate in close proximity.[31]
4.2.1. Topologically Activated Probes
Recent studies have investigated the potential use of NIR fluorescent probes in neuroimaging, particularly for the imaging of amyloid-beta (Aβ) plaques.[54], [2], [55] Optical probes for neuroimaging must meet certain requirements similar to the fluorescent probes already mentioned. However, they must also be able to rapidly cross the blood-brain barrier (BBB) after intravenous injection.[54] In order to enter the brain, the fluorescent optical imaging probes must be relatively small (approximately 600 Da) and relatively lipophilic (and therefore, should not bear ionic groups).[54], [55] To meet these criteria, small organic dyes having substantial conformational freedom while free in solution have been developed. The dye remains in its quenched “off” state while unbound and conformationally free. However, upon binding to Aβ, the dyes become conformationally restricted. The increase in rigidity of the dye upon binding decreases vibrational-rotational processes coupled to ground and excited states, decreases the radiationless decay rate, and increases the fluorescence quantum yield of the bound molecule.[54]
Using known Aβ-staining compounds such as Congo Red and Thioflavin T as guides, Bacskai et al. have designed small organic dyes suitable for in vivo imaging of Aβ by utilizing a push-pull architecture with terminal donor and acceptor moieties that are interconnected with a highly polarizable bridge.[54], [2] By inserting various donor and acceptor groups, they are able to manipulate the relative energies of the HOMO and LUMO; better donor and acceptor pairs led to a smaller HOMO-LUMO gap and to the desired absorption/emission bands.[54]
Other studies have found that modifications to the structure of Curcumin also led to fluorescent organic dyes that target Aβ plaques.[55] Incorporating a difluoroboronate group and N,N’-dimethyl groups in place of the phenolic hydroxyls of Curcumin, Ran et al. were able to synthesize an organic molecule with red-shifted absorption and emission upon binding to Aβ40 aggregates.[55]
Topologically activated probes have also been developed to image enzyme activity. For instance, Ras proteins play important roles in many cell signal transduction pathways including those involving cell division cycles, programmed cell death, and differentiation. It has been reported that misregulation of Ras proteins is responsible for 90% of pancreatic, 50% of colon, and 30% of lung and breast cancers.[56] The proteins must be localized to the inner surface of the plasma membrane which occurs via post-translational modification mediated by farnesyl protein transferase.[56] Pham et al. exploited this post-translational modification using dansyl and dapoxyl dyes that show strong fluorescence emissions in organic solvents, but very weak emissions in water. Studies showed that conjugation of the dapoxyl dye to the N-terminus of the Ki Ras-B peptide sequence followed by farnesylation in an enzyme assay resulted in a 2-fold increase in fluorescence signal compared to a control probe.[56]
4.2.2. FRET Probes
Small Molecule FRET Probes
A common approach to activatable probe development is combining a fluorophore (donor) and quencher (acceptor) (FRET mechanism). In the case of peptides, the fluorphore and quencher are attached to opposite ends of the peptide linking them. These probes are activated by target biomolecules that induce cleavage or recognition, which in turn generates amplified fluorescence signal by increasing the physical distance between the dye and quencher.[31], [33]
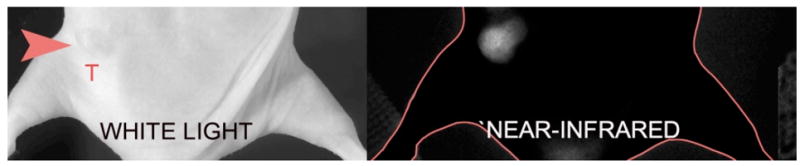
Imaging of protease activity seems to be the major use for FRET activated fluorescent imaging probes. Pham et al. recently designed a low molecular weight peptide-based NIR fluorescent probe designed primarily to sense tumor associated matrix metalloproteinase (MMP-7) activity (Fig. (15)).[1] Using a NIR fluorophore they had synthesized (NIRQ820)[12b], they found it was an efficient quencher for the commercially available fluorophore Cy5.5.[1] After attaching Cy5.5 and NIRQ820 onto a core MMP-7 substrate, they found that the probe was 7 times more fluorescent after enzymatic degradation in an MMP-7 biological model.[1] The Matrisian group built upon these concepts with larger molecules, which will be discussed later. Pham et al. also designed a caspase-3 activatable peptide-based probe by directly attaching a synthesized nonfluorescent azulene quencher (absorbance of 750 nm) and Alexa Fluor 680 onto a cleavable peptide substrate.[57] Treatment with caspase-3 induced over a four-fold increase in fluorescent signal while no activation was observed in the presence of a caspase-3 inhibitor.
Fig. (15).
“Smart” activated probe using FRET technology: imaging of MMP-7 activity in nude mice (unpublished results courtesy of Dr. Pham, image taken by Dr. Yongdoo Choi).
Most MMPs reside in the extracellular space. Therefore, MMP-activatable probes can be used to target the tumor microenvironment rather than the cells themselves.[33] In recent studies, cell-penetrating peptides (CPPs) have been used to image proteolytic activation in tumors.[58] These cell-permeable extracellular probes are selectively activated near cancer cells and then transported into near-by cells.[33] Tsien and colleagues used this strategy and designed activated CPPs (ACPPs) composed of a polycation and a polyanion that were fused together with a cleavable linker. The polycation sequence was able to penetrate the cell membrane along with attached cargo (in this case a Cy5 dye), but the attachment of a polyanion sequence effectively neutralized the effect via electrostatic interactions. When neutralized (in the absence of MMPs), uptake was effectively blocked. In the presence of MMPs the linker was cleaved, freeing the polycation from the polyanion, and the Cy5 payload was thus internalized by the cell. These ACPPs successfully identified tumors overexpressing MMP-2 and -9.[58] It was theorized that FRET pairs can be attached to the termini of the peptide with the quencher on the polyanionic sequence and the fluorophore on the polycation.
Similar studies have used intracellular protease-mediated fluorescence activation and retention as a molecular imaging strategy. This strategy is similar to the ACPPs discussed previously, except that the membrane permeable peptide remains in a quenched state free to travel into and out of cells in the absence of a target enzyme. Upon intracellular proteolysis, the charged fluorescent tag is cleaved from the permeation peptide leading to signal accumulation as the quencher is washed out of the cell into the extracellular space.[59]
The intracellular protease-mediated fluorescence activation strategy has found use in the imaging of cell death. Bullok et al. have recently reported the development of a permeation peptide fluorescent probe that is caspase activatable (TcapQ647).[60], [61] TcapQ647 is comprised of an all D-amino acid Tat-peptide-based permeation peptide sequence (comprised mostly of arginine) to afford cell penetration and an L-amino acid effector caspase recognition sequence. Flanking the caspase recognition sequence with a farred quencher, QSY21, and a fluorophore, Alexa Fluor 647, completed the probe. The efficacy of TcapQ647 was tested via recombinant enzyme assays that indicated TcapQ647 was preferentially cleaved by effector capases 3 and 7 by up to a 170-fold higher rate than initiator caspase 9.[60] In vivo experiments demonstrated the ability of TcapQ647 to detect amoeba-induced cell death in mice with bilateral colon xenografts.[61]
A similar permeation peptide, KcapQ, was developed by Maxwell and colleagues that uses a lysine-rich cell-penetrating sequence and the same caspase recognition sequence flanked by the same fluorophore (Alexa Fluor 647) and quencher (QSY21) used in the TcapQ647 probe.[62] Again, KcapQ was preferentially cleaved by effector caspases 3 and 7 while all initiator capases showed essentially no activity. KcapQ however displayed a unique increase in its absorbance at 605 nm when compared to the basic peptide conjugated to fluorophore alone. According to the researchers, this observation is consistent with strong Coulombic intramolecular interactions in the quencher-fluorophore pair, and data suggests that the quenching mechanism occurred through n-π or π-π stacking rather than classical FRET mechanisms.[62]
Rather than induction of fluorescence upon protease cleavage, quenched activity-based probes (qABPs) fluoresce after labeling an active protease.[63], [64] Bogyo and colleagues designed and synthesized qABPs that contain a fluorescent donor and acceptor in close proximity that are effectively quenched by FRET. qABPs are small molecules that are able to modify their enzyme target by forming specific covalent bonds with key catalytic residues. The covalent modifications release the quenching fluorescence acceptor, thereby increasing the observed fluorescence of the donor. The fluorescent qABPs are cell-permeable and label active cysteine proteases, which allow for real-time monitoring of protease activity within living cells. The fluorescently-labeled cysteine proteases were investigated using fluorescence microscopy and standard biochemical methods.[63] Expansion into the use of qABPs with NIR dyes (qNIRF-ABPs) enabled monitoring of cathepsin activity in vivo after intravenous injection of the probes into mice bearing grafted tumors.[64] The qNIRF-ABPs not only allowed for real-time imaging of target proteases, but the permanent nature of the probes also allowed for secondary ex vivo biochemical profiling. Such validation studies permitted the identification of specifically activated proteases and the correlation of their activity with whole body images.
Several classes of molecular probes have been developed for RNA detection. A very good review of these probes has been published recently.[65] The most commonly used probes are FRET-based oligonucleotide hairpin probes. These fluorescent probes are typically labeled at one end with a donor fluorophore and at the other with an acceptor/quencher. These probes are designed to form stem-loop hairpin structures in the absences of a complementary target, causing the fluorescence of the donor fluorophore to be quenched. Upon hybridization with a target nucleic acid, the hairpin opens and physically separates the donor fluorophore from the acceptor/quencher, allowing fluorescence to occur upon excitation.[65] These probes have very high signal-to-background ratios and their intensity can increase by greater than 200-fold upon opening and binding to a target.[66]
Macromolecular and Polymer-based FRET Probes
Low-molecular weight imaging probes often tend to be unstable, nonspecific, and rapidly cleared. A larger problem related to small molecules is that they generally lack sites for chemical modification without affecting their biological activities.[33] Modern polymer chemistry has provided opportunities to make polymer-based imaging probes that have large surface areas, increased plasma half-lives, enhanced stability, improved targeting, and reduced nonspecific binding.[33] Polymer chemistry advancements have provided many biocompatible polymer structures ranging from poly amino acids and dendrimers to multivalent, branched, graft, and block co-polymers.
In order to detect intracellular targets in vivo, fluorescent probes must not be cleared from the body rapidly in order to provide adequate time for the probe to exit blood vessels and accumulate in regions of interest, i.e. tumors. Weissleder and colleagues have developed a plethora of polymer-based probes that have found widespread applications. One example is the urokinase plasminogen-activator (uPA)-sensitive NIR fluorescent probe.[67] Increased levels of uPA have been found in many types of malignancies and the expression level of uPA has been correlated with prognosis in studies of breast and bladder cancer.[67] The fluorescent probe consists of three components: (1) a copolymer of L-lysine and methoxy poly(ethylene glycol) (MPEG) as the backbone for substrate attachment and tumor delivery, (2) a peptide substrate recognized and cleaved by uPA, and (3) terminally conjugated NIR fluorophores (Cy5.5 and Cy7). The fluorophores were assembled in close proximity resulting in effective FRET dye-dye quenching. The probe fluorescence was tested using fluorescence spectrophotometry and was used to detect urokinase in biological samples. When incubated with uPA, the Cy5.5 uPA-selective fluorescent probe showed an increase in fluorescence of 680% over time and reached a plateau after 2 hours.[67]
A related study investigated the use of fluorescent nanofibers as uPA-selective fluorescent probes. The nanofibers were comprised of a peptide substrate highly degradable by uPA conjugated to a fluorescein derivative (FITC) and flanked by a hydrophobic β-sheet segment at the C-terminus (consisting of D-amino acids to avoid nonspecific proteolytic digestion) and a MPEG hydrophilic polymer attached at the N-terminus (to prevent aggregation).[68] The nanofibers self assemble and can be sorted by size by passing them through a mini extruder to obtain fiber lengths of 100 nm and 200 nm. In the presence of uPA, a four-fold increase in fluorescence was observed.
Another study by Galande and Tung et al. used the multiple antigenic peptide (MAP) system, which is a small and discrete dendrimer scaffold.[69] The fluorescent probes they designed were based on a tetravalent, branched lysine core with dendritic arms that integrated a dipeptide as a substrate for cathepsin S. To make the probe more soluble, short and discrete poly(ethylene glycol) (PEG) groups were inserted between the peptides and the fluorophore, CyTE-777. They found that eight ethylene oxide units in the PEG chain (CyPEG-2) resulted in the most soluble probe. Activation studies conducted on CyPEG-2 with cathepsin S showed more than a 70-fold increase in fluorescence in pH 6.5 buffer.[69]
Another application for a poly-L-lysine backbone sterically protected by MPEG side chains is for the imaging of lymph nodes.[70] Researchers designed a poly-L-lysine backbone that contained Cy5.5 fluorophores conjugated to residues that were in close enough proximity to efficiently quench the fluorescent probe in its native state. After intravenous and subcutaneous injections, enzymatic cleavage of the unmodified lysines in the backbone released the fluorescent probe (Cy5.5) resulting in fluorescent signal increases of up to 30-fold.[70] Data indicated the probe was effective in imaging local and systemic lymph nodes in vivo since blood circulation of the probe was prolonged due to the chemical structure of the backbone. The longer residence time allowed for accumulation in the lymph nodes.
It has been noted that real-time imaging of cell death would assist in clinically managing cancer patients by determining whether or not their anticancer therapeutic regimen was effective.[33] Biocompatible NIR-fluorescent activatable polymeric nanoparticles have been devised to detect early signs of apoptosis.[71] Such a nanoparticle was generated via conjugation of a Cy5.5 caspase-3 cleavable peptide substrate onto a biocompatible polymeric nanoparticle prepared from a hydrophilic polymer [branched poly(ethyleneimine)] and a hydrophobic moiety (deoxycholic acid). This synthetic scheme resulted in spherical nanoparticles with diameters ranging from 80- to 100-nm; the particles were also cell-permeable. The Cy5.5 fluorophores were in a self-quenched state due to their close spatial proximity. However, NIR fluorescence intensified by ten-fold in the presence of caspase-3 in in vitro studies. Live cell studies using HeLa cells confirmed the fluorescent nanoparticle probe was able to image caspase-dependent apoptosis.[71]
Other macromolecular scaffolds, such as dendrimers, have found use as carriers for fluorescent optical dyes in biological applications. Poly(amido amine) (PAMAM) dendrimers have been used as scaffolds for fluorescent optical imaging probes that serve as proteolytic beacons to image MMP-7 activity.[72], [73] McIntyre and Matrisian et al. have engineered a generation 4 PAMAM dendrimer that incorporates a covalently-bound fluorescein (donor)-labeled peptide and tetramethylrhodamine (TMR, acceptor) [PB-M7VIS]. The fluorescein-labeled peptide serves as a selective optical sensor for MMP-7 activity and TMR serves as an internal standard to detect both uncleaved and cleaved reagents. Treatment of PB-M7VIS with MMP-7 resulted in a 17-fold enhancement in fluorescence versus non-treated control samples, and PB-M7VIS was 13-fold more selective for MMP-7 than MMP-3.[72] In vivo selectivity of PB-M7VIS was determined in a mouse xenograft model. Significant fluorescence enhancement was seen in MMP-7 expressing tumors compared to control tumors lacking MMP-7 expression.[72] A similar dendrimer probe was reported by Scherer and Matrisian et al. in which the fluorescein and TMR dyes were exchanged for NIR dyes to afford better in vivo imaging.[73] This proteolytic beacon still used a generation 4 PAMAM dendrimer scaffold, but incorporated a covalently coupled Cy5.5-labeled peptide and AF750 (PB-M7NIR). Here, the Cy5.5-labeled peptide was designed for selective cleavage by MMP-7 while the AF750 served as the internal standard. PB-M7NIR showed a five-fold increase in the Cy5.5 fluorescence signal when cleaved by MMP-7 in vitro. In vivo studies showed a 2.2-fold signal enhancement, while ex vivo studies showed a 300-fold signal enhancement in MMP-7 overexpressing tumors compared to nonexpressing tumors.[73] Both the PB-M7NIR and PB-M7VIS proteolytic beacons rely on quenching of the sensor fluorescence (fluorescein or Cy5.5) by both homotransfer self-quenching and by additional quenching afforded by FRET with the internal reference fluorophores (TMR or AF750).[72], [73]
FRET-based avidin and trastuzumab-activatable targeted optical probes have also been designed to detect cancer metastases in vivo.[74] Using TAMARA as the donor and QSY7 as the acceptor, the FRET pair was conjugated onto avidin [Av-TM-Q7 or Av-TM-Q7(CL)] to target the D-galactose receptor, or trastuzumab, a monoclonal antibody against the human epithelial growth factor receptor type2 (HER2/neu) (Traz-TM-Q7 or Traz-TM). The FRET quenched probes, Av-TM-Q7 and Traz-TM-Q7, showed fluorescent enhancements of 40-fold and 13-fold, respectively, when denatured by SDS during in vitro experiments.[74] In vivo studies demonstrated that the quenched activatable probes had high tumor-to-background ratios indicating that FRET dequenching is an effective mechanism for activating fluorescent probes.[74]
Monoclonal antibodies have also been utilized to make activatable fluorescent optical imaging probes. Specifically, trastuzumab has been conjugated to multiple self-quenching or FRET fluorophores to construct activatable targeted optical imaging probes.[75], [74] These studies show the power of activated versus targeted versions of optical imaging probes. While the activatable self-quenched probes employed the fluorophores Cy5.5 or Alexa Fluor 680 conjugated to trastuzumab at a ratio of approximately 7 dyes per antibody, the targeted probes had approximately one Cy5.5 or Alexa680 conjugated to trastuzumab.[75] In vivo imaging of the fluorescent probes in HER2+ and HER2- tumor-bearing mice showed that the activatable self-quenched Alexa680 probe had the highest tumor-to-background ratio and the targeted tumor, HER2+, showed the greatest fluorescence enhancement.[75]
5. CONCLUDING REMARKS
In the past decade we have witnessed remarkable achievements in the development and implementation of noninvasive optical imaging. In spite of advancements in this area, the chemical design of fluorescent dyes and reporter probes is perceived as a limiting step in imaging. In order to push the technology forward for use in studies with large primates or humans, it is necessary to design better dyes. On top of the prerequisites of being stable and safe, such NIR dyes would have to emit with extremely high fluorescence quantum yields. However, currently used dyes emit approximately less than 30% quantum yield. We predict that ideal fluorescent dyes for medical imaging should have 40-50% quantum yield if optical imaging is to become a useful tool in clinical trials. In summary, much effort is needed to synthesize dyes meeting these requirements. The result could be an effective, safer imaging modality with broad use in clinical settings.
Experience obtained from our work and that of others has led us to believe that there is no single imaging modality which can effectively provide information that is sufficiently robust to detect biological events with high sensitivity, deep tissue penetration, and quick data acquisition and processing. Therefore, the current trend in this area attempts to combine the imaging modalities necessary to provide superior imaging properties through synergistic enhancements unmatched by any single modality. Advancements in the design of multimodal imaging probes will bring the field of optical imaging one step closer to clinical applications. For instance, multimodal probes have already been designed that contain moieties for nuclear and optical imaging that are able to detect HER2[76], target αvβ3 integrin expressed in human melanoma[77] and interleukin 11 receptor alpha-chain[78], as well as image the lymphatic system in mice.[50] Other multimodal probes have been synthesized that contain MR and optical imaging moieties. These probes have been used in the surgical resection of brain tumors[79], the detection of apoptotic cells[80], and for the delivery and noninvasive imaging of siRNAs to tumors.[81] Combining optical imaging with other imaging modalities allows for the advantages of each technique to be realized in one study. Moreover, multimodality allows for more data to be acquired with one probe, which can lead to a better diagnosis of disease or provide feedback on treatment efficacy.
Acknowledgments
The authors gratefully acknowledge Dr. Samantha Nolting for her help in preparing the manuscript and Michiyo Koyama for figure preparation. The authors are partially supported by grants from the NIBIB (1R03EB009524-01) and NIA (AG026366).
ABBREVIATIONS
- NIR
Near-infrared
- FRET
fluorescence resonance energy transfer
- Cy
cyanine
- TAMRA
carboxytetramethylrhodamine
- FITC
fluorescein isothiocyanate
- PAMAM
polyamidoamine
- MMP
matrix metalloproteinase
- HOMO
highest occupied molecular orbital
- LUMO
lowest occupied molecular orbital
- FR
folate receptor
- DPA
dipicolylamine
- BBB
blood brain barrier
- CPPs
cell-penetrating peptides
- ACPPs
activated cell-penetrating peptides
- qABP
quenched activity-based probe
- uPA
urokinase plasminogen-activator
- MAP
multiple antigenic peptide
- PEG
polyethylene glycol
- PAMAM
polyamidoamine
- TMR
tetramethylrhodamine
- HER2
human epithelial growth factor receptor type2
- RNA
ribonucleic acid
- SiRNA
small interfering ribonucleic acid
- RGD
arginylglycylaspartic acid
- Aβ
amyloid-beta
Footnotes
CONFLICT OF INTEREST The authors report no conflicts of interest.
References
- 1.Pham W, Choi Y, Weissleder R, Tung CH. Developing a peptide-based near-infrared molecular probe for protease sensing. Bioconjug Chem. 2004;15(6):1403–7. doi: 10.1021/bc049924s. [DOI] [PubMed] [Google Scholar]
- 2.Raymond SB, Skoch J, Hills ID, Nesterov EE, Swager TM, Bacskai BJ. Smart optical probes for near-infrared fluorescence imaging of Alzheimer’s disease pathology. Eur J Nucl Med Mol Imaging. 2008;35(Suppl 1):S93–8. doi: 10.1007/s00259-007-0708-7. [DOI] [PubMed] [Google Scholar]
- 3.Haugland RB. Handbook of Fluorescent Probes. Mol Probes Inc. 1996 [Google Scholar]
- 4.Fabian J, Nakazumi H, Matsuoka M. Near-infrared absorbing dyes. Chemical Reviews (Washington, DC, United States) 1992;92(6):1197–1226. [Google Scholar]
- 5.Spiliadis A, Vladutiu L, Zaharia M. A study concering Fischer aldehyde condensation with aromatic amines. Rev Chim (Buc) 1975;26(11):868–901. [Google Scholar]
- 6.Gaspar CL, Baldea I, Panea I. Kinetics of the formation of hemicyanine dyes by the condensation of Fischer’s base aldehyde with anilines. Dyes and Pigments. 2006;69:45–53. [Google Scholar]
- 7.(a) Fluhler E, Burnham VG, Loew LM. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry. 1985;24(21):5749–55. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]; (b) Fromherz P, Mueller CO. Voltage-sensitive fluorescence of amphiphilic hemicyanine dyes in neuron membrane. Biochim Biophys Acta. 1993;1150:111–22. doi: 10.1016/0005-2736(93)90079-f. [DOI] [PubMed] [Google Scholar]; (c) Grinvald A, Hildesheim R, Farber IC, Anglister L. Improved fluorescent probes for the measurement of rapid changes in membrane potential. Biophys J. 1982;39(3):301–8. doi: 10.1016/S0006-3495(82)84520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konig W. Ber Dtsch Chem Ges B. 1922;55B:3293. [Google Scholar]
- 9.Konig W. Angew Chem. 1925:743. [Google Scholar]
- 10.Mujumdar RB, Ernst LA, Mujumdar SR, Lewis CJ, Waggoner AS. Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconjug Chem. 1993;4(2):105–11. doi: 10.1021/bc00020a001. [DOI] [PubMed] [Google Scholar]
- 11.Dyadyusha GG, Kachkovskii AD, Dekhtyar ML. Perturbatio theory of nonbonding orbitals in polymeric dyes. Theoretical and experimental chemistry. 1987;24:251256. [Google Scholar]
- 12.(a) Pham W, Weissleder R, Tung C-H. An azulene dimer as a near-infrared quencher. Angewandte Chemie, International Edition. 2002;41(19):3659–3662. doi: 10.1002/1521-3773(20021004)41:19<3659::AID-ANIE3659>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]; (b) Pham W, Lai WF, Weissleder R, Tung CH. High efficiency synthesis of a bioconjugatable near-infrared fluorochrome. Bioconjug Chem. 2003;14(5):1048–51. doi: 10.1021/bc034070h. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds GA, Drexhage KH. Stable heptamethine pyrylium dyes that absorb in the infrared. J Org Chem. 1977;42(5):885–8. [Google Scholar]
- 14.Mujumdar SR, Mujumdar RB, Grant CM, Waggoner AS. Cyanine-labeling reagents: sulfobenzindocyanine succinimidyl esters. Bioconjug Chem. 1996;7(3):356–62. doi: 10.1021/bc960021b. [DOI] [PubMed] [Google Scholar]
- 15.Pham W, Medarova Z, Moore A. Synthesis and application of a water-soluble near-infrared dye for cancer detection using optical imaging. Bioconjug Chem. 2005;16(3):735–40. doi: 10.1021/bc049700+. [DOI] [PubMed] [Google Scholar]
- 16.Shao F, Weissleder R, Hilderbrand SA. Monofunctional carbocyanine dyes for bio- and bioorthogonal conjugation. Bioconjug Chem. 2008;19(12):2487–91. doi: 10.1021/bc800417b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham W, Cassell L, Gillman A, Koktysh D, Gore JC. A near-infrared dye for multichannel imaging. Chem Commun (Camb) 2008;(16):1895–7. doi: 10.1039/b719028j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Kiyose K, Kojima H, Urano Y, Nagano T. Development of a ratiometric fluorescent zinc ion probe in near-infrared region, based on tricarbocyanine chromophore. J Am Chem Soc. 2006;128(20):6548–9. doi: 10.1021/ja060399c. [DOI] [PubMed] [Google Scholar]; (b) Peng X, Song F, Lu E, Wang Y, Zhou W, Fan J, Gao Y. Heptamethine cyanine dyes with a large stokes shift and strong fluorescence: a paradigm for excited-state intramolecular charge transfer. J Am Chem Soc. 2005;127(12):4170–1. doi: 10.1021/ja043413z. [DOI] [PubMed] [Google Scholar]
- 19.Kulinich AV, Ishchenko AA. Merocyanine Dyes: Synthesis, structure, properties and applications. Russ Chem Rev. 2009;78(2):141–164. [Google Scholar]
- 20.Berkovic G, Krongauz V, Weiss V. Spiropyrans and Spirooxazines for Memories and Switches. Chem Rev. 2000;100(5):1741–1754. doi: 10.1021/cr9800715. [DOI] [PubMed] [Google Scholar]
- 21.Shen Q, Cao Y, Liu S, Steigerwald ML, Guo XF. Conformation-induced electrostatic gating of teh conduction of spiropyran-coated organic thin-film transitors. J Phys Chem. 2009;113(24):10807–10812. [Google Scholar]
- 22.Horiuchi T, Miura H, Uchida S. Highly-efficient metal-free organic dyes for dye-sensitized solar cells. Chem Commun (Camb) 2003;(24):3036–7. doi: 10.1039/b307819a. [DOI] [PubMed] [Google Scholar]
- 23.Bossi M, Belov V, Polyakova S, Hell SW. Reversible red fluorescent molecular switches. Angew Chem Int Ed Engl. 2006;45(44):7462–5. doi: 10.1002/anie.200602591. [DOI] [PubMed] [Google Scholar]
- 24.Drexhage KH. J Res Natl Bur Stand. 1976;80A:421–428. [Google Scholar]
- 25.Beija M, Afonso CA, Martinho JM. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chemical Society reviews. 2009;38(8):2410–33. doi: 10.1039/b901612k. [DOI] [PubMed] [Google Scholar]
- 26.Chandran SS, Dickson KA, Raines RT. Latent fluorophore based on the trimethyl lock. J Am Chem Soc. 2005;127(6):1652–3. doi: 10.1021/ja043736v. [DOI] [PubMed] [Google Scholar]
- 27.Milstein S, Cohen LA. J Am Chem Soc. 1972;94:9158–9165. doi: 10.1021/ja00781a029. [DOI] [PubMed] [Google Scholar]
- 28.Riggs JL, Seiwald RJ, Burckhalter JH, Downs CM, Metcalf TG. Isothiocyanate compounds as fluorescent labeling agents for immune serum. Am J Pathol. 1958;34(6):1081–97. [PMC free article] [PubMed] [Google Scholar]
- 29.Lefevre C, Kang HC, Haugland RP, Malekzadeh N, Arttamangkul S, Haugland RP. Texas Red-X and Rhodamine Red-X, New Derivatives of Sulforhodamine 101 and Lissamine Rhodamine B with Improved Labeling and Fluorescence Properties. Bioconjugate Chem. 1996;7(4):482–489. doi: 10.1021/bc960034p. [DOI] [PubMed] [Google Scholar]
- 30.Lieberwirth U, Arden-Jacob J, Drexhage KH, Herten DP, Muller R, Neumann M, Schulz A, Siebert S, Sagner G, Klingel S, Sauer M, Wolfrum J. Multiplex dye DNA sequencing in capillary gel electrophoresis by diode laser-based time-resolved fluorescence detection. Anal Chem. 1998;70(22):4771–9. doi: 10.1021/ac980230k. [DOI] [PubMed] [Google Scholar]
- 31.Alford R, Ogawa M, Choyke PL, Kobayashi H. Molecular probes for the in vivo imaging of cancer. Mol Biosyst. 2009;5(11):1279–91. doi: 10.1039/b911307j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecchi M, Ottobrini L, Martelli C, Del Sole A, Lucignani G. Instrumentation and probes for molecular and cellular imaging. Q J Nucl Med Mol Imaging. 2007;51(2):111–26. [PubMed] [Google Scholar]
- 33.Lee S, Park K, Kim K, Choi K, Kwon IC. Activatable imaging probes with amplified fluorescent signals. Chem Commun (Camb) 2008;(36):4250–60. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 34.Bloch S, Lesage F, McIntosh L, Gandjbakhche A, Liang K, Achilefu S. Whole-body fluorescence lifetime imaging of a tumor-targeted near-infrared molecular probe in mice. J Biomed Opt. 2005;10(5):054003. doi: 10.1117/1.2070148. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen JC, Tan IC, Marshall MV, Fife CE, Sevick-Muraca EM. Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotechnol. 2009;20(1):74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bugaj JE, Achilefu S, Dorshow RB, Rajagopalan R. Novel fluorescent contrast agents for optical imaging of in vivo tumors based on a receptor-targeted dye-peptide conjugate platform. J Biomed Opt. 2001;6(2):122–33. doi: 10.1117/1.1352748. [DOI] [PubMed] [Google Scholar]
- 37.Bremer C, Ntziachristos V, Weissleder R. Optical-based molecular imaging: contrast agents and potential medical applications. Eur Radiol. 2003;13(2):231–43. doi: 10.1007/s00330-002-1610-0. [DOI] [PubMed] [Google Scholar]
- 38.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2009 doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 39.Moon WK, Lin Y, O’Loughlin T, Tang Y, Kim DE, Weissleder R, Tung CH. Enhanced tumor detection using a folate receptor-targeted near-infrared fluorochrome conjugate. Bioconjug Chem. 2003;14(3):539–45. doi: 10.1021/bc0340114. [DOI] [PubMed] [Google Scholar]
- 40.Li C, Greenwood TR, Glunde K. Glucosamine-bound near-infrared fluorescent probes with lysosomal specificity for breast tumor imaging. Neoplasia. 2008;10(4):389–98. doi: 10.1593/neo.07856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Greenwood TR, Bhujwalla ZM, Glunde K. Synthesis and characterization of glucosamine-bound near-infrared probes for optical imaging. Org Lett. 2006;8(17):3623–6. doi: 10.1021/ol060783e. [DOI] [PubMed] [Google Scholar]
- 42.Ye Y, Bloch S, Xu B, Achilefu S. Design, synthesis, and evaluation of near infrared fluorescent multimeric RGD peptides for targeting tumors. J Med Chem. 2006;49(7):2268–75. doi: 10.1021/jm050947h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly KA, Carson J, McCarthy JR, Weissleder R. Novel peptide sequence (“IQ-tag”) with high affinity for NIR fluorochromes allows protein and cell specific labeling for in vivo imaging. PLoS One. 2007;2(7):e665. doi: 10.1371/journal.pone.0000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosaka N, Ogawa M, Choyke PL, Karassina N, Corona C, McDougall M, Lynch DT, Hoyt CC, Levenson RM, Los GV, Kobayashi H. In vivo stable tumor-specific painting in various colors using dehalogenase-based protein-tag fluorescent ligands. Bioconjug Chem. 2009;20(7):1367–74. doi: 10.1021/bc9001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schellenberger EA, Bogdanov A, Jr, Petrovsky A, Ntziachristos V, Weissleder R, Josephson L. Optical imaging of apoptosis as a biomarker of tumor response to chemotherapy. Neoplasia. 2003;5(3):187–92. doi: 10.1016/S1476-5586(03)80050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ntziachristos V, Schellenberger EA, Ripoll J, Yessayan D, Graves E, Bogdanov A, Jr, Josephson L, Weissleder R. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc Natl Acad Sci U S A. 2004;101(33):12294–9. doi: 10.1073/pnas.0401137101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah C, Miller TW, Wyatt SK, McKinley ET, Olivares MG, Sanchez V, Nolting DD, Buck JR, Zhao P, Ansari MS, Baldwin RM, Gore JC, Schiff R, Arteaga CL, Manning HC. Imaging biomarkers predict response to anti-HER2 (ErbB2) therapy in preclinical models of breast cancer. Clin Cancer Res. 2009;15(14):4712–21. doi: 10.1158/1078-0432.CCR-08-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pham W, Xie J, Gore JC. Tracking the migration of dendritic cells by in vivo optical imaging. Neoplasia. 2007;9(12):1130–7. doi: 10.1593/neo.07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pham W, Zhao BQ, Lo EH, Medarova Z, Rosen B, Moore A. Crossing the blood-brain barrier: a potential application of myristoylated polyarginine for in vivo neuroimaging. Neuroimage. 2005;28(1):287–92. doi: 10.1016/j.neuroimage.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi H, Koyama Y, Barrett T, Hama Y, Regino CA, Shin IS, Jang BS, Le N, Paik CH, Choyke PL, Urano Y. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS Nano. 2007;1(4):258–64. doi: 10.1021/nn700062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leevy WM, Gammon ST, Jiang H, Johnson JR, Maxwell DJ, Jackson EN, Marquez M, Piwnica-Worms D, Smith BD. Optical imaging of bacterial infection in living mice using a fluorescent near-infrared molecular probe. J Am Chem Soc. 2006;128(51):16476–7. doi: 10.1021/ja0665592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leevy WM, Gammon ST, Johnson JR, Lampkins AJ, Jiang H, Marquez M, Piwnica-Worms D, Suckow MA, Smith BD. Noninvasive optical imaging of staphylococcus aureus bacterial infection in living mice using a Bis-dipicolylamine-Zinc(II) affinity group conjugated to a near-infrared fluorophore. Bioconjug Chem. 2008;19(3):686–92. doi: 10.1021/bc700376v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K, Nagano T. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J Am Chem Soc. 2005;127(13):4888–94. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]
- 54.Nesterov EE, Skoch J, Hyman BT, Klunk WE, Bacskai BJ, Swager TM. In vivo optical imaging of amyloid aggregates in brain: design of fluorescent markers. Angew Chem Int Ed Engl. 2005;44(34):5452–6. doi: 10.1002/anie.200500845. [DOI] [PubMed] [Google Scholar]
- 55.Ran C, Xu X, Raymond SB, Ferrara BJ, Neal K, Bacskai BJ, Medarova Z, Moore A. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-beta deposits. J Am Chem Soc. 2009;131(42):15257–61. doi: 10.1021/ja9047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pham W, Pantazopoulos P, Moore A. Imaging farnesyl protein transferase using a topologically activated probe. J Am Chem Soc. 2006;128(36):11736–7. doi: 10.1021/ja063599x. [DOI] [PubMed] [Google Scholar]
- 57.Pham W, Weissleder R, Tung CH. An azulene dimer as a near-infrared quencher. Angew Chem Int Ed Engl. 2002;41(19):3659–62. 3519. doi: 10.1002/1521-3773(20021004)41:19<3659::AID-ANIE3659>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 58.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101(51):17867–72. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bullok KE, Gammon ST, Violini S, Prantner AM, Villalobos VM, Sharma V, Piwnica-Worms D. Permeation peptide conjugates for in vivo molecular imaging applications. Mol Imaging. 2006;5(1):1–15. [PubMed] [Google Scholar]
- 60.Bullok K, Piwnica-Worms D. Synthesis and characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. J Med Chem. 2005;48(17):5404–7. doi: 10.1021/jm050008p. [DOI] [PubMed] [Google Scholar]
- 61.Bullok KE, Maxwell D, Kesarwala AH, Gammon S, Prior JL, Snow M, Stanley S, Piwnica-Worms D. Biochemical and in vivo characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. Biochemistry. 2007;46(13):4055–65. doi: 10.1021/bi061959n. [DOI] [PubMed] [Google Scholar]
- 62.Maxwell D, Chang Q, Zhang X, Barnett EM, Piwnica-Worms D. An improved cell-penetrating, caspase-activatable, near-infrared fluorescent peptide for apoptosis imaging. Bioconjug Chem. 2009;20(4):702–9. doi: 10.1021/bc800516n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blum G, Mullins SR, Keren K, Fonovic M, Jedeszko C, Rice MJ, Sloane BF, Bogyo M. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat Chem Biol. 2005;1(4):203–9. doi: 10.1038/nchembio728. [DOI] [PubMed] [Google Scholar]
- 64.Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007;3(10):668–77. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 65.Bao G, Rhee WJ, Tsourkas A. Fluorescent probes for live-cell RNA detection. Annu Rev Biomed Eng. 2009;11:25–47. doi: 10.1146/annurev-bioeng-061008-124920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14(3):303–8. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 67.Law B, Curino A, Bugge TH, Weissleder R, Tung CH. Design, synthesis, and characterization of urokinase plasminogen-activator-sensitive near-infrared reporter. Chem Biol. 2004;11(1):99–106. doi: 10.1016/j.chembiol.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 68.Law B, Weissleder R, Tung CH. Protease-sensitive fluorescent nanofibers. Bioconjugate Chem. 2007;18(6):1701–1704. doi: 10.1021/bc070054z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galande AK, Hilderbrand SA, Weissleder R, Tung CH. Enzyme-targeted fluorescent imaging probes on a multiple antigenic peptide core. J Med Chem. 2006;49(15):4715–20. doi: 10.1021/jm051001a. [DOI] [PubMed] [Google Scholar]
- 70.Wunderbaldinger P, Turetschek K, Bremer C. Near-infrared fluorescence imaging of lymph nodes using a new enzyme sensing activatable macromolecular optical probe. Eur Radiol. 2003;13(9):2206–11. doi: 10.1007/s00330-003-1932-6. [DOI] [PubMed] [Google Scholar]
- 71.Kim K, Lee M, Park H, Kim JH, Kim S, Chung H, Choi K, Kim IS, Seong BL, Kwon IC. Cell-permeable and biocompatible polymeric nanoparticles for apoptosis imaging. J Am Chem Soc. 2006;128(11):3490–1. doi: 10.1021/ja057712f. [DOI] [PubMed] [Google Scholar]
- 72.McIntyre JO, Fingleton B, Wells KS, Piston DW, Lynch CC, Gautam S, Matrisian LM. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochem J. 2004;377(Pt 3):617–28. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scherer RL, VanSaun MN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol Imaging. 2008;7(3):118–31. [PMC free article] [PubMed] [Google Scholar]
- 74.Ogawa M, Kosaka N, Longmire MR, Urano Y, Choyke PL, Kobayashi H. Fluorophore-Quencher Based Activatable Targeted Optical Probes for Detecting in Vivo Cancer Metastases. Mol Pharm. 2009 doi: 10.1021/mp800115t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogawa M, Regino CA, Choyke PL, Kobayashi H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8(1):232–9. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sampath L, Kwon S, Ke S, Wang W, Schiff R, Mawad ME, Sevick-Muraca EM. Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med. 2007;48(9):1501–10. doi: 10.2967/jnumed.107.042234. [DOI] [PubMed] [Google Scholar]
- 77.Houston JP, Ke S, Wang W, Li C, Sevick-Muraca EM. Quality analysis of in vivo near-infrared fluorescence and conventional gamma images acquired using a dual-labeled tumor-targeting probe. J Biomed Opt. 2005;10(5):054010. doi: 10.1117/1.2114748. [DOI] [PubMed] [Google Scholar]
- 78.Wang W, Ke S, Kwon S, Yallampalli S, Cameron AG, Adams KE, Mawad ME, Sevick-Muraca EM. A new optical and nuclear dual-labeled imaging agent targeting interleukin 11 receptor alpha-chain. Bioconjug Chem. 2007;18(2):397–402. doi: 10.1021/bc0602679. [DOI] [PubMed] [Google Scholar]
- 79.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63(23):8122–5. [PubMed] [Google Scholar]
- 80.Schellenberger EA, Sosnovik D, Weissleder R, Josephson L. Magneto/optical annexin V, a multimodal protein. Bioconjug Chem. 2004;15(5):1062–7. doi: 10.1021/bc049905i. [DOI] [PubMed] [Google Scholar]
- 81.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13(3):372–7. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]


