Abstract
Vacuum and extreme ultraviolet radiation from 8 – 24 eV generated at a synchrotron was used to postionize laser desorbed neutrals of antibiotic-treated biofilms and a modified fullerene using laser desorption postionization mass spectrometry (LDPI-MS). Results show detection of the parent ion, various fragments, and extracellular material from biofilms using LDPI-MS with both vacuum and extreme ultraviolet photons. Parent ions were observed for both cases, but extreme ultraviolet photons (16 – 24 eV) induced more fragmentation than vacuum ultraviolet (8 – 14 eV) photons.
Keywords: biofilm, mass spectrometry, postionization, synchrotron radiation, extreme ultraviolet, vacuum ultraviolet
1. Introduction
Vacuum ultraviolet (VUV) single photon ionization (SPI) of laser or ion desorbed neutrals has demonstrated sensitive detection and the capability for mass spectrometric imaging of atomic and molecular analytes [1–4]. Laser desorption VUV postionization mass spectrometry (LDPI-MS) of biofilms showed improved sensitivity above 8 eV photon energy with optimal signal to noise at 10.5 eV, given constant photon flux [5]. By contrast, multiple photon ionization of fullerenes and organic compounds showed increased spectral complexity [6,7] with the absorption of many photons imparting excess energy and causing an inability to mitigate channels of dissociation and ionization [1]. Prior researchers argue that extreme ultraviolet (EUV) radiation near 26 eV may reduce fragmentation for single photon ionization of van der Waals and H-bonded clusters [8,9,10,11] with spectra appearing similar to those obtained at 10.5 eV [12]. Yet, considerable molecular fragmentation of small molecules was present in these investigations [8,9,10]. The role of protonated clusters in these experiments to enhance ionization, reduce fragmentation, or facilitate cooling is unclear [11]. LDPI-MS data was collected here to compare postionization by VUV and EUV radiation. Two different thin film samples were examined: a pure fullerene derivative and a bacterial biofilm with dissolved antibiotic rifampicin.
2. Experimental
Studies were performed with a modified commercial secondary ion mass spectrometer (TOF.SIMS 5, ION TOF Inc., Münster, Germany) coupled to a 349 nm Nd:YLF desorption laser (Explorer, Newport Corp, 5 ns pulse length, 300 & 25 m spot, 2 – 100 MW/cm2) and tunable, monochromatized 8 – 24 eV synchrotron radiation at the Chemical Dynamics Beamline of the Advanced Light Source (Lawrence Berkeley National Laboratory, Berkeley, CA). Mass spectrum were averaged from 80,000 to 200,000 laser shots. The experimental apparatus and parameters were similar to those described previously [3,5,13]. [6.6] diphenyl C62 bis(butyric acid methyl ester) (C62, mol. wt. 1100 Da, Sigma-Aldrich) was deposited on coated glass microscope slides of indium tin oxide (ITO, Sigma-Aldrich). Biofilms were prepared from bacterial stock solutions of Staphylococcus epidermidis (ATCC 35984, Manassas, VA, USA) [14]. Drip flow biofilms were grown on ITO slides by methods previously described [5,15]. Each sample was scanned for direct ionization by laser desorption (LD, no VUV) and synchrotron background (SB, no LD). EUV spectra were not normalized to the beam flux to account for differences in synchrotron fill and beamline transmission at different wavelengths and hence an absolute comparison to the VUV data cannot be performed.
3. Results & Discussion
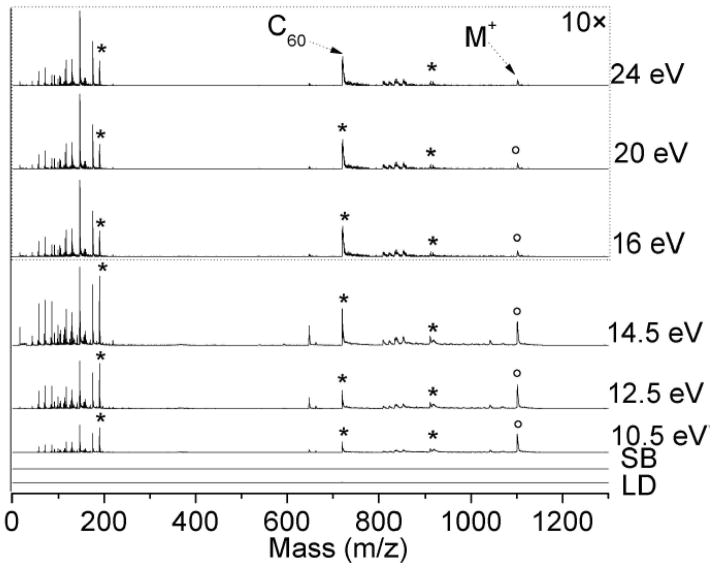
LDPI-MS of neat [6.6] diphenyl C62 bis(butyric acid methyl ester), referred to here as C62, are displayed in Figure 1, using both VUV and EUV single photon ionization. The parent ion peak (M+) at m/z 1100 was clearly observed at all six photon energies in the VUV and EUV. Three known fragments included m/z 190, 720, and 910, which correspond to the loss of one or two of the 190 Da side chains to the 720 Da C60 core, as well as the core itself (chemical structure shown in Figure 2). Fragmentation of the ligand becomes more apparent at higher photon energies as the abundance of peaks from m/z 57 to 175 increase with respect to m/z 190. Prominent peaks at m/z 648 and 660 were observed at all photon energies, including at 7.87 and 8.5 eV (data not shown), indicating they are probably impurities of C62. Mass resolution was ~1100 at m/z 720 (C60) in the VUV and limited by the temporal energy distribution of ion extraction through a continuous source. Neither direct ionization by laser desorption (LD, no VUV) and nor synchrotron background signal (SB, no LD) were observed.
Figure 1.
LDPI-MS with VUV (<15 eV) as well as EUV photon energies (>15 eV) photon energies of neat C62 displaying parent ion (M+). Asterisks indicate known fragments at m/z 190, 720, and 910, and circles indicate M+. EUV spectra were scaled by 10. Synchrotron background (SB) was recorded at 20 eV.
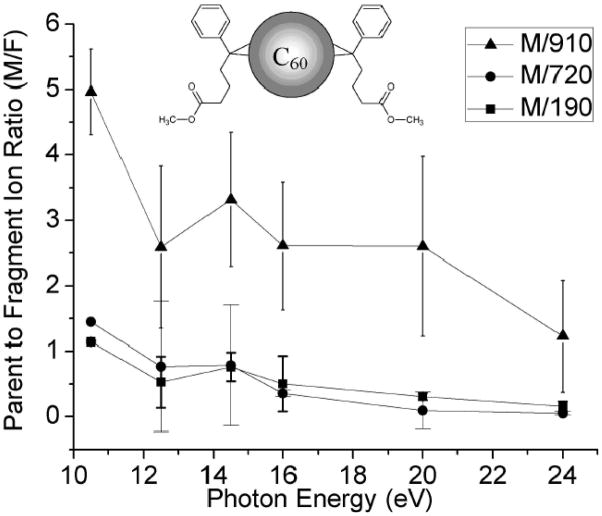
Figure 2.
Parent to fragment ion ratios (M/F) for known C62 fragments at m/z 190 (C12H24O2), 720 (C60), and 910 (C60 + 190).
Parent to fragment ion ratios (M/F) for m/z 190, 720, and 910 for the data from Figure 1 and another data set are shown in Figure 2. All M/F ratios decreased as the photon energy increased. From 10.5 to 24 eV, all three M/F values reduce to ~33%. The relative abundance of m/z 910 was about 3 times less than either m/z 190 or 720 at most photon energies.
Rifampicin was doped at a concentration of 120 M into a bacterial biofilm and measured by EUV LDPI-MS. Figure 3 shows a comparison of the result to similar spectra recorded at VUV (8.0 to 12.5 eV) photon energies [5]. The rifampicin parent ion at m/z 823 was barely observable above the noise in the EUV spectra, while it was readily observed in prior VUV spectra. Fragment ions at m/z 84, 284, 510, 524, 641, and 655 (indicated by asterisks) were observed at all three EUV photon energies, as previously identified by VUV LDPI-MS. Mass resolution was between 200 and 400 at major rifampicin fragment peaks.
Figure 3.
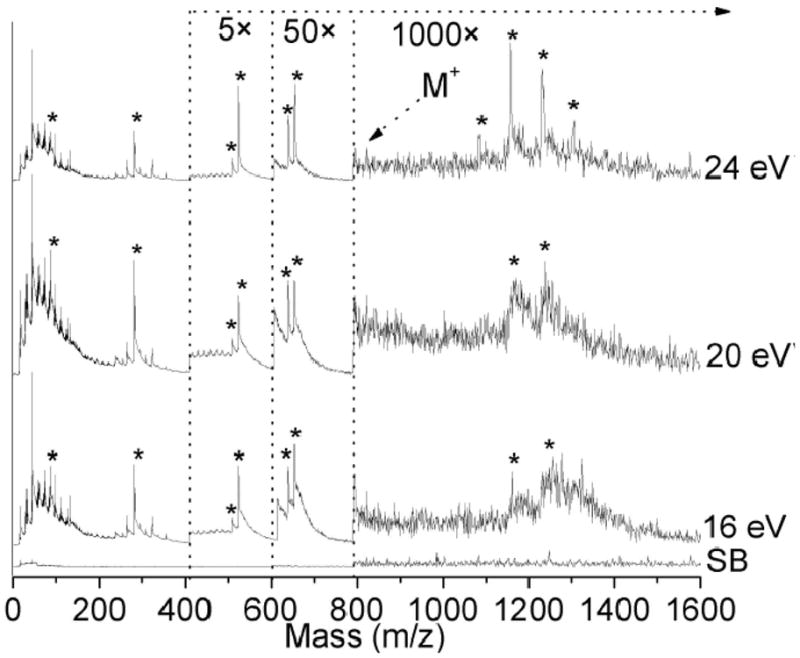
LDPI-MS with EUV radiation (16, 20, and 24 eV) of 120 M concentration of rifampicin doped biofilm. Synchrotron background (SB) was recorded at 24 eV. Fragment ions are denoted by asterisks.
Peaks observed from m/z 1000 – 1400 derived from the biofilm response to rifampicin were also observed by both EUV and VUV LDPI-MS, as previously discussed [5]. Increasing the photon energy from 20 to 24 eV enhanced the signal to noise ratio for these peaks. Mass resolution ranged from 100 to 300 at m/z 1157 with the best resolved peaks at 24 eV.
Table 1 shows parent to fragment ion ratios (M/F) for LDPI-MS of rifampicin doped biofilm at known antibiotic fragments m/z 84, 284, and 655 at 10.5 and 20 eV photon energies. Sensitivity decreases rapidly above m/z 800 at 20 eV and is reflected in the M/F values. Table 1 shows the abundance of fragments increased 10 – 200 times from 10.5 to 20 eV. Yet, fragments m/z 84 and 284 increased and remain prominent at 20 eV. Studies by Nuutinen et al. suggest this may result due to stabilization by a piperazine group, represented in these two rifampicin fragments, and dissociation from its methyl group [16]. Similar VUV work with neat rifampicin observed the prominence of fragments containing methylated piperazine [5].
Table 1.
Values of parent to fragment ion ratios (M/F) for LDPI-MS at 10.5 and 20 eV of rifampicin doped biofilm using fragments m/z 84, 284, and 655.
| M/F | 10.5 eV | 20 eV | ||
|---|---|---|---|---|
| <x> | σ | <x> | σ | |
| M/84 | 0.028 | 0.009 | 0.0011 | 0.0006 |
| M/284 | 0.22 | 0.18 | 0.0012 | 0.0007 |
| M/655 | 0.31 | 0.24 | 0.030 | 0.02 |
4. Conclusion
Single photon ionization with EUV radiation was effective for the two organic/biological systems examined here, but did induce more fragmentation compared with VUV radiation. However, fragmentation is sometimes an unavoidable consequence of the laser desorption process, and addition of matrix compounds or other methods for cooling and stabilizing the desorbed species prior to postionization might mitigate this effect. Further work is clearly required to explore whether the enhanced fragmentation observed here with EUV radiation occurs in other examples of laser desorption and if any such enhancement is offset by the overall increase in ionization efficiency for EUV vs. VUV radiation.
Acknowledgments
This work is supported by the National Institute of Biomedical Imaging and Bioengineering via grant EB006532. MA, LT, JZ and the ALS are supported by the Director, Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division of the U.S. Department of Energy under contract No. DE-AC02-05CH11231. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanley L, Zimmermann R. Anal Chem. 2009;81:4174–4182. doi: 10.1021/ac8013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhmetov A, Moore JF, Gasper GL, Koin PJ, Hanley L. J Mass Spectrom. 2010;45:137–145. doi: 10.1002/jms.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi LK, Zhou J, Wilson KR, Leone SR, Ahmed M. J Phys Chem A. 2009;113:4035–4044. doi: 10.1021/jp810408v. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Takahashi LK, Wilson KR, Leone SR, Ahmed M. Anal Chem. 2010;82:3905–3913. doi: 10.1021/ac1004629. [DOI] [PubMed] [Google Scholar]
- 5.Gasper GL, Takahashi LK, Zhou J, Ahmed M, Moore JF. L Hanley Anal Chem. 2010;82:7472–7478. doi: 10.1021/ac101667q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wurz P, Lykke KR. J Phys Chem. 1992;96:10129–10139. [Google Scholar]
- 7.Pallix JB, Schuhle U, Becker CH, Huestis DL. Anal Chem. 1989;61:805–811. [Google Scholar]
- 8.Dong F, Heinbuch S, Rocca JJ, Bernstein ER. J Chem Phys. 2006;125:154317-1–9. doi: 10.1063/1.2348878. [DOI] [PubMed] [Google Scholar]
- 9.Heinbuch S, Dong F, Rocca JJ, Bernstein ER. J Chem Phys. 2006;125:154316-1–7. doi: 10.1063/1.2348877. [DOI] [PubMed] [Google Scholar]
- 10.Dong F, Heinbuch S, Rocca JJ, Bernstein ER. J Chem Phys. 2006;124:224319-1–17. doi: 10.1063/1.2202314. [DOI] [PubMed] [Google Scholar]
- 11.Heinbuch S, Dong F, Rocca JJ, Bernstein ER. J Chem Phys. 2007;126:244301-1–11. doi: 10.1063/1.2746036. [DOI] [PubMed] [Google Scholar]
- 12.Dong F, Heinbuch S, Xie Y, Bernstein ER, Rocca JJ, Wang Z, Ding X, He S. J Am Chem Soc. 2009;131:1057–1066. doi: 10.1021/ja8065946. [DOI] [PubMed] [Google Scholar]
- 13.Heimann PA, Koike M, Hsu CW, Blank D, Yang XM, Suits AG, Lee YT, Evans M, Ng CY, Flaim C, Padmore HA. Rev Sci Instrum. 1997;68:1945–1951. [Google Scholar]
- 14.Gasper GL, Carlson R, Akhmetov A, Moore JF, Hanley L. Proteom. 2008;8:3816–3321. doi: 10.1002/pmic.200701142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu KD, Stewart PS, Xia F, Huang CT, McFeters GA. Appl Environ Microbiol. 1998;64:4035–4039. doi: 10.1128/aem.64.10.4035-4039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuutinen JMJ, Ratilainen J, Rissanen K, Vainiotalo P. J Mass Spectrom. 2001;36:902–910. doi: 10.1002/jms.191. [DOI] [PubMed] [Google Scholar]