Abstract
Severely hypoxic regions in tumors result from a combination of rapid cell division and aberrant angiogenesis. The Hypoxia Inducible Factors (HIFs) mediate transcriptional responses to localized hypoxia in normal tissues and in cancers, and can promote tumor progression by altering cellular metabolism and stimulating angiogenesis. Recently, HIFs have been shown to stimulate specific signaling pathways and transcription factors, including Notch and Oct4, that control stem cell self-renewal and multipotency. As many cancers are thought to develop from a small number of transformed, self-renewing and multipotent “cancer stem cells”, these results suggest new roles for HIFs in tumor progression.
Introduction
It has long been recognized that solid tumors contain poorly vascularized regions characterized by severe hypoxia (oxygen deprivation), acidosis and nutrient starvation (Carmeliet and Jain, 2000; Pouyssegur et al., 2006). Tumor hypoxia is typically associated with poor patient prognosis, partly because low oxygen levels reduce the effectiveness of radiation therapy, which kills tumor cells by generating reactive oxygen species (ROS). Over the past decade, work from many laboratories has indicated that hypoxic microenvironments contribute to cancer progression by activating adaptive transcriptional programs that promote cell survival, motility and tumor angiogenesis. Recent reports describing molecular connections between oxygen-regulated transcription factors and pathways known to control stem cell function have suggested a new mechanism whereby hypoxia-induced transcription factors may drive tumor growth; namely, through the generation or expansion of tumor initiating cells, or “cancer stem cells”. In this review, we will discuss how these results add an important new facet to our traditional view of hypoxia and cancer.
Many of the cellular responses to hypoxia are mediated through changes in gene expression. The transcription factors primarily responsible for these changes are the Hypoxia Inducible Factors (HIFs), the biology of which has been reviewed elsewhere (Pouyssegur et al., 2006; Semenza, 2003). Briefly, HIFs are members of the bHLH-PAS family of proteins, and bind to canonical DNA sequences (hypoxia regulated elements, or HREs) in the promoters or enhancers of target genes. They consist of an alpha (HIF-α) and a beta (HIF-β, or ARNT) subunit, and activate the expression of at least 150 genes encoding proteins that regulate cell metabolism, survival, motility, basement membrane integrity, angiogenesis, hematopoiesis, and other functions. Regulation of HIF activity is mediated primarily through the stability of the alpha subunit: under conditions of abundant oxygen (>8–10%), HIF-α proteins are translated but rapidly degraded. HIF-α degradation is triggered by the hydroxylation of two key proline residues in its highly conserved oxygen-dependent degradation domain (ODD). These hydroxylation events, catalyzed by specific proline hydroxylase (PHD) enzymes, are necessary and sufficient for binding to the Von Hippel-Lindau tumor suppressor protein (pVHL), the recognition component of an E3-ubiquitin ligase that targets the HIFs to the 26S proteasome for destruction. As oxygen levels decrease below 8–10%, HIF-α proteins become increasingly stabilized, although the nature of the oxygen-sensing mechanisms regulating these events remains controversial. Once stabilized, HIF-α proteins bind to constitutively expressed ARNT (HIF-β) subunits in the nucleus, bind DNA and activate transcription through interactions with co-activators, including CBP/p300. Interestingly, binding to CBP/p300 is regulated by hydroxylation of a conserved asparagine residue in the HIF-α C-terminal domain (Pouyssegur et al., 2006).
HIF-1α and HIF-2α share a high degree of sequence identity, underscored by their shared ability to heterodimerize with ARNT and bind HREs to activate transcription of common, as well as some unique, target genes (Raval et al., 2005). Whereas HIF-1α is expressed in an apparently ubiquitous fashion, HIF-2α expression is restricted to particular cell types, including vascular endothelial cells, neural crest cell derivatives, lung type II pneumocytes, liver parenchyma, cardiomyocytes, and interstitial cells in the kidney (Wiesener et al., 2003). Genetic ablation experiments in mice have demonstrated that all HIF subunits tested to date are essential for embryonic development and survival. These analyses have led to the view that oxygen gradients develop as a function of limited diffusion in rapidly growing tissues. The inability to mount proper transcriptional responses to physiological hypoxia in HIF-deficient embryos results in developmental arrest and death. The specific phenotypes observed in mutant embryos differ depending on which HIF subunit is mutated, but alterations in cell survival, differentiation and tissue angiogenesis have been reported for mice lacking ARNT, HIF-1α or HIF-2α (Ramirez-Bergeron and Simon, 2001).
In contrast to the exquisitely regulated HIF activation observed in embryos, the highly disorganized vascular supply of solid tumors typically produces regions of severe hypoxia or anoxia closely abutting well perfused areas (Pouyssegur et al., 2006). The consequent stabilization of HIF proteins in hypoxic cancer cells is thought to promote tumor progression, in large part by inducing the localized expression of specific target genes encoding vascular endothelial growth factor (VEGF), glycolytic enzymes (PGK, ALDA), glucose transporters (GLUT1), and proteins regulating motility (lysl oxidase) and metastasis (CXCR4, E-cadherin), among others (Semenza, 2003). Many tumor studies support this view: for example, subcutaneous fibrosarcomas generated from HIF-1α deficient, Ras-transformed murine embryonic fibroblasts (MEFs) grew more slowly than their HIF-replete controls (Ryan et al., 2000). Similar xenograft experiments with ARNT-deficient hepatoma cells also showed a clear decrease in tumor growth compared to ARNT-expressing counterparts (Maxwell et al., 1997). HIF activity can also be induced or enhanced in some transformed cells through oxygen-independent oncogenic signaling pathways, including those regulated by IGF2/IGF1R, TGF-α/EGFR, and PI3K/Akt (Semenza, 2003). Expression of the HIF-α proteins in human tumor cells is often correlated with poor prognosis: for example, high-grade pediatric astrocytomas display greater HIF-2α expression than do corresponding low-grade tumors (Khatua et al., 2003). Interestingly, HIF-1α and HIF-2α share some target genes, including those encoding VEGF, GLUT1, ADM-1, whereas genes encoding glycolytic enzymes (PGK1, ALDA) are unique HIF-1α targets and those encoding TGF-α and cyclin D1 appear to be unique HIF-2α targets, at least in certain cell types (Raval et al., 2005). Although much remains to be determined, extensive analyses have solidified the central dogma that HIF activity in cancer cells drives tumor progression by inducing the expression of genes that promote hypoxic adaptation. The degree to which HIF activation in tumor stromal cells, such as infiltrating macrophages and neutrophils, contributes to tumor angiogenesis and progression is an important question currently under investigation.
Evidence from a variety of experimental systems has shown that hypoxia also regulates the proliferation and differentiation of multiple stem cell populations, including ES cells, neuronal and neural crest stem cells, hematopoietic stem cells, and trophoblast stem cells. The direct role of HIFs in controlling these effects has been demonstrated in some, but as yet not all, of these stem cell types. The hypoxic responses of different stem cell populations is consistent with the idea that oxygen levels may be an important component of particular stem cell niches, and that HIF activity can regulate the defining features of stem cells, including self-renewal and multipotency. In the following sections, we will discuss the mechanisms by which hypoxia and HIFs mediate these effects, and discuss their implications for cancer biology. Finally, we propose a molecular model for how HIFs may promote the adoption of stem cell characteristics by differentiated hypoxic tumor cells.
Hypoxia, HIFs and stem cells
Stem cell “niches” are defined as particular locations or microenvironments that maintain the combined properties of stem cell self-renewal and multipotency. In Drosophila and C. elegans, germ stem cell niches have been described with remarkable single-cell resolution. Germ stem cells in the Drosophila ovariole and testis require physical interaction with supporting cap or hub cells, respectively, to retain stem cell identity (Ohlstein et al., 2004). In the C. elegans gonad, the niche consists primarily of a single distal tip cell whose long cytoplasmic processes make extensive physical contact with germ stem cells (Morrison and Kimble, 2006). In mammals, spatially defined stem cell niches have also been identified in multiple tissues, including the gonad, skin, intestine, and subventricular zone (SVZ) of the brain, although in some cases the cells comprising the niche have not yet been explicitly identified (Joseph and Morrison, 2005). A combination of genetic and molecular analyses have identified a number of molecular factors, typically supplied by the supporting cells of the niche, that control stem cell identity. These factors include components of the BMP, Notch, Wnt, JAK-STAT and Shh signaling pathways, which provide intercellular cues that regulate stem cell identity and differentiation (Joseph and Morrison, 2005; Ohlstein et al., 2004). These signaling functions have been highly conserved through evolution. For example, altered Notch signaling affects the function of a variety of mammalian stem cells (hematopoietic, intestinal, and skin), as well as intestinal stem cells in Drosophila, and germ stem cells in C. elegans (Joseph and Morrison, 2005; Ohlstein et al., 2004; Ohlstein and Spradling, 2006). Coordinate regulation of genes controlling stem cell function is achieved in part through the activity of chromatin remodeling factors, including Bmi-1 and PRC2 (Joseph and Morrison, 2005; Lee et al., 2006).
Local oxygen concentrations can directly influence stem cell self-renewal and differentiation. One attractive hypothesis is that stem cells, particularly in long-lived animals, might benefit from residing in hypoxic niches where oxidative DNA damage may be reduced. Direct measurement of oxygen levels has revealed that bone marrow is, in general, quite hypoxic (~1–2% O2) (Cipolleschi et al., 1993). Given the ongoing debate as to whether HSCs in bone marrow are associated with osteoblasts or sinusoidal endothelial cells (or both) (Kiel et al., 2005), it will be interesting to determine the oxygen concentrations in specific bone marrow subdomains, although such an experiment remains technically challenging. Wherever HSCs reside, their proliferation and function is clearly affected by oxygen. Danet et al. demonstrated that culturing human bone marrow HSCs under hypoxic conditions (1.5% O2) promoted their ability to engraft and repopulate the hematopoietic compartment of NOD/SCID mice (Danet et al., 2003). Similar results were obtained for hematopoietic progenitors isolated from embryonic yolk sacs or generated from ES cells grown in three-dimensional embryoid bodies in vitro (Ramirez-Bergeron and Simon, 2001). These oxygen mediated effects are not unique to HSCs: culturing neural crest stem cells or neuronal stem cells under hypoxic conditions (~5% O2) promotes their proliferation and skews cellular differentiation toward specific fates (Morrison et al., 2000a; Studer et al., 2000). Differentiation of human placental cytotrophoblast cells is also directly influenced by hypoxia (Genbacev et al., 1997). Finally, Pahlman and colleagues have demonstrated that hypoxic culture confers a more immature phenotype on human neuroblastoma and breast cancer cells (Axelson et al., 2005).
Some of the effects of hypoxia on stem cell function are directly mediated by the HIF proteins. Targeted mutation of the ARNT subunit eliminates both HIF-1α and HIF-2α function, and results in a decreased number of progenitors of all hematopoietic lineages in Arnt−/− embryonic yolk sacs. This phenotype is recapitulated when Arnt−/− ES cells are induced to form hematopoietic progenitors in embryoid bodies in vitro (Ramirez-Bergeron and Simon, 2001). Although Arnt-deficient mouse embryos display a variety of developmental abnormalities, they die at E9.5–E10.5 from defective placental function. Analysis of Arnt−/− (or HIF-1α −/−, HIF-2α−/− double) mutant placentas revealed that HIF activity influences the differentiation of trophoblastic stem cells into either spongiotrophoblasts, which occupy a particularly hypoxic zone, or into trophoblast giant cells, which lie close to the oxygen-rich maternal spiral arteries (Cowden Dahl et al., 2005). The effects of HIF activity on trophoblast cell fate determination have also been recapitulated using TS cell lines cultured in vitro. These experiments implicate the HIF proteins in the control of HSC and trophoblastic SC function; future work will be necessary to determine their specific functions in other stem cell populations. To date, only a few HIF target genes that might confer these effects have been identified. Expression of VEGF, in particular, accounted for many of the HIF-mediated effects on hematopoietic progenitors (Ramirez-Bergeron and Simon, 2001), but there is little doubt that other factors and signaling pathways are involved.
Recent reports have identified new molecular mechanisms by which HIFs directly modify cellular differentiation and stem cell function. Lendahl, Poellinger and colleagues reported that hypoxia blocked the differentiation of myogenic satellite cells, a myogenic cell line (C2C12), and primary neural stem cells in a Notch-dependent manner (Gustafsson et al., 2005). When Notch receptors interact with the Jagged or Delta family of ligands, two proteolytic cleavage events result in the release of the Notch intracellular domain (ICN) from the plasma membrane and its transport to the nucleus, where it forms a DNA-binding complex with other coactivators including MAML, CSL, and p300, and activates target gene expression. Using neurogenic rat embryonic carcinoma cells, the authors demonstrated that hypoxic treatment increased stabilization of the transcriptionally active ICN and stimulation of Notch target genes Hes-1 and Hey-2. Chromatin immunoprecipitation (ChIP) experiments revealed that HIF-1α was physically recruited to a DNA-binding complex containing ICN in hypoxic cells. Hypoxic induction of Notch target genes was dependent on ICN, and also required the functional C-terminal transactivation domain of HIF-1α, which interacts directly with p300/CBP. Moreover, it appears that this property was not unique to HIF-1α, as HIF-2α also augmented Notch target gene expression in hypoxic A-498 renal carcinoma cells. Exactly how HIF-α proteins integrate into the ICN:MAML:CSL complex is not yet understood, nor is it known whether this response modulates the expression of all Notch target genes, or only a subset (Gustafsson et al., 2005).
Notch pathway signaling has profound effects on cellular differentiation in Drosophila, C. elegans, and mammals, making the direct connection to HIF factors particularly intriguing. The results from Gustafsson et al. suggest that altered Notch signaling may underlie some of the developmental defects observed in HIF deficient embryos, and in adult cells and tissues (such as the chondrocyte growth plate) from which HIF-1α has been selectively deleted (Schipani et al., 2001). It is also striking that a primary effect of hypoxia, acting through Notch, was to inhibit the differentiation of a variety of cell types. Notch signaling is critical for the maintenance of undifferentiated stem and progenitor cell populations in the mammalian intestinal crypt, and also influences differentiation of mature enterocytes (Wilson and Radtke, 2006). Forced Notch activation in hematopoietic bone marrow or T cell progenitor cells also blocks differentiation, and results in T cell acute lymphoblastic leukemia (T-ALL) (Pear and Aster, 2004). It is interesting to note, however, that bone marrow-specific deletion of Jaggedl and Notchl function does not deplete HSCs or disrupt hematopoiesis (Mancini et al., 2005), raising the possibility that other Notch receptors and/or ligands are active in these cells. It is tempting to speculate that a stem cell residing in an hypoxic niche may require HIF-α proteins to fully activate Notch target genes that inhibit differentiation, thereby contributing to stem cell self-renewal and multipotency. Testing this hypothesis directly will entail selective inactivation of HIF-α or Notch in specific stem cell populations in vivo. It is important to remember, however, that the effects of Notch signaling can be cell type-dependent, as Notch activation actually promotes terminal differentiation in epidermal keratinocytes and certain neural stem cells (Morrison et al., 2000b; Wilson and Radtke, 2006).
Our laboratory recently reported that hypoxia regulates stem cell function through direct activation of specific HIF target genes. To determine the functional redundancy between HIF-1α and HIF-2α in embryonic development, we targeted a HIF-2α cDNA into the HIF-1α locus in murine ES cells, thereby replacing HIF-1α expression with HIF-2α This “knock-in” (HIF-2α KI) allele was designed to test the degree to which expanded HIF-2α expression, under the regulatory control of the HIF-1α locus, could complement a HIF-1α null mutation (Covello et al., 2006). As HIF-1α deficient embryos die at E9.5–E10.5, we predicted that if HIF-2α was completely incapable of complementing HIF-1α function, a similar embryonic lethality would be observed in homozygous HIF-2 KI embryos. Surprisingly, HIF-2α KI homozygotes were resorbed between E3.5 and E7.5. The few embryos recovered between E6.5 and E7.5 displayed gross developmental abnormalities marked by aberrant tissue patterning and marker gene expression. Subsequent analysis revealed that these phenotypes correlated to expanded expression of Oct4, a critical transcriptional regulator controlling ES cell identity. Interestingly, the ability of HIF-2α to regulate Oct4 expression is not shared with HIF-1α, as ChIP analysis revealed binding of only HIF-2α to hypoxic regulatory elements in the murine Oct4 promoter.
Oct4, Sox2 and Nanog form a transcriptional network that regulates a large number of genes associated with cellular differentiation in ES cells (Lee et al., 2006; Loh et al., 2006). Analyses of human ES cells revealed that all three factors occupy and activate genes that promote ES cell growth and self-renewal, while simultaneously repressing genes that promote differentiation (Lee et al., 2006). In vivo, Oct4 is expressed in the inner cell mass of blastocysts, from which ES cells are derived, and in the murine epiblast. Oct4 expression is downregulated in somatic cells around the time of gastrulation, but retained in primordial germ cells and, apparently, some adult stem cell populations (Tai et al., 2005). Subtle changes in Oct4 protein levels have dramatic effects on ES cell differentiation: a two-fold decrease in Oct4 expression induces ES cells to differentiate into trophectoderm, whereas a two-fold increase induces differentiation of mesodermal cell types (Niwa et al., 2000). Sustained Oct4 expression is incompatible with terminal differentiation of somatic cells, suggesting that expanded HIF-2α expression elevates Oct4 activity in homozygous HIF-2α KI embryos and cells, thereby contributing to the observed phenotypes. Interestingly, HIF-2α deficient embryos have severely reduced numbers of primordial germ cells, which require Oct4 for survival (Kehler et al., 2004), consistent with a normal in vivo role of HIF-2α in regulating Oct4 expression and stem cell function.
The links between the HIFs, Notch and Oct4 reveal specific molecular mechanisms whereby oxygen responses can inhibit differentiation and, possibly, promote stem cell identity. They also raise the possibility of cross-talk between hypoxia and other stem cell signaling pathways. Direct connections between BMPs or Shh pathways and the HIFs have not yet been demonstrated, although TGF-β has been reported to induce HIF-α stabilization by inhibiting PHD2, one of the proline hydroxylases that target HIF-α subunits for normoxic degradation (McMahon et al., 2006). In addition, a recent paper describing physical interaction between β-catenin and HIF-1α suggests at least one mechanism by which Wnt signaling might affect HIF activity in stem cells (Kaidi et al., 2007). Determining the degree of cross-talk, if any, between the HIFs and these important signaling networks in stem cells is an exciting prospect for future research.
Hypoxia and cancer stem cells – a new role for HIFs?
A number of experiments over the past decade support the idea that cancers can grow from a discrete subpopulation of malignant cells with stem cell properties (cancer stem cells) (Huntly and Gilliland, 2005; Reya et al., 2001). These transformed cells are formally similar to normal stem cells in that they self-renew and produce more committed progenitor or “transit amplifying” cells whose progeny differentiate, albeit aberrantly, to produce the bulk of the tumor. To date, cells with these and other stem cell properties have been identified in human hematopoietic, brain and breast cancers, and are likely to be found in other tumors (Huntly and Gilliland, 2005; Reya et al., 2001; Vescovi et al., 2006).
Cancer stem cells typically represent a small fraction of the total tumor, can be enriched on the basis of cell-surface marker expression, and generate serially transplantable tumors in recipient immunodeficient mice (Huntly and Gilliland, 2005). Some cancer stem cells also express ABC glycoprotein transporters at the cell surface, a trait shared with normal hematopoietic stem cells. These transporters effectively pump out vital dyes, resulting in a characteristic unlabeled “side-population” of cells detected in FACs plots. Unfortunately, these transporters also eliminate chemotherapeutic drugs, thereby promoting the multidrug resistance (MDR) observed in a large number of cancer cell lines (Comerford et al., 2002). In CML patients, the expression of ABC transporters may explain the persistence of Gleevec-resistant transformed stem cells despite years of Gleevec treatment and disease remission (Michor et al., 2005). The source of cancer stem cells is not entirely clear, and may differ depending on the specific disease. Some experimental results are consistent with the idea that cancer stem cells can be derived from normal stem cells that have undergone oncogenic transformation, as described for human AML leukemic stem cells (Huntly and Gilliland, 2005). In contrast, it is possible that malignant progenitor cells, or even differentiated cells, can be induced to express the properties of self-renewal and mulpotency through mutation or altered gene activation (Krivtsov et al., 2006).
Two recent papers support the idea that stem cell characteristics can be imposed on more differentiated cell types through a surprisingly small number of genetic manipulations. Takahashi and Yamanaka demonstrated that directed expression of only four transgenes can convert normal murine fibroblasts (embryonic and adult) into cells closely resembling embryonic stem (ES) cells (Takahashi and Yamanaka, 2006). Particular fibroblast cell clones engineered to express KLF4, Sox2, Oct4 and c-Myc transgenes gained a striking ES-cell like morphology, expressed genes characteristic of ES cells, and (unlike fibroblasts) failed to senesce during extended in vitro culture. When injected into wild type murine blastocysts, these modified fibroblasts contributed to multiple differentiated cell types in developing embryos, although none of these chimeric animals survived to birth. While not bona fide ES cells, it is clear that the modified fibroblasts are multipotent and have gained the ability to self-renew, at least in vitro.
In a separate report, Armstrong and colleagues expressed an MLL-AF9 oncogenic fusion protein in highly purified committed granulocyte macrophage progenitor (GMP) cells to test their ability to produce leukemia when injected into mice (Krivtsov et al., 2006). Remarkably, this single genetic alteration produced an oligoclonal AML disease characterized by a small number (<1%) of transplantable leukemia initiating GMP (L-GMP) cells in the bone marrow. Transcript profiling on these L-GMPs, as well as normal HSCs and GMPs, revealed a group of 363 genes that are highly expressed in HSCs, downregulated in normal GMPs, but re-activated in tumor initiating L-GMPs. These genes appear to be arranged in a hierarchical relationship, so that activation of a few regulatory proteins and transcription factors may account for the overall self-renewal transcript signature observed. Which of the 363 genes contribute directly to self-renewal and/or other stem cell functions remains to be determined. Together, the work from Yamanaka’s and Armstrong’s groups demonstrates that self-renewal and multipotency programs can be activated in differentiated cells through a small and discrete number of genetic alterations (Krivtsov et al., 2006; Takahashi and Yamanaka, 2006).
It is striking that two of the four factors identified by Takahashi and Yamanaka are directly activated by HIF-2α, albeit by different mechanisms. As described above, superphysiological induction of Oct4 expression in HIF-2α KI cells correlates with profound effects on embryonic development, hematopoietic differentiation, and tumor growth (Covello et al., 2006). Interestingly, Oct4 expression has been detected in a variety of cancer cell lines, and is induced by hypoxia in a HIF-2α expressing renal carcinoma cell line (Tai et al., 2005). These results suggest that the Oct4 locus, which is not expressed in normal differentiated somatic cells, can be derepressed in cancer cells and may promote a undifferentiated cellular phenotype. In support of this idea, Jaenisch and colleagues demonstrated that inducible expression of Oct4 in transgenic mice produced reversible epithelial dysplasia, a characteristic of premalignant lesions (Hochedlinger et al., 2005). Together, these data support a role for Oct4 in promoting the proliferation of undifferentiated progenitor and/or stem cells, thereby contributing to tumor growth. In fact, RNAi mediated inhibition of Oct4 expression in HIF-2α KI teratomas reduced tumor size in allograft assays (Covello et al., 2006). It is interesting that the dysplastic Oct4-expressing murine epithelia, as well as the HIF-2α KI teratomas, showed elevated levels of β-catenin protein, suggesting that Oct4 expression may stimulate Wnt pathway signaling in some cells. The degree to which Oct4 contributes to the growth of human tumors is unknown, although it is clearly implicated in testicular germ cell tumors (Gidekel et al., 2003). Presumably, Oct4 expression would not have to be reactivated in cancer stem cells derived from direct oncogenic transformation of germ stem cells, or possibly in other adult stem cells.
Interestingly, HIF proteins also modulate the activity of c-Myc, an oncogene of central importance to many cancers. Huang and colleagues demonstrated that HIF-1α antagonizes c-Myc activity by competing for binding to Sp-1 under hypoxic conditions, with consequent inhibition of c-Myc dependent cell cycle progression (Koshiji et al., 2004). Surprisingly, HIF-2α has the opposite effect on c-Myc, promoting cell cycle progression and transformation by enhancing the transcriptional effects of c-Myc on both activated and repressed target genes in multiple cancer cell lines, mouse embryo fibroblasts, and embryonic cell lines (Gordan et al., 2007). HIF-2α potentiates c-Myc activity by enhancing its physical association with Sp1, Miz1 and Max, although the precise mechanisms regulating these events are not yet fully understood. These effects may partly explain the observation that HIF-2α specifically promotes the growth of pVHL deficient renal clear cell carcinoma cells in xenograft experiments, whereas HIF-1α does not (Seagroves and Johnson, 2002).
HIF regulation of Notch activity may also contribute to cancer stem cell formation. In mammals, Notch appears to have oncogenic effects in some contexts (T-ALL, intestinal tumors), and tumor suppressor effects in others (keratinocytes) (Weng and Aster, 2004; Wilson and Radtke, 2006). These different properties may reflect the complexity of gene families encoding the Notch receptor and Delta and Jagged ligands, and their differential regulation in specific cell types. Moreover, the Notch pathway is known to interact with other pathways that control stem cell function. For example, Wnt and Shh signaling are repressed in murine epidermal cells in a Notch-dependent manner (Wilson and Radtke, 2006). Interestingly, Notch has recently been shown to activate the expression of c-Myc, suggesting an indirect mechanism whereby HIF-1α may regulate Notch signaling (Weng et al., 2006).
Finally, proteins regulating other stem cell functions have been identified as HIF targets. The human gene encoding the ABC glycoprotein transporter MDR1, which confers multidrug resistance on a variety of cancer cells, is a direct HIF target (Comerford et al., 2002). Another HIF-regulated ABC transporter, Bcrp/ABCG2, is also expressed in a number of stem cell types and is implicated in chemotherapeutic drug resistance in breast cancers (Krishnamurthy et al., 2004). Finally, the gene encoding the enzymatic component of human telomerase (hTERT) is induced by hypoxia in a HIF dependent manner (Nishi et al., 2004). Expression of ABC transporters and sustained telomerase activity are thought to be important features of stem cell function.
We propose that HIF stabilization in hypoxic tumor cells may promote the adoption of stem cell properties, including self-renewal and multipotency, by stimulating the expression or activity of Oct4, Notch, and other critical signaling pathways. If true, it suggests that hypoxic tumor tissues could be a breeding ground for cancer stem cells, although these could certainly be derived from oncogenic transformation of extant adult stem cells. The results summarized in this review suggest multiple mechanisms by which tumor hypoxia could contribute to the conversion of differentiated tumor cells into cancer stem cells. For example, disrupted epigenetic silencing in a transformed cell may result in derepression of the Oct4 locus, rendering it susceptible to regulation by HIF-2α (Covello et al., 2006). Potentiation of c-Myc activity by HIF-2α could also promote proliferation, and operate in concert with Oct4 to activate a self-renewal gene expression program (Gordan et al., 2007). To date, there are no reports in the literature suggesting that KLF4 or Sox2 are direct (or indirect) HIF targets, although it is certainly possible. Given the opposing effects of HIF-1α and HIF-2α on c-Myc transcriptional activity, the functional outcome of hypoxic c-Myc regulation may depend on the relative expression levels of HIF-1α and HIF-2α in a given cancer cell type (Gordan et al., 2007; Koshiji et al., 2004). Stabilization of HIF-1α could also enhance Notch function, the effects of which might include inhibition of differentiation, modulation of other stem cell signaling pathways (Wnt and Shh), and possibly induction of c-Myc expression (Gustafsson et al., 2005; Wilson and Radtke, 2006). Lastly, hypoxic induction of MDR1, ABCG2 and hTERT expression in these cells could confer other important stem cell characteristics (Comerford et al., 2002; Krishnamurthy et al., 2004; Nishi et al., 2004). The combined effects of hypoxia and the HIF proteins may ultimately impose attributes of stem cell identity on more differentiated transformed cells. It should be emphasized that this outcome would likely be a rare event: only those hypoxic tumor cells which express a particular level of the relevant stem cell factors, particularly Oct4 and Notch, would be expected to gain stem cell characteristics.
The proposed model suggests that the effects of tumor hypoxia extend beyond its critically important role in driving angiogenesis and modulating cancer cell metabolism and survival. As eradicating cancer stem cells is increasingly recognized as an important goal in curing cancer, the HIF pathways are even more attractive as targets of therapeutic intervention (Semenza, 2003). Reducing HIF activity in cancer stem cells may promote their differentiation, thereby reducing their ability to repopulate tumors after chemo- and radiation therapies. To test this idea, it will be necessary to ablate the function of HIF-1α, HIF-2α, Notch, Oct4, c-Myc and other pathway components in rigorously defined tumor models.
Figure 1.
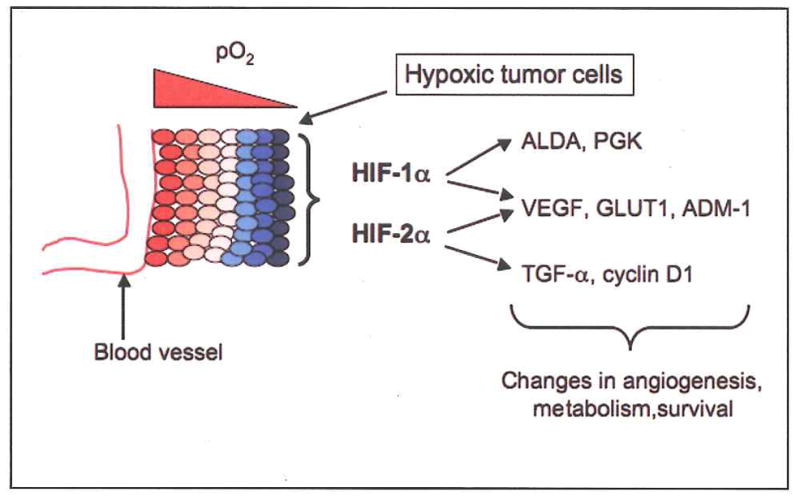
Traditional view of HIFs in tumor progression. Tumor cells residing closer to blood vessels are relatively well oxygenated (red), whereas those at more distant sites become hypoxic (blue). Stabilization of HIF-α proteins in these cells stimulates the expression of numerous target genes encoding factors that mediate adaptation to the hypoxic stress. Some target genes are regulated specifically by HIF-1α, such as those encoding glycolytic enzymes ALDA and PGK, whereas others are specific targets of HIF-2α, such as those encoding TGF-α and cyclin D1. Most HIF target genes are regulated by both HIF-1α and HIF-2α, including those encoding the angiogenic cytokine VEGF and the glucose transporter GLUT1 (Raval et al., 2005, and references therein).
Figure 2.
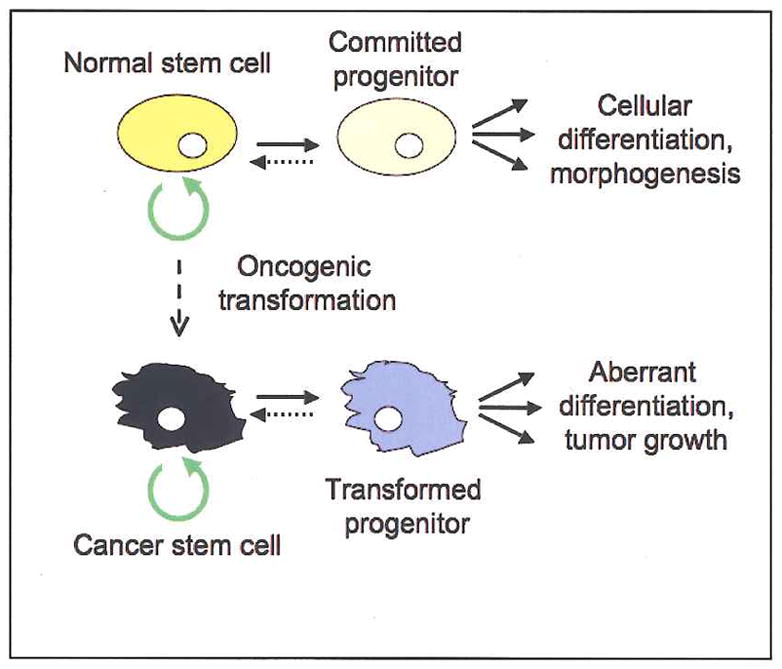
One mode of cancer stem cell generation. Normal stem cells (top) typically divide slowly, but retain the capacity for apparently limitless self-renewal (circular green arrow). Asymmetric division of a multipotent stem cell produces one daughter stem cell and one committed progenitor (or transit amplifying) cell that undergoes a limited, if rapid, series of divisions. The progeny of this transit amplifying cell differentiate to produce a tissue or tissues. A stem cell may undergo oncogenic transformation (vertical dashed line) and lose important homeostatic control mechanisms: the resultant transit amplifying cell and its progeny may consequently fail to differentiate normally or exhibit normal growth controls, thereby generating a tumor. Whereas the transformed cancer stem cell retains the property of self-renewal and can produce new tumors in serial transplantation experiments, its progeny do not (Huntly and Gilliland, 2005). Growing evidence suggests that progenitor cells can, under certain circumstances, regain stem cell properties (horizontal dashed lines) (Krivstov et al., 2006, Morrison and Kimble, 2006).
Figure 3.
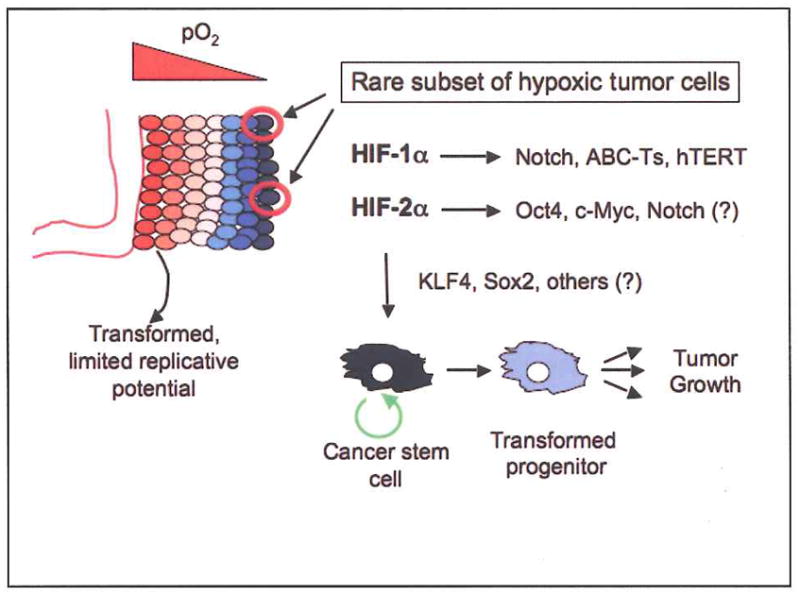
Proposed model for HIF activity in generating cancer stem cells. Cells in hypoxic tumor regions stabilize HIFs and activate adaptive gene expression (see Figure 1). HIF activity in a rare subset of hypoxic tumor cells could also enhance the activity or expression of Notch, Oct4, c-Myc, ABC transporters (ABC-Ts), and telomerase. Increased expression of KLF4, Sox2, and other factors could promote further dedifferentiation and confer stem cell-like properties, such as self-renewal (circular green arrow), on what was originally a transformed cell with limited replicative potential. Inhibition of HIF activity in the resultant cancer stem cells might block, or reverse, this effect.
Acknowledgments
We apologize to our many colleagues whose work we were unable to cite, or could cite only indirectly, due to space limitations. We thank members of the Simon lab for critical comments on the manuscript. Some work cited in this article was supported by NIH grant HL66130 (BK and MCS). MCS is an Investigator of the Howard Hughes Medical Institute.
Contributor Information
Brian Keith, Email: bkeith@mail.med.upenn.edu.
M. Celeste Simon, Email: celeste2@mail.med.upenn.edu.
References
- Axelson H, Fredlund E, Ovenberger M, Landberg G, Pahlman S. Hypoxia-induced dedifferentiation of tumor cells--a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin Cell Dev Biol. 2005;16:554–563. doi: 10.1016/j.semcdb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, Carmeliet P, Simon MC. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl A, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-Myc transcriptional activity. Cancer Cell. 2007 doi: 10.1016/j.ccr.2007.02.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- Joseph NM, Morrison SJ. Toward an understanding of the physiological function of Mammalian stem cells. Dev Cell. 2005;9:173–183. doi: 10.1016/j.devcel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomeli H, Nagy A, McLaughlin KJ, Scholer HR, Tomilin A. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004;5:1078–1083. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatua S, Peterson KM, Brown KM, Lawlor C, Santi MR, LaFleur B, Dressman D, Stephan DA, MacDonald TJ. Overexpression of the EGFR/FKBP12/HIF-2alpha pathway identified in childhood astrocytomas by angiogenesis gene profiling. Cancer Res. 2003;63:1865–1870. [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1 alpha induces cell cycle arrest by functionally counteracting Myc. Embo J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2342. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor betal induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000a;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000b;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Nishi H, Nakada T, Kyo S, Inoue M, Shay JW, Isaka K. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT) Mol Cell Biol. 2004;24:6076–6083. doi: 10.1128/MCB.24.13.6076-6083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Pear WS, Aster JC. T cell acute lymphoblastic leukemia/lymphoma: a human cancer commonly associated with aberrant NOTCH 1 signaling. Curr Opin Hematol. 2004;11:426–433. doi: 10.1097/01.moh.0000143965.90813.70. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Ramirez-Bergeron DL, Simon MC. Hypoxia-inducible factor and the development of stem cells of the cardiovascular system. Stem Cells. 2001;19:279–286. doi: 10.1634/stemcells.19-4-279. [DOI] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1 alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1 alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves T, Johnson RS. Two HIFs may be better than one. Cancer Cell. 2002;1:211–213. doi: 10.1016/s1535-6108(02)00048-x. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- Weng AP, Aster JC. Multiple niches for Notch in cancer: context is everything. Curr Opin Genet Dev. 2004;14:48–54. doi: 10.1016/j.gde.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H, Tobias J, et al. c-Myc is an important direct target of Notchl in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Wamecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. Faseb J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 2006;580:2860–2868. doi: 10.1016/j.febslet.2006.03.024. [DOI] [PubMed] [Google Scholar]


