Abstract
The role of iron anode on electrochemical dechlorination of aqueous trichloroethylene (TCE) is evaluated using batch mixed-electrolyte experiments. A significantly higher dechlorination rate, up to 99%, is reported when iron anode and copper foam cathodes are used. In contrast to the oxygen-releasing inert anode, the cast iron anode generates ferrous species, which regulate the electrolyte to a reducing condition (low ORP value) and favor the reduction of TCE. The main products of TCE electrochemical reduction on copper foam cathode include ethene and ethane. The ratio of these two hydrocarbons gases varied with the electrolyte ORP condition and current density as more ethane gas generates at more reducing electrolyte condition and at higher current condition. A pseudo-first order model is used to describe the degradation of TCE, the first order rate constant (k) increased with the current applied, but exhibits a negative relation with initial concentration. Depending on the current, electrolysis by iron anode causes a reduction in the ORP and an increase in the pH of the mixed electrolyte. Enhanced reaction rates in this investigation indicate that the electrochemical reduction using copper foam and iron anode may be a promising process for remediation of groundwater contaminated with chlorinated organic compounds.
Keywords: TCE, redox control, electrochemical dechlorination, iron anode, copper foam cathode
Introduction
Trichloroethene, a widely used organic solvent, is one of the most pervasive soils and groundwater contaminants. Several processes have been developed to remove or transform TCE from environmental media, including phase-transfer processes,1,2 chemical transfer processes,3 electrochemical transformation 4–8 and biotransformation 9, 10. Electrochemical transformation is one of the processes that have received considerable interest for its effectiveness, environmental friendliness and versatility.11, 12 It has been explored for degradation of chlorinated hydrocarbons through two routes. One route is by cathodic reduction, which is extensively reported in the literature. Most chlorinated hydrocarbons, such as tetrachloroethylene (PCE), TCE and tetrachloride, can be dehalogenated by electroreduction on the cathode surface. Another route is by electrochemical oxidation on the anode surface. Some anodes with high oxygen evolution reaction (OER) overpotential, such as boron-doped diamond (BDD), 7 PbO2 13 or TiOx ceramic electrode, 8 produce highly oxidative hydroxyl radicals on the anode surface that can cleave the carbon-halogen and carbon-carbon bond in chlorinated hydrocarbons. In comparison with anodic oxidation, cathodic reduction has intrinsic advantages, including (i) cathodes have longer service life and are more accessible than high OER overpotential anodes; and (ii) dechlorination on the cathode surface is usually faster and more cost-effective than that by anodic oxidation. 8, 13, 14 Only BDD electrode was reported to show comparable anodic electrocatalytic performance to chlorinated hydrocarbons with respect to the electroreduction process.15
However, due to fact that electrochemical processes always involve mass transfer processes for target compound and the concentration of chlorinated compounds in natural aqueous media is relatively low, the efficiency of electrochemical reduction treatment is still not acceptable for real application. Over the past few decades, methods to improve the efficiency of electrochemical reduction of chlorinated hydrocarbons can be summarized by two aspects: (i) the use of high-performance electrodes and electrolysis cells (ii) optimizing the operating condition of the cell. Selecting specific electrocatalytic cathode material for reductive dechlorination is a typical research topic for enhanced electrochemical dechlorination. Palladized materials have proven to be effective cathodes for reductive dechlorination of TCE.4, 16 Low-cost materials such as iron, copper, zinc, carbon material, metallized-carbon and mixed metal oxide were also tested as cathode for reductive dechlorination of a variety of chlorinated hydrocarbons.4, 5, 17, 18 Using three-dimensional (3D) electrodes, i.e., porous electrode, is another method that is evaluated to improve dechlorination rate. Due to the large specific surface area, 3D electrodes improve the mass transfer of contaminants, thus enhance the removal rate of target compounds. Up to 80% reductive dehalogenation of carbon tetrachloride is reported by a porous copper cathode, with a residence time of 10 min even in low conductivity solutions.19 A study on chlorinated hydrocarbons transformation on several metallized-carbon fiber electrodes showed that Ag modified electrode exhibits the best electrocatalytic activity.17 New and modified cell designs, such as multi-phase iron oxides-packed column cell,16 concentric 3D copper reactor,19 and granular graphite-packed reactor 6 lead to improved transformation.
Studies on reductive dechlorination of chlorinated hydrocarbons also show that the removal efficiency or rate is greatly affected by the operating conditions, such as current density, electrolyte flow rate, electrolyte composition and pH.4–6, 20–22 For example, a current density under 4 mA cm−2 is reported to be sufficient for reasonable PCE removal rate on lead electrode.20, 21 Acidic and neutral pH conditions also appear to favor the decholrination of TCE.22 There is an optimal value or range for operating parameters for a specific cell in terms of dechlorination rate, specific energy consumption or current efficiency.
This study evaluates a new electrolysis approach for electrochemical reductive dechlorination, in which the anodic reaction are utilized for regulating redox conditions and significantly improve the reduction of chlorinated compound (TCE). Oxidation of iron anodes produces ferrous ions leading to a highly reducing environment, when compared to conventional non-reactive anodes. In a mixed electrolyte, the highly reducing environment and presence of ferrous ions coupled with electrolytic reduction at the cathode accelerate the transformation of chlorinated compounds. The objective of this paper is to evaluate the effect of using iron anode with different cathode types on the transformation rates and efficiency of TCE in a bicarbonate solution. Bicarbonate solution is selected because it represents groundwater conditions at many aquifers, including karstic aquifers, which are difficult to remediate.
Experimental Section
Materials
The chemicals used include TCE (99.5%, Sigma-Aldrich), cis-dichloroethylene (cis-DCE, 97%, Sigma-Aldrich), vinyl chloride (VC, analytical standard, 200 μg mL−1 in methanol, Supelco), hydrocarbon gas standard (analytical standard, 1% (w/w) methane, ethene, acetylene in nitrogen, Supelco), and NaHCO3 (analytic grade, JT Baker). Excess TCE was dissolved into 18MΩ high-purity water to form a TCE saturated solution (20°C, saturated dissolution rate is 1.07 mg mL−1), which was used as stock solution for preparing TCE aqueous solutions. The conductive materials investigated as cathode include copper foam (60 pores per inch (PPI), 99.5% purity, Aibixi Ltd., China), iron foam (45 PPI, 98% iron and 2% nickel, Aibixi Ltd., China), nickel foam (60 PPI, 99.9% purity, Lyrun Ltd., China), and vitreous carbon foam (100 PPI, ERG, USA ). The materials were cut into the same size with a working geometry of 4 cm length, 1 cm width and 0.3 cm thickness. Copper plate (99.9% purity, VWR) and high-purity iron plate (3N5 purity, ESPI metals, USA) electrodes (4 cm length, 1 cm width, 0.1 cm thickness) were also investigated as cathode materials. Three anode materials were compared in this study: cast gray iron (Macmaster-Carr, USA), mixed metal oxide (MMO, mesh type, 3N International, USA) and lead dioxide. The lead dioxide electrode was fabricated in our laboratory using electrodeposition method (40 mA cm−2 current density) as described in the previous work.23 Before each experiment, the iron electrode was polished with coarse emery cloth, etched by diluted HCl solution (10 wt%) and washed with distilled water. The copper foam electrode was also rinsed with diluted H2SO4 solution (3 wt%), 2% Micro-90 cleaning solution (Cole Parmer, USA ) and distilled water prior to assembly.
Analytical Methods
Concentrations of TCE, cis-DCE and VC were measured using 8610GC instrument with purge-trap system (SRI, USA), photoionization detector and MXT-VOL stationary column. The purge-trap autosampler was equipped with carbon-sieve trap and Tenax™ trap, allowing the detection of highly volatile VC. 50 μL of water sample was injected in 5 mL deionized water in glass tubes, and loaded into the 10-port autosampler. The GC was programmed at 40°C for 6 minutes, then ramped to 60°C in 2 minutes, held at 60°C for 10 minutes. Hydrocarbon gases (methane, ethene, ethane and acetylene) in headspace of electrolytic cell were analyzed through a Model 310 GC (SRI, USA) with flame ionization detector and Haysep-T column. 100 μL of headspace gas was sampled and injected from an on-column port. The temperature program applied was: heat column from 40 to 140°C at a rate of 15°C min−1, hold 140°C for 1 minute, and cool to 40°C at a rate of 20°C min−1. Chloride ion concentration was analyzed by Dionex DX-120 ion chromatograph. After each experiment, an aliquot 0.2 to 0.5 ml of supernatant was transferred into 5 mL vials which had been pre-filled with de-ionized water (> 18 M3), then filtered by 0.45μm pore size filter paper prior to final analysis. pH, conductivity and oxidation-reduction potential (ORP) of the electrolyte were measured by pH meter, conductivity meter and ORP meter with corresponding microprobes (Microelectro, USA). The microprobes allow the measurement on these parameters using small amount of liquid (≈0.2 mL).
Procedure
The electrochemical transformation experiments were conducted in an undivided glass electrolytic cell (see Figure S1 in the Supporting Information (SI)) at ambient temperature (25±1°C). The temperature variation of the electrolyte during electrolysis is less than 2.6 °C for all experiments. A 150 mL syringe was connected to the cell, allowing gas expansion during electrolysis when the inside pressure is above 2 kg force cm−2 (no gas expansion was observed under this pressure in all experiments). The anode and cathode were placed in parallel position with 1.7 cm distance. During electrolysis, the electrolyte was stirred using a Teflon-coated, one inch magnetic stirring bar (500 rpm). For each trial, 110 mL 0.01M sodium bicarbonate solution was transferred into the cell and 5 mL of TCE saturated water solution was added. The solution was stirred for 30 minutes to allow equilibrium of TCE in the aqueous solution. The electric current was then applied and TCE concentration was routinely measured. The aqueous solution was also sampled for ORP measurements (sample volume was 0.2 mL). For the headspace gas (initial headspace above solution was 67 mL), gas sampling (100 μL) was done in some experiments. After electrolysis, the final pH and conductivity of the solution were measured.
Results and discussion
Effect of anode type
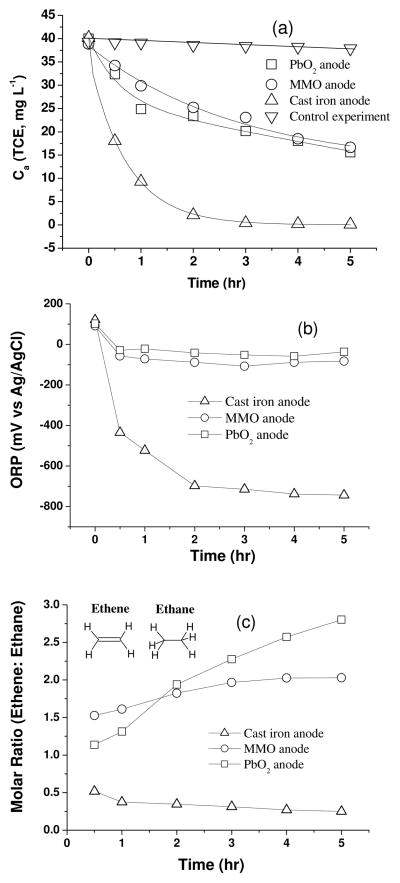
The effects of three types of anodes; MMO, PbO2 and cast iron, on TCE transformation by constant current electrolysis in a mixed-electrolyte cell are compared. The decay of aqueous TCE in the cells using copper foam cathode and these anodes is presented in Figure 1a. Cast iron anodes caused faster and sustainable transformation of aqueous TCE compared to other anodes. Within 0.5 hour, the aqueous TCE concentration decreased by 50% and after 5 hours the concentration decreased to below detection limits of the analytic method used in this study (0.1 mg L−1). In contrast, the concentrations of aqueous TCE when PbO2 and MMO anodes are used decreased to 15.6 and 16.6 mg L−1, respectively, after 5 hours. PbO2, as a high OER overpotential anode, did not show considerable improvement in TCE degradation, suggesting that the effect of oxidation pathway on the anode surface is limited. During the electrolysis, cis-DCE and VC, as possible degradation intermediates of TCE, were monitored. However, no visible accumulation of these chlorinated intermediates was found in three cells. On the other hand, hydrocarbon gases (ethene, ethane and methane) were detected immediately after electrolysis started. Figure S2 in the SI shows hydrocarbon gases concentrations in the headspaces after 0.5 hour. The much higher concentrations of ethene, ethane and methane in the cell using cast iron anode support the phenomenon that faster TCE degradation occurred in this cell.
Figure 1.
(a) Decay of aqueous TCE concentration, (b) time profiles of electrolyte ORP, and (c) time profiles of molar ratio of headspace gases (ethene to ethane) in the cells using copper foam cathode and different anodes. Electrolysis current was 90 mA. Initial TCE concentration was around 39 mg L−1. Control experiment was conducted without applying electrical current.
The anodic reaction with cast iron causes the oxidation of iron since the standard potential of Fe0-Fe2+ redox couple is only −0.44 V vs SHE (Eq 1).24, 25 Ferrous ions generated from the iron anode could combine with the hydroxyls, forming amorphous ferrous hydroxide (Eq 2). 24
| (1) |
| (2) |
To understand the enhancing effect from cast iron anode, the electrolyte ORP was monitored during electrolysis and their profiles are shown in Figure 1(b). The ORP of the electrolyte in the cell using cast iron anode decreased sharply within 0.5 hours followed by a progressively stable ORP value around −800 mV. In contrast, the other anodes showed a much higher ORP value (between 0 and −100 mV), indicating a less reducing environment in the electrolyte compared to the iron anode. Because the primary reaction at the iron anode is formation of ferrous ions, rather than the oxygen evolution, the subsequently formed ferrous species (ferrous hydroxide, ferrous carbonate, ferrous complexes, etc.) have reduction potential to consume the oxidative substances in the electrolyte, such as dissolved oxygen, creating a very reducing mixed electrolyte. Thus, except for the chemical recombination through Eq 4, the atomic hydrogens (Eq 3) at the cathode mostly contribute to the reduction of TCE (Eq 5) and a faster degradation rate is achieved.26 In the cells with inert anode (MMO or PbO2), oxygen gas is continuously generated at the anode which possibly limits the TCE transformation. Furthermore, the reduction potential of O2 is 1.229 V vs SHE (Eq 6),25 higher than the reduction potential of TCE (0.42 V vs SHE from TCE to cis-DCE),27 O2 is much more vunerable to reduction than TCE (see the calculation using Nernst equation in the SI). As a result, very different TCE transformation behavior under 90 mA and even lower electrolysis current (e.g. 30 mA) is observed in these cells.
| (3) |
| (4) |
| (5) |
| (6) |
The findings can be further verified by comparing the degradation products in the three cells. The molar ratios of ethene (with four hydrogen atoms in the molecular structure) to ethane (with six hydrogen atoms in the molecular structure) in the headspace gas during electrolysis are compared in Figure 1c. Less ethene was generated, with ethene to ethane ratio of less than 1, in the cell using iron anode. However, the cells using inert anodes behave very differently with higher ratios of ethene to ethane. Under similar mass transfer and electrolysis conditions, less ethane production means atomic hydrogen is relatively insufficient for TCE reduction, suggesting that some of the atomic hydrogens have been consumed by other reactions or processes.
Effect of cathode type
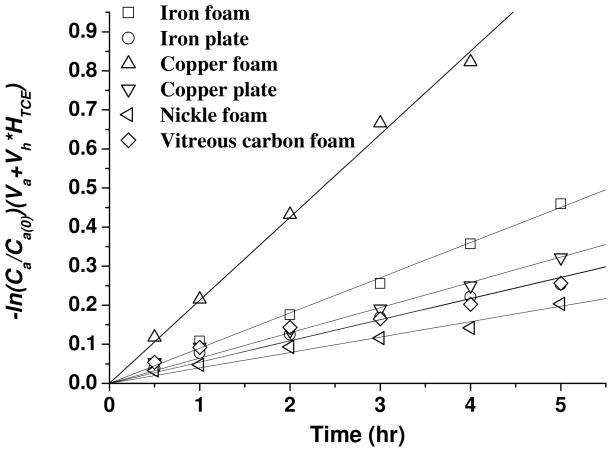
Four foam materials and two planar materials are compared as cathode for electrochemically reductive dechlorination of TCE with cast iron anode. A current of 90 mA current was used for all electrolysis experiments. Under this current, gas bubbles generation on electrodes confirmed that all the cathodes worked at a hydrogen-releasing potential. Based on the exponential decay of TCE when iron anode and copper cathode were used (Figure 1a), a pseudo-first order model is proposed to describe the transformation kinetics of TCE (model development is given in the SI) 22:
| (7) |
where Ca(0) and Ca(t) are TCE concentration in aqueous solution at time=0 and time=t (mg L−1), respectively; Va is the volume of aqueous solution (L); Vh is the volume of headspace (dm3); k is first-order rate constant of TCE degradation by electrochemical process (L h−1); g is the headspace gas expansion rate (dm3 h−1); HTCE is the dimensionless Henry law constant of TCE; and t is the electrolysis time (hour). Assuming g= 0, which is valid based on the results, Eq 7 could be further represented as Eq 8.
| (8) |
Plots of -ln(Ca(t)/Ca(0))(Va+VhHTCE) versus time for different cathodes are presented in Figure 2, and the corresponding k values are listed in Table 1. The copper foam cathode, followed by iron foam and copper plate, exhibit the best performance for transformation of TCE with iron anodes. Even with high specific surface area, the vitreous carbon and nickel foam cathode did not result in high dechlorination rates, being less effective than copper plate cathode, but comparable to iron plate cathode. Relatively little information is available about the electrocatalytic reactions of TCE on different cathodes. No specific influence for the electrode materials (carbon, copper and lead) was reported on the electrochemical degradation of PCE.21 Hydrogenation efficiencies close to 100% were reported for Ag, Zn, Cu and Pb cathodes in studies on electroreduction of chloroform.18 Our results with iron anodes show that although there is no difference in transformation speciation, TCE transformation rate in the presence of iron anodes is also dependent on the type of cathode material. Moreover, the transformation rate could be further improved by adopting high specific surface area electrodes. Copper and iron foams both show better performance than that of the corresponding plate material. The vitreous carbon foam did not show superior performance, which is in agreement with other investigators’ results on aqueous dechlorination.20
Figure 2.
TCE degradation kinetics in the electrolyte containing 39 mg L−1 TCE. Slopes of linear regression lines shown for each cathode data set represent the pseudo-first-order rate constants for TCE reduction (k).
Table 1.
Experimental results for the batch electrolysis experiments with different anodes and cathodes (current = 90 mA)
| NO. | Cathode | Anode | CA (mg L−1) (ini-fin) | ζ (μS cm−1) (ini-fin) | pH (ini-fin) | Chloride (mg L−1) | Cl− mass recovery (%) | FDEa (%) | C mass recovery (%) (0.5h-fin)b | K (×10−3 L h −1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Copper foam | Cast iron | 39.9-<0.1 | 862-1368 | 7.7-11.3 | 32.7 | 80.2 | >99.7c | 90-25 | 212.5±3.8 |
| 2 | Copper foam | MMO | 38.9-16.7 | 853-840 | 7.7-7.1 | 16.2 | 89.2 | 57.2 | 102-70 | NA |
| 3 | Copper foam | PbO2 | 40.0-16.5 | 853-911 | 7.6-6.9 | 11.7 | 74.3 | 61.0 | 104-71 | NA |
|
| ||||||||||
| 4 | Copper plate | Cast iron | 38.6-4.3 | 845-970 | 7.6-10.1 | 30.5 | 80.3 | 88.8 | 90-23 | 64.6± 2.2 |
| 5 | Pure iron plate | Cast iron | 39.7-7.1 | 850-866 | 7.5-9.7 | 19.6 | 72.1 | 82.1 | 89-33 | 54.4± 2.2 |
| 6 | Iron foam | Cast iron | 40.2-1.7 | 863-950 | 7.7-10.1 | 19.5 | 57.7 | 95.6 | 94-20 | 90.1± 1.6 |
| 7 | Nickle foam | Cast iron | 40.3-10.1 | 854-858 | 7.7-9.5 | 13.2 | 63.2 | 74.9 | 91-36 | 39.5± 1.6 |
| 8 | Vitreous carbon foam | Cast iron | 40.4-7.1 | 855-1393 | 7.6-11.8 | 22.8 | 80.6 | 82.4 | 86-30 | 54.1± 3.7 |
FDE(Final degradation Efficiency)= (Ca(0)−Ca(5h)/Ca(0)) ×100%
Carbon mass recovery only considered the carbon mass contribution from the spiked TCE.
>99.7% means the concentration of aqueous TCE was below the detection limitation of the analytic method (0.1 mg L−1) after 5-hours electrolysis. 0.1 mg L−1 was used as final TCE concentration for FDE calculation. Same for other silimar expressions in this table.
A summary of other relevant information from the experiments is presented in Table 1. A considerable pH rise is observed in all the experiments using iron anode. When inert anode is used, the protons generated from anode neutralize the hydroxyl ions generated from cathode, maintaining a relatively neutral pH. However, when iron anode is used, hydroxyl ions produced by cathodic reactions will not completely combine with ferrous ions to form precipitate (Ksp of Fe(OH)2 is 7.08E-16),28 promoting the pH to increase. In addition, other chemical processes such as the formation of FeCO3(s), Fe(HCO3)+ could consume some ferrous ions and further improve the concentration of free hydroxyl ions. 29–30 Although alkaline electrolyte is not preferred for electrochemical reductive dechlorination,22 the pH is obviously a less important factor as compared to ORP and electrode type since the neutral pH did not bring high dechlorination rate in this study (in the cell using inert anodes). As for the chloride ions, higher final concentrations were detected in the experiments with high TCE removal rates, which is in agreement with expectations. However, comparing chloride ions mass recovery shows relatively lower rates (57.7%) when iron anode is used. These low values may be in part explained by absorption on ferrous hydroxide and formation of ferrous and ferric chloride complexes in solution.28–30 Additionally, relatively good carbon mass recovery rates are obtained 0.5 hour after electrolysis. However, carbon mass recovery rates decrease to low values after 5 hours of testing, especially when iron anodes were used (20% to 36%). In the literatures, carbon mass recovery varied from around 30% to above 90% when TCE and PCE bulk electrolysis were conducted in a closed system similar to the setup used in this study.6, 13, 14 It is possible that the relatively low carbon mass recoveries after 5 hours are due to the buildup of gas pressure in the headspace, which could produce inaccuracies in the absolute concentration of hydrocarbons gas in headspace gas. However, using the ratio of these hydrocarbon gases as an index to analyze the degradation behavior of TCE is reasonable and reliable since ethane and ethene have similar physiochemical properties.
Effect of current and initial TCE concentration
For a specific electrochemical system, current and substrate concentration are two important variables that could impact the process. Six levels of current and three levels of initial TCE concentration are investigated. The results, including final degradation efficiency (FDE) and k values, are summarized in Table S1 (SI).
As presented in Table S1, FDE of TCE (measured after 5 h of electrolysis) increases to more than 99% with increasing the electric current at three levels of initial concentration. As the initial concentration increase, it becomes relatively harder to achieve more than 99% TCE transformation after 5-hours electrolysis. For the pseudo-first order rate constant (k, L h−1), the first observation is that it increases with increasing the applied current. This trend appears clear for the case of 74 mg L−1. The second observation is that rate constant k is dependent on TCE concentration and current (or current density), but does not follow a consistent trend with concentration and current. For the electrochemical dechlorination of TCE on iron electrodes, the reaction rate (rTCE) is believed to be associated with adsorbed hydrogen and adsorbed TCE, as given by,26
| (9) |
where ΘH and ΘTCE represent the surface coverage of adsorbed hydrogen (or atomic hydrogen) and adsorbed TCE, respectively, and n is the reaction order of hydrogen in the hydrodechlorination reaction. Assuming the reduction of TCE on copper proceeds with the same mechanism, the change in k can be explained. When the current density increases, more atomic hydrogens cover the real surface of cathode, so the absorbed TCE molecules are more likely to acquire atomic hydrogens. This explains the trend that k increases with increasing current. Other investigators also reported faster TCE reduction rates at more negative cathode potentials.14 Their results also can be explained by the effect of ΘH. On the other hand, increasing TCE concentration has an adverse effect on k values, suggesting that the TCE coverage is not proportional to the concentration of TCE in the bulk electrolyte. The reduction of TCE on the cathode proceeds with a chemisorption process, rather than a physical adsorption process, and the chemisorption process is the controlling process for the overall rate of dechlorination.26
In spite of the low TCE reduction rates at low currents, application of low current results in a better efficiency. Eq 10 is used to calculate the average current efficiency (ACE(t)) of TCE reduction from the start (time=0 ) to a given time t (time = t):
| (10) |
where F is the Faraday’s constant (96485 C mol−1), nj is the number of electrons transferred from TCE to hydrocarbon compound j, χj is the molar percentage of species j in the total hydrocarbon gases, MTCE is the molecular weight of TCE (131.4 g mol−1), i and t represent current (ampere) and electrolysis time (second). Eq 10 assumes that hydrocarbon gases, including ethene, ethane, acetylene and methane, are the exclusive final products of TCE electrolytic reduction. In our case, acetylene is not detected and the amount of methane is negligible, so only ethene (n=6) and ethane (n=8) are considered for calculation (6 ≤ Σnjχj≤8). The molar percentage of these two gases were obtained by measuring the headspace gas at time t. Figure S3 in the SI demonstrates the ACE at different times for 39 mg L−1 initial concentration experiment set. The lowest current, 5 mA, always exhibits the highest current efficiency during the 5 hours electrolysis, ranging from 43.3% at 0.5 h to 14.7% at 5 h. In contrast, 90 mA showed 11.0% ACE after 0.5 h and constantly decreased to 2.0% after 5 hours. Generally, the basic observation is that the current efficiency for TCE reduction decreases with increasing the current due to the mass transfer limitation. Considering the rising cell voltage at higher currents, the unit energy consumption climbs to a higher value. Thus, from a practical point of view, the requirement of faster dechlorination or energy efficiency will determine the current levels that should be applied. In our batch electrolysis experiments, 30 mA seems the threshold value that achieves FDE value above 95%, k value around 100×10−3 L h−1 for three different initial concentrations. Assuming a specific surface area of 5000 m2 m−3 and a uniform current density distribution on the electrode, 30 mA reflects 0.5 mA cm−2 current density on copper foam.
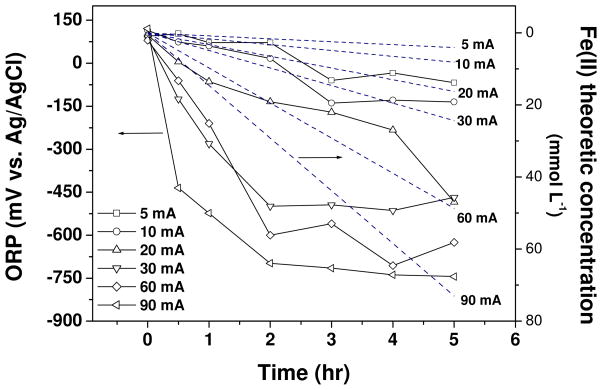
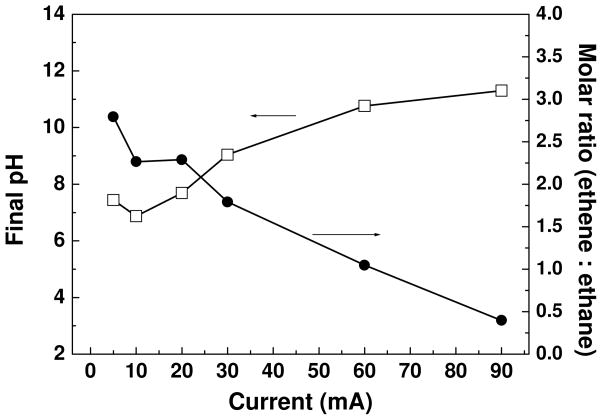
Other information regarding the effect of current on electrolysis can be found in Figure 3 and Figure 4. In Figure 3, the ORP of the electrolyte at different current conditions are presented. The ORP shifts to negative values once the electrolysis starts, and the change rate depends on the current. For 5 and 10 mA currents, the ORP of the electrolyte decreases gradually and levels off above −150 mV. By contrast, 90 mA current result in a rapid drop of ORP value, showing the buildup of a very reducing electrolyte condition within 0.5 hour. Assuming the current efficiency for cast iron dissolution is 100%, the molar concentrations of Fe(II) species (including all soluble and dissolved ferrous species) in the electrolyte should increase linearly with respect to the electrolysis time (the dash line shown in Figure 3), and constant decrease of ORP is supposed to occur for all current conditions. However, the profiles of electrolyte ORP in Figure 3 gradually stabilize at late stages of electrolysis, instead of dropping down constantly. This suggests that the precipitation of ferrous hydroxide and other possible side reactions on the anode (such as passivation, or O2 evolution)31 will prevent the continuous decrease in ORP. The effect of current density on electrolyte final pH and ratio of ethene to ethane are depicted in Figure 4. The pH vs. current plot shows that higher current results in higher final pH value. Considering that maintaining neutral pH is essential for groundwater remediation, choosing an appropriate current that does not induce a significant pH rising, but still attain reasonable TCE removal rate, is an important design issue. In our case, 30 mA seems the optimum value that could balance these two aspects. As for the final products composition, the current (or current density) also plays an important role. At higher currents, the sufficient atomic hydrogens on cathode and ferrous species both favor the generation of ethane, being lower ratio of ethene to ethane.
Figure 3.
Time profiles of electrolyte ORP at different electrolysis currents (39 mg L−1 initial TCE concentration). The Fe(II) theoretic concentrations (dash line) were calculated based on Faraday’s law using 100% current efficiencies (this is hypothetical and actual efficiencies are less than 100%). Copper foam cathode and cast iron anode. The arrows in the figure refer to the relevant Y-axis.
Figure 4.
Effect of current on final pH of electrolyte and headspace gas composition (molar ratio of ethene to ethane). Initial concentration of aqueous TCE was around 39 mg L−1 in all experiments.
Implications for in-situ remediation
The results presented here provide a new concept that can be utilized for remediation of groundwater contaminated with chlorinated solvents. Previously dechlorination was achieved when anode and cathode were separated with ionic conductivity membrane or porous frit. Separated cell design prevents any impact from anodic reactions on reductive dechlorination and allows maintaining a reducing condition in cathodic reaction zone. However, for in-situ remediation of groundwater, separated cell design is not only complicated, but also not cost-effective, and causes higher energy consumption and maintenance problems. The present study show that fast dechlorination of chlorinated compounds can be obtained in a mixed-electrolyte reactor, by coupling cast iron anode and high specific surface area cathode. This finding is mainly attributed to the unique anodic reaction on cast iron: ferrous species generated through iron anode dissolution, instead of oxygen gas formation on inert anode. Therefore, a reducing environment for groundwater remediation can be realized using mixed-electrolyte cell design, which helps to meet a variety of technical and economic challenges that electrochemical remediation method may face.
On the other hand, there are possible adverse effects of iron electrolysis that should be explored. The precipitate and possible pH increase from the process may impact the physical, chemical and biological characteristics of the groundwater and aquifer. The successful application of this technology will depend on a variety of conditions, such as groundwater chemistry, current intensity with respect to groundwater flow rate, the physical and chemical properties of the aquifer. Research is ongoing to evaluate these effects and assess the practicability of this remedial strategy in karstic aquifers.
Supplementary Material
Acknowledgments
The project described was supported by Award Number P42ES017198 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Pseudo-first order model, reduction potential calculation, the schema of experiment setup, a figure depicting the headspace hydrocarbon gases concentration, a figure describing the ACE at different currents, and a table containing the k values and FDE under different currents and initial concentrations.
References
- 1.Heron G, Van Zutphen M, Christensen TH, Enfield CG. Soil heating for enhanced remediation of chlorinated solvents: A laboratory study on resistive heating and vapor extraction in a silty, low-permeable soil contaminated with trichloroethylene. Environ Sci Technol. 1998;32(10):1474–1481. [Google Scholar]
- 2.Rabideau AJ, Blayden JM, Ganguly C. Field performance of air sparging system for removing TCE from groundwater. Environ Sci Technol. 1999;33(1):157–162. [Google Scholar]
- 3.Tsitonaki A, Petri B, Crimi M, Mosbaek H, Siegrist RL, Bjerg PL. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A Review. Crit Rev Environ Sci Technol. 2010;40(1):55–91. [Google Scholar]
- 4.Li T, Farrell J. Reductive dechlorination of trichloroethene and carbon tetrachloride using iron and palladized-iron cathodes. Environ Sci Technol. 2000;34(1):173–179. [Google Scholar]
- 5.Petersen MA, Sale TC, Reardon KF. Electrolytic trichloroethene degradation using mixed metal oxide coated titanium mesh electrodes. Chemosphere. 2007;67(8):1573–1581. doi: 10.1016/j.chemosphere.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 6.Al-Abed SR, Fang YX. Use of granular graphite for electrolytic dechlorination of trichloroethylene. Environ Eng Sci. 2007;24(6):842–851. [Google Scholar]
- 7.Carter KE, Farrell J. Electrochemical Oxidation of Trichloroethylene Using Boron-Doped Diamond Film Electrodes. Environ Sci Technol. 2009;43(21):8350–8354. doi: 10.1021/es9017738. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Betterton EA, Arnold RG. Electrolytic oxidation of trichloroethylene using a ceramic anode. J Appl Electrochem. 1999;29(8):961–970. [Google Scholar]
- 9.Pant P, Pant S. A review: Advances in microbial remediation of trichloroethylene (TCE) J Environ Sci-China. 2010;22(1):116–126. doi: 10.1016/s1001-0742(09)60082-6. [DOI] [PubMed] [Google Scholar]
- 10.Aulenta F, Canosa A, De Roma L, Reale P, Panero S, Rossetti S, Majone M. Influence of mediator immobilization on the electrochemically assisted microbial dechlorination of trichloroethene (TCE) and cis-dichloroethene (cis-DCE) J Chem Technol Biotechnol. 2009;84(6):864–870. [Google Scholar]
- 11.Mouli PC, Mohan SV, Reddy SJ. Electrochemical processes for the remediation of wastewater and contaminated soil: emerging technology. J Sci Ind Res India. 2004;63(1):11–19. [Google Scholar]
- 12.Juttner K, Galla U, Schmieder H. Electrochemical approaches to environmental problems in the process industry. Electrochim Acta. 2000;45(15–16):2575–2594. [Google Scholar]
- 13.Saez V, Vicente MDE, Frias-Ferrer AJ, Bonete P, Gonzalez-Garcia J. Electrochemical degradation of perchloroethylene in aqueous media: An approach to different strategies. Water Res. 2009;43(8):2169–2178. doi: 10.1016/j.watres.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Betterton EA, Arnold RG, Ela WP. Electrolytic reduction of trichloroethylene and chloroform at a Pt- or Pd-coated ceramic cathode. J Appl Electrochem. 2003;33(2):161–169. [Google Scholar]
- 15.Scialdone O, Galia A, Gurreri L, Randazzo S. Electrochemical abatement of chloroethanes in water: Reduction, oxidation and combined processes. Electrochim Acta. 2010;55(3):701–708. [Google Scholar]
- 16.Roh Y, Cho KS, Lee S. Electrochemical remediation of trichloroethene-contaminated groundwater using palladized iron oxides. J Environ Sci Health, Part A: Toxic/Hazard Subst Environ Eng. 2001;36(6):923–933. doi: 10.1081/ese-100104121. [DOI] [PubMed] [Google Scholar]
- 17.Sonoyama N, Sakata T. Electrochemical continuous decomposition of chloroform and other volatile chlorinated hydrocarbons in water using a column type metal impregnated carbon fiber electrode. Environ Sci Technol. 1999;33(19):3438–3442. [Google Scholar]
- 18.Sonoyama N, Hara K, Sakata T. Reductive electrochemical decomposition of chloroform on metal electrodes. Chem Lett. 1997;(2):131–132. [Google Scholar]
- 19.He JH, Ela WP, Betterton EA, Arnold RG, Saez AE. Reductive dehalogenation of aqueous-phase chlorinated hydrocarbons in an electrochemical reactor. Ind Eng Chem Res. 2004;43(25):7965–7974. [Google Scholar]
- 20.Saez V, Esclapez MD, Tudela I, Bonete P, Gonzalez-Garcia J. Electrochemical Degradation of Perchloroethylene in Aqueous Media: Influence of the Electrochemical Operational Variables in the Viability of the Process. Ind Eng Chem Res. 2010;49(9):4123–4131. [Google Scholar]
- 21.Saez V, Esclapez MD, Frias-Ferrer A, Bonete P, Gonzalez-Garcia J. Electrochemical Reduction of Perchloroethylene in Aqueous Media: Influence of the Electrode Material. J New Mater Electrochem Syst. 2008;11(4):287–295. [Google Scholar]
- 22.Al-Abed SR, Fang YX. Influences of pH and current on electrolytic dechlorination of trichloroethylene at a granular-graphite packed electrode. Chemosphere. 2006;64(3):462–469. doi: 10.1016/j.chemosphere.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Mao XH, Tian F, Gan FX, Lin A, Zhang XJ. Comparison of the performances of Ti/SnO2-Sb, Ti/SnO2-Sb/PbO2, and Nb/BDD anodes on electrochemical degradation of azo dye. Russ J Electrochem. 2008;44(7):802–811. [Google Scholar]
- 24.Sengil IA, Ozacar M. Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. J Hazard Mater. 2006;137(2):1197–1205. doi: 10.1016/j.jhazmat.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2. John Wiley & Sons; New York: 2001. [Google Scholar]
- 26.Li T, Farrell J. Electrochemical investigation of the rate-limiting mechanisms for trichloroethylene and carbon tetrachloride reduction at iron surfaces. Environ Sci Technol. 2001;35(17):3560–3565. doi: 10.1021/es0019878. [DOI] [PubMed] [Google Scholar]
- 27.Arnold WA, Roberts AL. Pathways of chlorinated ethylene and chlorinated acetylene reaction with Zn(0) Environ Sci Technol. 1998;32(19):3017–3025. [Google Scholar]
- 28.Cornell RM, Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. 2. Wiley-VCH; Weinheim, Germany: 2003. [Google Scholar]
- 29.Langmuir D. Aqueous Environmental Geochemistry; Prentice Hall; Upper Saddle River, NJ, USA: 1997. [Google Scholar]
- 30.Roh Y, Lee SY, Elless MP. Characterization of corrosion products in the permeable reactive barriers. Environ Geol. 2000;40(1–2):184–194. [Google Scholar]
- 31.Hansen HK, Nunez P, Raboy D, Schippacasse I, Grandon R. Electrocoagulation in wastewater containing arsenic: Comparing different process designs. Electrochim Acta. 2007;52(10):3464–3470. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.