Abstract
Chronic itch could be a presenting sign of malignancy. Pruritus of lymphoma is the common prototype of paraneoplastic itch and can precede other clinical signs by weeks and months. Paraneopalstic pruritus has also been associated with solid tumors and is an important clinical symptom in paraneoplastic skin diseases such as erythroderma, Grovers disease, malignant acanthosis nigricans, generalized granuloma annulare, Bazex syndrome and dermatomyositis. In any case with high index of suspicion a thorough work-up is required. This review highlights the association between itch and malignancy and presents new findings related to pathophysiological mechanisms and the treatment of itch associated with malignancy. Combinative therapies reducing itch sensitization and transmission using selective serotonin and neuroepinephrine reuptake inhibitors, Kappa opioids and Neuroleptics are of prime importance in reducing this bothersome symptom.
Keywords: paraneoplastic itch, Itch pathophysiology, neuroleptics, Kappa opioids
Introduction
Chronic pruritus is defined as itch which last more than 6 weeks (1). It has been linked to internal malignancy and in particular lymphoproliferative disease(2). Paraneoplastic itch is defined as : 1. itch that occurs early during the natural process or even precedes the clinical evidence of the malignancy. 2. It is not caused by the neoplastic mass invasion or compression and 3. subsides after the removal of the tumor. Pruritus of lymphoma is the common prototype of paraneoplastic itch and can precede other clinical signs by weeks and months(2). Although generalized idiopathic pruritus has been often linked to internal malignancy(3), there are few studies that examined the prevalence of itch in malignancies. Itch associated with malignancy could range from mild to intractable, which is defined as a chronic itch state in which the cause cannot be removed or treated, and no relief or cure has been found in the generally accepted course of medical practice (1).
The purpose of this review is to highlight the association between itch and malignancy and to present new findings related to the pathophysiological mechanisms and the treatment of itch associated with malignancy.
Epidemiology
Prevalence of chronic itch has been reported decades ago as high as 30% in patients with Hodgkin's disease(4). A small-scale study showed that approximately 15% of patients with non-Hodgkin's disease suffered from generalized pruritus(5). According to a retrospective study recently conducted at MD Anderson (6), the incidence of itch is about 19% in patients with Hodgkin's disease who were referred to dermatology.
Itch has been reported to be a preceding sign in patients with multiple myeloma (7), however there is no data on its prevalence.
Several small scale studies examined the underlying etiology of idiopathic generalized pruritus and found that malignancy is a cause in less than 10% of the patients. Lymphoma and leukemia were the most common malignancies (8-10).
Pruritus as a paraneoplastic sign in solid tumors of different types has been anecdotally reported (11). Pruritus was found in one out of the 9 patients with paraneoplastic syndromes among 68 patients who suffered with non-small cell lung carcinoma (12). In patients with extrahepatic cholestatic, itch may be caused by obstructive tumor in the pancreatic head and primary sclerosing cholangitis (2). Intractable pruritus has been reported as an initial presentation of insulinoma (13).
Although the pruritus may occasionally be present years before the tumor becomes detectable, a full investigation for a causative solid tumor is probably not warranted, unless other skin manifestations or clinical signs suggestive of malignancy exist.
Clinical characteristics of pruritus in malignancy
Itch can present with ichthyosiform skin changes on the extremities (2) or as a new-onset eczema lesions with Hodgkin's disease (6). Severe intractable itch has been reported in lymphoma patients. Some of the most severe pruritic cases in our practice suffer from lymphoma. Nocturnal itch is common in all forms of chronic itch (14). Patients with paraneoplastic itch often suffer from severe intractable itch and present with secondary skin lesions as a result of the malicious itch-scratch cycle that include excoriations, hyperpigmentation or hypopigmentation, lichenification, prurigo nodules and scars.
Aquagenic pruritus is itch that develops minutes after contact with water of any temperature with no visible skin rash or urticaria. It is more commonly known to be associated with polycythemia vera, however several reports demonstrate an association with other lymphoproliferative diseases (15-16). Aquagenic pruritus can precede the development of T cell lymphoma or myelodysplasia by several years.
Paraneoplastic pruritus could present as part of a primary epidermal and dermal skin diseases associated with malignancy. Recently, we reported 2 cases of generalized granuloma annulare with severe itch as the initial presentation of chronic myelomonocytic leukemia (17). Generalized granuloma annulare has been associated with lymphoma and leukemia (18). Pruritus is a common manifestation in dermatomyositis (19), an average of 18-32% of patients with dermatomyositis have or will develop malignancy (20).
Transient acantholytic dermatosis (Grovers Disease) is an extremely pruritic papulovesicular disease that occurs mainly on the trunk in adult men. It has been reported to be associated with hematological malignancies, although it is not clear whether the association is related to B symptoms of sweat and fever that induce this rash (21-24). Erythroderma is another paraneoplastic skin manifestation that is extremely itchy and is commonly associated with hematologic malignancies (25). Recently paraneopalstic pemphigus presented as severe erythrodermic pruritic rash (26).
Eruptive seborrheic keratosis with pruritus (Lesser Trelat sign) has been reported to be associated with adenocarcinoma of the gut and hematopoietic malignancies in more than a century, however the validity of this sign is debated (27-28).
Malignant acanthosis nigricans (MAN) is highly associated with adenocarcinoma of the gut and is frequently associated with generalized itch in 41% of the patients in a large survey of 90 patients with MAN (29).
Bazex syndrome (acrokeratosis paraneoplastica) is a rare acral papulosquamous eruption that has been reported to be pruritic. It occurs predominantly in males and is associated with squamous cell carcinomas of the head and neck and aerodigestive ways (30).
Investigations
In any patients with suspected paraneoplastic pruritus, a thorough history and a complete physical examination including an exam for lymph nodes are central to the evaluation of pruritus. Diagnostic testing is directed by the clinical evaluation. Laboratory tests should include a complete blood cell count (CBC), LDH and liver function tests. Radiological tests including CT of chest and abdomen are recommended to rule out lymphoma. A detailed history and exam will be helpful to direct further investigation; for example, a patient with bone pain should undergo blood and urine tests to rule out myeloma. As mentioned above, aquagenic pruritus can precede the development of T cell lymphoma or myelodysplasia by several years. Therefore, it is our recommendation that in any patient who suffers from aquagenic pruritus, a complete blood cell count (CBC) as well as chest X ray to be performed followed by yearly blood tests and chest X rays.
Pathophysiological mechanisms
The pathophysiological mechanisms of pruritus associated with malignancy remains poorly understood. Recently major advancements have been achieved in understanding the neurobiology of itch both peripherally and centrally. In the following section we will briefly review recent findings on itch neural pathway receptors and mediators and treatment strategies that could play a role in chronic itch associated to malignancy.
Itch-selective and specific neural pathway
It has been for long time suggested that nonhistaminergic pathway likely plays a role in the transmission of chronic itch(31-32). In the last three years, several groups were able to identify a distinct group of nerve fibers that transmit non-histaminergic itch in the spinal cord of monkeys, as well as in humans(33-35). These fibers were found to respond to Mucuna pruriens, a plant reported by Shelley decades ago to induce itch without flare, which contains a protease named mucunanin (36-37). Mucunanin induces itch by activation of C nerve fibers that do not respond to histamine, but respond to mechanical stimuli. (33-35). The role of this newly identified itch pathway in chronic itch and systemic diseases remains to be elucidated. Sun and Chen (38-39) have recently identified an itch specific gene, a G protein (GRPR) within the spinal cord of mice that mediates pruritus but does not respond to painful stimuli. GRPR gene is a member of the bombesin family, bombesin is an amphibian protein widely distributed in gastrointestinal tract and central nervous system. It is abundant in malignant gastric and lung tumors in humans (39). However, these cancers commonly are not associated with itch in humans. The role of GRPR in the pathogenesis of chronic itch in humans remains to be determined.
Endogenous opioids and chronic itch
Intense, generalized itch is known to be one of the most common side effects of analgesic therapy with exogenous mu-opioids such as morphine. Endogenous opioids are important players in the pathogenesis of itch per se, as well as itch related to systemic disease. It has been proposed that chronic systemic itch is related to an imbalance between mu and kappa opioids (31, 40)
The role of endogenous opioid system in the pathogenesis of pruritus in lymphoma has not been studied yet. However, the efficacy of butorphanol (a kappa-opioid agonist and mu-opioid antagonist) in the treatment of pruritus in a non-Hodgkin lymphoma patient indicated the possible involvement of opioids in the modulation of this type of pruritus (41).
Cytokines and itch
Cytokines such as IL-6 and IL-8, which have been reported to increase in chronic kidney disease associated itch and atopic dermatitis respectively (42-43) are closely related to pathophysiology of lymphoma (44-45). Their roles in pathogenesis of itch in lymphoma are worthy of further investigation. The recent discoveries of IL-31 (a TH2 cell-derived cytokine) capable of eliciting itch, and increased IL-31 level in atopic dermatitis and prurigo nodularis, indicated a potential role of this cytokine in the modulation of chronic itch (46). An IL-31 antibody could effectively reduce scratching behavior in an atopic dermatitis-like murine model during the onset of clinical skin manifestations, suggesting the potential therapeutic role of IL-31 antibody in treatment of chronic itch (47). A mutation in the in the OSMR gene, which encodes oncostatin M receptor beta (OSMRbeta), an interleukin (IL)-6 family cytokine receptor has been recently discovered in patients with familial lichen amyloidosis, a severe form of localized itch (48). Therefore assessing the role of IL31and interleukin 6 in itch associated to lymphoproliferative diseases is a timely topic.
New Treatment options
Recently nalfurafine (TRK-820), a kappa-receptor agonist, has been launched in Japan as the first oral non histaminergic oral anti pruritic in the treatment of severe ESRD-associated pruritus (49). It has also been shown to successfully relieve pruritus in an animal model with cholestasis (50). However, TRK-820 is not available commercially yet in the US. Butorphanol is a commercially available analgesic; it works as an antagonist of mu-receptors and an agonist kappa-receptor, which was showed to be efficient in the treatment of intractable pruritus associated with lymphoma and other systemic diseases (41).
Aprepitant is a neurokinin 1 receptor antagonist widely used as antiemetic agent in chemotherapy and has recently been reported to be an effective antipruritic agent in 3 cases of Sezary syndrome with severe itch (51). The recommended dose was 80 mg per day. Future controlled trials are required to clarify the role of this expensive drug as an anti-pruritic for lymphoma.
Current Treatment Strategies
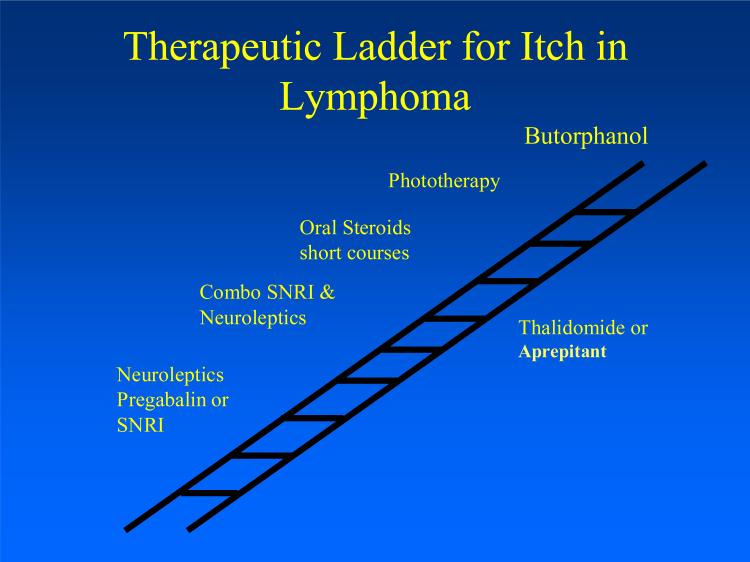
The focuses of current strategies are to reduce the intensity of itch and to block afferent transmission via peripheral and central neural mechanisms. There are a limited number of studies examining the efficacy of these agents for the treatment of itch in malignancy and most of the data presented is based on case series or small-scale studies. The purpose of this section is to review the rational systemic therapeutic ladders for pruritus associated with malignancy and in particular with lymphoproliferative diseases (see figure 1, table 2).
Figure 1.
Therapeutic ladder of treatment of pruritus in lymphoma.
Table 2.
Recommended systemic treatment for paraneoplastic associated pruritus.
| Drug names | Antipruritic mechanisms | Suggested doses |
|---|---|---|
| Hydroxyzine | H1-antihistamine and sedating | 50-100 mg/day |
| Doxepin | H1-antihistamine and tricyclic antidepressant | 25-75 mg/day |
| Naltrexone | Mu-opioid antagonist | 25-50 mg/day |
| Butorphanol | Mu-antagonist and kappa-agonist | 1-4 mg/day intranasally |
| Gabapentin | Central and peripheral itch pathway inhibitor | 300 mg up to 3200 mg/day |
| Pregabalin | Central and peripheral itch pathway inhibitor | 150 mg/day to 300 mg/day |
| Mirtazapine | Selective α2 adrenergic receptor antagonist | 15 mg at night |
| Paroxetine | Selective serotonin reuptake inhibitor | |
| Thaldiomide | Central and peripheral itch pathway inhibitor Anti TNF, antiangiogenic | 100mg/day up to 200mg |
| Aprepitant | Neurokinin 1 inhibitor | 80 mg at day time |
Antidepressants for itch
Selective serotonin reuptake inhibitors (SSRIs)
SSRIs such as sertraline and paroxetine, act by selective inhibition of serotonin reuptake. Their beneficial effect is working presumably via alteration of neurotransmitters’ concentrations within the central nervous system. A randomized, controlled study addressing paraneoplastic pruritus has shown that paroxetine significantly reduces itch (52). A recent open-labeled study showed paroxetine and fluvoxamine to be efficient in the treatment of different types of chronic itch including itch associated to systemic lymphoma and solid carcinoma (53). The antipruritic effect takes 2-3 weeks from commencement of treatment to become effective.
Selective Neuroepinephrine Re-Uptake Inhibitors (SNRI)
SNRIs relieve itch possibly by reducing central sensitization to itch due to their effects on both serotonin and noradrenergic α2 receptors, as they do in treatment of neuropathic pain (54). We found mirtazapine to be particularly effective in treatment of nocturnal pruritus (55). Mirtazapine has been reported to be effective in the treatment of itch associated with lymphoma (56). The new SNRIs such as venlafaxine and duloxetine do not seem to have significant antipruritic effects in our experience.
Neuroleptics
Gabapentin and pregabalin are structural analogues of the neurotransmitter gamma-aminobutyric acid (GABA). The exact mechanisms of their antipruritic effect are not clear. They probably inhibit central itch pathways, as they do in neuropathic pain (57).
Sedating antihistamines
Sedating antihistamines still have a role in treating itch, especially nocturnal itch (54). Sedating antihistamines such as hydroxyzine at high dose commonly are used to treat pruritus in patients with systemic itch probably by exerting a beneficial effect through their soporific properties. Doxepin, a tricyclic antidepressant, has a similar effect as SSRI and is also used to treat nocturnal itch, probably amid its potent antihistamine H1 receptor property.
Thalidomide
Thalidomide has been used for more than decade as an antipruritic and may exert its effect by several mechanisms including suppression of excessive tumor necrosis factor alpha production, as well as a peripheral and central nerve depressant. Pruritus disappears in a period of 2-3 weeks. The major adverse effects are peripheral neuropathy and its high teratogenic effect that requires mandatory monitoring in women of reproductive potential in the US in the STEPS program. In the last 5 years, Thalidomide became an important part of chemotherapy of hematologic malignancies including myeloma, lymphoma and solid tumors. Interestingly, the less neurotoxic derivative of thalidomide Lenalidomide that has been recently approved has been reported to induce pruritus (58).
Ultraviolet (UV) therapy
UVB is well known to relieve pruritus in systemic diseases particularly in chronic renal disease. The role of UV therapy including both narrow band UV-B and Psoralen plus ultraviolet (UV) A (PUVA) is well established for cutaneous T cell lymphoma (59), but has not been reported to our knowledge for itch associated with systemic lymphoma and other malignancies.
Treatment of pruritus in lymphoma (see figure 1)
A recent case series reported that the combination of low dose of mirtazapine (7.5 to 15 mg at evening) and gabapentin (300 mg at night up to 900-2400 mg per day) was effective in treatment of the itch of cutaneous of T-cell lymphoma (60). Butorphanol has been used to treat intractable itch in lymphoma with dose of 3-4 mg/day. Another option that we found helpful to alleviate intractable lymphoma itch is oral prednisone 40 mg tapering down gradually in 3 weeks(2). Thalidomide the costly drug is a second line anti pruritic agent that could be considered and can also be part of the chemotherapeutic regimen.
Pruritus resolves rapidly after successful removal and treatment of the underlying malignancy.
Conclusion
Chronic itch could be a presenting sign of malignancy. In any case with high index of suspicion a thorough work-up is required. The underlying mechanisms responsible for this type of itch are still largely unclear. The recent advances in understanding specific itch pathways and mediators will hopefully lead to novel therapies. Combinative therapies reducing itch sensitization and transmission are of prime importance.
Table 1.
Skin paraneoplastic syndromes that itch.
| Paraneoplastic syndrome | Associated malignancies |
|---|---|
| erythroderma | Lymphoma, leukemia |
| Lesser Trelat | Adenocarcinoma of gut, hematologic malignancies |
| Generalized granuloma annulare | Lymphoma, leukemia, |
| Grovers Disease | Hematological malignancies |
| Dermatomyositis | carcinoma of colon in men breast ovaries cancer in women Nasopharangyeal carcinoma in Asians |
| Bazex syndrome | head and neck upper airway digestive tract carcinoma (pharynx, larynx, esophagous) |
| Malignant Acanthosis Nigricans | Adenocarcinoma of gut |
Acknowledgments
Dr Yosipovitch is supported by NIH Grant 1R01AR055902-01A1
References
- 1.Yosipovitch G, Greaves MW. Definitions of itch. In: Yosipovitch G, Greaves MW, Fleischer AB, McGlone F, editors. Itch: basic mechanisms and therapy. Marcel Dekker; New York: 2004. p. 2. [Google Scholar]
- 2.Wang H, Yosipovitch G. New Insights into the pathophysiology of chronic itch in patients with end stage renal failure, chronic liver disease and lymphoma. Int J Dermatol. 2010;49:1–12. doi: 10.1111/j.1365-4632.2009.04249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiramanek N. Itch: a symptom of occult disease. Aust Fam Physician. 2004 Jul;33(7):495–9. [PubMed] [Google Scholar]
- 4.Gobbi PG, Attardo-Parrinello G, Lattanzio G, et al. Severe pruritus should be a B-symptom in Hodgkin's disease. Cancer. 1983;51:1934–1936. doi: 10.1002/1097-0142(19830515)51:10<1934::aid-cncr2820511030>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SS, Kuruvilla M, Pai GS, et al. Cutaneous manifestations of non-Hodgkin's lymphoma. Indian J Dermatol Venereol Leprol. 2003 Jan-Feb;69(1):12–5. [PubMed] [Google Scholar]
- 6.Rubenstein M, Duvic M. Cutaneous manifestations of Hodgkin's disease. Int J Dermatol. 2006;45:251–256. doi: 10.1111/j.1365-4632.2006.02675.x. [DOI] [PubMed] [Google Scholar]
- 7.Erskine J G, Rowan R M, Alexander J O, Sekoni GA. Pruritus as a presentation of myelomatosis. Br Med J. 1977;1:687–688. doi: 10.1136/bmj.1.6062.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polat M, Oztas P, Ilhan MN, et al. Generalized pruritus: a prospective study concerning etiology. Am J Clin Dermatol. 2008;9(1):39–44. doi: 10.2165/00128071-200809010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Zirwas MJ, Seraly MP. Pruritus of Unknown origin. J Am Acad Dermatol. 2001;45(6):892–6. doi: 10.1067/mjd.2001.117732. [DOI] [PubMed] [Google Scholar]
- 10.Goon TJ, Yosipovitch G, Chan YH, Goh CL. Clinical characteristics of generalized idiopathic pruritus in patients from a tertiary center in Singapore. Int j Dermatol. 2007;46:1023–1026. doi: 10.1111/j.1365-4632.2007.03152.x. [DOI] [PubMed] [Google Scholar]
- 11.Krajnik M, Zylicz Z. Pruritus accompanying solid tumors. In: Zylicz Z, Twycross R, Jones EA, editors. Pruritus in advanced disease. Oxford Press; 2004. pp. 97–106. [Google Scholar]
- 12.Campanella N, Moraca A, Pergolini M, Daher W, Fianchini A, Sabbatini A, Brunelli A, Al-Refai M. syndromes in 68 cases of resectable non-small cell lung carcinoma: can they help in early detection? Paraneoplastic. Med Oncol. 1999;16:129–33. doi: 10.1007/BF02785846. [DOI] [PubMed] [Google Scholar]
- 13.King NK, Siriwardana HP, Coyne JD. Siriwardena AK Intractable pruritus associated with insulinoma in the absence of multiple endocrine neoplasia: a novel paraneoplastic phenomenon. Scand J Gastroenterol. 2003;38:678–80. doi: 10.1080/00365520310001950. [DOI] [PubMed] [Google Scholar]
- 14.Patel T, Ishiuji Y, Yosipovitch G. Nocturnal itch: why do we itch at night? Acta Derm Venereol. 2007;87(4):295–8. doi: 10.2340/00015555-0280. [DOI] [PubMed] [Google Scholar]
- 15.Khalifa N, Singer CR, Black AK. Aquagenic pruritus in a patient associated with myelodysplasia and T-cell non-Hodgkin's lymphoma. J Am Acad Dermatol. 2002;46:144–5. doi: 10.1067/mjd.2001.117391. [DOI] [PubMed] [Google Scholar]
- 16.Ratnaval RC, Burrows NP, Marcus RE, Norris PG. Aquagenic pruritus and acute lymphoblastic leukaemia. Br J Dermatol. 1993 Sep;129(3):348–9. doi: 10.1111/j.1365-2133.1993.tb11868.x. [DOI] [PubMed] [Google Scholar]
- 17.Hinckley MR, Walsh SN, Molnár I, Sheehan DJ, Sangueza OP, Yosipovitch G. Generalized granuloma annulare as an initial manifestation of chronic myelomonocytic leukemia: a report of 2 cases. Am J Dermatopathol. 2008 Jun;30(3):274–7. doi: 10.1097/DAD.0b013e318166ea1a. [DOI] [PubMed] [Google Scholar]
- 18.Li A, Hogan DJ, Sanusi ID, Smoller BR. Granuloma annulare and malignant neoplasms. Am J Dermatopathol. 2003 Apr;25(2):113–6. doi: 10.1097/00000372-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Shirani Z, Kucenic MJ, Carroll CL, Fleischer AB, Jr, Feldman SR, Yosipovitch G, Jorizzo JL. Pruritus in adult dermatomyositis. Clin Exp Dermatol. 2004;29:273–6. doi: 10.1111/j.1365-2230.2004.01510.x. [DOI] [PubMed] [Google Scholar]
- 20.Vieugels RA, Callen JP. Dermatomyositis. In: Callen JP, Jorizzo JL, editors. Dermatological Signs of Internal Disease. 4th ed. Elsevier China; 2009. pp. 11–20. [Google Scholar]
- 21.Garçon N, Karam A, Lemasson G, Metges JP, Misery L. Paraneoplastic transient acantholytic dermatosis (Grover's disease) along Blaschko lines. Eur J Dermatol. 2009;19:405–6. doi: 10.1684/ejd.2009.0702. [DOI] [PubMed] [Google Scholar]
- 22.Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009 Sep;133(9):1490–4. doi: 10.5858/133.9.1490. [DOI] [PubMed] [Google Scholar]
- 23.Davis MD, Dinneen AM, Landa N, Gibson LE. Grover's disease: clinicopathologic review of 72 cases. Mayo Clin Proc. 1999 Mar;74(3):229–34. doi: 10.4065/74.3.229. [DOI] [PubMed] [Google Scholar]
- 24.Fujita Y, Sato-Matsumura KC, Ohnishi K. Transient acantholytic dermatosis associated with B symptoms of follicular lymphoma. Clin Exp Dermatol. 2007;32:752–4. doi: 10.1111/j.1365-2230.2007.02471.x. [DOI] [PubMed] [Google Scholar]
- 25.Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007 May;48(5):855–65. doi: 10.1080/10428190601137336. [DOI] [PubMed] [Google Scholar]
- 26.Fukumoto T, Shiroyama Y, Niizeki H, Kobayashi N, Asada H, Ishii N, Hashimoto T, Miyagawa S. Paraneoplastic pemphigus presenting as erythrodermic lichenoid dermatitis with concomitant features of pemphigus foliaceus. J Dermatol. 2007;34:645–9. doi: 10.1111/j.1346-8138.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 27.Holdiness MR. On the classification of the sign of Leser-Trélat. J Am Acad Dermatol. 1988 Jan;18(1 Pt 1):149. doi: 10.1016/s0190-9622(88)80058-6. No abstract available. [DOI] [PubMed] [Google Scholar]
- 28.Moore R, Devere TS. Epidermal manifestations of internal malignancy. Deramtol Clin. 2008;26:17–29. doi: 10.1016/j.det.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Brown J, Winkelmann RK. Acanthosis nigricans: a study of 90 cases. Medicine (Baltimore) 1968 Jan;47(1):33–51. [PubMed] [Google Scholar]
- 30.Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica). An analytic review. Medicine (Baltimore) 1991;70:269–80. doi: 10.1097/00005792-199107000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Yosipovitch G, Carstens E, McGlone F. Chronic itch and chronic pain. Analogous mechanisms. Pain. 2007;131:4–7. doi: 10.1016/j.pain.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology ofitch. Nat Rev Neurosci. 2006 Jul;7(7):535–47. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 33.Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007 Sep 12;27(37):10007–14. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namer B, Carr R, Johanek LM, et al. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008 Oct;100(4):2062–9. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johanek LM, Meyer RA, Friedman RM, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008 Jul 23;28(30):7659–69. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shelley WB, Arthur RP. Studies on cowhage (Mucuna pruriens) and its pruritogenic proteinase, mucunain. AMA Arch Derm. 1955 Nov;72(5):399–406. doi: 10.1001/archderm.1955.03730350001001. [DOI] [PubMed] [Google Scholar]
- 37.Reddy B. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008 Apr 23;28(17):4331–5. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007 Aug 9;448(7154):700–3. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 39.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009 Sep 18;325:1531–4. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumagai H, Saruta T, Matsukawa S, et al. Prospects for a novel kappa-opioid receptor agonist, TRK-820, in uremic pruritus. In: Yosipovitch G, Greaves MW, Fleischer JA, McGlone F, editors. Itch, Basic Mechanisms and Therapy. Dekker; New York, NY: 2004. pp. 279–286. [Google Scholar]
- 41.Dawn AG, Yosipovitch G. Butorphanol for treatment of intractable pruritus. J Am Acad Dermatol. 2006;54:527–531. doi: 10.1016/j.jaad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Kimata H, Lindley I. Detection of plasma interleukin-8 in atopic dermatitis. Arch Dis Child. 1994 Feb;70(2):119–22. doi: 10.1136/adc.70.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant. 2006;21:749–55. doi: 10.1093/ndt/gfi204. [DOI] [PubMed] [Google Scholar]
- 44.Biggar RJ, Johansen JS, Smedby KE, et al. Serum YKL-40 and interleukin 6 levels in Hodgkin lymphoma. Clin Cancer Res. 2008;14:6974–8. doi: 10.1158/1078-0432.CCR-08-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HL, Eom HS, Yun T, et al. Serum and urine levels of interleukin-8 in patients with non-Hodgkin's lymphoma. Cytokine. 2008;43(1):71–5. doi: 10.1016/j.cyto.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006 Feb;117(2):411–7. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Grimstad O, Sawanobori Y, Vestergaard C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009 Jan;18(1):35–51. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka A, Arita K, Lai-Cheong JE, Palisson F, Hide M, McGrath JA. New insight into mechanisms of pruritus from molecular studies on familial primary localized cutaneous amyloidosis. Br J Dermatol. 2009 Dec;161(6):1217–24. doi: 10.1111/j.1365-2133.2009.09311.x. [DOI] [PubMed] [Google Scholar]
- 49.Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2009 Nov 19; doi: 10.1093/ndt/gfp588. [DOI] [PubMed] [Google Scholar]
- 50.Inan S, Cowan A. Nalfurafine. A kappa opioid receptor agonist, inhibits scratching behavior secondary to cholestasis induced by chronic ethynylestradiol injections in rats. Pharmacol Biochem Behav. 2006 Sep;85(1):39–43. doi: 10.1016/j.pbb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Duval A, Dubertret L. Aprepitant as an antipruritic agent? N Engl J Med. 2009 Oct 1;361(14):1415–6. doi: 10.1056/NEJMc0906670. [DOI] [PubMed] [Google Scholar]
- 52.Zylicz Z, Krajnik M, Sorge AA, Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom.Manage. 2003;26:1105–1112. doi: 10.1016/j.jpainsymman.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Ständer S, Bockenholt B, Schürmeyer-Horst F, et al. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45–51. doi: 10.2340/00015555-0553. [DOI] [PubMed] [Google Scholar]
- 54.Summey BT, Jr, Yosipovitch G. Pharmacologic advances in the systemic treatment of itch. Dermatol Ther. 2005 Jul-Aug;18(4):328–32. doi: 10.1111/j.1529-8019.2005.00035.x. [DOI] [PubMed] [Google Scholar]
- 55.Hundley JL, Yosipovitch G. Mirtazapine for reducing nocturnal itch in patients with chronic pruritus: a pilot study. J Am Acad Dermatol. 2004 Jun;50(6):889–91. doi: 10.1016/j.jaad.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 56.Davis MP, Frandsen JL, Walsh D, et al. Mirtazapine for pruritus. J Pain Symptom Manage. 2003 Mar;25(3):288–91. doi: 10.1016/s0885-3924(02)00645-0. [DOI] [PubMed] [Google Scholar]
- 57.Yosipovitch G, Samuel L. Neuropathic and psychogenic itch. Dermatol Ther. 2008 Jan-Feb;21(1):32–41. doi: 10.1111/j.1529-8019.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 58.Bonkowski JJ, Vermeulen LC, Kolesar JM. The clinical utility of lenalidomide in multiple myeloma and myelodysplastic syndromes. J Oncol Pharm Pract. 2009 Nov 12; doi: 10.1177/1078155209351967. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Carter J, Zug KA. Phototherapy for cutaneous T-cell lymphoma: online survey and literature review. J Am Acad Dermatol. 2009;60:39–50. doi: 10.1016/j.jaad.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 60.Demierre MF, Taverna J. Mirtazapine and gabapentin for reducing pruritus in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2006;55:543–4. doi: 10.1016/j.jaad.2006.04.025. [DOI] [PubMed] [Google Scholar]