Abstract
The dorsal raphe nucleus (DR) contains the majority of serotonin (5-hydroxytryptamine, 5-HT) neurons in the brain that regulate neural activity in forebrain regions through their widespread projections. DR function is linked to stress and emotional processing, and is implicated in the pathophysiology of affective disorders. Glutamatergic drive of the DR arises from many different brain areas with the capacity to inform the nucleus of sensory, autonomic, endocrine and metabolic state as well as higher order neural state. Imbalance of glutamatergic neurotransmission could contribute to maladaptive 5-HT neurotransmission and represents a potential target for pharmacotherapy. Within the DR, glutamate-containing axon terminals can be identified by their content of one of three types of vesicular glutamate transporter, VGLUT1, 2 or 3. Each of these transporters is heavily expressed in particular brain areas such that their content within axons correlates with the afferent's source. Cortical sources of innervation to the DR including the medial prefrontal cortex heavily express VGLUT1 whereas subcortical sources primarily express VGLUT2. Within the DR, many local neurons responsive to substance P contain VGLUT3, and these provide a third source of excitatory drive to 5-HT cells. Moreover VGLUT3 is present, with or without 5-HT, in output pathways from the DR. 5-HT and non-5-HT neurons receive and integrate glutamatergic neurotransmission through multiple subtypes of glutamate receptors that have different patterns of expression within the DR. Interestingly, excitatory drive provided by glutamatergic neurotransmission is closely opposed by feedback inhibition mediated by 5-HT1A receptors or local GABAergic circuits. Understanding the intricacies of these local networks and their checks and balances, may help identify how potential imbalances could cause psychopathology and illuminate strategies for therapeutic manipulation.
Keywords: serotonin, excitatory, vesicular glutamate transporter, VGLUT1, VGLUT2, VGLUT3, neurokinin1, substance P, AMPA, kainite, mGluR
1. Relevance of glutamate neurotransmission in the DR
Glutamate neurotransmission both in cortical areas and within the dorsal raphe nucleus (DR) has been implicated in depressive illness in humans (Paul and Skolnick, 2003), and represents a novel target for drug development and pharmacotherapy of affective disorders. Indeed, in humans and animal models, glutamate receptor antagonists have been reported to be effective antidepressants (Trullas and Skolnick, 1990; Maj et al., 1992; Papp and Moryl, 1996; Yilmaz et al., 2002). In particular, the NMDA receptor antagonist ketamine has garnered interest for its rapid antidepressant effects in treatment-resistant depressed patients (Berman et al., 2000; Zarate et al., 2006; Krystal, 2007). Selective NR2B-subunit and metabotropic glutamate receptor ligands are also currently of interest for therapeutic use with fewer cognitive effects and less abuse potential than ketamine (Maeng et al., 2008; Preskorn et al., 2008; Yasuhara and Chaki, 2010; Li et al., 2011).
The prefrontal cortex (PFC) is often referred to as a likely site of action of glutamatergic ligands with respect to antidepressant activity. PFC volume is lost in depressed patients (Drevets, 1998) and there is evidence for altered function of the PFC in rodent models of depression (Rajkowska et al., 1999; Liu and Aghajanian, 2008). One target of glutamatergic outflow from the PFC is the DR, and it is possible that glutamatergic innervation of the DR may also be an important locus for the etiology and treatment of depression. Specifically blocking NMDA-type glutamate receptors within the DR attenuates the development of learned helplessness behaviors that may represent a depression-like state in rats (Grahn et al., 2000). In addition, evidence specifically implicates the pathway from the PFC to the DR as important for modulating the harmful effects of stress (Amat et al., 2005).
In addition to the PFC, there are many additional sources of glutamate neurotransmission within the DR that likely have the capacity to control serotonin (5-Hydroxytriptamine, 5-HT) function during normal and stressful conditions. Indeed, the magnitude of this innervation can be visualized by localizing the postsynaptic density protein PSD-95, a protein particularly enriched at glutamatergic synapses (Figure 1).
Figure 1. PSD-95 immunolabeling within the mouse DR visualized by array tomography.
A. 3D image of the DR rendered from a stack of 28 ultrathin (70nm) serial sections showing immunolabeling for PSD-95 (red), a marker of excitatory synapses, and tryptophan hydroxylase (TPH) (green), to identify serotonin cells. Tissue sections were immunolabeled with rabbit anti-PSD-95, (1:200, Cell Signaling Technologies) and sheep anti-TPH, (1:200, Millipore). B. Arrowheads in A point to same elements in B at higher magnification, showing the exquisitely discrete labeling of the synaptic marker with total absence of out of focus light achieved in array tomography. Scale bars = 50 um in A, and 20 um in B.
Tract-tracing studies have identified the medial PFC, several hypothalamic areas, the lateral habenula, periaqueductal gray and medullary regions including parabrachial nuclei as glutamatergic afferent sources to the DR (Kalén et al., 1985; Lee et al., 2003). These diverse brain areas are associated with many functions, for example hypothalamic sites are linked to endocrine and metabolic function, the lateral habenula to reward state, medullary areas to autonomic function, visceral and somatic sensation, and the PFC with conscious perception and decision making. The diversity of these afferent sources is consonant with the observation that similarly diverse classes of stimuli have the capacity to influence mood and motivated behavior associated with 5-HT function.
2. Populations of Glutamate Axons: VGLUT1 and VGLUT2
The identification of the transporters that fill synaptic vesicles with glutamate has provided an excellent tool to identify glutamate-containing axon terminals in the brain and specifically in the DR (Hisano et al., 2000; Fremeau et al., 2001; Herzog et al., 2001; Takamori et al., 2001; Fremeau et al., 2002; Kaneko and Fujiyama, 2002; Kaneko et al., 2002; Varoqui et al., 2002; Gras et al., 2002; Herzog et al., 2004; Commons, 2009). Three different types of vesicular glutamate transporters (VGLUT1-3) are selectively, but not exclusively, expressed in different anatomical areas (Fremeau et al., 2001; Herzog et al., 2001; Kaneko et al., 2002; Ziegler et al., 2002; Fremeau et al., 2004). Thus, VGLUT types have been used as markers to neurochemically identify different populations of glutamate axons innervating to the DR (Figure 2). Cortical neurons mostly express VGLUT1 and therefore axons arising from the medial PFC in the DR would preferentially contain VGLUT1. A complementary distribution of VGLUT2-containing neurons to those cells expressing VGLUT1 has been described. Hypothalamic regions, the lateral habenula, as well as adjacent areas to the DR such as the periaqueductal gray and parabrachial nucleus all heavily express VGLUT2 and are afferent sources to the DR (Hisano et al., 2000; Fremeau et al., 2001; Herzog et al., 2001; Kaneko and Fujiyama, 2002; Kaneko et al., 2002; Varoqui et al., 2002). Thus glutamate neurons sending projections from these areas would primarily express VGLUT2.
Figure 2. Schematic illustration of major glutamatergic afferents to the DR.
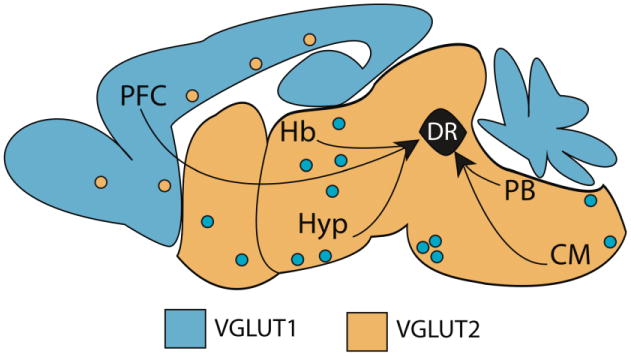
Black arrows indicate brain regions that provide glutamatergic innervation to the DR including the prefrontal cortex (PFC), lateral habenula (Hb) multiple subregions of the hypothalamus (Hyp), the parabrachial nucleus (PB) and areas in the caudal medulla (CM). As a rule, VGLUT1 (light blue) or VGLUT2 (orange) are predominant in cortical and subcortical domains respectively. There are however exceptions to this rule, depicted as polka-dotted colors (Kaneko et al., 2002; Ziegler et al., 2002).
3. VGLUT1 and VGLUT2 in Emotional Processing
Consistent with the cortical expression of VGLUT1 and its likely presence in projections from the PFC to the DR (Figure 3), some evidence implicates this transporter specifically in emotional behavior. Attenuated expression of VGLUT1 in a heterozygous knockout mouse increases anxiety-related behavior and vulnerability to depressive-like behavior (Tordera et al., 2007; Garcia-Garcia et al., 2009). In addition, a course of treatment with antidepressants increases VGLUT1 expression levels (Tordera et al., 2005) suggesting a change in the activity state of these neurons.
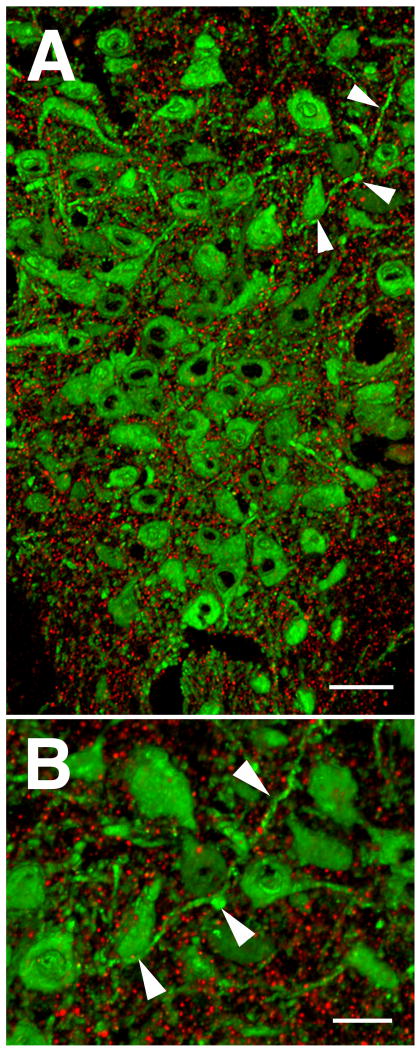
Figure 3. Cortical afferents to the DR.
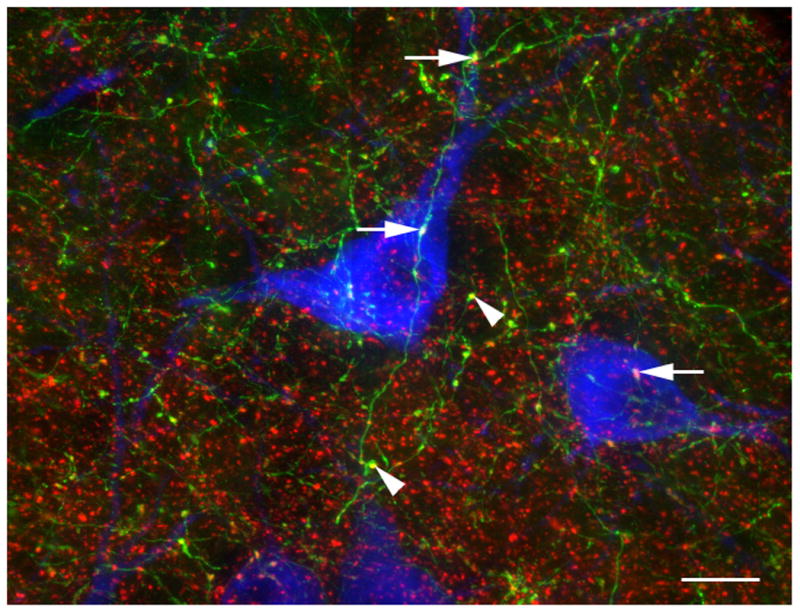
A subset of the axons that originate in the cortex were identified by the presence of EGFP using piGAP cre-dependent reporter (Badaloni et al., 2007) and Emx1-cre expressing mice (Gorski et al., 2002). EGFP (green) was detected by immunolabeling for GFP (chicken anti-GFP, 1:1000, Aves Labs) and VGLUT1 (red) was detected using guinea pig anti-VGLUT1 (1:1000, Millipore). VGLUT1 is present in the axon varicosities and a few of the double-labeled boutons are indicated with arrowheads. EGFP is only expressed in some cortical neurons (due to mosaic expression of the piGAP reporter) and therefore many VGLUT1 axons (red) lack EGFP. A few VGLUT1-containing EGFP-labeled axons are in proximity to 5-HT cells (arrows). 5-HT cells were identified by immunolabeling for tryptophan hydroxylase 2 (blue; rabbit 1:1000, Novus Biologicals). Scale bar = 12 um.
Evidence also suggests the importance of VGLUT2-containing pathways in regulating the DR in pathophysiological states. Glutamate axons originating in the lateral habenula likely express VGLUT2 (Hisano et al., 2000; Varoqui et al., 2002) and influence the activity of DR networks (Kalén et al., 1989; Varga et al., 2003). Altered activity of the lateral habenula has been associated with depression and drug addiction (Sartorius and Henn, 2007; Hikosaka, 2010). A recent study using different rat models of depression has shown that lesioning the habenula alleviates depression-like symptoms and normalizes the extracellular levels of 5-HT as well as its turnover in the DR (Yang et al., 2008). Previously, the habenula-DR pathway had been implicated in mediating the effects of drugs of abuse on 5-HT neurons (Paris and Cunningham, 1994). In addition, VGLUT2 coexists to some extent with the neuropeptide corticotropin releasing factor (CRF) in the DR, and CRF neurotransmission has been of intense interest for its role in regulating DR activity during stress, which is a contributing factor to many psychopathological states (Waselus and Van Bockstaele, 2007).
Immunofluorescence labeling has shown a widespread and punctate distribution of VGLUT1- and VGLUT2-containing axon terminals within the DR, however VGLUT2-positive terminals appeared to be more abundant than those containing VGLUT1 (Commons et al., 2005). In addition, ultrastructural analysis revealed that both types of axonal boutons preferentially establish Gray's type 1 or asymmetric synaptic contacts either with tryptophan hydroxylase (TPH)- or non-TPH-labeled cells (Commons et al., 2005). Interestingly, postsynaptic targets of VGLUT1- and VGLUT2-containing axonal boutons differ in morphology. Specifically, VGLUT1-labeled terminals predominantly synapse onto small-caliber dendrites (< 0.5 μm diameter), and therefore at locations distal from the soma, or even onto dendritic spines. In contrast VGLUT2-containing axons preferentially synapse onto larger caliber dendritic shafts (> 0.5 μm diameter) proximal to the cell soma (Commons et al., 2005) (Figure 4). Thus, the two sets of afferent inputs could have different influence on action potential generation in the postsynaptic cell. That is, in the simple scenario of passive decay of postsynaptic potentials as they summate near the soma, more distal inputs provided by VGLUT1-containing axons may have a more modulatory role, while proximal inputs containing VGLUT2 may have a direct influence on neuronal excitability.
Figure 4. Schematic view of the most likely location of VGLUT1 and VGLUT2 axons in the DR, with respect to their postsynaptic targets.
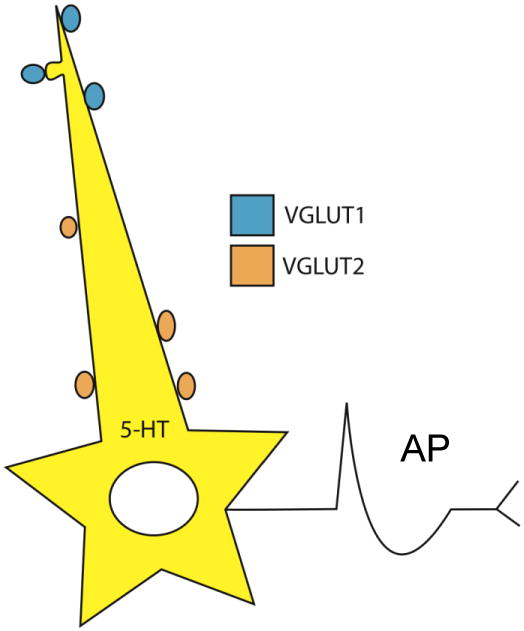
VGLUT1-containing afferents are more often associated with distal dendrites and spines, whereas VGLUT2 afferents target proximal dendritic shafts and cell bodies. Thus, the two sets of afferent inputs could have different influence on action potential (AP) generation in the postsynaptic cell.
For technical reasons, dendritic spines are difficult to study in the DR; however, 5-HT neurons are known to have dendritic spines, sparsely on their primary and the secondary dendrites but with progressively high density on higher order dendrites (Li et al., 2001). Dendritic spines are of particular interest for their association with synaptic plasticity. The more common association of VGLUT1 than VGLUT2 with dendritic spines would raise the possibility that there could be differences in the mechanisms of plasticity at each of these types of synapses (Commons et al., 2005). Overall, the anatomical arrangement of VGLUT1- and VGLUT2-containing axons in the DR parallels the potentially modulatory influence of cortical function, whereas information regarding physical state conveyed through subcortical routes may have a more exigent influence on 5-HT neurotransmission.
4. VGLUT3-containing cell bodies and axons
In addition to VGLUT1 and VGLUT2, the DR is heavily invested with axons containing VGLUT3 and many of these likely arise from local VGLUT3 expressing neurons. When VGLUT3 was cloned, it was quickly determined to be expressed in many regions that were not thought to use glutamate as a neurotransmitter including the DR. Many years previously, Ottersen and Storm-Mathisen (1984) developed antisera to glutamate and described its distribution throughout the brain using immunohistochemistry. They discovered intermediate levels of glutamate-immunolabeling in the DR as well as other unexpected locations, and suspected that this immunolabeling perhaps represented a metabolic pool of glutamate. Similar to Ottersen and Storm-Mathisen, the surprising observation of VGLUT3 expression in areas such as the DR led to the suspicion of a false positive finding: did these cells with VGLUT3 actually use glutamate as a neurotransmitter?
Accumulating evidence now exists that VGLUT3 is sufficient to confer glutamate neurotransmission (Gillespie et al., 2005; Seal et al., 2008; Varga et al., 2009). Within the DR, the emerging picture is that there are different populations of VGLUT3 neurons. The first population of VGLUT3-containing cells also contains 5-HT (“VGLUT3-5-HT cells”). Evidence suggests that these cells may have the capacity to co-release 5-HT and glutamate, but a complete understanding of the co-transmitter role of this population is still emerging. Only about a third of neurons in the DR contain 5-HT however (Descarries et al., 1982), and some of neurons that lack 5-HT contain VGLUT3, comprising a separate population of neurons (“VGLUT3-glutamate cells”). These neurons have both local axon collaterals and forebrain projections and in both these terminal fields evidence suggests they release the neurotransmitter glutamate.
4.1. VGLUT3-5-HT containing neurons
While evidence supports the co-neurotransmission of glutamate with other neurotransmitter systems such as dopamine (Hnasko et al., 2010; Stuber et al., 2010; Tecuapetla et al., 2010) or GABA (Zander et al., 2010), the extent and importance of the 5-HT co-transmission with glutamate conferred by VGLUT3 remain to be resolved. One factor that has confused the issue of co-neurotransmission of 5-HT and glutamate via VGLUT3 is the discrepancy between colocalization of VGLUT3 and 5-HT in axons terminals versus cell bodies (Figure 5). Several studies have carefully investigated using either in situ hybridization or immunohistochemical techniques the co-distribution of VGLUT3 within 5-HT cell soma, and it is substantial (Gras et al., 2002; Hioki et al., 2004; Mintz and Scott, 2006; Shutoh et al., 2008; Hioki et al., 2010). Perhaps every 5-HT cell in the DR expresses some level of VGLUT3 mRNA and protein. This observation however does not match with the observed colocalization between VGLUT3 and 5-HT in axon terminals. Colocalization in axons is overall modest, substantially less than rates of colocalization in cell bodies, and it varies depending on the brain area examined. Therefore with respect to 5-HT neurons, only a minority contain VGLUT3 within their axons while the majority do not. These appear as binary categories, but they could also be two extremes on a gradient of axon terminal content.
Figure 5. There is a dissociation between colocalization of VGLUT3 and 5-HT in cell bodies vs. axons.
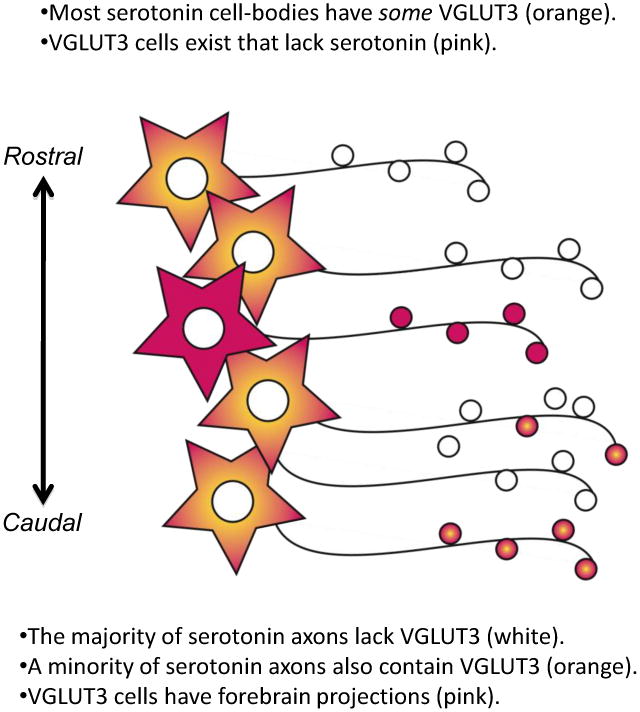
Many 5-HT cell bodies have some detectable VGLUT3-immunolabeling (orange), whereas the majority of 5-HT axons in the forebrain lack detectable VGLUT3-immunolabeling (white). Axons containing both VGLUT3 and 5-HT (orange boutons) vary in abundance by brain region. Several areas richly invested with axons containing both VGLUT3 and 5-HT are projection sites of the caudal DR. In addition, there are many VGLUT3 containing cells in the DR that lack 5-HT (pink). These “VGLUT3-glutamate cells” also contribute to ascending projections from the DR (pink). Thus, axons arising from the DR contain 5-HT, VGLUT3, or a combination of both together.
The caudal component of the DR, including the B6 group of 5-HT cells, particularly distinguishes itself as an area containing many VGLUT3-5-HT neurons. In the caudal DR, a group of cells at the base of the aqueduct provide innervation to the epithelial lining of the ventricles, interdigitating between both microvilli and ciliary protrusions in the supraependimal plexus. Almost every varicosity within the supraependimal plexus contains both markers for 5-HT and VGLUT3 (Shutoh et al., 2008; Commons, 2009). Axons innervating the dorsal part of the lateral septum also have high levels of colocalization and these axons probably also arise from neurons located in the caudal part of the DR (Waselus et al., 2006). In contrast, in the adult rat, only a small fraction of other 5-HT axons contain VGLUT3 in the cortex, hippocampus, amygdala, medial septum, caudate putamen and nucleus accumbens (Boulland et al., 2004; Shutoh et al., 2008). Within the DR, there is a high level of co-existence between VGLUT3 and 5-HT only in the re-current axon collaterals within the caudal DR, while in the other areas of the DR, VGLUT3-containing axons do not colocalize with any other neurotransmitter marker (Commons, 2009).
With respect to axon terminals however, perhaps the most direct evidence that 5-HT and glutamate are co-released and indeed populate the same vesicles comes from evidence suggesting that glutamate transport by VGLUT3 into vesicles facilitates the concurrent filling with 5-HT (Amilhon et al., 2010). In addition, recently an elegant study using optogenetic techniques clearly demonstrated the fast glutamatergic component of the raphe-hippocampal pathway (Varga et al., 2009). However, a second factor has confused the issue of co-neurotransmission between 5-HT and glutamate, and that is the presence of many VGLUT3-containing cells that lack 5-HT in the DR, and the observation that these VGLUT3 cells contribute to ascending DR projections (Jackson et al., 2009; Yamakawa and Antle, 2010; Hioki et al., 2010).
4.2. VGLUT3-glutamate Cells
VGLUT3-glutamate cells in the DR are located essentially in the center of the DR, particularly in the shell of the dorsal DR and extending into the area between the dorsal and ventral clusters of 5-HT cells at mid-rostrocaudal levels of the DR (Gras et al., 2002; Commons, 2009; Hioki et al., 2010) (Figure 6). Recently, we showed that the majority of VGLUT3 cells, at least 70%, also contain the receptor for substance P, neurokinin 1 (NK1), which has a similar distribution through the DR (Barbaresi, 1998; Commons and Valentino, 2002; Commons, 2009). Substantial colocalization of NK1 receptors and VGLUT3 is important because previously it had been established NK1 receptor bearing neurons in the DR are functionally glutamatergic (Liu et al., 2002; Valentino et al., 2003). NK1 receptors are dendritically localized to cells resident to the DR that contain immunolabeling for glutamate (Commons and Valentino, 2002). Crucially, activation of NK1 receptors by substance P increases glutamatergic postsynaptic potentials onto 5-HT neurons (Liu et al., 2002). These two observations, that substance P activates glutamate neurotransmission and VGLUT3 cells contain the receptor for substance P, identified these VGLUT3 cells as neurons using glutamate as a neurotransmitter within the DR.
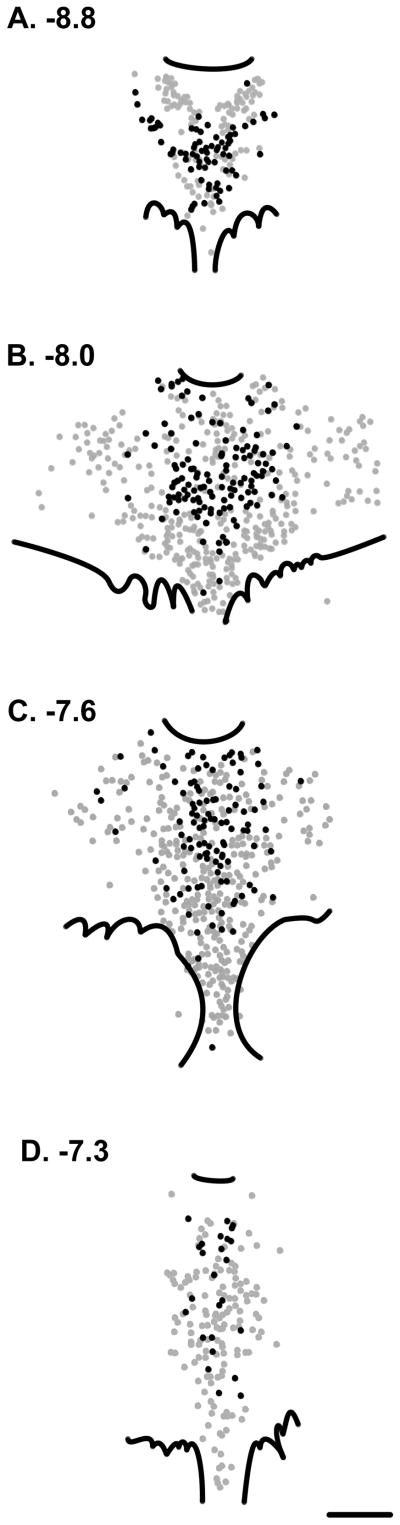
Figure 6. Mapping of VGLUT3-glutamate cells (black dots) in the rat DR from the caudal (A) to rostral (D) pole of the DR.
Distances from bregma according to the atlas of Paxinos and Watson (1998) are noted. Figure adapted from (Commons, 2009) reprinted with permission. For each section, a black dot indicates a cell body with immunolabeling for VGLUT3 but not 5-HT; a “VGLUT3-glutamate cell”. Grey dots indicate the location of 5-HT immunolabeled cells (most of which also contain detectable VGLUT3-immunolabeling as depicted in Figure 5). VGLUT3-glutamate cells are located in the center of the nucleus, with a preferential distribution toward the midline as well as mid-dorsoventral locations. Scale bar = 500 um.
Additional information is known about how NK1 receptor bearing neurons, now identified as VGLUT3-glutamate cells, may participate in DR networks. That is, VGLUT3 cells directly innervate 5-HT cells and drive 5-HT release leading to subsequent activation of 5-HT1A receptors (Figure 7A). Using in vivo extracellular single-unit recordings, where local neural networks remain intact, we found that activation of NK1 drives a broad (multisynaptic) inhibition of 5-HT neurons via 5-HT1A receptors (Valentino et al., 2003). As a consequence, substance P released in the DR leads to decreases in 5-HT release in the forebrain (Guiard et al., 2007). These effects are blocked by glutamate receptor antagonists in the DR, providing another line of evidence that NK1/VGLUT3 cells use glutamate as a neurotransmitter. Speculatively, these data could suggest that an acute function of VGLUT3-glutamate cells is as a trigger or amplifier of 5-HT feedback inhibition. However, constitutive function of NK1/VGLUT3 cells seems to be important to maintain 5-HT1A-receptor sensitivity. Indeed, knockout mice lacking either functional NK1 receptors or functional VGLUT3 have a common phenotype of desensitized 5-HT1A receptors in the DR (Froger et al., 2001; Amilhon et al., 2010).
Figure 7. Schematic representation of network interactions of VGLUT3-glutamate cells and 5-HT neurons.
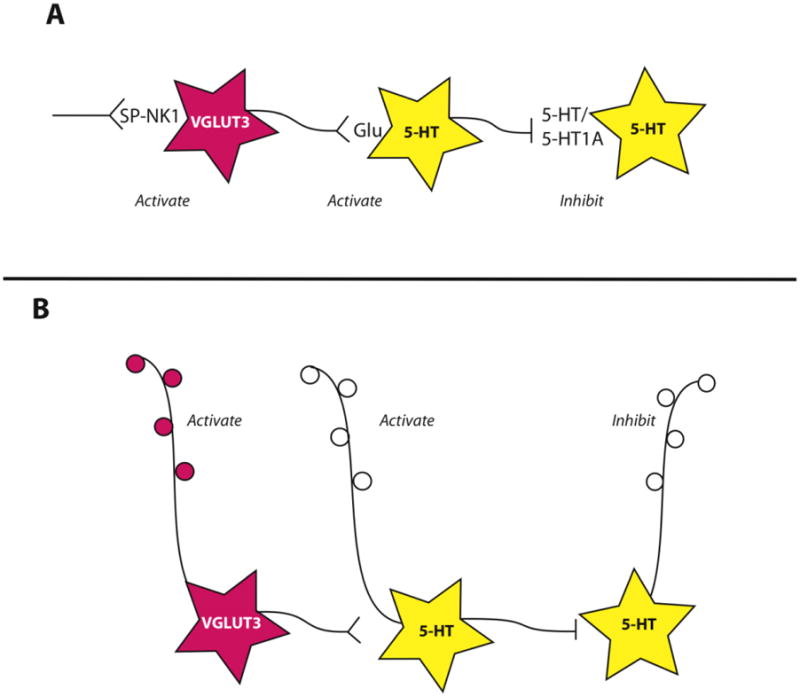
see text for references. A. Substance P (SP) acting at its receptor, neurokinin 1 (NK1) has the capacity to activate VGLUT3-glutamate neurons (pink). These neurons in turn release glutamate onto some 5-HT neurons (yellow), driving their activation and 5-HT release. This subsequently triggers inhibition of other serotonin neurons via 5-HT1A receptors. This scheme explains experimental observations in the rostral and middle portion of the DR, different relationships may exist in the caudal DR. B. VGLUT3-glutamate neurons also contribute to output projections of the DR, raising the possibility of reciprocal activation states between different output pathways.
This network relationship of NK1 receptor to VGLUT3- and 5-HT-containing neurons explains many experimental observations in the middle and rostral levels of the DR. Evidence suggests a twist in the interactions between these components in the caudal DR. As mentioned, the caudal DR appears to have a higher proportion neurons containing both VGLUT3 and 5-HT in their axons. Moreover, NK1 receptors have been reported on 5-HT neurons themselves in the caudal DR (Lacoste et al., 2006; Lacoste et al., 2009). These rostro-caudal distinctions in the DR provide intriguing evidence for functional differences that remain to be fully understood.
Recent evidence suggests that not only do VGLUT3-glutamate cells participate in local circuits, but they also contribute to ascending projections. The contribution of non-5-HT neurotransmitters to the output of the DR has long been known. In almost every retrograde tract-tracing study, projecting neurons from the DR often lack dual immunolabeling for 5-HT. Furthermore, there is a population of projections identified with anterograde tract-tracing that lack 5-HT immunolabeling (Aznar et al. 2004) and remain after 5,7-dihydroxytryptamine lesion of 5-HT neurons (Halberstadt and Balaban, 2008). Many VGLUT3-glutamate cells in the DR, as well as the median raphe (MR), contribute to these non-5-HT ascending projections (Jackson et al., 2009; Yamakawa and Antle, 2010; Hioki et al., 2010). Therefore, parallel efferent projection pathways arise from midbrain raphe nuclei: one arises from 5-HT neurons (with or without VGLUT3) and another from VGLUT3-glutamatergic cells.
One target of VGLUT3-glutamate cells of the DR, and more predominantly VGLUT3 cells in the MR, is the hippocampal formation (Jackson et al., 2009). Indeed, as mentioned previously, physiological evidence also supported the glutamate nature of ascending projections to the hippocampus (Varga et al., 2009). This glutamatergic innervation from the raphe nuclei may be mediated by VGLUT3-glutamate projections and/or VGLUT3-5-HT projections.
Additional areas receiving innervation from raphe VGLUT3-glutamate cells include several nuclei within the hypothalamus, the ventral tegmental area, the substantial nigra pars compacta, and the pre-optic area (Hioki et al., 2010). Since VGLUT3-glutamate cells project to several forebrain targets, and concurrently are associated with activation of 5-HT1A receptors, this raises the interesting possibility that glutamate and at least a certain population of 5-HT afferents have reciprocal activation states. When NK1-receptor bearing VGLUT3-glutamate cells are active, that is associated with an inhibition of 5-HT cells. One would predict that while glutamate is released in several forebrain areas by VGLUT3-containing ascending projections, 5-HT release would be dampened (Figure 7B).
5. Postsynaptic Response to Glutamate in DR 5-HT and non-5-HT cells
Glutamatergic afferent drive of the DR is closely linked to feedback inhibitory mechanisms, often involving interactions between 5-HT neurons mediated by 5-HT1A receptors. In addition the glutamatergic activation of local GABA neurotransmission may be important in regulating the activity of the DR. Afferents from the PFC directly innervate both 5-HT and GABAergic neurons such that activation of glutamatergic afferents from the PFC is often associated with an inhibition of 5-HT neurons (Jankowski and Sesack, 2004; Celada et al., 2001). Moreover, some evidence suggests that 5-HT vs. non-5-HT cells may be selectively innervated by different populations of glutamatergic axons or these axons may be under separate control mechanisms. Specifically, glutamatergic innervation of DR 5-HT cells appears differentially affected by stress exposure in comparison to non-5-HT cells (Kirby et al., 2007).
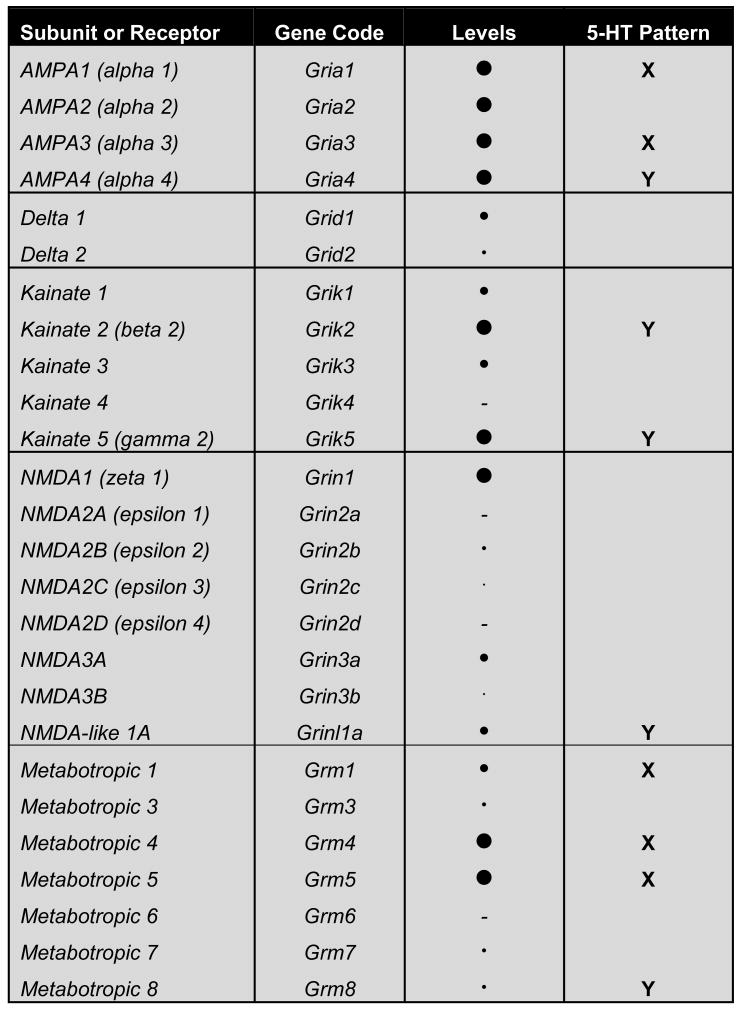
Several studies have examined postsynaptic responses to glutamate and have reported neurotransmission at both NMDA and AMPA/Kainate receptors (Pan and Williams, 1989; Pallotta et al., 1998; Celada et al., 2001; Gartside et al., 2007) as well as metabotropic receptors (Kawashima et al., 2005). In order to further identify the specific glutamate receptor subtypes and subunits that may mediate signaling in the DR, we surveyed their expression patterns within the DR using the Allen Brain Institute's Mouse Atlas (Figures 8 and 9; Lein et al., 2007). A subjective estimate of expression level was scored, as was the pattern of expression in comparison to that of tryptophan hydroxylase 2 (TPH2), used as a marker for 5-HT cells, and GAD2 (GAD65), a marker for GABAergic neurons. That is, we evaluated if genes appeared selectively enriched or reduced in midline areas where 5-HT cells would lie. Reduced expression along the midline would suggest expression in non-TPH cells resident to the DR. This analysis revealed that AMPA receptor subunits 1-4 are well represented in the DR, with Gria2 likely present and Gria4 appearing selectively enriched in the location of 5-HT cells. Kainate receptor subunits are less widely available but two of them have no expression, Grik2 and Grik5, and these appear particularly enriched in the area of 5-HT cells. Some of the metabotropic receptors (mGluR) are represented in the DR, in particular mGluR1, 4 and 5. However, only mGluR8, which has very low level of expression, appears selectively enriched in the area of 5-HT cells. Overall, these observations would suggest that perhaps 5-HT and non-5HT neurons in the DR use different repertoires of glutamate receptor signaling complexes to receive and integrate synaptic information, echoing the likelihood of unique characteristics of glutamatergic drive of 5-HT vs. non-5-HT cells within the DR (Kirby et al., 2007).
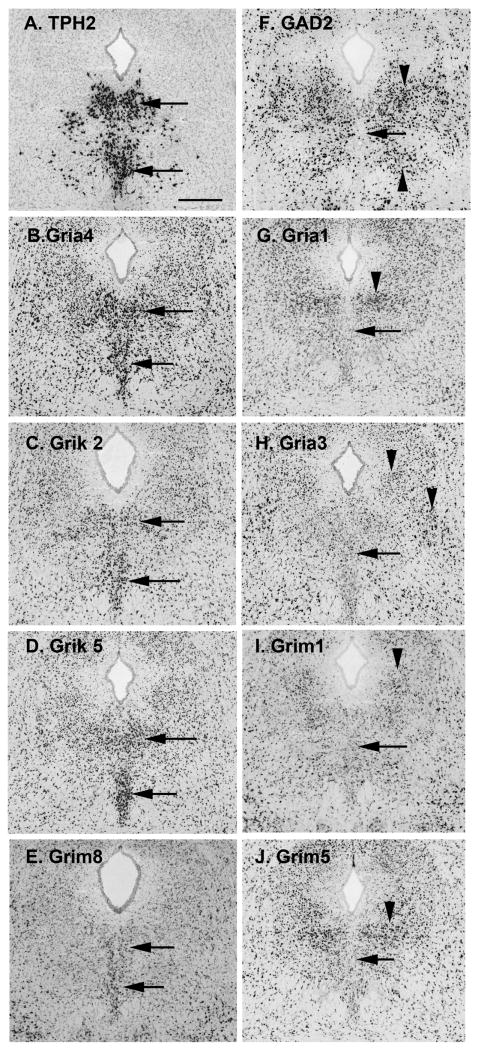
Figure 8. Images of glutamate-receptor gene expression in the mouse DR from the Allen Mouse Brain Atlas, Allen Institute for Brain Science, Seattle WA. ©2009. Available from: http://mouse.brain-map.org.
Gene expression of ionotropic and metabotropic glutamate receptors in the DR were surveyed and compared to the distribution 5-HT and GABAergic neurons (data summarized in Figure 9). A. Identifying the location of 5-HT cells, TPH2 (tryptophan hydroxylase 2) expression is intense along the midline (arrows). B-E. Glutamate receptors genes that have more expression on the midline than laterally, similar to the pattern of TPH2 expression. F. GAD2 (glutamate decarboxylase-2 or GAD65) expression reveals the distribution of GABAergic neurons in the DR. Cells on the midline (arrow) have lower expression levels than clusters of cells laterally (arrowheads). G-J. Glutamate receptor subunits that, similar to GAD2, show lower expression on the midline (arrows), and higher expression laterally (arrowheads). All panels same scale, bar in A = 400 microns.
Figure 9. Summary of the expression pattern and level of glutamate receptor subunits in the DR as evaluated with the Allen Mouse Brain Atlas, Allen Institute for Brain Science, Seattle WA. ©2009. Available from: http://mouse.brain-map.org (example images depicted in Figure 8).
A dash represents no detectable expression, while circles in increasing sizes proportional to relative abundance when present. The “5-HT Pattern” indicates if the gene was scored as enriched (check mark) or reduced (cross mark) in the regions where 5-HT cells are located. Equivalent expression in 5-HT cell pattern and neighboring areas are unmarked.
6. Conclusions
Glutamatergic innervation of DR arises from cortical, subcortical and local sources and these correlate with axons containing VGLUT1, VGLUT2 and VGLUT3 respectively. Appropriate development and functioning of these axon populations is likely important for regulating 5-HT release across the forebrain. Future work should lead to a greater understanding the potential role of each of these populations in psychopathology associated with 5-HT dysfunction.
Highlight.
Glutamatergic neurotransmission in the dorsal raphe nucleus (DR) regulates the serotonin (5-Hydroxytryptamine, 5-HT) neurotransmission, both are linked to pathophysiology of affective disorders.
Glutamate-axons arising from different brain areas heavily express one of three types of vesicular glutamate transporter: VGLUT1, VGLUT2 or VGLUT3.
We discuss how these glutamate excitatory inputs may regulate DR circuits and their implications in emotional processing.
Acknowledgments
Supported by the National Institutes of Health grant DA-021801.
Footnotes
Conflicts of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar S, Qian ZX, Knudsen GM. Non-serotonergic dorsal and median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience. 2004;124:573–581. doi: 10.1016/j.neuroscience.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Badaloni A, Bonanomi D, Albieri I, Givogri I, Bongarzone E, Valtorta F, Consalez GG. Transgenic mice expressing a dual, CRE-inducible reporter for the analysis of axon guidance and synaptogenesis. Genesis. 2007;45:405–412. doi: 10.1002/dvg.20307. [DOI] [PubMed] [Google Scholar]
- Barbaresi P. Immunocytochemical localization of substance P receptor in rat periaqueductal gray matter: a light and electron microscopic study. J Comp Neurol. 1998;398:473–490. doi: 10.1002/(sici)1096-9861(19980907)398:4<473::aid-cne2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Valentino RJ. Cellular basis for the effects of substance P in the periaqueductal gray and dorsal raphe nucleus. J Comp Neurol. 2002;447:82–97. doi: 10.1002/cne.10228. [DOI] [PubMed] [Google Scholar]
- Commons KG, Beck SG, Bey VW. Two populations of glutamatergic axons in the rat dorsal raphe nucleus defined by the vesicular glutamate transporters 1 and 2. Eur J Neurosci. 2005;21:1577–1586. doi: 10.1111/j.1460-9568.2005.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3) J Chem Neuroanat. 2009;38:273–281. doi: 10.1016/j.jchemneu.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel FR, Galante M, Habbas S, McLean H, Daniel H. Role of the Vesicular Transporter VGLUT3 in Retrograde Release of Glutamate by Cerebellar Purkinje Cells. J Neurophysiol. 2010 doi: 10.1152/jn.00736.2010. in press. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Froger N, Gardier AM, Moratalla R, Alberti I, Lena I, Boni C, De Felipe C, Rupniak NM, Hunt SP, Jacquot C, Hamon M, Lanfumey L. 5-hydroxytryptamine (5-HT)1A autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization. J Neurosci. 2001;21:8188–8197. doi: 10.1523/JNEUROSCI.21-20-08188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Elizalde N, Matrov D, Harro J, Wojcik SM, Venzala E, Ramírez MJ, Del Rio J, Tordera RM. Increased vulnerability to depressive-like behavior of mice with decreased expression of VGLUT1. Biol Psychiatry. 2009;66:275–282. doi: 10.1016/j.biopsych.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Cole AJ, Williams AP, McQuade R, Judge SJ. AMPA and NMDA receptor regulation of firing activity in 5-HT neurons of the dorsal and median raphe nuclei. Eur J Neurosci. 2007;25:3001–3008. doi: 10.1111/j.1460-9568.2007.05577.x. [DOI] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn RE, Watkins LR, Maier SF. Impaired escape performance and enhanced conditioned fear in rats following exposure to an uncontrollable stressor are mediated by glutamate and nitric oxide in the dorsal raphe nucleus. Behav Brain Res. 2000;112:33–41. doi: 10.1016/s0166-4328(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Guilloux JP, Reperant C, Hunt SP, Toth M, Gardier AM. Substance P neurokinin 1 receptor activation within the dorsal raphe nucleus controls serotonin release in the mouse frontal cortex. Mol Pharmacol. 2007;72:1411–1418. doi: 10.1124/mol.107.040113. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Selective anterograde tracing of nonserotonergic projections from dorsal raphe nucleus to the basal forebrain and extended amygdala. J Chem Neuroanat. 2008;35:317–325. doi: 10.1016/j.jchemneu.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Holmgren C, Härtig W, Qureshi T, Chaudhry FA, Storm-Mathisen J, Dobszay MB, Berghuis P, Schulte G, Sousa KM, Fremeau RT, Jr, Edwards RH, Mackie K, Ernfors P, Zilberter Y. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: involvement of vesicular glutamate transporter 3. J Neurosci. 2004;24:4978–4988. doi: 10.1523/JNEUROSCI.4884-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki H, Fujiyama F, Nakamura K, Wu SX, Matsuda W, Kaneko T. Chemically specific circuit composed of vesicular glutamate transporter 3- and preprotachykinin B-producing interneurons in the rat neocortex. Cereb Cortex. 2004;14:1266–1275. doi: 10.1093/cercor/bhh088. [DOI] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Hisano S, Hoshi K, Ikeda Y, Maruyama D, Kanemoto M, Ichijo H, Kojima I, Takeda J, Nogami H. Regional expression of a gene encoding a neuron-specific Na(+)-dependent inorganic phosphate cotransporter (DNPI) in the rat forebrain. Brain Res Mol Brain Res. 2000;83:34–43. doi: 10.1016/s0169-328x(00)00194-7. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Bland BH, Antle MC. Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse. 2009;63:31–41. doi: 10.1002/syn.20581. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Kalén P, Karlson M, Wiklund L. Possible excitatory amino acid afferents to nucleus raphe dorsalis of the rat investigated with retrograde wheat germ agglutinin and D-[3H]aspartate tracing. Brain Res. 1985;360:285–297. doi: 10.1016/0006-8993(85)91244-2. [DOI] [PubMed] [Google Scholar]
- Kalén P, Strecker RE, Rosengren E, Björklund A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989;492:187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Karasawa J, Shimazaki T, Chaki S, Okuyama S, Yasuhara A, Nakazato A. Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neurosci Lett. 2005;378:131–134. doi: 10.1016/j.neulet.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pan YZ, Freeman-Daniels E, Rani S, Nunan JD, Akanwa A, Beck SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32:712–723. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH. Ketamine and the potential role for rapid-acting antidepressant medications. Swiss Med Wkly. 2007;137:215–216. doi: 10.4414/smw.2007.11932. [DOI] [PubMed] [Google Scholar]
- Lacoste B, Riad M, Descarries L. Immunocytochemical evidence for the existence of substance P receptor (NK1) in serotonin neurons of rat and mouse dorsal raphe nucleus. Eur J Neurosci. 2006;23:2947–2958. doi: 10.1111/j.1460-9568.2006.04833.x. [DOI] [PubMed] [Google Scholar]
- Lacoste B, Riad M, Ratté MO, Boye SM, Lévesque D, Descarries L. Trafficking of neurokinin-1 receptors in serotonin neurons is controlled by substance P within the rat dorsal raphe nucleus. Eur J Neurosci. 2009;29:2303–2314. doi: 10.1111/j.1460-9568.2009.06775.x. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Li H, Kaneko T, Mizuno N. Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 2001;900:110–118. doi: 10.1016/s0006-8993(01)02272-7. [DOI] [PubMed] [Google Scholar]
- Liu R, Ding Y, Aghajanian GK. Neurokinins activate local glutamatergic inputs to serotonergic neurons of the dorsal raphe nucleus. Neuropsychopharmacology. 2002;27:329–340. doi: 10.1016/S0893-133X(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Maj J, Rogóz Z, Skuza G, Sowińska H. Effects of MK-801 and antidepressant drugs in the forced swimming test in rats. Eur Neuropsychopharmacol. 1992;2:37–41. doi: 10.1016/0924-977x(92)90034-6. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Scott TJ. Colocalization of serotonin and vesicular glutamate transporter 3-like immunoreactivity in the midbrain raphe of Syrian hamsters (Mesocricetus auratus) Neurosci Lett. 2006;394:97–100. doi: 10.1016/j.neulet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984;229:374–392. doi: 10.1002/cne.902290308. [DOI] [PubMed] [Google Scholar]
- Pallotta M, Segieth J, Whitton PS. N-methyl-d-aspartate receptors regulate 5-HT release in the raphe nuclei and frontal cortex of freely moving rats: differential role of 5-HT1A autoreceptors. Brain Res. 1998;783:173–178. doi: 10.1016/s0006-8993(97)01333-4. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J Neurophysiol. 1989;61:719–726. doi: 10.1152/jn.1989.61.4.719. [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E. Antidepressant-like effects of 1-aminocyclopropanecarboxylic acid and D-cycloserine in an animal model of depression. Eur J Pharmacol. 1996;316:145–151. doi: 10.1016/s0014-2999(96)00675-9. [DOI] [PubMed] [Google Scholar]
- Paris JM, Cunningham KA. Habenula lesions decrease the responsiveness of dorsal raphe serotonin neurons to cocaine. Pharmacol Biochem Behav. 1994;49:555–560. doi: 10.1016/0091-3057(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101, 606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutoh F, Ina A, Yoshida S, Konno J, Hisano S. Two distinct subtypes of serotonergic fibers classified by co-expression with vesicular glutamate transporter 3 in rat forebrain. Neurosci Lett. 2008;432:132–136. doi: 10.1016/j.neulet.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Smith GS, Savery D, Marden C, López Costa JJ, Averill S, Priestley JV, Rattray M. Distribution of messenger RNAs encoding enkephalin, substance P, somatostatin, galanin, vasoactive intestinal polypeptide, neuropeptide Y, and calcitonin gene-related peptide in the midbrain periaqueductal grey in the rat. J Comp Neurol. 1994;350:23–40. doi: 10.1002/cne.903500103. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordera RM, Pei Q, Sharp T. Evidence for increased expression of the vesicular glutamate transporter, VGLUT1, by a course of antidepressant treatment. J Neurochem. 2005;94:875–883. doi: 10.1111/j.1471-4159.2005.03192.x. [DOI] [PubMed] [Google Scholar]
- Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, Del Rio J. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) Eur J Neurosci. 2007;25:281–290. doi: 10.1111/j.1460-9568.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bey V, Pernar L, Commons KG. Substance P Acts through local circuits within the rat dorsal raphe nucleus to alter serotonergic neuronal activity. J Neurosci. 2003;23:7155–7159. doi: 10.1523/JNEUROSCI.23-18-07155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Kocsis B, Sharp T. Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur J Neurosci. 2003;17:280–286. doi: 10.1046/j.1460-9568.2003.02465.x. [DOI] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schäfer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, Staines WA, McGeer EG, Fibiger HC. Transmitters contained in the efferents of the habenula. Brain Res. 1980;195:479–484. doi: 10.1016/0006-8993(80)90084-0. [DOI] [PubMed] [Google Scholar]
- Waselus M, Galvez JP, Valentino RJ, Van Bockstaele EJ. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanat. 2006;31:233–242. doi: 10.1016/j.jchemneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Waselus M, Van Bockstaele EJ. Co-localization of corticotropin-releasing factor and vesicular glutamate transporters within axon terminals of the rat dorsal raphe nucleus. Brain Res. 2007;1174:53–65. doi: 10.1016/j.brainres.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa GR, Antle MC. Phenotype and function of raphe projections to the suprachiasmatic nucleus. Eur J Neurosci. 2010;31:1974–1983. doi: 10.1111/j.1460-9568.2010.07228.x. [DOI] [PubMed] [Google Scholar]
- Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Yasuhara A, Chaki S. Metabotropic glutamate receptors: potential drug targets for psychiatric disorders. Open Med Chem J. 2010;4:20–36. doi: 10.2174/1874104501004020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–344. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- Zander JF, Münster-Wandowski A, Brunk I, Pahner I, Gómez-Lira G, Heinemann U, Gutiérrez R, Laube G, Ahnert-Hilger G. Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J Neurosci. 2010;30:7634–7645. doi: 10.1523/JNEUROSCI.0141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]