Abstract
Polycystic kidney disease (PKD) is characterized by cardiovascular irregularities, including hypertension. Dopamine, a circulating hormone, is implicated in essential hypertension in humans and animal models. Vascular endothelial primary cilia are known to function as mechano-sensory organelles. Though both primary cilia and dopamine receptors play important roles in vascular hypertension, their relationship has never been explored. To determine the roles of the dopaminergic system and mechanosensory cilia, we studied the effects of dopamine on ciliary length and function in wild-type (WT) and mechano-insensitive polycystic mutant cells (Pkd1−/− and Tg737orpk/orpk). We show for the first time that mouse vascular endothelia exhibit dopamine receptor-type 5 (DR5), which co-localizes to primary cilia in cultured cells and mouse arteries in vivo. DR5 activation increases cilia length in arteries and endothelial cells through cofilin and actin polymerization. DR5-activation also restores cilia function in the mutant cells. In addition, silencing DR5 completely abolishes mechano-ciliary function in WT cells. We find that DR5 plays very important roles in ciliary length and function. Furthermore, the chemosensory function of cilia can alter the mechanosensory function through changes in sensitivity to fluid-shear stress. We propose that activated ciliary DR5 has a functional mechanosensory role in endothelial cells.
Keywords: Dopamine receptors, polycystic kidney disease, primary cilia, vascular endothelia
Introduction
Primary cilium is a small hair-like projection present on the apical membrane of most cells. By virtue of its shape and location, the primary cilium is able to act as an antenna, sensing and transmitting information from the extracellular matrix to the cell interior. To assist with its unique sensory roles, a high density of specialized proteins such as receptors, ion channels, kinases, phosphatases, secondary messengers and other signaling modules are localized in the ciliary membrane, cilioplasm, or at the ciliary base1. These proteins enable the primary cilia to act as chemosensors and mechanosensors.
Dysfunctional cilia have been associated with a large number of diseases such as polycystic kidney disease (PKD) and various other diseases, which have been collectively referred to as “ciliopathies”. Improper structure and function of the primary cilia has been reported in patients suffering from PKD2–4. In addition to renal cyst formation, PKD is also characterized by non-cystic manifestations such as hypertension, left ventricular hypertrophy, cardiac valve abnormalities, intracranial aneurysms and abdominal wall hernias, among others5,6. Furthermore, hypertension in PKD has been associated with abnormal mechanosensory cilia function and structure7,8.
Dopamine is an endogenous neuronal hormone, known to produce a wide range of cardiovascular and renal effects. Various subtypes of dopamine receptors are known to be present in different parts of the cardiovascular system9. Hence, dopamine is known to regulate systemic blood pressure, renal hemodynamics and electrolyte balance. In humans, activation of dopamine receptors within the blood vessels can cause vasodilation10. Most importantly, circulating dopamine mediates vasodilation through both endothelium-dependent (60%) and endothelium-independent (40%) mechanisms11. Within the vascular endothelial cells, dopamine receptors type-1 (DR1) and -5 (DR5) are involved in endothelium-dependent relaxation12. Because of this, any abnormality in dopamine metabolism and/or receptor function has been implicated in essential hypertension in humans13–15 and animal models16–18.
Despite the fact that cilia and dopamine play critical roles in hypertension, their relationship has not been explored. All we know from the clinical study is that PKD patients with borderline hypertension are better managed with DOPA (a dopamine precursor) than with angiotensin converting enzyme (ACE) inhibitors19. Our current studies show for the first time that the dopaminergic system regulates sensory cilia structure and function. Activation of ciliary dopamine receptor increases cilia length. To examine the relationship between dopamine and cilia within PKD, we further used mechano-insensitive Pkd1−/− and Tg737orpk/orpk endothelial cells, previously derived from Pkd mouse models7,8. We show that ciliary dopamine activation can restore mechanosensory cilia function in response to fluid-shear stress. We propose that localization of dopamine receptor to cilia plays important chemosensory and mechanosensory roles in vascular endothelial cells.
Materials and Methods
The use of endothelial cells and other biohazard reagents was approved by the Institutional Biosafety Committee of The University of Toledo. The use of animal tissues was approved by The University of Toledo animal care and use committee. The details of this section on pharmacological agents, sequences for primers and siRNAs are available online at http://hyper.ahajournals.org (online supplement).
Results
DR5 localizes to and regulates length of primary cilia
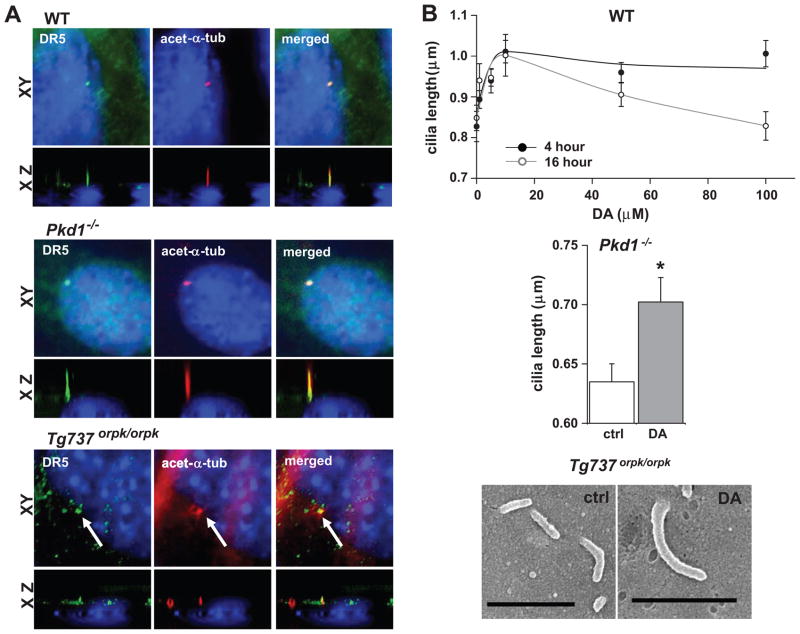
We show for the first time that dopamine receptor (DR)-type 5 is localized to the primary cilia of cultured endothelial cells and femoral artery in vivo. Using well-characterized mouse endothelial cells, expressions of DR type-3 and 5 (DR3 and DR5) are detected at the transcript level (SuppFig1a). Subcellular localization of these receptor subtypes was studied three-dimensionally using DR3- and DR5-specific antibodies (Fig1a). DR5 is localized to primary cilia of wild-type and Pkd1−/− endothelial cells. DR5 is also localized in short, stubby cilia of Tg737orpk/orpk cells. DR5 cilia localization was observed widely in a monolayer of endothelial cells and also in endothelia of femoral artery in vivo (SuppFig1b). No specific localization of DR3 was observed in the cilia (not shown).
Figure 1. Dopamine receptor-type 5 (DR5) localizes to and regulates length of primary cilia.
a. Immunolocalization study using specific antibody to DR5 confirms ciliary expression in monolayer wild-type (WT) and mechano-insensitive (Pkd1−/− and Tg737orpk/orpk) vascular endothelial cells. The XY and XZ fluorescence images show localization of DR5 (green) to the cilia (acetylated-α-tubulin; red). Arrows indicate abnormal cilia formation in Tg737orpk/orpk cells. b. Dopamine treatment for 4 or 16 hours increases ciliary length in a dose dependent manner in wild-type cells. Dopamine also increases cilia length in Pkd1−/− cells as shown in the bar graph or induces cilia formation in Tg737orpk/orpk cells as shown in the representative electron micrographs. Asterisks denote p<0.05. Bar = 1μm. N>3 independent experiments.
Dopamine treatment for 4 or 16 hours increases cilia length in a dose-dependent manner (Fig1b). Concentration of dopamine to induce maximal increase in cilia length is optimal at 10 μmol/L for both 4 and 16 hours. Activation of DR5 is sufficient to increase cilia length (SuppFig2a). To further confirm that DR3 activation does not play a role in cilia length regulation, we used DR3 inhibitor in the presence of dopamine. Observation with immunofluorescence and electron microscopy techniques shows that DR5 activation, either with dopamine or DR5-specific agonist, increases in cilia length (SuppFig2b). To further verify this finding, we isolated and treated mouse femoral arteries with either vehicle or 10 μmol/L dopamine for 16 hours (SuppFig3a). As expected, dopamine increases cilia length ex vivo comparable to that of cultured cells. Because the femoral artery contains smooth muscle cells, which also have primary cilia20,21, the artery was laid down in such a way that only the first layer of cells from the intima was observed through both immunofluorescence and electron microscopy techniques (SuppFig3b).
To understand the functional relevance of ciliary DR5 in PKD, we examined DR5 activation in Pkd1−/− and Tg737orpk/orpk endothelial cells (Fig1b). Interestingly, cilia length is also increased significantly in Pkd1−/− cells treated with dopamine. Because of their small and stubby cilia, we were not able to accurately determine the cilia length measurement in Tg737orpk/orpk cells. However, it is surprising that the length of cilia in Tg737orpk/orpk cells tends to be longer or occasionally restored as seen in wild-type cells. In all genotypes, receptor activation with dopamine does not show an apparent subcellular redistribution of DR5 (not shown).
Dopamine increases cilia length through cellular actin differentiation via cofilin dephosphorylation
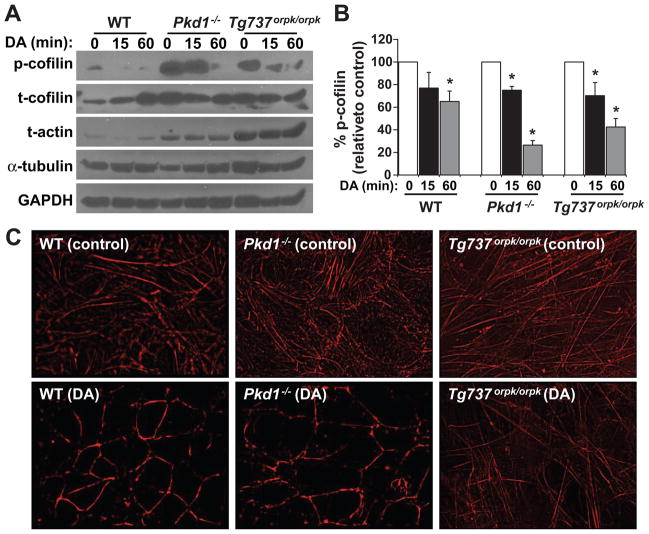
Inhibition of actin polymerization has been shown to play an important role in ciliogenesis22–24. Furthermore, dephosphorylated or activated form of cofilin has been shown to inhibit actin polymerization25,26. To examine this possibility in our system, we measured phosphorylated cofilin before and after treatment with dopamine for 15 and 60 minutes (Fig2a). Supporting our idea, a significant decrease of phosphorylated cofilin is observed in dopamine-treated cells (Fig2b). Throughout our Western blot analyses, we also consistently observed the expression level of total actin to be greater in Pkd1−/− and Tg737orpk/orpk than in wild-type cells. Please note that we denoted the total actin as globular actin (G-actin) and filamentous actin (F-actin) because we reduced and monomerized F-actin during our sample preparation. Thus, we next analyzed F-actin only to further understand the effects of dopamine in actin polymerization (Fig2c). To our surprise, dopamine induces actin re-arrangement in all cell types. Although the effect on Tg737orpk/orpk cells is not as substantial, dopamine induces redistribution of stress actin fibers to cortical actin. This actin redistribution has been associated with shear-induced cellular differentiation27, a characteristic of mechanical-induced cilia activation28.
Figure 2. Dopamine increases cilia length through cellular actin differentiation via cofilin dephosphorylation.
a. Wild-type (WT), Pkd1−/− and Tg737orpk/orpk cells were analyzed for the ratio of phosphorylated cofilin (p-cofilin) to total cofilin (t-cofilin) before and after 10 μmol/L dopamine (DA) treatment for 15 or 60 minutes. Both α-tubulin and GAPDH were used as loading controls, while total actin (t-actin) consistently expresses at a higher level in the mutant cells. b. Dopamine significantly decreases levels of phosphorylated cofilin in wild-type, Pkd1−/− and Tg737orpk/orpk cells. c. Dopamine induces cellular differentiation as evidenced from cytoskeletal actin rearrangement. Stress actin fibers and cortical actin formation are respectively presented before (control) and after 60 minutes dopamine treatment. N=3 independent experiments. Asterisks denote p<0.05.
To further confirm the roles of cofilin in regulating cilia length, we used calyculin to increase the basal phosphorylation level of cofilin by blocking protein phosphatase-1 (PP1). Thus, calyculin would be constitutively inactivated. Blocking cofilin sufficiently and significantly decreases cilia length in the presence or absence of dopamine (SuppFig4a). When the F-actin was analyzed, the association between actin rearrangement and cilia length was further confirmed (SuppFig4b). We found that calyculin could block dopamine-induced actin re-arrangement.
Activation of ciliary dopamine receptor partially restores mechanosensory function of endothelial cilia in Pkd1−/− and Tg737orpk/orpk cells
Because primary cilia have been proposed to be chemosensory organelles29,30 and to further verify the functional specificity of DR5 in the cilia, we challenged wild-type endothelial cells with dopamine, DR5-and DR3-specific agonists (SuppFig5a). Our data show that DR3 activation has no functional implication, at least in cytosolic calcium increases. Most important is that the agonists-induced cytosolic calcium studies validate the involvement of DR5 in cilia function. We also challenged Pkd1−/− and Tg737orpk/orpk cells with dopamine (SuppFig5b). As in wild-type cells, the chemosensory role of dopamine receptor in the cilia was verified in these mutant cells. Due to shorter cilia and thus lower DR5 expression level to cilia, a much smaller increase in cytosolic calcium was observed in Tg737orpk/orpk cells.
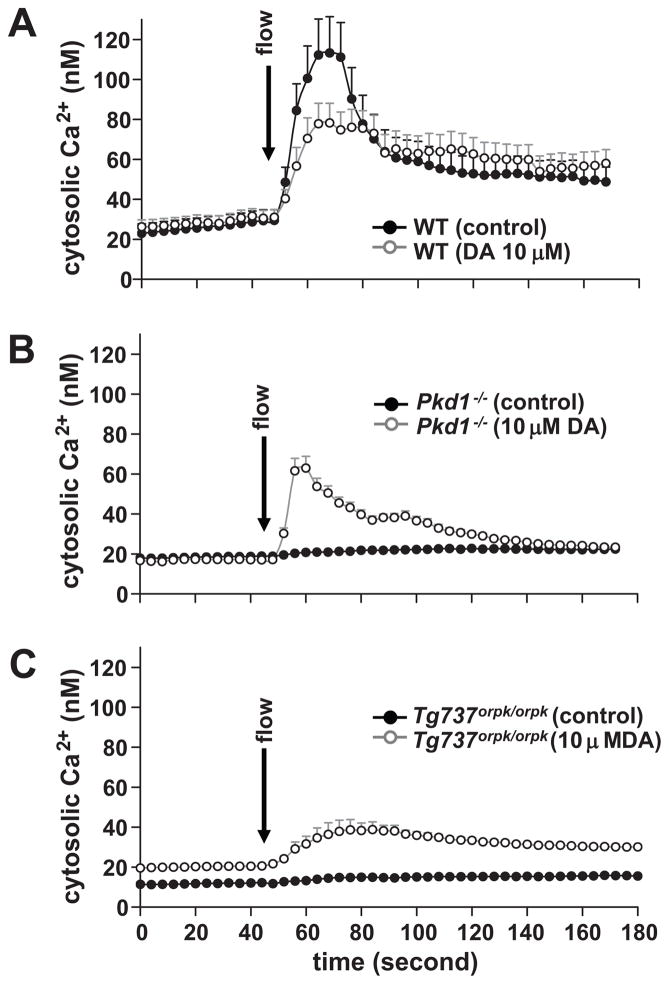
Vascular endothelial cilia have also been proposed to function as mechanosensory organelles31,32. To examine the correlation between cilia length and function, we performed fluid-shear stress experiments to analyze cilia function in wild-type cells treated with vehicle or 10 μmol/L of DA for 16 hours (Fig3a). We found that the averaged area under the curve in the presence and absence of dopamine were not significantly different. Thus, while dopamine significantly increases cilia length in wild-type endothelial cells, its effect on cilia function is minimal. Because dopamine also increases cilia length in Pkd1−/− as well as Tg737orpk/orpk cells, we next examined whether dopamine could have an effect on the mechanosensory function of cilia. Unexpectedly, the mechanosensory role in Pkd1−/− cells was restored in the presence of dopamine (Fig3b). More surprisingly, the mechanosensory role in Tg737orpk/orpk cells was also restored in the presence of dopamine (Fig3c).
Figure 3. Activation of ciliary dopamine receptor partially restores mechanosensory function of endothelial cilia in Pkd1−/− and Tg737orpk/orpk cells.
a. Mechanosensory function of cilia is determined by studying changes in intracellular calcium in response to fluid-flow shear stress. Untreated wild-type (WT) cells and cells treated with dopamine (DA) for 16 hours do not exhibit significant changes in cytosolic calcium in response to fluid-flow shear stress. b. Pkd1−/− cells are incapable of responding to fluid-flow shear stress and hence an increase in intracellular calcium is not observed in Pkd1−/− in response to fluid flow. However, after treatment with dopamine for 16 hours, Pkd1−/− cells regain their ability to sense fluid flow, with a significant increase in intracellular calcium. c. Tg737orpk/orpk cells, which have short or stubby cilia, are incapable of sensing fluid flow shear stress. After dopamine treatment, these cells exhibit a small but significant rescue of the ciliary function. N=5–9 independent experiments; each represents an average of 100–150 cells.
Ciliary DR5 is a mechanosensory receptor
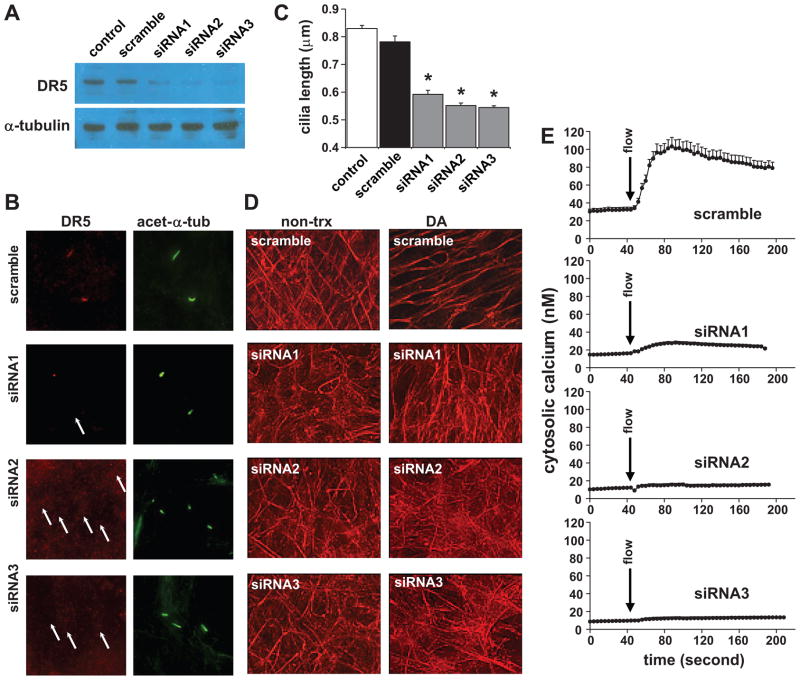
To further confirm DR5 as a mechanosensory receptor, we transfected wild-type endothelial cells with various GFP-tagged siRNAs, thereby knocking down DR5 level in the cells. Protein analysis shows substantially repressed DR5 expressions in siRNA treated cells compared to untreated cells or transfected cells with scramble siRNA (Fig4a). As expected, no DR5 was detected in the cilia of DR5-specific siRNA treated cells (Fig4b). Furthermore, DR5 knockdown cells have significantly shorter cilia, confirming the role of dopamine in regulating cilia length (Fig4c). Consistent with our data showing that dopamine regulates cilia length through actin distribution, dopamine-induced actin distribution is abolished in DR5 knockdown cells (Fig4d). To examine the mechanosensory function of cilia, we next challenged the cells with fluid-shear stress (Fig4e). Cells treated with siRNA became insensitive to fluid flow, supporting the role of DR5 as a mechanosensory protein in vascular endothelial cells. Unlike the regulatory system of cilia length, however, blocking cofilin through PP1 antagonist does not have any substantial effect on mechanosensory cilia function (SuppFig6). This indicates that regulatory pathways of cilia length and function may be differentially regulated.
Figure 4. Ciliary DR5 is a mechanosensory receptor.
a. The presence of dopamine receptor type 5 (DR5) was analyzed by western blot in wild-type vascular endothelial cells transfected with lipofectamine only (control), scramble RNA, siRNA1, siRNA2 or siRNA3. α-tubulin was used as a loading control. b. The presence of ciliary dopamine receptor was verified in transfected cells, and acetylated-α-tubulin was used as a ciliary marker. White arrows indicate the absence of dopamine receptor in the cilia. c. Cilia length decreases in siRNA transfected cells compared to non-transfected (control) or scramble siRNA transfected cells. Asterisks denote p<0.05. d. Cytoskeletal actin filament was analyzed in the transfected cells before treatment (non-trx) and after 16 hour-dopamine treatment (DA). e. Mechanosensory function of cilia was analyzed in the transfected cells. N>3 independent experiments; each represents an average of 100–150 cells.
Discussion
For the first time, we show that dopamine receptor type 5 (DR5) is localized to primary cilia in wild-type as well as in ciliary abnormal Pkd1−/− and Tg737orpk/orpk endothelial cells. We further provide evidence supporting the ciliary roles of DR5 as chemo- and mechano-sensors. In addition, we show that endothelial cells can alter their sensitivity to fluid-shear stress through chemosensory function of cilia. The regulation of cilia structure involves dephosphorylated cofilin, which controls cellular cytoskeleton actin filament rearrangement. This cellular actin differentiation is required for extension of cilia length, including in Tg737orpk/orpk cells. Most interesting is that activation of ciliary DR5 would promote and restore cilia function, especially in Pkd1−/− and Tg737orpk/orpk endothelial cells. We propose that ciliary DR5 within the vascular endothelia can provide a substantial implication in cilia-related diseases, such as hypertension and polycystic kidney disease (PKD).
In the present study, we find that DR5 is specifically localized to vascular endothelial cilia in cultures and arteries in vivo. Dopamine through activation of DR5 increases cilia length in a concentration-dependent manner within 4 hours in cultures and mouse femoral arteries ex vivo. We did not perform in vivo study in mice, because the dose-response study in vivo is still largely a challenge due to a drop in blood pressure caused by dopamine. More specifically, dopamine induces vasodilation in vivo, compromising an overall collapse of the cardiovascular system. Regardless, the ciliary DR5 in cultured cells or blood vessels is a functional receptor, because specific activation of DR5 shows responses in both ciliary length and cytosolic calcium increase.
Recently, we have proposed that endothelial cilia are mechanosensory organelles that play a major role in pathogenesis of hypertension7,8. Abnormalities in dopamine synthesis or dopamine receptor function have also been implicated in essential hypertension in humans13–15 and animal models16–18. For example, mouse genetic model with aberrant DR5 exhibits severe hypertension with unopposed sympathetic activity18. Furthermore, it was previously unknown how hypertensive PKD patients could be managed more successfully with dopamine precursor than other anti-hypertensive therapies19.
To examine functional relevance of DR5 within PKD, we used Pkd1−/− and Tg737orpk/orpk endothelial cells previously derived from Pkd mouse models. These mutant cells have abnormal mechanosensory cilia function and structure, respectively7,8. Similar to wild-type cells, activation of DR5 also increases cilia length in Pkd1−/− and Tg737orpk/orpk endothelial cells. Because most Tg737orpk/orpk endothelial cells have very short stubby cilia, if any, we were not able to quantify the magnitude of cilia length increase. Nonetheless, the ciliogenesis in Tg737orpk/orpk cells implies that a mechanism other than the intraflagellar transport exists. Most importantly, this mechanism can be activated with dopamine to increase cilia length.
A primary cilium is structurally composed of acetylated and detyrosinated microtubules, which connect to the sub-membraneous actin network at its basal body. Ciliogenesis in Tg737orpk/orpk cells has been observed in the presence of cytocalasin D, an actin polymerization inhibitor22–24. Pharmacological agents which disrupt microtubule polymerization, like nocadazole, promote the formation of actin stress fibers. On the other hand, agents that stabilize microtubules, such as taxol, inhibit assembly of actin filaments. Thus, any activation or inhibition of actin polymerization could affect the microtubule-based cilium.
Patients with PKD exhibit significant connective tissue abnormalities involving vascular and airway smooth muscle cells21, suggesting a possible disruption of cellular actin dynamics. In addition, vascular cells derived from a mouse Pkd model exhibit higher levels of cofilin when activated by the adrenergic receptor agonist33, which further confirms the role of actin dynamics in cilia dysfunction model. Cofilin, a small ubiquitous protein that binds to actin cytoskeleton, can promote the rate of monomer disassociation and sever actin filament, thereby inhibiting actin-polymerization25,26.
To further examine the roles of activated cofilin by dopamine, we also measured phosphorylated cofilin in our cells. Compared to wild-type cells, higher basal levels of inactivated cofilin in Pkd1−/− and Tg737orpk/orpk cells were consistently observed. This probably results in inhibition of cilia length and/or function. Consistent with this view, a consistently higher level of total actin is needed for F-actin polymerization in the mutant cells. We further show that dopamine-induced cofilin activation can promote cortical F-actin formation, which has been used as a differentiation marker in neurons34. Overall, our data show that dopamine can induce cofilin activation, through dephosphorylation by PP135. Consistent with this view, when we inhibited PP1 with calyculin, cilia length was significantly decreased. Calyculin, downstream to dopamine, is also able to reverse the increase in cilia length and actin rearrangement observed in cells treated with dopamine. In addition, no significant change in intracellular calcium response was observed in the presence of fluid-flow shear stress, reinforcing the cellular functions of actin reorganization in ciliogenesis and DR5 in the mechanosensory property of primary cilia.
Not only do our data show the functionality of DR5 as a chemosensor in cilia, our studies also demonstrate the role of DR5 as a ciliary mechanosensor. We and others have shown that Pkd1−/− and Tg737orpk/orpk cells lack mechanosensory cilia function36,37. To our surprise, dopamine not only induces an increase in cilia length, but it also restores the mechanosensory roles in both Pkd1−/− and Tg737orpk/orpk cells. Interestingly, in wild-type cells with normal mechanosensory cilia function, activation of DR5 does not initiate a significant change in cilia function, although cilia length is significantly increased. We propose that activated DR5 in the cilia can have a functional sensory role in endothelial cells.
To further investigate the mechanosensory capacity of DR5 in wild-type cells, we next knocked down DR5 expression. Inhibition of DR5 expression in cilia was achieved using different siRNA constructs, which happened to have a similar efficacy to that verified by our Western blot and immunolocalization studies. Dopamine-induced cofilin activation was ceased in siRNA-transfected cells as evidence from the discontinuation of F-actin rearrangement. Most surprising is the loss of mechanosensory function in DR5 knockdown cells. The length of the cilia in knockdown cells was similar to the length of those treated with calyculin. However, while the cilia in DR5-knockdown cells no longer possessed their mechanosensory capabilities, cells treated with calcyculin, which still exhibited DR5 co-localized to the cilia retained their mechanosensory abilities. Thus, we propose that ciliary DR5 in endothelial cells have dual chemosensory and mechanosensory roles.
Primary cilia have been proposed to regulate cardiovascular functions, including blood pressure7,8. Given the facts that DR5 mutations in mice17,18 or abnormal dopaminergic system in humans13–15 leads to hypertension, we suggest that dysfunctional DR5 is associated with cellular function of cilia. As such, dysfunction in cilia and DR5 could result in a similar hypertension phenotype as observed in PKD patients5,6 and patients associated with dopaminergic system13–15. Thus, the chemosensory and mechanosensory roles of primary cilia are equally important for the maintenance of proper cardiovascular homeostasis and vascular tone.
Supplementary Material
Perspectives.
Previous clinical study indicates that hypertension in PKD patients is better managed with a dopamine precursor19. However, it was not immediately understood why and how it is more useful than other anti-hypertensive agents. We show here that activation of peripheral dopamine receptor can regulate cilia length and restore cilia function in PKD. Overall, our study helps explain dopamine receptor agonism as a potential therapeutic option in hypertensive PKD patients.
Acknowledgments
Authors would like to thank Maki Takahashi and Blair Mell for their technical support. Charisse Montgomery is acknowledged for her editorial assistance.
Sources of Funding
NIH DK080640, DK080640-01S1 and DK080640-02S1
Footnotes
Conflict of Interest
N/A
References
- 1.Nauli SM, Haymour HS, AbouAlaiwi WA, Lo ST, Nauli AM. Primary Cilia are Mechanosensory Organelles in Vestibular Tissues. Mechanosensitivity and Mechanotransduction. 2011;4:317–350. [Google Scholar]
- 2.Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol. 2006;17:1015–1025. doi: 10.1681/ASN.2005080830. [DOI] [PubMed] [Google Scholar]
- 3.Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. American journal of physiology. 2007;292:F930–945. doi: 10.1152/ajprenal.00285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. American journal of physiology. 2009;296:F1464–1476. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nature reviews. 2009;5:221–228. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris PC, Torres VE. Polycystic kidney disease. Annual review of medicine. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circulation research. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–569. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johns DW, Ayers CR, Carey RM. The dopamine agonist bromocriptine induces hypotension by venous and arteriolar dilation. Journal of cardiovascular pharmacology. 1984;6:582–587. doi: 10.1097/00005344-198407000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi M, Kobayashi Y, Shimoura K, Hattori K, Nakase A. Endothelium-dependent and -independent relaxation by dopamine in the rabbit pulmonary artery. Clinical and experimental pharmacology & physiology. 1992;19:401–410. doi: 10.1111/j.1440-1681.1992.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohlstein EH, Zabko-Potapovich B, Berkowitz BA. Studies on vascular dopamine receptors with the dopamine receptor agonist: SK&F 82526. The Journal of pharmacology and experimental therapeutics. 1984;229:433–439. [PubMed] [Google Scholar]
- 13.Sato M, Soma M, Nakayama T, Kanmatsuse K. Dopamine D1 receptor gene polymorphism is associated with essential hypertension. Hypertension. 2000;36:183–186. doi: 10.1161/01.hyp.36.2.183. [DOI] [PubMed] [Google Scholar]
- 14.Sowers JR, Nyby M, Jasberg K. Dopaminergic control of prolactin and blood pressure: altered control in essential hypertension. Hypertension. 1982;4:431–437. doi: 10.1161/01.hyp.4.3.431. [DOI] [PubMed] [Google Scholar]
- 15.Thomas GN, Tomlinson B, Critchley JA. Modulation of blood pressure and obesity with the dopamine D2 receptor gene TaqI polymorphism. Hypertension. 2000;36:177–182. doi: 10.1161/01.hyp.36.2.177. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, Sibley DR, Westphal HJ, Jose PA. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. The Journal of clinical investigation. 1996;97:2283–2288. doi: 10.1172/JCI118670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asico L, Zhang X, Jiang J, Cabrera D, Escano CS, Sibley DR, Wang X, Yang Y, Mannon R, Jones JE, Armando I, Jose PA. Lack of Renal Dopamine D5 Receptors Promotes Hypertension. J Am Soc Nephrol. 2010;22:82–89. doi: 10.1681/ASN.2010050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, 3rd, Westphal H, Jose PA, Sibley DR. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22:10801–10810. doi: 10.1523/JNEUROSCI.22-24-10801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barendregt JN, Florijn KW, Muizert Y, Chang PC. Borderline hypertensive autosomal dominant polycystic kidney disease patients have enhanced production of renal dopamine. Normalization of renal haemodynamics by DOPA infusion. Nephrol Dial Transplant. 1995;10:1332–1341. [PubMed] [Google Scholar]
- 20.Lu CJ, Du H, Wu J, Jansen DA, Jordan KL, Xu N, Sieck GC, Qian Q. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney & blood pressure research. 2008;31:171–184. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Du H, Wang X, Mei C, Sieck GC, Qian Q. Characterization of primary cilia in human airway smooth muscle cells. Chest. 2009;136:561–570. doi: 10.1378/chest.08-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitaval A, Tseng Q, Bornens M, Thery M. Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. The Journal of cell biology. 2010;191:303–312. doi: 10.1083/jcb.201004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK. Soluble levels of cytosolic tubulin regulate ciliary length control. Molecular biology of the cell. 2011;22:806–816. doi: 10.1091/mbc.E10-03-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriyama K, Iida K, Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- 26.Nebl G, Meuer SC, Samstag Y. Dephosphorylation of serine 3 regulates nuclear translocation of cofilin. The Journal of biological chemistry. 1996;271:26276–26280. doi: 10.1074/jbc.271.42.26276. [DOI] [PubMed] [Google Scholar]
- 27.Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, Weinbaum S. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11418–11423. doi: 10.1073/pnas.0804954105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AbouAlaiwi WA, Ratnam S, Booth RL, Shah JV, Nauli SM. Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation. Human molecular genetics. 2011;20:354–367. doi: 10.1093/hmg/ddq470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, Larusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G725–734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AbouAlaiwi WA, Lo ST, Nauli SM. Primary Cilia: Highly Sophisticated Biological Sensors. Sensors. 2009;9:7003–7020. doi: 10.3390/s90907003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- 33.Du H, Wang X, Wu J, Qian Q. Phenylephrine induces elevated RhoA activation and smooth muscle alpha-actin expression in Pkd2+/− vascular smooth muscle cells. Hypertens Res. 2010;33:37–42. doi: 10.1038/hr.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuen EY, Yan Z. Dopamine D4 receptors regulate AMPA receptor trafficking and glutamatergic transmission in GABAergic interneurons of prefrontal cortex. J Neurosci. 2009;29:550–562. doi: 10.1523/JNEUROSCI.5050-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graziane NM, Yuen EY, Yan Z. Dopamine D4 Receptors Regulate GABAA Receptor Trafficking via an Actin/Cofilin/Myosin-dependent Mechanism. The Journal of biological chemistry. 2009;284:8329–8336. doi: 10.1074/jbc.M807387200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature genetics. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 37.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. American journal of physiology. 2006;290:F1320–1328. doi: 10.1152/ajprenal.00463.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.