Abstract
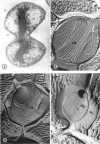
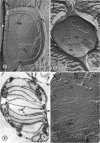
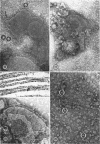
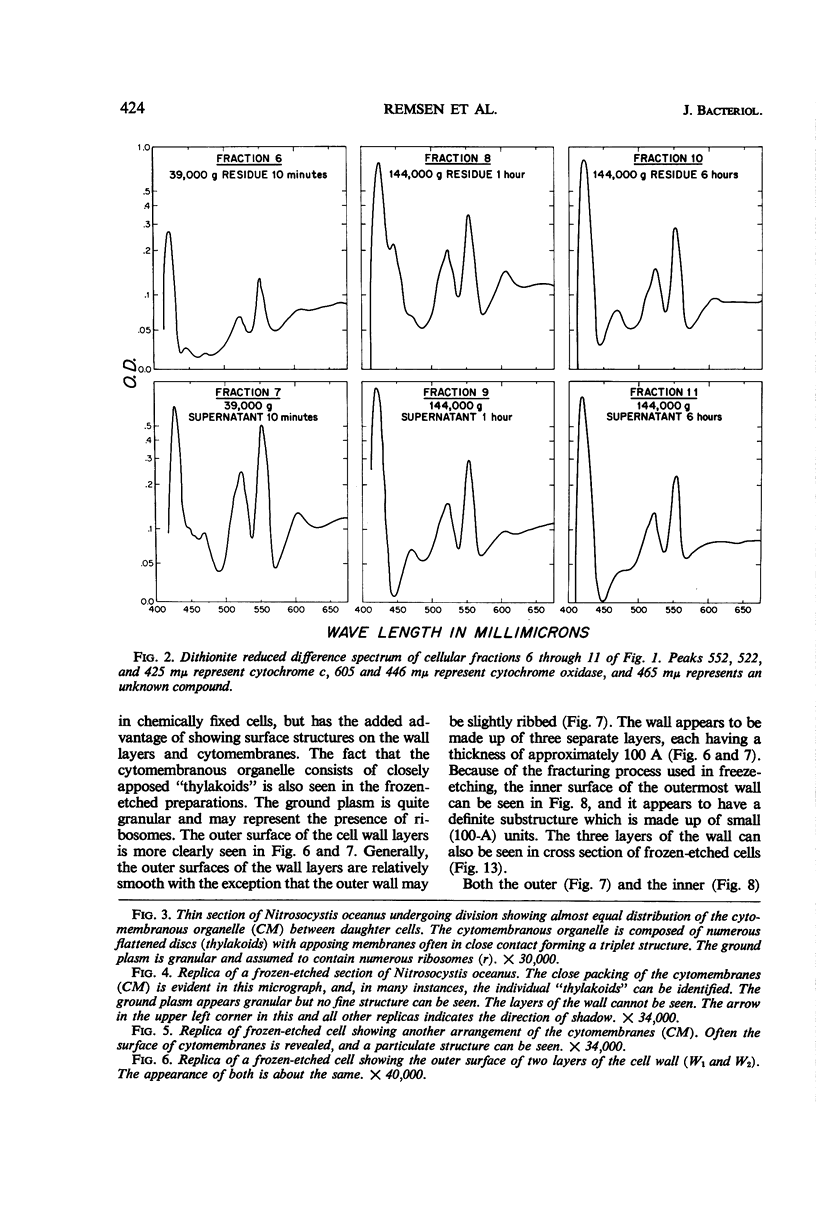
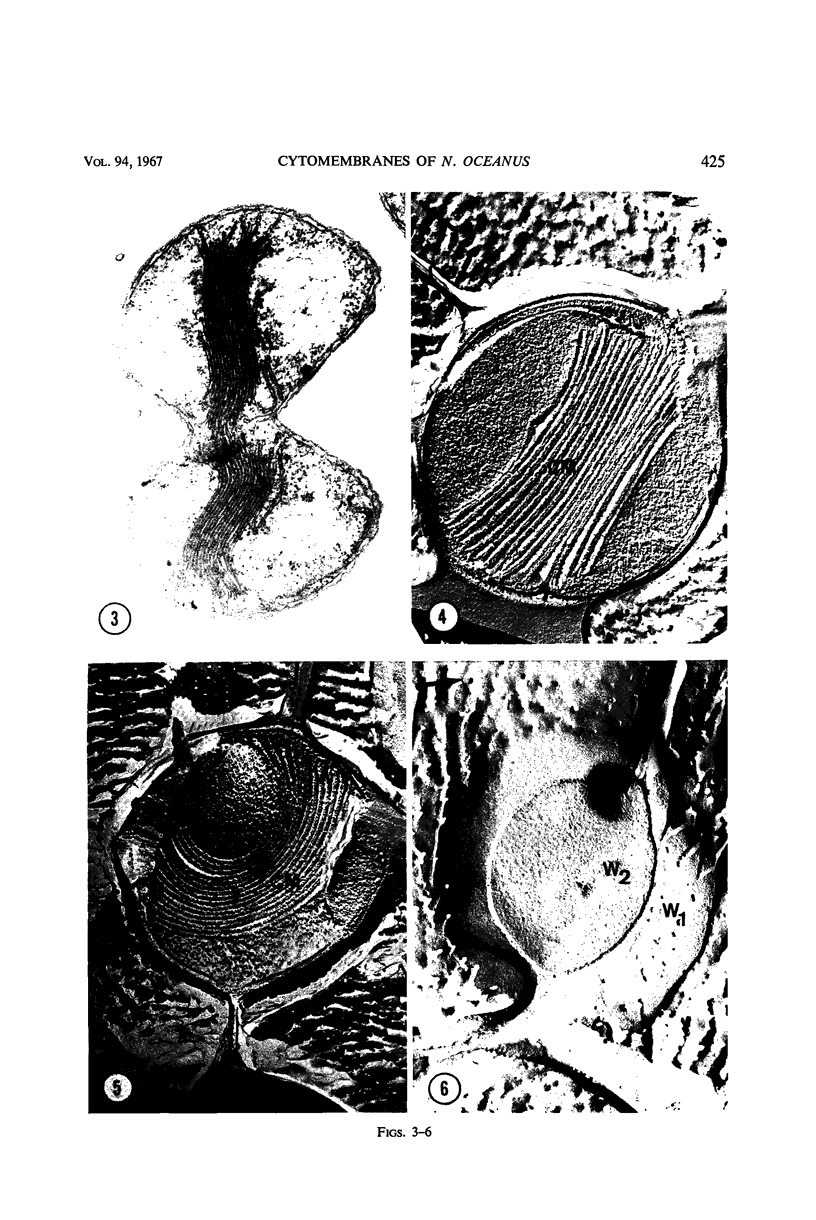
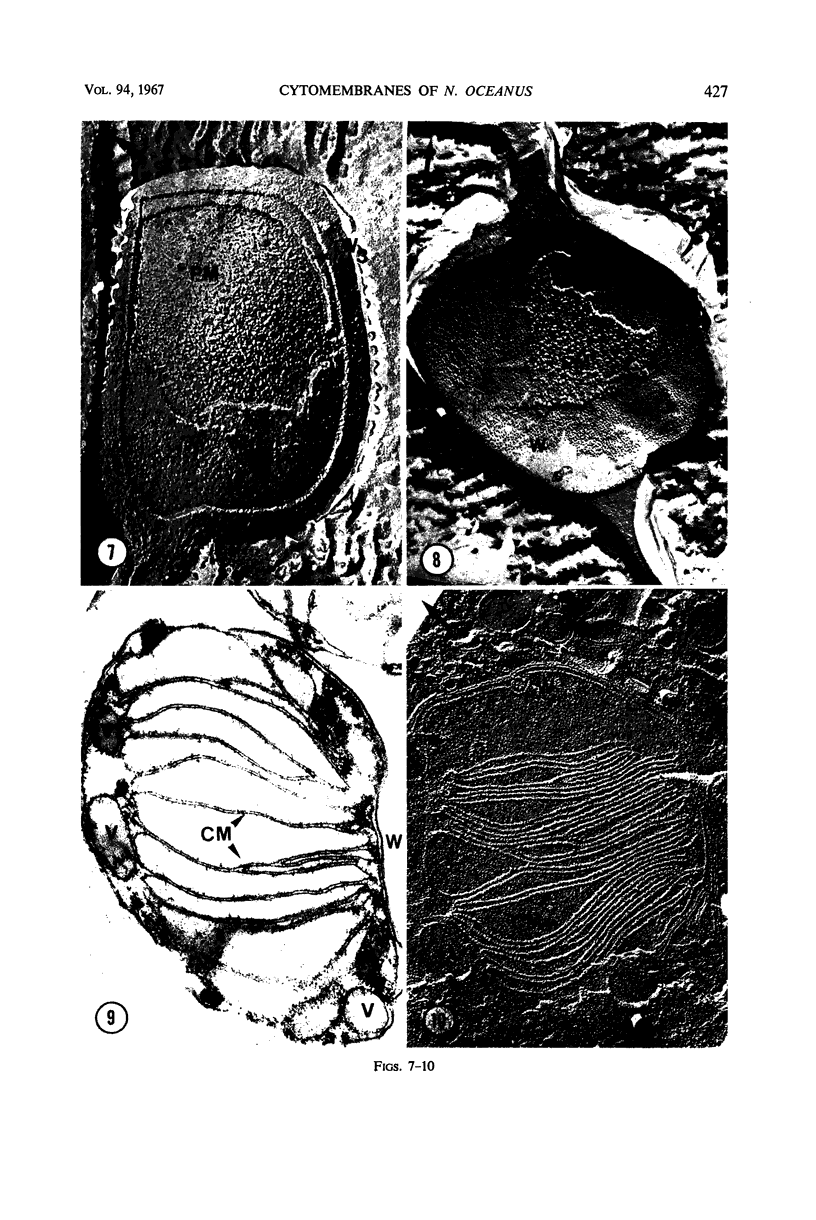
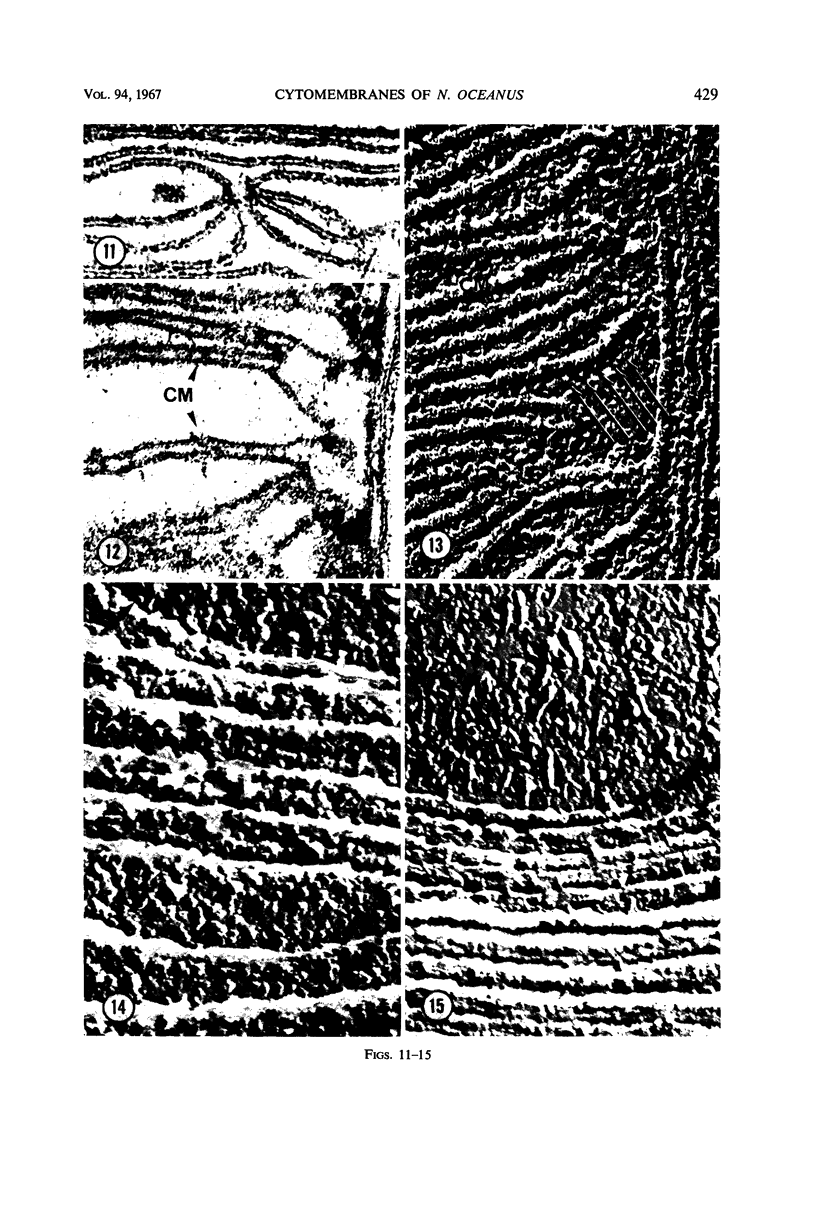
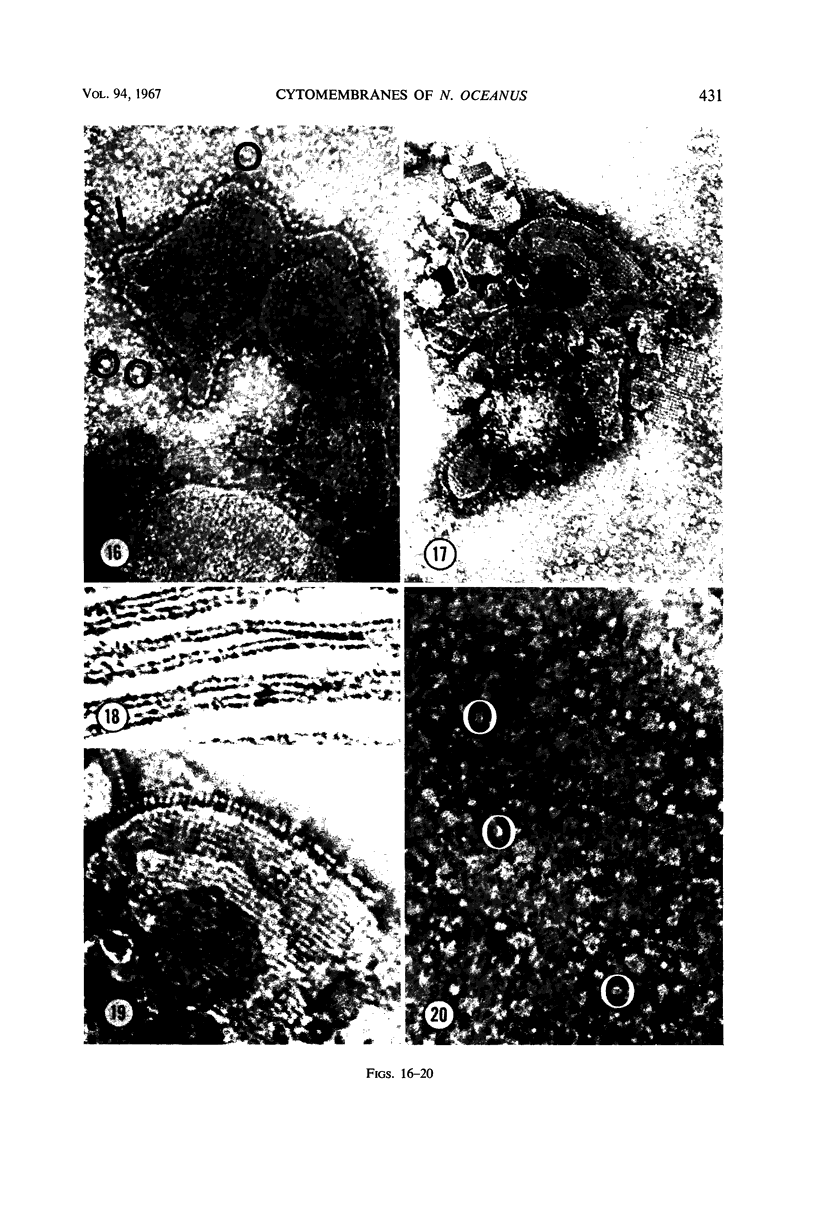
Thin-sectioned, negatively stained, and freeze-etched preparations of Nitrosocystis oceanus cytomembranes were compared. The cytomembranes in freeze-etched cells were covered with 80- to 120-A particles. When cells were disrupted and differentially centrifuged, various membrane and particle fractions were obtained. Negatively stained membrane fragments from the pellet centrifuged at 3,000 × g showed 70- to 80-A stalked particles, whereas those from the pellet centrifuged at 39,000 × g exhibited a crystalline array of subunits with a 30- to 40-A periodicity. High-speed supernatant and pellet fractions centrifuged at greater than 39,000 × g contained 40- to 120-A free particles but no membranes. In chemically fixed cells, 40-A particles were found embedded in the matrix of membranes. Results suggest that the larger 80- to 120-A particles are enzyme complexes, whereas the smaller 30- to 40-A particles represent a structural protein or a lipoprotein of the membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann E., Allmann D. W., Green D. E. The membrane systems of the mitochondrion. I. The S fraction of the outer membrane of beef heart mitochondria. Arch Biochem Biophys. 1966 Jul;115(1):153–164. doi: 10.1016/s0003-9861(66)81051-2. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Emmelot P. Electron microscopic observations on negatively stained plasma membranes isolated from rat liver. J Cell Biol. 1965 Jul;26(1):299–305. doi: 10.1083/jcb.26.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., PARSONS D. F., WILLIAMS G. R. CYTOCHROME CONTENT OF MITOCHONDRIA STRIPPED OF INNER MEMBRANE STRUCTURE. Science. 1964 Jan 10;143(3602):136–139. doi: 10.1126/science.143.3602.136. [DOI] [PubMed] [Google Scholar]
- CONTI S. F., HIRSCH P. BIOLOGY OF BUDDING BACTERIA. 3. FINE STRUCTURE OF RHODOMICROBIUM AND HYPHOMICROBIUM SPP. J Bacteriol. 1965 Feb;89:503–512. doi: 10.1128/jb.89.2.503-512.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPETRIS S. ULTRASTRUCTURE OF THE CELL WALL OF ESCHERICHIA COLI. J Ultrastruct Res. 1965 Apr;12:247–262. doi: 10.1016/s0022-5320(65)80098-3. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ MORAN H., ODA T., BLAIR P. V., GREEN D. E. A MACROMOLECULAR REPEATING UNIT OF MITOCHONDRIAL STRUCTURE AND FUNCTION. CORRELATED ELECTRON MICROSCOPIC AND BIOCHEMICAL STUDIES OF ISOLATED MITOCHONDRIA AND SUBMITOCHONDRIAL PARTICLES OF BEEF HEART MUSCLE. J Cell Biol. 1964 Jul;22:63–100. doi: 10.1083/jcb.22.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-MORAN H. Cell-membrane ultrastructure. Low-temperature electron microsopy and x-ray diffraction studies of lipoprotein components in lamellar systems. Circulation. 1962 Nov;26:1039–1065. doi: 10.1161/01.cir.26.5.1039. [DOI] [PubMed] [Google Scholar]
- GIBBS S. P. The ultrastructure of the chloroplasts of algae. J Ultrastruct Res. 1962 Dec;7:418–435. doi: 10.1016/s0022-5320(62)90038-2. [DOI] [PubMed] [Google Scholar]
- GIESBRECHT P., DREWS G. [Electron microscope studies on the development of "chromatophores" by Rhodospirillum molischianum Giesberger]. Arch Mikrobiol. 1962;43:152–161. [PubMed] [Google Scholar]
- GREEN D. E. THE MITOCHONDRION. Sci Am. 1964 Jan;210:67–74. [PubMed] [Google Scholar]
- Green D. E., Perdue J. F. Correlation of mitochondrial structure and function. Ann N Y Acad Sci. 1966 Jul 14;137(2):667–684. doi: 10.1111/j.1749-6632.1966.tb50189.x. [DOI] [PubMed] [Google Scholar]
- Green D. E., Tzagoloff A. The mitochondrial electron transfer chain. Arch Biochem Biophys. 1966 Sep 26;116(1):293–304. doi: 10.1016/0003-9861(66)90036-1. [DOI] [PubMed] [Google Scholar]
- HICKMAN D. D., FRENKEL A. W. OBSERVATIONS ON THE STRUCTURE OF RHODOSPIRILLUM MOLISCHIANUM. J Cell Biol. 1965 May;25:261–278. doi: 10.1083/jcb.25.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICKMAN D. D., FRENKEL A. W. OBSERVATIONS ON THE STRUCTURE OF RHODOSPIRILLUM RUBUM. J Cell Biol. 1965 May;25:279–291. doi: 10.1083/jcb.25.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLT S. C., MARR A. G. LOCATION OF CHLOROPHYLL IN RHODOSPIRILLUM RUBRUM. J Bacteriol. 1965 May;89:1402–1412. doi: 10.1128/jb.89.5.1402-1412.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess W. M. Fixation and staining of fungus hyphae and host plant root tissues for electron microscopy. Stain Technol. 1966 Jan;41(1):27–35. doi: 10.3109/10520296609116276. [DOI] [PubMed] [Google Scholar]
- Jacob F., Ryter A., Cuzin F. On the association between DNA and membrane in bacteria. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):267–278. doi: 10.1098/rspb.1966.0029. [DOI] [PubMed] [Google Scholar]
- MOOR H. DIE GEFRIER-FIXATION LEBENDER ZELLEN UND IHRE ANWENDUNG IN DER ELEKTRONENMIKROSKOPIE. Z Zellforsch Mikrosk Anat. 1964 Apr 28;62:546–580. [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney R. P., Edwards M. R. Fine Structure of Thiobacillus thiooxidans. J Bacteriol. 1966 Aug;92(2):487–495. doi: 10.1128/jb.92.2.487-495.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. The ultrastructure of cell membranes and their derivatives. Biochem Soc Symp. 1959;16:3–43. [PubMed] [Google Scholar]
- Rees M., Nason A. A P-450-like cytochrome and a soluble terminal oxidase identified as cytochrome o from Nitrosomonas europaea. Biochem Biophys Res Commun. 1965 Nov 8;21(3):248–256. doi: 10.1016/0006-291x(65)90279-2. [DOI] [PubMed] [Google Scholar]
- Remsen C. C. The fine structure of frozen-etched Bacillus cereus spores. Arch Mikrobiol. 1966 Sep 8;54(3):266–275. doi: 10.1007/BF00408999. [DOI] [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOECKENIUS W. Some observations on negatively stained mitochondria. J Cell Biol. 1963 May;17:443–454. doi: 10.1083/jcb.17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin A. Die Ultrastruktur der Zellwand und des Chloroplasten von Chlorella. Z Zellforsch Mikrosk Anat. 1966;74(3):325–350. [PubMed] [Google Scholar]