Abstract
The first synthesis of dolabelide C (1), a cytotoxic marine macrolide, is reported utilizing a phosphate tether-mediated approach. Bicyclic phosphates (S,S,SP)-5 and (R,R,RP)-5 serve as the central building blocks for the construction of two major 1,3-anti-diol subunits in 1 through selective cleavage pathways, regioselective olefin reduction and cross-metathesis. Overall, phosphate-mediated processes provided copious amounts of both major subunits allowing for a detailed RCM macrocyclization study to the 24-membered macrolactone 1.
I. Introduction: The Dolabelide Family
In 1995, the isolation and structural characterization of two new 22-membered macrolides, dolabelides A and B, from the sea hare Dolabella auricularia was reported (Figure 1). These compounds exhibited cytotoxicity against cervical cancer HeLa-S3 cells with IC50 values of 6.3 and 1.3 μg/mL, respectively.1 Two years later, dolabelides C and D,2 24-membered macrolides, were isolated from the same source and were found to possess cytotoxicity toward HeLa-S3 cells with IC50 values of 1.9, and 1.5 μg/mL, respectively. To the best of our knowledge, the mechanism of action of these compounds remains unknown to date.
Figure 1.
The dolabelide family, isolated from Dollabella auricularia.
Common features among the dolabelide family are 11 stereogenic centers, 8 of which bear oxygen, and two E-configured trisubstituted olefins. Other structural features possessed by this family of macrolactones include 1,3-anti-diol fragments found at C7/C9 and C19/C21, along with an accompanying 1,3-syn-diol at C9/C11 and polypropionate fragments at C1/C4 and C21/C23. The stereochemical complexity and biological profile of this class of compounds has attracted synthetic interest from several groups3 and in 2006, the first total synthesis of dolabelide D was reported by Leighton and coworkers.4
Dolabelide C (1) can be disconnected into C1-C14 and C15-C30 subunits, 2 and 3, respectively (Scheme 1). The endgame for this approach is similar to Leighton's strategy towards dolabelide D,4 employing a macrocyclization sequence to install the C14/C15 trisubstituted olefin through a late stage ring-closing metathesis (RCM) reaction. Macrocyclization, via RCM, is preceded by Yamaguchi coupling between the C1 carboxylic acid of the northern subunit 2 and the C23 carbinol center in the southern subunit 3. Central to this approach are the 1,3 anti-diol motifs at C7/C9 and C19/C21, which can be assembled and elaborated from bicyclic phosphates (R,R,RP)-5 and (S,S,SP)-5, respectively, utilizing a phosphate tether-mediate approach.
Scheme 1. Retrosynthesis of Dolabelide C.
Results and Discussion
II. Construction of P-Chiral, Nonracemic Bicyclo[4.3.1]phosphates (R,R,RP)-5 and (S,S,SP)-5
The enantiomeric phosphate triester building blocks (R,R,RP)-5 and (S,S,SP)-5 (Scheme 2) were assembled via a phosphate tether, RCM desymmetrization approach5 inspired by Burke and coworkers.6 In this method, a phosphate tether effectively serves to mediate the tripodal coupling of anti-diol 87 with an allylic alcohol component via either a phosphoryl monochloride or through a one-step coupling/oxidation sequence from commercially available allyl tetraisopropylphosphorodiamidite to yield pseudo-C2-symmetric triene 9. Desymmetrization8 by ring-closing metathesis (RCM) using Grubbs catalyst [(IMesH2)(PCy3)(Cl)2Ru=CHPh (cat-B)]9,10,11 affords P-chiral bicyclo[4.3.1]phosphate (S,S,SP)-5 and is based on the premise that only the terminal olefin cis to the phosphate-tethered olefin reacts to generate 5 possessing two sterically differentiated olefins.
Scheme 2. Tether-Mediated Desymmetrization of C2-Symmetric 1,3-anti-Diol 8a.
aReagents and Conditions: (a) Allyl tetraisopropylphosphorodiamidite, 1-H-tetrazole, MeCN, 2 h, rt, then m-CBPA, 1 h, 64%; (b) cat-B, CH2Cl2, 85-90%.
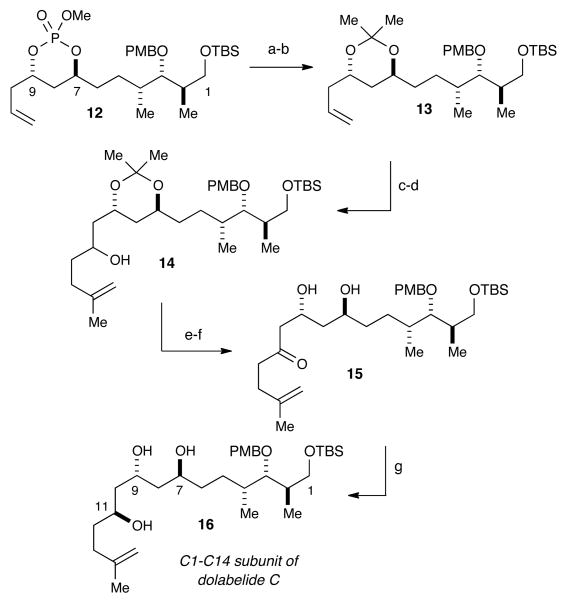
III. Construction of C1–C14 Subunit
We embarked upon the synthesis of the C1–C14 subunit of dolabelide beginning with an elaborate cross methesis (CM) between bicyclic phosphate (R,R,Rp)-5 possessing a Type III exocyclic terminal olefin and Type I olefin 10.12 As shown previously,13 CM of (R,R,Rp)-5 is high yielding with both Type I and Type II olefins in the presence of the Hoveyda-Grubbs catalyst (cat-C).14 Various conditions for the desired CM of 10 and (R,R,Rp)-5 were probed and it was found that employing 6 mol% Hoveyda-Grubbs catalyst at 90 °C in DCE gave the CM adduct 4 in 72% yield (Scheme 3). This notable CM event between two complex olefins assembles in a single operation five of the six stereocenters contained within the C1–C14 subunit of dolabelide C. Moreover, excess amounts of 10, a Type II CM partner, could be recovered in near quantitative yield and recycled in future CM events.
Scheme 3. Phosphate-Mediated Sequence for Assembly of the C1–C14 Subunita.
aReagents and Conditions: (a) cat-C (6 mol %), DCE, 90 °C, 72%; (b) o-NO2C6H5SO2NHNH2, Et3N, CH2Cl2, 72%; (c) Pd(OAc)2 (5 mol %), HCO2H, Et3N, DCE, 40 °C, then MeOH, TMSCHN2, 87%. Abbreviations: DCE = dichloroethane.
Two related regioselective processes were next investigated. The first involved a regioselective removal of the exocyclic C5–C6 olefin in the CM adduct 4 in the presence of the C10–C11 internal olefin, which sets the stage for subsequent regioselective hydride opening of the bicyclic system. Upon probing several hydrogenation conditions, (Wilkinson's catalyst, Crabtree's catalyst, Pd/C) it was found that a mild diimide reduction, generated in situ from o-nitrobenzenesulfonyl-hydrazine,15 provided the necessary hydrogenated phosphate moiety 11 with near complete regioselectivity for the exocyclic olefin. In comparison, other diimide conditions (tosylhydrazine, NaOAc, H2O, DCE, 90 °C) gave drastically lower yields, likely due to bicyclic phosphate instability under basic medium.16
Having achieved the regioselectively-hydrogenated product 11, a regioselective opening with hydride was probed as an additional method to unmask the phosphate tether. Initial studies focused on allylic copper hydride addition using various reagents (Stryker's reagent, CuCN•2LiCl/PhSiH3, CeCl3•7H2O/NaBH4) (Scheme 3). Unfortunately, all conditions probed provided only unreacted starting material or total decomposition of the reaction mixture. Pd-catalyzed formate reductions were next investigated for generation of the requisite terminal olefin.17 Employment of 1.5 equivalents of formic acid and 5 mol % Pd(OAc)2 at 40 °C in DCE selectively opened phosphate 11 to provide the desired terminal olefin in 12. Methylation of the phosphate acid intermediate showed that a highly regioselective process was operative (>20:1 ratio of regioisomers as evident by 31P NMR analysis). Purification provided phosphate 12 in 87% yield. The remarkable regioselectivity reveals another feature of the phosphate tether, whereby orthogonal orbital alignment within 11 allows for selective Pd(0)-catalyzed ionization of C12 over the C9 allylic phosphate position. This ionization allows Pd to deliver the hydride selectively at the internal C10 position to provide the desired terminal olefin. Addition of the hydride at the terminal C12 position would afford an allylic phosphate anion that is capable of additional ionization events with the C9 phosphate. Ultimately, the success of this reaction results in a net olefin transposition expediting the route to the C1–C14 subunit, as well as showcasing an additional facet of the phosphate-mediated methodology.
Upon completion of the synthesis of phosphate 12, work began toward the installation of the C11–C14 fragment (Scheme 4). Cleavage of the phosphate was achieved using LiAlH4, which generated a diol that was subsequently protected (PPTS, 2,2-dimethoxypropane, CH2Cl2) to yield acetonide 13. Subsequent ozonolysis (O3, pyridine, CH2Cl2:MeOH 1:1, Me2S) of the terminal olefin produced the requisite aldehyde, which was subjected to the corresponding Grignard18 derived from 1-iodo-3-methyl-3-butene affording 14 in a 95% yield. Dess-Martin periodinane (DMP, NaHCO3, CH2Cl2) oxidation of the free alcohol in 14 produced the corresponding ketone in 90% yield. Attempts to selectively reduce the acetonide-protected ketone, using an assortment of reducing agents, failed to give any diastereoselectivity at C11. This problem was circumvented by deprotection of the acetonide and subsequent syn reduction utilizing the C9 alcohol. Selective removal of the acetonide was achieved by the addition of CeCl3•7H2O and water,19 which efficiently (86% yield) cleaved the acetonide-protecting group without loss of the primary TBS group to provide diol 15. Final reduction of ketone 15 using Evan's conditions (Et2BOMe, NaBH4)20 afforded triol 16 in 60% (95% brsm) with excellent diastereoselectivity (ds ≥ 20:1).
Scheme 4. Synthesis of C1–C14 Carbon Frameworka.
aReagents and conditions: (a) LiAlH4, Et2O, 75%; (b) PPTS, 2,2-DMP, CH2Cl2, 96%; (c) O3, pyridine, 1:1 MeOH:CH2Cl2, -78 °C, then Me2S, 72%; (d) 1-Iodo-3-methyl-butene, Mg, Et2O, -78 °C, 95%; (e) Dess-Martin periodinane, NaHCO3, CH2Cl2, 90%; (f) CeCl3•7H2O, H2O/MeCN (1:7), 87%; (g) Et2BOMe, NaBH4, THF:MeOH 4:1, -78 °C, ds ≥ 20:1, 60% (95% brsm).
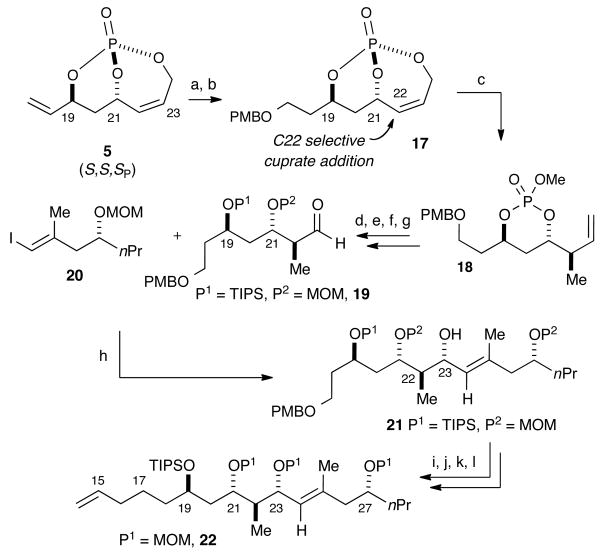
IV. First Generation Synthesis of the C15–C30 Subunit of Dolabelide C
The construction of the C15–C30 subunit of dolabelide began with the enantiomeric bicyclic phosphate (S,S,SP)-5 possessing unique orbital symmetry of the bicyclic phosphate (Scheme 5).21 Initial studies probed the possibility of an oxidation of the exocyclic olefin of 5 in the presence of the cyclic olefin. After testing various conditions, a chemoselective hydroboration of (S,S,SP)-5 was achieved using 9-BBN followed by mild NaBO3 oxidation to yield the primary alcohol. Due to the aforementioned instability of (S,S,SP)-5 to basic hydrolysis, a mild perborate oxidation protocol developed by Burke and coworkers was implemented. Burke has shown this protocol to be compatible with multiple acetate protecting groups;22 optimization of this hydroboration reaction with phosphate 5 found the reaction to be highly dependent on the amount of oxidant, equivalents of H2O, and reaction time. Subsequent PMB ether formation using p-methoxybenzyl trichloroacetimidate produced 17 in good yields demonstrating the acid stability of bicyclic phosphate (S,S,SP)-5. Employing the previously reported regio- and diastereoselective cuprate addition protocol, displacement of 17 (CuCN•2LiCl, ZnMe2, THF, -30 °C to rt) afforded the SN2′ displaced phosphate acid exclusively (ds ≥ 20:1), which upon methylation (TMSCHN2 and MeOH) produced cyclic phosphate ester 18 in excellent overall yield (87%). This reaction again highlights the remarkable orbital alignment of the bicyclic phosphate system and its concave nature, where only one of four possible products for this SN2′ cuprate reaction is generated. Reductive cleavage, followed by sequential protection of the diol systems with TIPS and MOM groups, and final ozonolysis of the olefin afforded aldehyde 19 in good yield.
Scheme 5. First Generation Synthesis of the C15–C30 Subunita.
aReagents and conditions: (a) 9-BBN, then H2O, NaBO3•4H2O, 80%; (b) p-OMeC6H4OCH2OC=NH(CCl3), PPTS, CH2Cl2, 89%; (c) 1. CuCN•2LiCl, Me2Zn, THF, -30 °C to rt; 2. TMSCHN2, MeOH, 87%; (d) LiAlH4, Et2O, 0 °C, 96%; (e) TIPSCl, imidazole, DMAP, CH2Cl2, 86%; (f) MOMCl, iPr2NEt, CH2Cl2, 91%; (g) O3, pyridine, -78 °C, Me2S, 75%; (h) t-BuLi, ZnBr2, 20, then n-BuLi, (R,S,)-NME, then 19, 65%, 11:1 dr; (i) MOMCl, iPr2NEt, DCE, 82%; (j) DDQ, pH 7 buffer, CH2Cl2, 92%; (k) TsCl, DABCO, CH2Cl2, 90%; (l) allylMgBr, CuI, -20 °C to 0 °C, 89%. Abbreviations: 9-BBN = 9-borabicyclo(3.3.1)nonane; TMS = trimethylsilyl; TIP = triisopropylsilyl; MOM = methoxymethyl; DMAP = 4-(dimethylamino)pyridine; NME = N-methylephedrine; DDQ = 2,3-dichloro-5,6-dicyanobenzoquinone; Ts = tosyl; DABCO = 1,4-diazabicyclo(2.2.2)octane.
With aldehyde 19 and vinyl iodide 20 in hand, studies aimed at a diastereoselective addition to aldehyde 19 to set the C23 stereogenic carbinol center began (Scheme 5). Initial efforts to generate the C23 stereocenter by lithiate additions gave predominately the undesired 1,2-Felkin product. To overcome this problem, investigation focused on reagent-controlled, ephedrine-based asymmetric vinylzincate additions, described by Marshall.23 Aldehyde 19 reacted with 20 under these conditions to obtain the desired 1,3-syn isomer 21 in an 11:1 ratio of diastereomers, in 65% yield. Successful formation of 21 provided the advance intermediate bearing the requisite stereochemistry of the C15-C30 subunit. With 21 in place, only the installation of the C14–C15 terminal olefin was needed to complete the C15–C30 subunit of dolabelide C. MOM-protection of the C23 alcohol, DDQ removal of the PMB ether, tosylation, and cuprate displacement all proceeded in good yield to afford the terminal olefin 22 and complete the C15–C30 subunit of dolabelide C. Overall, a 12-step sequence to 22 from 5 was achieved, bearing the requisite stereochemistry for the C15–C30 subunit of dolabelide C.
V. Second Generation Synthesis of the C15–C30 Subunit of Dolabelide C
A second-generation synthesis was next developed when attempts to remove the three MOM-protecting groups from 22 proved problematic. To our dismay, all conditions tested for cleavage of these groups in the presence of the more labile TIPS-protecting groups provided unreacted starting material or total decomposition of the substrate (Scheme 6). The difficulty in removing these protecting groups prompted a reevaluation of protecting groups to access a suitable C15–C30 subunit of dolabelide. This alternative strategy coincided with a planned installation of the C23 carbinol at the last step of the sequence to streamline the route.
Scheme 6. MOM-deprotection Conditions.
The alternative approach to the C15–C30 subunit began by employing previously established CM/reduction methodology (Scheme 7).12a As anticipated, 5 underwent CM with 23 in the presence of 6 mol % cat-C14 (DCE, 90 °C) providing E-configured (>20:1) product in 82% yield. Selective reduction of the external olefin was again achieved using o-nitrobenzenesulfonyl-hydrazine15 furnishing 24 in 75% yield.24 Compound 24 was also synthesized through a one-pot, sequential cross-metathesis/olefin reduction protocol in 59% overall yield utilizing the same reagents shown in Scheme 6 This yield averages to 77% per synthetic step over the 2-step combined transformation. Regio- and diastereoselective methyl cuprate addition into 24 and subsequent phosphate cleavage produced diol 25 in good yield.21 Diol 25 was protected as the acetonide (PPTS, 2,2-dimethoxypropane) in 96% yield.
Scheme 7. Second Generation Synthesis of C15–C30 Subunita.
aReagents and conditions: (a) cat-C (6 mol %), 23, DCE, 90 °C, 82%; (b) o-NO2C6H5SO2NHNH2, Et3N, CH2Cl2, 75%; (c) 1. CuCN•2LiCl, Me2Zn, THF, -30 °C to rt; 2. TMSCHN2, MeOH, 91%; (d) LiAlH4, Et2O, 0 °C, 92%; (e) 2,2-DMP, PPTS, CH2Cl2, 96%; (f) OsO4, NMO, t-BuOH/THF/ H2O, then NaIO4, Phosphate Buffer pH 7, then NaBH4, EtOH, 0 °C, 81%; (g) TBSCl, pyridine, 95%; (h) H2, Pd/C, EtOAc, NaHCO3, 90%; (i) Ph3P, I2, imidazole, then t-BuOK, THF, 94%; (j) TBAF, THF, 98%; (k) (COCl)2, DMSO, Et3N, CH2Cl2, -78 °C to rt; (l) t-BuLi, Et2O, 29, -78 °C to 0 °C, 30 min, 28, -78 °C, ∼1:1 syn:anti, 79% over 2 steps; (m) Dess-Martin, CH2Cl2, 85% n) NaBH4, MeOH, 0 °C, 89%, ∼2.7:1 syn:anti. Abbreviations: DMP = dimethoxypropane; PPTS = pyridinium p-toluenesulfonate; NMO = N-methylmorpholine N-oxide; TBS = tert-butyldimethylsilyl; TBAF = tetra-n-butylammonium fluoride.
The terminal olefin was next converted into primary alcohol 26 by an oxidative cleavage/reduction sequence in good yields. TBS-protection of alcohol 26 proceeded in 86% yield and was followed by removal of the PMB-ether to provide the corresponding primary alcohol. Conversion of the alcohol to a terminal olefin through an iodination/elimination sequence occurred in excellent yield over the two-step sequence. Achievement of the C14/C15 olefin left only a deprotection/oxidation/nucleophilic addition sequence to obtain the necessary C15–C30 subunit. TBAF removal of the TBS-protecting group to 27 and Swern oxidation provided aldehyde 28 necessary for the addition of the C24–C30 fragment.
Despite previous success with the Marshall asymmetric zincate addition protocol,21 difficulties in reaction reproducibility and low product yields, also recently noted by Marshall,23 prompted investigation of an alternative addition sequence. Thus, vinyl iodide 29 was converted to the lithiate with 2 equivalents of tBuLi followed by the addition of aldehyde 28 to afford a 1:1 mixture of C23 epimers of alcohol 30 in 79% yield. The two diastereoisomers of 30 were easily separated by column chromatography, allowing for isolation of the correct stereoisomer of alcohol 30 as well as facile recycling (oxidation/reduction) of the undesired diastereomer (dr = 2.7:1).25 Overall, this alternative 13-step route to 30 from phosphate (S,S,SP)-5 provided a C15–C30 fragment ready to couple with the C1–C14 subunit of dolabelide C.
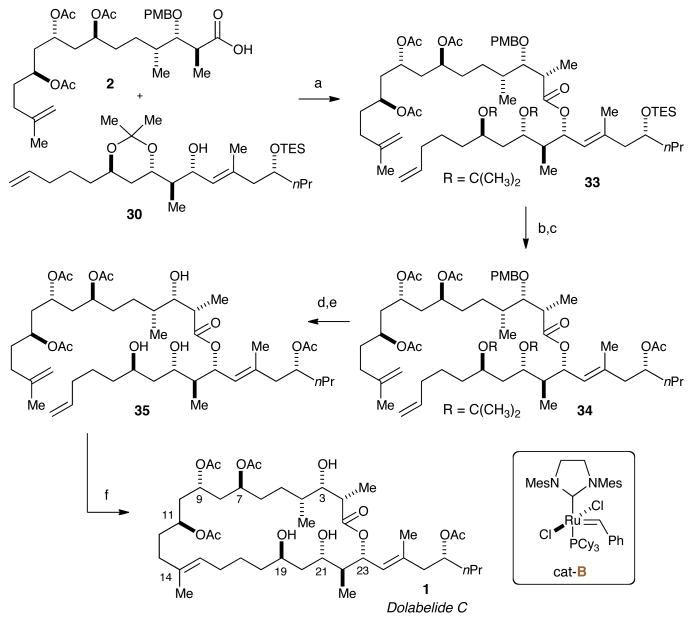
VI. Completion of the Total Synthesis
Studies toward the completion of dolabelide C next commenced with the complete acetylation of triol 16 to install the proper acetylation pattern for C1–C14 subunit of dolabelide C (Scheme 8). This was accomplished by adding acetic anhydride and pyridine to triol 16 to afford triacetate 31 in excellent yield. Deprotection of the TBS protecting group provided alcohol 32 in 93% yield. Swern oxidation of 32 generated the desired aldehyde that was prone to epimerization and was taken on without purification. Pinnick oxidation of the aldehyde provided carboxylic acid 2 in 81% yield over the two-step sequence, which was ready for coupling with the C15–C30 subunit.
Scheme 8. Generation of ready-to-couple C1–C14 Subunita.
aReagents and conditions: (a) Ac2O, DMAP, pyridine, 95%; (b) TBAF, THF, 93%; (c) (COCl)2, DMSO, Et3N, CH2Cl2; (d) NaClO2, 2-methyl-2-butene, H3PO4, 81% over 2 steps.
Final coupling of the C1–C14 carboxylic acid 2 and the C15–C30 alcohol 30 was achieved using Yamaguchi conditions,26 as previously described by Leighton and coworkers (Scheme 9).4 The addition of 2,4,6-trichlorobenzoyl chloride, Et3N, and DMAP at -78 °C for 21 hours avoided epimerization at C2 and yielded the desired coupled 33 in 77% yield. Deprotection of the C27-TES protecting group was achieved with TBAF in 94% yield. Subsequent acylation provided 34 in 98% yield. The final two protecting groups were removed using PPTS in MeOH, followed by treatment with DDQ to provide metathesis precursor 35 in excellent yield over two steps. Efforts to close the ring were attempted prior to PMB ether removal and provided the desired RCM product as observed by HRMS, albeit in poor overall conversion. As a result, subsequent investigations focused on RCM of the deprotected triol 35. Portion-wise addition of 20 mol % of (IMesH2)(PCy3)(Cl)2Ru=CHPh (cat-B)11 to triol 35 afforded approximately a 1:1 E/Z mixture of dolabelide C 1 and its (Z)-isomer in a 57-60% yield.
Scheme 9. Completion of Dolabelide C (1)a.
aReagents and conditions: (a) 2,4,6-trichlorobenzoyl chloride, DMAP, Et3N, toluene, 77%; (b) TBAF, 94%; (c) Ac2O, pyridine, DMAP, 98%; (d) PPTS, 83%; (e) DDQ, Phosphate Buffer pH = 7, CH2Cl2, 95%; (f) cat-B (20 mol %), CH2Cl2 (0.5 mM), 57%, E:Z = ∼1:1.
VII. Purification Attempts and RCM/Isomerization Side Reaction
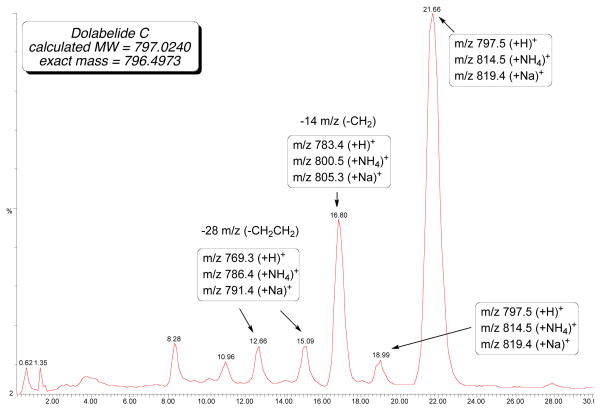
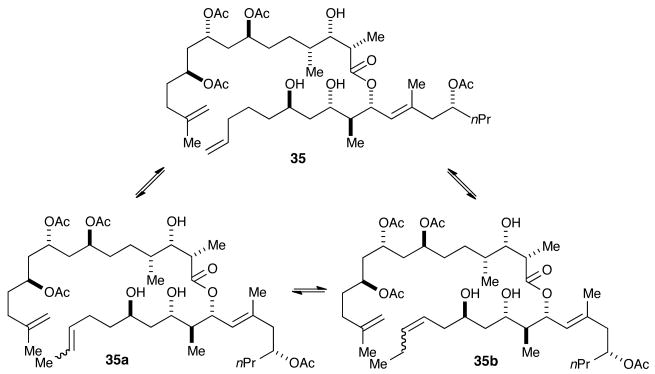
Initial attempts utilizing repeated standard normal-phase chromatography removed the Z-isomer (higher Rf), leaving what was believed to be pure dolabelide C (1) as a single spot on TLC. 1H NMR analysis, however, revealed impurities, which were originally presumed to be the Z-isomer. Attempted purification using preparative reverse-phase LC-MS revealed the major by-product, as seen by 1H NMR to be a demethylenated analog (M-14), along with trace amounts of a constitutional isomer and two by-products resulting from formal loss of an ethylene (M-28) as seen in the total-ion chromatogram (Figure 2). These by-products (35a and 35b) are presumed to occur from isomerization of 35 followed by RCM, resulting in the smaller macrocycles (Scheme 10).27 Numerous reports are consistent with this observation28 and to the best of our knowledge; this is the first report of a detailed LC-MS analysis of the aforementioned side reaction.
Figure 2.
LC-MS analysis of mixture from final RCM.
Scheme 10. Possible Isomerization Pathways from Metathesis Step.
In an attempt to understand and optimize the final RCM sequence, the final metathesis precursor was scaled-up. Both syntheses to each subunit proved to be scalable, providing 300 mg of 30 and 175 mg of 32.29 This material was carried through the same endgame steps (Scheme 9) affording 160 mg of RCM precursor 35 to apply towards the goal of optimizing the C14/15 E:Z ratio and minimizing the amount of deleterious side reactions occurring in the final RCM step. For this study metathesis catalyst cat-B and three other candidates shown in Figure 3 were screened.30
Figure 3.
Metathesis Catalysts Screened on Final RCM Step.
Initially, the conditions shown from Scheme 9 were reproduced (Table 1, entry 1), where LC-MS analysis showed nearly complete conversion and a similar ratio to what was previously observed. Screening of other catalysts by varying the phosphine or NHC-ligand showed a significant increase of by-product formation (entries 3-5). While no significant changes in E:Z ratio were obtained from catalyst screening, rigorous degassing and purification of the solvent were shown to reduce deleterious side products.31
Table 1.
Catalyst Screening of Final RCM.
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Conversion | E:Za | E:Z: By-Pdtsa |
| 1 | cat-B | >99% | 1:1 | 1:1:0.17 |
| 2 | cat-Bb | 87% | 1.2:1 | 1.2:1:0.26 |
| 3 | cat-D | 100% | 1:1 | 1:1:0.80 |
| 4 | cat-E | 100% | 1:1.1 | 1:1.1:0.45 |
| 5 | cat-F | 87% | 1.2:1 | 1.2:1:0.61 |
Ratios determined through peak area integration from LC-MS analysis of crude mixtures.
Purified newly purchased catalyst through SiO2 plug in 10:1 hexanes:EtOAc.
All reactions were run with stepwise addition of 20 mol % catalyst over 6 h at 40 °C in 0.5 μM CH2Cl2.
Due to the scalability of this synthesis,32 ample material was provided for characterization, where all NMR spectra matched with the previous reported data.2,33 In addition, sufficient quantities of the unnatural C14/15 Z-isomer34 were generated for both NMR analysis and collection of biological data to determine its bioactivity and potential potency against cervical cancer.35
Conclusion
In conclusion, dolabelide C (1) and its non-natural C14–C15 Z-diastereomer were produced and isolated from a scalable phosphate-mediated synthesis. A complex mixture was generated in the final RCM step resulting in by-products, which arose from a net loss of CH2 and C2H4, that proved to be difficult to separate via repeated flash chromatography (8:1 CH2Cl2:acetone). Since the material produced at the end of the first synthesis of 1 was sparse, a re-synthesis provided 175 mg of the C1–C14 subunit (32), 300 mg of the C15–C30 subunit (30) and 160 mg of RCM precursor 35. This allowed a detailed optimization study through a screening of various metathesis catalysts, concluding with the originally developed conditions (20 mol % cat-B, 0.5 μm, 40 °C) provided the optimum results. Overall, 14 mg (21% yield) of pure dolabelide C and 10 mg (15% yield) of the pure Z-isomer were produced in a 24-step longest linear sequence (LLS) from commercially available material utilizing the (R,R,RP) antipode of 5 (sequence streamlined to a 22-step LLS using one-pot, sequential protocols).
Experimental Section
General Methods
All reactions were carried out in oven- or flame-dried glassware, under an argon atmosphere, using standard gastight syringes, cannulae, and septa. Stirring was achieved with oven-dried magnetic stir bars. Et2O, THF, and CH2Cl2 were passed through a purification system employing activated Al2O3. Et3N was eluted through basic alumina and stored over KOH. Butyl lithium was titrated prior to use. All olefin metathesis catalysts were obtained commercially and used without further purification. All 1H and 13C NMR spectra were recorded in CDCl3 or C5D5N at 400 MHz or 500 MHz and 126 MHz, respectively, and calibrated to the solvent peak. All mass spectra were obtained using electrospray ionization (ESI) (MeOH) coupled to high-resolution mass spectrometry (HRMS). Observed rotations at 589 nm were measured using an automatic polarimeter. Infrared spectra were obtained using a Fourier transform infrared (FTIR) spectrometer.
(4S,6R)-4-((R)-But-3-en-2-yl)-6-(5-(4-methoxybenzyloxy)pentyl)-2,2-dimethyl-1,3-dioxane (SI-1)
Diol 25 (2.00 g, 5.95 mmol) was dissolved in CH2Cl2 (8 mL) at rt. 2,2-Dimethoxypropane (8 mL) and PPTS (150 mg, 0.595 mmol) were added respectively and the clear solution was stirred until completion. The reaction was quenched with saturated NaHCO3 (15 mL) and diluted with CH2Cl2. The aqueous layer was extracted with CH2Cl2 (3 × 25 mL) and the combined organic layers were washed once with brine (30 mL), dried (Na2SO4) and concentrated under reduced pressure. Compound SI-1 was isolated using flash chromatography (19:1 hexanes/EtOAc) as a clear oil (2.18 g, 98%); [α]D = -36.3 (c = 0.40, CH2Cl2); FTIR (neat) 2983, 2935, 2856, 1612, 1512, 819 cm -1; 1H NMR (500 MHz, CDCl3) δ 7.25 (d, J = 8.4 Hz, 2H), 6.87 (d, J = 8.6 Hz, 2H), 5.83 (ddd, J =17.4, 10.5 and 7.3 Hz, 1H), 5.03 (ddd, J = 17.5, 11.0 and 2.6 Hz, 2H), 4.43 (s, 2H), 3.80 (s, 3H), 3.74–3.66 (m, 1H), 3.63 (ddd, J = 9.7, 6.3 and 6.3 Hz, 1H), 3.45 (t, J = 6.6 Hz, 2H), 2.18–2.26 (m, 1H), 1.71–1.64 (m, 1H), 1.63–1.53 (m, 2H), 1.55–1.35 (m, 6H), 1.32 (s, 6H), 1.30–1.24 (m, 1H), 0.98 (d, J = 6.9 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 159.1, 140.9, 130.7, 129.3, 114.4, 113.7, 100.3, 72.5, 70.1, 70.0, 66.8, 55.3, 42.1, 36.3, 35.9, 29.7, 26.2, 25.3, 24.7, 24.4, 15.3; HRMS calcd for C23H36NaO4 (M+Na)+ 399.2511; found 399.2498 (ESI).
(R)-2-((4S,6R)-6-(5-(4-Methoxybenzyloxy)pentyl)-2,2-dimethyl-1,3-dioxan-4-yl)propan-1-ol (26)
Olefin SI-1 (1.50 g, 3.99 mmol) was dissolved in t-BuOH:THF:H2O (10:2:1, 20 mL) at rt. N-Methyl morpholine N-oxide (933 mg, 7.98 mmol) and OsO4 (0.19 mL, 0.08 mmol, 4% aq) were added and the reaction was stirred for approximately 12 h until olefin was completely consumed. The mixture was then diluted with phosphate buffer pH 7 (twice the volume of t-BuOH) and NaIO4 was added (3.41 mg, 16.0 mmol). The reaction was stirred vigorously for approximately 2 h until the diol was completely consumed (monitored by TLC). The reaction was quenched with solid Na2SO3 (2.0 g) and acetone was removed under reduced pressure. The residue was partitioned with EtOAc (20 mL) and H2O (10 mL) and the aqueous layer was extracted with EtOAc (3 × 20 mL). The collected organics were washed once with brine (20 mL), dried (Na2SO4) and filtered. After concentrating under reduced pressure, the crude product was purified using flash chromatography (5:1 hexanes/EtOAc) to generate intermediate aldehyde as a yellow oil.
The resultant aldehyde was dissolved in EtOH (16 mL) and cooled to 0 °C. NaBH4 (303 mg, 7.98 mmol) was added and the reaction was slowly brought back to rt. Upon completion (∼45 min), the solution was partitioned with 2:1, Et2O:H2O (40 mL), the aqueous layer was extracted with Et2O (3 × 5 mL) and the organic layers were combined, washed with brine (30 mL), dried (MgSO4), filtered and concentrated under reduced pressure. Purification with flash chromatography (1:2 hexanes/EtOAc) afforded 26 (88% over two steps, 1.35 mg) as a clear oil; [α]D = - 0.26 (c = 0.18, CH2Cl2); FTIR (neat) 3442, 2933, 2856, 1612, 1512, 819 cm -1; 1H NMR (500 MHz, CDCl3) δ 7.25 (d, J = 8.5 Hz, 2H), 6.87 (d, J = 8.6 Hz, 2H), 4.43 (s, 2H), 3.81 (s, 3H), 3.79–3.73 (m, 1H), 3.68 (ddd, J = 9.2, 6.3 and 6.3 Hz, 1H), 3.58 (d, J = 5.1 Hz, 2H), 3.43 (t, J = 6.6 Hz, 2H), 3.08 (s, 1H), 1.20–1.80 (m, 11H), 1.38 (s, 3H), 1.33 (s, 3H), 0.82 (d, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 159.1, 130.8, 129.2, 113.7, 100.5, 73.1, 72.5, 70.1, 68.3, 66.6, 55.3, 40.6, 37.9, 35.8, 29.7, 26.2, 25.2, 24.6, 24.6, 12.7; HRMS calcd for C22H36NaO5 (M+Na)+ 403.2460; found 403.2413 (ESI).
tert-Butyl((R)-2-((4S,6R)-6-(5-(4-methoxybenzyloxy)pentyl)-2,2-dimethyl-1,3-dioxan-4-yl)propoxy)dimethylsilane (SI-2)
Alcohol 26 (1.34 mg, 3.53 mmol) was dissolved in CH2Cl2 (23 mL) at rt. Imidazole (720 mg, 10.6 mmol), DMAP (10 mg, 0.08 mmol) and TBSCl (800 mg, 5.29 mmol) were added, respectively. The reaction was quenched upon completion (∼90 min, monitored by TLC) with saturated NH4Cl (25 mL) and diluted with Et2O (50 mL). The aqueous layer was extracted with Et2O (3 × 25 mL) and the organic layers were washed with brine (25 mL). The combined organic layers were dried (Na2SO4) and concentrated under reduced pressure. Purification with flash chromatography (20:1 hexanes/EtOAc) afforded SI-2 (1.70 g, 97%) as a yellow oil; [α]D = -16.6 (c = 0.35, CH2Cl2); FTIR (neat) 2933, 2856, 2881, 1247, 835 cm -1; 1H NMR (500 MHz, CDCl3) δ 7.26 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 8.6 Hz, 2H), 4.24 (s, 2H), 3.80 (s, 3H), 3.74–3.66 (m, 2H), 3.56 (d, J = 4.4 Hz, 2H), 3.44 (t, J = 6.6 Hz, 2H), 1.37–1.67 (m, 11H), 1.31 (s, 6H), 0.89 (s, 9H), 0.85 (d, J = 6.8 Hz, 3H), 0.02 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 159.0, 130.7, 129.2, 113.7, 100.1, 72.5, 70.1, 67.0, 66.8, 64.1, 55.2, 40.5, 36.6, 35.9, 29.7, 26.1, 25.9, 25.3, 24.6, 24.5, 18.2, 12.2, -5.5, -5.5; HRMS calcd for C28H50NaO5Si (M+Na)+ 517.3325; found 517.3334 (ESI).
5-((4R,6S)-6-((R)-1-(tert-Butyldimethylsilyloxy)propan-2-yl)-2,2-dimethyl-1,3-dioxan-4-yl)pentan-1-ol (SI-3)
PMB ether SI-2 (1.65 g, 3.34 mmol) was dissolved in EtOAc (16 mL) at rt. A catalytic amount of 10% Pd/C (50 mg) and NaHCO3 (280 mg, 3.34 mmol) were added sequentially and the flask was pressurized with a H2 balloon. After 10 h the mixture was filtered through pad of Celite® and rinsed thoroughly with EtOAc. The filtrate was concentrated under reduced pressure and purified with flash chromatography (10:1 hexanes/EtOAc) to yield alcohol SI-3 (1.12 g, 90% yield) as a clear oil; [α]D = -0.11 (c = 0.40, CH2Cl2); FTIR (neat) 3357, 2933, 2858, 1379, 1251, 1224, 835, 775 cm-1; 1H NMR (500 MHz, CDCl3)36a δ 3.75–3.68 (m, 2H), 3.63 (t, J = 6.6 Hz, 2H), 3.55 (dd, J = 4.7 and 1.3 Hz, 2H), 1.70–1.38 (m, 10H), 1.33 (s, 6H), 1.28–1.22 (m, 1H), 0.89 (s, 9H), 0.84 (d, J = 6.9 Hz, 3H), 0.04 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 100.2, 67.1, 66.8, 64.1, 63.0, 40.5, 36.6, 35.9, 32.7, 25.9 (3), 25.7, 25.3, 24.6, 24.5, 18.2, 12.2, -5.5, -5.5; HRMS calcd for C20H42NaO4Si (M+Na)+ 397.2750, found 397.2773 (ESI).
(R)-2-((4S,6R)-2,2-Dimethyl-6-(pent-4-enyl)-1,3-dioxan-4-yl)propan-1-ol (27)
Alcohol SI-3 (1.12 g, 2.99 mmol) was dissolved in THF (30 mL) at rt. Triphenylphosphine (941 mg, 3.59 mmol) and imidazole (477 mg, 6.59 mmol) were added, respectively, and the solution was cooled to 0 °C. I2 (912 mg, 3.59 mmol) was added and the reaction was stirred for approximately 30 min (monitored by TLC). The solution was diluted with hexane and filtered through a pad of silica, while washing with hexane, and concentrated under reduced pressure. The crude product was taken onto the next step.
The iodo compound was dissolved in THF (35 mL) at rt followed by stepwise addition of t-BuOK (1.0 g, 8.98 mmol). The reaction was stirred for ∼30 min. and was quenched with H2O. The aqueous layer was extracted with EtOAc (3 × 20 mL portions) and the organic layers were combined, washed with brine (20 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Purification with flash chromatography (20:1 hexanes/EtOAc) yielded the resultant terminal olefin (1.0 g, 94%) as a clear oil.
The resultant silyl ether (1.00 g, 2.81 mmol) was dissolved in THF (10 mL) and cooled to 0 °C. A solution of TBAF in THF (8.5 mL, 1.0 M in THF) was added dropwise. The reaction was stirred at 0 °C until completion (∼45 min), quenched with saturated NH4Cl (10 mL) and the aqueous layer was extracted with Et2O (3 × 20 mL). The organic layers were combined, washed with brine (20 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Purification using flash chromatography (10:1 hexanes/EtOAc) afforded 27 (670 mg, 98%) as a clear oil; [α]D = -78.6 (c = 0.50, CH2Cl2); FTIR (neat) 3446, 2983, 2935, 2879, 1379, 1224, 908 cm-1; 1H NMR (500 MHz, CDCl3) δ 5.86–5.75 (m, 1H), 5.07–4.93 (m, 2H), 3.82–3.76 (m, 1H), 3.69 (ddd, J = 9.2, 9.2 and 6.2 Hz, 1H), 3.61–3.55 (m, 2H), 3.08 (s, 1H), 2.06 (dd, J = 14.1 and 7.1 Hz, 2H), 1.80–1.40 (m, 7H), 1.38 (s, 3H), 1.33 (s, 3H), 0.81 (d, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 138.7, 114.7, 100.5, 73.0, 68.2, 66.6, 40.6, 37.8, 35.3, 33.6, 24.7, 24.6, 24.6, 12.6; HRMS calcd for C14H27O3 (M+H)+ 243.1960, found 243.2895 (ESI).
(2R,3R,7R,E)-2-((4S,6R)-2,2-Dimethyl-6-(pent-4-enyl)-1,3-dioxan-4-yl)-5-methyl-7-(triethylsilyloxy)dec-4-en-3-ol (30)
A solution of oxalyl chloride (0.158 mL, 1.86 mmol) in CH2Cl2 (4.8 mL) was cooled to -78 °C and DMSO (0.220 mL, 3.01 mmol) was added slowly by syringe (gas evolution). After stirring for 10 min, a solution of alcohol 27 (300 mg, 1.24 mmol) in CH2Cl2 (3.0 mL) was added by cannula and rinsed with CH2Cl2 (2 × 0.5 mL). The cloudy mixture was stirred at -78 °C for 15 min at which time Et3N (0.700 mL, 4.96 mmol) was added dropwise. The reaction mixture was stirred for 1 h at -78 °C, quenched cold with saturated NaHCO3 (5 mL) and allowed to warm to rt. After diluting with CH2Cl2, the layers were separated and the aqueous layer was re-extracted with CH2Cl2 (3 × 12 mL). The organic layer was dried (Na2SO4), filtered through a silica plug and rinsed (3 × 25 mL) with a EtOAc/CH2Cl2(1:1). The filtrate was concentrated under reduced pressure to give aldehyde 28 as a yellow oil. The crude aldehyde was taken immediately to the next reaction without further purification.
To a solution of the vinyl iodide 29 (1.01 mg, 2.75 mmol) in Et2O (10 mL) at −78 °C was added t-BuLi (1.7 M in pentane, 3.40 mL, 5.75 mmol), and the reaction was immediately warmed to 0 °C for 25 min. The reaction was recooled to -78 °C, and the aldehyde 28 was slowly added via syringe in Et2O (2.5 mL, 0.60 mL rinse). After 1 h, the reaction was quenched at -78 °C with saturated NH4Cl, warmed to rt, and the layers were separated. The aqueous layer was extracted with Et2O (2 × 10 mL), and the combined organic layers washed with brine (15 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Flash chromatography (10:1 hexanes/EtOAc) afforded a 1:1 mixture of 1,3-syn and 1,3-anti 30 (ratio determined by 1H NMR analysis of crude reaction mixture, 464 mg, combined yield of diastereomers 77% over two steps).
Oxidation/Reduction Sequence
The 1,3-anti diastereomer (65 mg, 0.135 mmol) of 30 was dissolved in CH2Cl2 (2.6 mL) at rt. Dess-Martin periodinane (115 mg, 0.270 mmol) was added to the stirring solution, where upon completion (monitored by TLC), the reaction was diluted with Et2O (5 mL). The organic layer was washed with saturated NaHCO3 (2 × 5 mL) and dried (Na2SO4). After filtration, the solvent was removed under reduced pressure and the residual oil was purified through a short plug of SiO2 (1:1 hexanes/EtOAc) providing a clear oil (40 mg, 85%).
The ketone (6 mg, 0.0125 mmol) was dissolved in MeOH and cooled to 0 °C. NaBH4 (11 mg, 0.035 mmol) was added slowly and the mixture was stirred until the ketone was completely consumed (monitored by TLC). The mixture was partitioned with H2O:Et2O (1:1, 10 mL) and the resultant aqueous layer was extracted with Et2O (3 × 5 mL). The collected organic layers were washed with brine (5 mL) and dried (Na2SO4). The epimeric ratio of the crude material was determined by 1H NMR analysis after filtration and removal of solvent under reduced pressure, (∼2.7:1). Flash chromatography (5:1 hexanes/EtOAc) provided both isomers (4 mg, 89%) as a clear oil; [α]D = -8.1 (c = 1.3, CH2Cl2); FTIR (neat) 3456, 2954, 2935, 2875, 1458, 1379, 1224, 908 cm-1; 1H NMR (500 MHz, CDCl3) δ 5.82 (dddd, J = 16.9, 10.1, 6.7 and 6.7 Hz, 1H), 5.16 (d, J = 9.1 Hz, 1H), 5.01 (ddd, J = 17.1, 3.4 and 1.5 Hz, 1H), 4.95 (d, J = 10.2 Hz, 1H), 4.27 (t, J = 8.7 Hz, 1H), 3.99 (s, 1H), 3.84–3.74 (m, 3H), 2.27 (dd, J =14.1 and 4.3 Hz, 1H), 2.16 (dd, J = 8.7 and 3.0 Hz, 1H), 2.06 (q, J = 7.0 Hz, 2H), 1.70 (s, 3H), 1.41 (s, 3H), 1.35 (s, 3H), 1.72–1.20 (m, 11H), 0.96 (t, J = 8.2 Hz, 9H), 0.88 (t, J = 7.3 Hz, 3H), 0.71 (d, J = 6.9 Hz, 3H), 0.59 (q, J = 8.2 Hz, 6H); 13C NMR (126 MHz, CDCl3) δ 138.6, 136.0, 128.8, 114.6, 100.6, 72.9, 72.4, 70.7, 66.6, 48.5, 44.1, 38.7, 38.0, 35.2, 33.6, 24.7, 24.6, 24.5, 18.4, 17.3, 14.2, 11.6, 7.0, 5.0; HRMS calcd for C28H54NaO4Si (M+Na)+ 505.3689; found 505.3674 (ESI).
(5S,7R,9S,12R,13S,14R)-15-(tert-Butyldimethylsilyloxy)-13-(4-methoxybenzyloxy)-2,12,14-trimethylpentadec-1-ene-5,7,9-triyl triacetate (31)
To a solution of triol 16 (132 mg, 0.232 mmol) in CH2Cl2 (3.3 mL) was added DMAP (3 mg, 0.023 mmol), pyridine (0.750 mL, 9.30 mmol), and acetic anhydride (0.45 mL, 4.65 mmol). The reaction was stirred until disappearance of starting material at rt (∼2 h). The reaction was diluted with EtOAc (5 mL), quenched with saturated NH4Cl (5 mL), and the aqueous layer was re-extracted with EtOAc (3 × 10 mL). The organic layer was washed with brine (10 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Flash chromatography (5:1 hexanes/EtOAc) provided 31 (151 mg, 94%) as a clear oil; [α]D = +12.4 (c = 0.50, CH2Cl2); FTIR (neat) 2956, 2929, 2883, 2856, 1739, 1514, 1461, 1247 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.26 (d, J = 8.7 Hz, 2H), 6.87 (d, J = 8.7 Hz, 2H), 4.97 (dddd, J = 9.5, 6.2, 6.2 and 3.1 Hz, 1H), 4.89 (m, 2H), 4.71 (s, 1H), 4.66 (s, 1H), 4.53 (d, J = 10.9 Hz, 1H), 4.46 (d, J = 10.9 Hz, 1H), 3.80 (s, 3H), 3.71 (dd, J = 9.7 and 5.3 Hz, 1H), 3.63 (dd, J = 9.7 and 3.3 Hz, 1H), 3.25 (dd, J = 8.7 and 2.4 Hz, 1H), 2.07–1.99 (m, 2H), 2.05 (s, 3H), 2.03 (s, 3H), 1.99 (s, 3H), 1.92 (dddd, J = 18.7, 10.2, 4.4 and 4.4 Hz, 1H), 1.84–1.68 (m, 5H), 1.71 (s, 3H), 1.64–1.54 (m, 4H), 1.46–1.38 (m, 1H), 1.34–1.22 (m, 1H), 0.92 (s, 9H), 0.90 (d, J = 2.3 Hz, 3H), 0.88 (d, J = 2.3 Hz, 3H), 0.06 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 170.8, 170.7, 170.6, 159.1, 144.9, 131.7, 129.3, 113.9, 110.5, 83.3, 74.6, 70.9, 70.3, 67.5, 65.1, 55.4, 39.2, 38.7, 38.6, 35.4, 33.5, 33.1, 32.3, 30.5, 26.1, 22.6, 21.3, 21.3, 21.2, 18.5, 14.8, 13.6, -5.2, -5.2; HRMS calcd for C38H64NaO9Si (M+Na)+ 715.4217; found 715.4213 (ESI).
(5S,7R,9S,12R,13S,14R)-15-Hydroxy-13-(4-methoxybenzyloxy)-2,12,14-trimethylpentadec-1-ene-5,7,9-triyl triacetate (32)
To a solution of 31 (150 mg, 0.216 mmol) in THF (2.3 mL) was added TBAF (0.70 mL, 1.0 M in THF). The reaction was stirred until disappearance of starting material at rt (∼3 h). The reaction was diluted with EtOAc (3 mL), quenched with saturated NH4Cl (5 mL), and the aqueous layer was re-extracted with EtOAc (2 × 5 mL). The organic layer was dried (Na2SO4), filtered and concentrated under reduced pressure. Flash chromatography (2:1 hexanes/EtOAc) provided 32 (118 mg, 94%) as a clear oil; [α]D = +13.1 (c = 2.4, CH2Cl2); FTIR (neat) 3502, 3072, 2964, 2935, 2875, 1737, 1514, 1454, 1245 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.28 (d, J = 8.5 Hz, 2H), 6.88 (d, J = 8.6 Hz, 2H), 4.98 (dddd, J = 9.6, 6.3, 6.3 and 3.3 Hz, 1H), 4.99–4.85 (m, 2H), 4.73 (s, 1H), 4.67 (s, 1H), 4.58 (d, J = 10.6 Hz, 1H), 4.51 (d, J = 10.6 Hz, 1H), 3.81 (s, 3H), 3.62–3.67 (m, 2H), 3.25 (dd, J = 7.6, 3.3 Hz, 1H), 2.71 (s, 1H), 2.07–1.99 (m, 2H), 2.06 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H), 1.95–1.86 (m, 2H) 1.84–1.68 (m, 6H), 1.72 (s, 3H), 1.64–1.56 (m, 2H), 1.54–1.44 (m, 1H), 1.34–1.22 (m, 1H), 0.96 (d, J = 6.9 Hz, 3H), 0.94 (d, J = 7.1 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 170.7, 170.6, 170.5, 159.3, 144.7, 130.4, 129.4 (2), 113.9 (2), 110.4, 87.6, 74.8, 70.6, 70.0, 67.3, 66.5, 55.3, 39.0, 38.4, 37.7, 36.1, 33.3, 32.8, 32.2, 30.0, 22.4, 21.2, 21.1, 21.1, 15.4, 14.3; HRMS calcd for C32H50NaO9 (M+Na)+ 601.3353; found 601.3354 (ESI).
(2S,3S,4R,7S,9R,11S)-7,9,11-Triacetoxy-3-(4-methoxybenzyloxy)-2,4,14-trimethylpentadec-14-enoic acid (2)
A solution of oxalyl chloride (0.046 mL, 0.539 mmol) in CH2Cl2 (1.67 mL) was cooled to -78 °C and DMSO (0.077 mL, 1.08 mmol) was added slowly by syringe (gas evolution). After stirring for 10 min a solution of alcohol 32 (125 mg, 0.21 mmol) in CH2Cl2 (2.0 mL) was added by cannula and rinsed with CH2Cl2 (2 × 0.2 mL). The cloudy mixture was stirred at -78 °C for 15 min at which time Et3N (0.18 mL, 1.29 mmol) was added dropwise. The reaction mixture was stirred for 2 h at -78 °C. The reaction was quenched at -78 °C with saturated NaHCO3 (3 mL) and allowed to warm to rt. The reaction was diluted with CH2Cl2, and the layers were separated. The aqueous layer was re-extracted with CH2Cl2 (3 × 5 mL). The organic layer was dried (Na2SO4), filtered and concentrated under reduced pressure to give aldehyde as a yellow oil. The crude aldehyde was taken immediately to the next reaction with further purification.
To a solution of crude aldehyde was added t-butanol (4.5 mL) and 2-methyl-2-butene (1.5 mL). A solution of NaClO2 (390 mg, 4.30 mmol) and sodium dihydrogen phosphate (470 mg, 3.01 mmol) in H2O (2.0 mL) was prepared and added to the reaction mixture by syringe. The yellow solution was stirred vigorously for 2 h at rt, diluted with Et2O (15 mL) and poured into H2O (9 mL). The layers were separated and the aqueous phase was extracted with Et2O (3 × 10 mL). The combine organic layers were dried (Na2SO4), filtered and concentrated under reduced pressure. Flash chromatography (1:1 hexanes:EtOAc) provided 2 (104 mg, 81% over two steps) as a clear oil; [α]D = +8.13 (c = 0.16, CH2Cl2); FTIR (neat) 3251, 3076, 2964, 2923, 2854, 1737, 1714, 1512, 1454, 1245 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.24 (d, J = 8.5 Hz, 2H), 6.85 (d, J = 8.6 Hz, 2H), 5.10–4.85 (m, 3H), 4.73 (s, 1H), 4.67 (s, 1H), 4.56 (d, J = 10.7 Hz, 1H), 4.50 (d, J = 10.6 Hz, 1H), 3.79 (s, 3H), 3.57 (dd, J = 7.2 and 3.5 Hz, 1H), 2.78 (dddd, J = 14.33, 7.1, 7.1 and 7.1 Hz, 1H), 2.07–1.99 (m, 2H), 2.06 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H), 1.95–1.86 (m, 2H), 1.84–1.64 (m, 5H), 1.72 (s, 3H), 1.64–1.40 (m, 3H), 1.39–1.20 (m, 2H), 1.17 (d, J = 7.1 Hz, 3H), 0.93 (d, J = 6.7 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 179.9, 170.8, 170.7, 170.5, 159.4, 144.7, 130.0, 129.5 (2), 113.9 (2), 110.3, 84.2, 74.4, 70.8, 69.9, 67.3, 55.3, 42.3, 39.0, 38.1, 35.6, 33.3, 32.5, 32.3, 28.9, 22.4, 21.2, 21.1, 21.1, 14.7, 14.4; HRMS calcd for C32H48NaO10 (M+Na)+ 615.3145; found 615.3131 (ESI).
(5S,7R,9S,12R,13S,14S)-15-((2S,3R,7R,E)-2-((4S,6R)-2,2-Dimethyl-6-(pent-4-enyl)-1,3-dioxan-4-yl)-5-methyl-7-(triethylsilyloxy)dec-4-en-3-yloxy)-13-(4-methoxybenzyloxy)-2,12,14-trimethyl-15-oxopentadec-1-ene-5,7,9-triyl triacetate (33)
To a solution of alcohol 30 (77 mg, 0.159 mmol), carboxylic acid 2 (106 mg, 0.175 mmol), and DMAP (975 mg, 7.98 mmol) in toluene (32 mL) at -78 °C was added Et3N (0.5 mL, 3.61 mmol) dropwise followed by the slow addition of 2,4,6-trichlorobenzoyl chloride (0.56 mL, 3.58 mmol), which caused the white solution to thicken. The mixture was stirred for 21 h at -78 °C ensuring that the bath temperature did not rise above -65 °C. The reaction flask was then moved to a dry ice/CH3CN bath and stirred for 2.5 h maintaining the temperature between -30 °C to -42 °C. At the end of the 2.5 h the solution was slowly allowed to warm to rt in the bath over 1 h. The flask was placed in an ice bath for 2 h while being stirred. The reaction was quenched by the addition of sataturated NaHCO3 (15 mL). The layers were separated and the aqueous layer was back extracted with Et2O (25 mL). The combined organic layers were dried (Na2SO4), filtered, and concentrated under reduce pressure. Purification by flash chromatography (5:1 hexanes/EtOAc) provided ester 33 (130 mg, 77%) as a colorless oil; [α]D +3.63 (c = 0.28, CH2Cl2); FTIR (neat) 3076, 2954, 2935, 2875, 1739, 1515, 1442, 1244 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.19 (d, J = 8.7 Hz, 2H), 6.83 (d, J = 8.7 Hz, 2H), 5.82 (dddd, J = 17.0, 10.2, 6.8 and 6.8 Hz, 1H), 5.68 (dd, J = 9.9 and 5.7 Hz, 1H), 5.17 (d, J = 9.8 Hz, 1H), 5.02 (ddd, J = 17.1, 3.2 and 1.6 Hz, 1H), 5.00–4.93 (m, 3H), 4.94–4.85 (m, 2H), 4.74 (s, 1H), 4.67 (s, 1H), 4.51 (d, J = 10.8 Hz, 1H), 4.34 (d, J = 10.8 Hz, 1H), 3.79 (s, 3H), 3.70–3.80 (m, 2H), 3.69–3.60 (m, 3H), 2.69 (dt, J = 14.2 and 7.1 Hz, 1H), 2.20–1.89 (m, 8H), 2.06 (s, 3H), 2.04 (s, 3H), 2.00 (s, 3H), 1.80–1.20 (m, 18H), 1.76 (s, 3H), 1.73 (s, 3H), 1.34 (s, 3H), 1.29 (s, 3H), 1.08 (d, J = 7.1 Hz, 3H), 0.96 (t, J = 7.9 Hz, 9H), 0.93–0.84 (m, 9H) 0.58 (q, J = 7.9 Hz, 6H); 13C NMR (126 MHz, CDCl3) δ 175.3, 170.6, 170.6, 170.5, 158.9, 144.7, 139.6, 138.8, 131.2, 129.0, 122.7, 114.6, 113.6, 110.3, 100.1, 83.1, 73.9, 71.4, 70.7, 70.2, 70.0, 67.3, 67.1, 66.5, 55.2, 53.5, 48.8, 43.5, 42.1, 39.1, 38.5, 38.5, 35.4, 34.7, 33.7, 33.3, 32.1, 30.3, 29.9, 29.7, 24.9, 24.9, 24.8, 22.4, 21.2, 21.1, 21.1, 18.3, 17.6, 15.3, 14.8, 14.2, 13.2, 9.8, 7.0 (3), 5.0 (3); HRMS calcd for C60H100NaO13Si (M+Na)+ 1079.6831; found 1079.7115 (ESI).
(5S,7R,9S,12R,13S,14S)-15-((2S,3R,7R,E)-2-((4S,6R)-2,2-Dimethyl-6-(pent-4-enyl)-1,3-dioxan-4-yl)-7-hydroxy-5-methyldec-4-en-3-yloxy)-13-(4-methoxybenzyloxy)-2,12,14-trimethyl-15-oxopentadec-1-ene-5,7,9-triyl triacetate (SI-4)
Ester 33 (75 mg, 0.071 mmol) was dissolved in THF (1 mL) and cooled to 0 °C. A solution of TBAF in THF (0.214 mL, 1.0 M in THF) was added dropwise. The reaction stirred at 0 °C until completion (approximately 45 min). The reaction was quenched with saturatred NH4Cl and the aqueous layer was extracted with EtOAc (3 × 10 mL). The organic layers were combined, washed with brine (5 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Purification by flash chromatography (2:1 hexanes/EtOAc) afforded SI-4 (63 mg, 94%) as a clear; [α]D = -11.4 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.22 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 5.81 (dddd, J = 17.0, 10.2, 6.7 and 6.7 Hz, 1H), 5.64 (dd, J = 9.9 and 5.2 Hz, 1H), 5.20 (d, J = 9.6 Hz, 1H), 5.01 (ddd, J = 17.1, 3.4 and 1.6 Hz, 1H), 5.00–4.93 (m, 2H), 4.93–4.85 (m, 2H), 4.72 (s, 1H), 4.67 (s, 1H), 4.53 (d, J = 10.7 Hz, 1H), 4.38 (d, J = 10.7 Hz, 1H), 3.79 (s, 3H), 3.76–3.69 (m, 1H), 3.66–3.59 (m, 1H), 3.60–3.54 (m, 1H), 3.53 (dd, J = 8.4, 3.0 Hz, 1H), 2.70 (dt, J = 15.2 and 7.2 Hz, 1H), 2.15–1.87 (m, 8H), 2.06 (s, 3H), 2.04 (s, 3H), 2.00 (s, 3H), 1.80–1.20 (m, 21H), 1.76 (s, 3H), 1.73 (s, 3H), 1.33 (s, 3H), 1.28 (s, 3H), 1.10 (d, J = 7.1 Hz, 3H), 0.93–0.84 (m, 9H); 13C NMR (126 MHz, CDCl3) δ 175.1, 170.7, 170.6, 170.5, 158.9, 144.7, 139.6, 138.7, 131.2, 128.8, 123.1, 114.6, 113.5, 110.3, 100.2, 83.7, 73.8, 71.8, 70.7, 70.0, 68.4, 67.3, 66.4, 55.2, 53.5, 48.1, 43.4, 42.1, 39.2, 39.1, 38.5, 36.3, 35.4, 35.1, 33.7, 33.3, 32.8, 32.2, 29.8, 29.7, 24.8, 24.6, 22.4, 21.2, 21.1, 21.0, 18.9, 17.5, 14.8, 14.2, 13.6, 9.9; HRMS calcd for C54H86NaO13 (M+Na)+ 965.5966; found 965.5897 (ESI).
(5S,7R,9S,12R,13S,14S)-15-((2S,3R,7R,E)-7-Acetoxy-2-((4S,6R)-2,2-dimethyl-6-(pent-4-enyl)-1,3-dioxan-4-yl)-5-methyldec-4-en-3-yloxy)-13-(4-methoxybenzyloxy)-2,12,14-trimethyl-15-oxopentadec-1-ene-5,7,9-triyl triacetate (34)
To a solution of SI-4 (58 mg, 0.062 mmol) in CH2Cl2 (3.0 mL) was added DMAP (1 crystal), pyridine (0.2 mL, 2.46 mmol) and acetic anhydride (0.117 mL, 1.23 mmol). The reaction was stirred at rt until disappearance of starting material (∼2 h). The reaction was diluted with EtOAc (3 mL), quenched with saturated NH4Cl (3 mL) and the aqueous layer was re-extracted with EtOAc (3 × 5 mL). The organic layer was washed with brine (5 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Flash chromatography (1.5:1 hexanes/EtOAc) provided 34 (60 mg, 98%) as a clear oil; [α]D = +2.2 (c = 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.18 (d, J = 8.6 Hz, 2H), 6.83 (d, J = 8.6 Hz, 2H), 5.80 (dddd, J = 16.9, 10.2, 6.7 and 6.7 Hz, 1H), 5.67 (dd, J = 9.9 and 5.6 Hz, 1H), 5.16 (d, J = 9.7 Hz, 1H), 5.00 (ddd, J = 17.1, 3.4 and 1.6 Hz, 1H), 5.00–4.92 (m, 3H), 4.92–4.84 (m, 2H), 4.73 (s, 1H), 4.66 (s, 1H), 4.50 (d, J = 10.9 Hz, 1H), 4.35 (d, J = 10.9 Hz, 1H), 3.79 (s, 3H), 3.75–3.68 (m, 1H), 3.62–3.55 (m, 2H), 2.68 (dt, J = 16.0 and 6.9 Hz, 1H), 2.15–1.87 (m, 8H), 2.06 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H), 2.00 (s, 3H), 1.80–1.20 (m, 20H), 1.77 (s, 3H), 1.73 (s, 3H), 1.33 (s, 3H), 1.27 (s, 3H), 1.06 (d, J = 7.1 Hz, 3H), 0.93–0.84 (m, 9H); 13C NMR (126 MHz, CDCl3) δ 175.1, 170.6, 170.6, 170.6, 170.5, 158.9, 144.7, 138.7, 138.6, 131.3, 128.8, 122.5, 114.6, 113.5, 110.3, 100.2, 83.2, 73.8, 72.1, 71.2, 70.7, 70.0, 67.3, 66.4, 55.2, 53.5, 44.2, 42.1, 39.2, 39.1, 35.9, 35.4, 33.7, 33.3, 32.2, 31.9, 31.6, 29.9, 24.8, 24.8, 22.7, 21.3, 21.2, 21.1, 21.0, 18.4, 17.8, 14.7, 14.2, 14.2, 14.0, 13.2, 9.7; HRMS calcd for C56H88NaO14 (M+Na)+ 1007.6072; found 1007.6210 (ESI).
(5S,7R,9S,12R,13S,14S)-15-((4R,8R,9S,10S,12R,E)-4-Acetoxy-10,12-dihydroxy-6,9-dimethylheptadeca-6,16-dien-8-yloxy)-13-(4-methoxybenzyloxy)-2,12,14-trimethyl-15-oxopentadec-1-ene-5,7,9-triyl triacetate (SI-5)
To a solution of tetraacetate 34 (60 mg, 0.061 mmol) in MeOH (6 mL) was added PPTS (2.5 mg, 0.03 mmol). The reaction was stirred until disappearance of starting material at rt (∼ 4 h). The reaction was diluted with EtOAc, quenched with saturated NaHCO3 and the aqueous layer was re-extracted with EtOAc (3 × 10 mL). The organic layer was washed with brine (10 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Flash chromatography (1.5:1 hexanes/EtOAc) provided SI-5 (47 mg, 82%) as a clear oil; [α]D = +8.97 (c = 1.7, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.21 (d, J = 8.3 Hz, 2H), 6.85 (d, J = 8.4 Hz, 2H), 5.82 (dddd, J = 15.8, 12.1, 5.4 and 5.4 Hz, 1H), 5.69 (dd, J = 9.7 and 6.0 Hz, 1H), 5.18 (d, J = 9.8 Hz, 1H), 5.05–4.85 (m, 6H), 4.73 (s, 1H), 4.67 (s, 1H), 4.51 (d, J = 10.9 Hz, 1H), 4.34 (d, J = 11.0 Hz, 1H), 3.92–3.86 (m, 1H), 3.80 (s, 3H), 3.71 (m, 1H), 3.58 (dd, J = 8.4, 2.0 Hz, 1H), 2.73 (dddd, J = 14.2, 6.8, 6.8 and 6.8 Hz, 1H), 2.50–1.19 (m, 28H), 2.07 (s, 3H), 2.03 (s, 3H), 2.02 (s, 3H), 1.82 (s, 3H), 1.72 (s, 3H), 1.09 (d, J = 7.0 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H), 0.86–0.81 (m, 9H); 13C NMR (126 MHz, CDCl3) δ 175.1, 170.7, 170.6, 170.6, 170.5, 158.9, 144.7, 138.7, 138.6, 131.1, 128.4, 123.4, 114.7, 113.7, 110.3, 83.5, 73.8, 73.5, 72.5, 70.7, 70.1, 68.8, 67.3, 60.4, 55.2, 44.3, 43.4, 43.0, 39.5, 39.1, 38.5, 37.1, 36.3, 34.9, 33.7, 33.3, 32.2, 31.6, 29.8, 25.1, 22.7, 21.3, 21.2, 21.1, 21.1, 18.4, 17.8, 14.2, 14.0, 13.6, 10.8; HRMS calcd for C53H84NaO14 (M+Na)+ 967.5759; found 967.5789 (ESI).
(5S,7R,9S,12R,13S,14S)-15-((4R,8R,9S,10S,12R,E)-4-Acetoxy-10,12-dihydroxy-6,9-dimethylheptadeca-6,16-dien-8-yloxy)-13-hydroxy-2,12,14-trimethyl-15-oxopentadec-1-ene-5,7,9-triyl triacetate (35)
Ester SI-5 (105 mg, 0.112 mmol) was taken up in CH2Cl2 (5.0 mL) followed by the addition of pH = 7 buffer solution (5.0 mL) and DDQ (51 mg, 0.224 mmol) at rt. Upon completion (∼0.5 h, monitored by TLC), CH2Cl2 (13 mL) was added followed by saturated NaHCO3 (1 mL). The layers were separated, and the aqueous layer extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with brine (10 mL), dried (anhydrous Na2SO4), filtered, and concentrated under reduced pressure. Flash chromatography (2:1 hexanes/EtOAc) afforded 35 (89 mg, 97%) as a clear oil. [α]D = +4.4 (c = 0.50, CHCl3); 1H NMR (400 MHz, CDCl3) δ 5.79 (dddd, J = 17.0, 10.2, 6.7 and 6.7 Hz, 1H), 5.19 (dd, J = 9.7 and 9.5 Hz, 1H), 5.07–4.87 (m, 6H), 4.73 (s, 1H), 4.66 (s, 1H), 4.15–4.09 (m, 2H), 3.96–3.89 (m, 1H), 3.71 (dd, J = 9.9 and 1.2 Hz, 1H), 2.54 (dddd, J = 14.1, 7.0, 7.0 and 7.0 Hz, 1H), 2.17–1.19 (m, 28H), 2.07 (s, 3H), 2.03 (s, 3H), 2.02 (s, 3H), 1.82 (s, 3H), 1.72 (s, 3H), 1.08 (d, J = 7.0 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H), 0.86–0.81 (m, 9H); 13C NMR (126 MHz, CDCl3) δ 174.0, 172.4, 170.8, 170.8, 170.6, 144.6, 138.7, 138.0, 125.3, 114.6, 110.4, 72.6, 72.6, 72.4, 71.9, 70.7, 68.1, 67.1, 66.9, 45.3, 44.2, 42.7, 39.0, 37.6, 37.3, 36.9, 36.0, 33.7, 33.6, 33.3, 32.3, 31.5, 29.0, 25.3, 22.4, 21.4, 21.3, 21.2, 21.1, 18.5, 17.8, 13.9, 13.6, 12.5, 9.5; HRMS calcd for C45H76NaO13 (M+Na)+ 847.5184; found 847.5183 (ESI).
Dolabelide C (1)
To a refluxing solution of ester 23 (70 mg, 0.085 mmol) in degassed CH2Cl2 (175 mL) was added Grubbs II catalyst (8.0 mg, 0.0085 mmol). The reaction was refluxed 2 h with the addition of more catalyst (4.0 mg, 0.00425 μmol) after 2 h. A third portion of catalyst (4.0 mg, 0.00425) was had after 2 more hours; the reaction was refluxed for 6 hours (monitored by TLC and LC-MS). The solution was cooled to rt and concentrated under reduced pressure. The resultant residue was purified via flash chromatography through two sequential columns (8:1 CH2Cl2/acetone) and (5:1 pentane/EtOAc) afforded 1, (14.0 mg, 21% yield) as an analytically pure sample and its C14–C15 Z-configured diastereomer (10.0 mg, 15% yield) (vide infra); [α]D = +2.9 (c = 0.63, CHCl3); 1H NMR (500 MHz, pyridine-d5)36b δ 6.10-5.90 (br m, 1H), 5.70 (t, J = 9.3 Hz, 1H), 5.67 (s, 1H), 5.40 (d, J = 9.5 Hz, 1H), 5.38–5.31 (m, 2H), 5.30–5.23 (m, 2H), 5.16–5.10 (m, 1H), 4.88–4.82 (m, 1H), 4.37–4.32 (m, 1H), 4.03 (br d, J = 9.1 Hz, 1H), 2.93–2.85 (m, 1H), 2.52–2.48 (m, 1H), 2.32 (dd, J = 14.0 and 7.9 Hz, 1H), 2.28 (dd, J = 13.9 and 5.4 Hz, 1H), 2.21–2.15 (m, 1H), 2.11–2.00 (m, 6H), 2.08 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 2.05 (s, 3H), 1.98 (s, 3H), 1.96–1.92 (m, 2H), 1.90–1.80 (m, 4H), 1.79–1.58 (m, 7H), 1.59 (s, 3H), 1.53–1.49 (m, 3H), 1.32–1.28 (m, 2H), 1.19 (d, J = 7.3 Hz, 3H), 1.16 (d, J = 7.0 Hz, 3H), 0.90 (d, J = 6.6 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H); 13C NMR (126 MHz, pyridine-d5) δ 173.9, 170.6, 170.5, 170.4, 170.3, 136.7, 132.6, 127.3, 127.2, 74.3, 73.5, 71.8, 69.9, 69.9, 68.0, 67.9, 67.3, 46.4, 44.5, 43.7, 38.8, 38.5, 38.0, 37.2, 36.3, 35.2, 34.1, 31.8, 31.6, 29.3, 28.0, 27.0, 21.1, 21.0, 20.9, 20.9, 18.8, 17.6, 15.2, 14.0, 13.8, 12.6, 11.0; 1H NMR (500 MHz, CDCl3) δ 5.35 (t, J = 9.1 Hz, 1H), 5.10–5.05 (m, 2H), 5.04–5.00 (m, 1H), 4.98–4.92 (m, 1H), 4.88–4.82 (m, 2H), 4.08 (s, 1H), 3.93 (s, 1H), 3.57 (s, 1H), 3.24 (s, 1H), 2.60–2.54 (m, 1H), 2.54–2.47 (m, 1H), 2.25 (dd, J = 13.8 and 7.1 Hz, 1H), 2.21 (dd, J = 14.1 and 5.7 Hz, 1H), 2.07 (s, 3H), 2.03 (s, 3H), 2.02 (s, 3H), 2.01 (s, 3H), 1.90–1.83 (m, 2H), 1.81 (s, 3H), 1.78–1.64 (m, 3H), 1.64–1.62 (m, 4H), 1.58 (s, 3H), 1.56 (m, 9H), 1.43–1.27 (m, 5H), 1.25 (s, 3H), 1.23–1.20 (m, 1H), 1.07 (d, J = 7.1 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H), 0.84 (m, 6H); 13C NMR (126 MHz, CDCl3) δ 173.8, 171.2, 171.0, 170.7, 170.4, 137.9, 133.1, 126.1, 125.0, 74.6, 73.1, 72.2, 69.8, 69.2, 68.7, 68.1, 67.7, 45.1, 44.3, 42.7, 39.1, 36.8, 36.1, 35.1, 34.6, 31.9, 31.7, 29.7, 28.3, 26.7, 25.0, 21.2, 21.2, 21.2, 21.1, 18.5, 17.7, 15.2, 13.9, 13.6, 12.6, 10.6; HRMS calcd for C43H72NaO13 (M+Na)+ 819.4871; found 819.4858 (ESI).
Non-Natural C14-C15 Z-Isomer of 1
[α]D = +10.0 (c = 0.30, CHCl3); 1H NMR (500 MHz, pyridine-d5)36c δ 6.28 (br s, 1H), 6.20–6.05 (br m, 1H), 5.85 (t, J = 9.4 Hz, 1H), 5.51 (d, J = 8.8 Hz, 1H), 5.48–5.40 (m, 2H), 5.38–5.29 (m, 2H), 5.27–5.18 (m, 1H), 4.88–4.84 (m, 1H), 4.53–4.46 (m, 1H), 4.14 (br d, J = 7.4 Hz, 1H), 3.03–2.97 (m, 1H), 2.64–2.56 (m, 1H), 2.43 (dd, J = 7.5, 13.8 Hz, 1H), 2.37 (dd, J = 5.3, 13.1 Hz, 1H), 2.34–2.23 (m, 3H), 2.22–2.19 (m, 1H), 2.18–2.14 (m, 12H), 2.13–2.12 (m, 2 H), 2.08 (s, 3 H), 2.06–2.02 (m, 1 H), 2.02–2.00 (m, 1 H), 1.99–1.95 (m, 2 H), 1.95–1.91 (m, 1 H), 1.91–1.86 (m, 2 H), 1.84–1.78 (m, 2 H), 1.77 (s, 3 H), 1.75–1.66 (m, 3 H), 1.64–1.58 (m, 3 H); 1.49–1.31 (m, 4 H), 1.29 (d, J = 7.0 Hz, 6 H), 1.04 (d, J = 6.6 Hz, 3 H), 0.94 (t, J = 7.3 Hz, 3 H); 13C NMR (126 MHz, pyridine-d5) δ 174.2, 170.9, 170.9, 170.7, 170.6, 137.2, 134.3, 127.3, 126.9, 75.2, 73.6, 72.2, 71.8, 70.1, 68.3, 68.2, 68.1, 46.6, 44.8, 44.3, 39.9, 39.2, 39.0, 37.6, 36.7, 34.4, 32.8, 32.0, 29.3, 28.4, 27.9, 27.5, 23.3, 21.4, 21.4, 21.3, 21.3, 19.1, 17.9, 14.3, 14.3, 13.0, 11.4; 1H NMR (500 MHz, CDCl3)36d δ 5.13–5.07 (m, 2H), 5.03–4.97 (m, 3H), 4.96–4.91 (m, 1H), 4.90–4.85 (m, 1H), 4.27 (s, 1H), 4.05–4.01 (m, 1H), 3.68 (d, J = 9.7 Hz, 1H), 2.54 (tt, J = 7.0 Hz, 1H), 2.31–2.20 (m, 4H), 2.13–2.11 (m, 1H), 2.11–2.08 (m, 1H), 2.06–2.04 (m, 2H), 2.04–2.03 (m, 6H), 2.02 (s, 3H), 2.01 (s, 3H), 2.00–1.97 (m, 1H), 1.97–1.95 (m, 1H), 1.82 (s, 3H), 1.81–1.76 (m, 2H), 1.76–1.74 (m, 1H), 1.74–1.71 (m, 1H), 1.66 (s, 3H), 1.61 (s, 1H), 1.58–1.56 (m, 1H), 1.48–1.46 (m, 2H), 1.45–1.43 (m, 2H), 1.42–1.39 (m, 2H), 1.38–1.25 (m, 6H), 1.01 (d, J = 7.0 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H), 0.82 (d, J = 7.0 Hz, 3H), 0.79 (d, J = 6.7 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 173.6, 171.6, 171.3, 170.7, 170.4, 137.6, 133.9, 125.9, 125.8, 74.0, 72.8, 72.3, 71.3, 70.8, 68.0, 67.0, 66.6, 45.7, 44.1, 42.9, 38.0, 37.4, 37.0, 35.9, 34.9, 33.6, 32.2, 31.1, 28.2, 26.7, 25.9, 22.8, 21.4, 21.2, 21.2, 21.2, 21.2, 18.5, 17.8, 13.9, 13.4, 11.5, 9.36; HRMS calcd for C43H72NaO13 (M+Na)+ 819.4871; found 819.4877 (ESI).
HPLC-MS Analysis of 1
HPLC data was collected using the following gradient over 35 min:
| Time (min) | A% (99:1 H2O:MeCN) | B% (99:1 MeCN: H2O) | Flow Rate (mL/min) |
| 0.00 | 95.0 | 5.0 | 1.000 |
| 1.00 | 40.0 | 60.0 | 1.000 |
| 31.00 | 30.0 | 70.0 | 1.000 |
Supplementary Material
Acknowledgments
This investigation was kindly supported by funds provided by NSF CHE-0503875, NIH-NIGMS RO1 GM077309, and the NIH Dynamic Aspects in Chemical Biology Training Grant (A.W., J.D.W.). The authors thank the University of Kansas Honors department for a KU Undergraduate Research Award (J.N., KU-UGRA), Dr. Marc Anderson and Dr. Todd D. Williams for LC-MS analysis, Dr. Justin T. Douglas and Sarah Neuenswander for assistance in NMR analysis, and Materia, Inc. for supplying catalyst and helpful discussion. The authors also thank Dr. Kigoshi, Dr. Ojika, and Dr. Suenaga for supplying us with a natural sample of dolabelide C.
Footnotes
Supporting Information Available. Experimental details and spectroscopic data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ojika M, Nagoya T, Yamada K. Tetrahedron Lett. 1995;36:7491–7494. [Google Scholar]
- 2.Suenaga K, Nagoya T, Shibata T, Kigoshi H, Yamada K. J Nat Prod. 1997;60:155–157. [Google Scholar]
- 3.(a) Grimaud L, Rotulo D, Ros-Perez R, Guitry-Azam L, Prunet J. Tetrahedron Lett. 2002;43:7477–7479. [Google Scholar]; (b) Grimaud L, de Mesmay R, Prunet J. Org Lett. 2002;4:419–421. doi: 10.1021/ol017122w. [DOI] [PubMed] [Google Scholar]; (c) Desroy N, Le Roux R, Phansavath P, Chiummiento L, Bonini C, Genêt JP. Tetrahedron Lett. 2003;44:1763–1766. [Google Scholar]; (d) Schmidt DR, Park PK, Leighton JL. Org Lett. 2003;5:3535–3537. doi: 10.1021/ol035431b. [DOI] [PubMed] [Google Scholar]; (e) Le Roux R, Desroy N, Phansavath P, Genêt JP. Synlett. 2005:429–432. [Google Scholar]; (f) Keck GE, McLaws MD. Tetrahedron Lett. 2005;46:4911–4914. doi: 10.1016/j.tetlet.2005.04.146. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Vincent A, Prunet J. Synlett. 2006:2269–2271. [Google Scholar]; (h) Roche C, Desroy N, Haddad M, Phansavath P, Genêt JP. Org Lett. 2008;10:3911–3914. doi: 10.1021/ol801493w. [DOI] [PubMed] [Google Scholar]
- 4.Park PK, O'Malley SJ, Schmidt JDR, Leighton L. J Am Chem Soc. 2006;128:2796–2797. doi: 10.1021/ja058692k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Both antipodes of the bicyclic phosphate 5 and diol 7 have been previously constructed, see: Whitehead A, McReynolds MD, Moore JD, Hanson PR. Org Lett. 2005;7:3375–3378. doi: 10.1021/ol0512886. Recently a 4-step synthesis from commercially available 2,4-pentanedione (see reference 5a) has been developed to bicyclic phosphate 5 see: Venukadasula PKM, Chegondi R, Maitra S, Hanson PR. Org Lett. 2010;12:1556–1559. doi: 10.1021/ol1002913. For additional methods to 1,3-anti-diols, see: Bode SE, Wolberg M, Müller M. Synthesis. 2006:557–588.
- 6.Burke SD, Muller N, Beaudry CM. Org Lett. 1999;1:1827–1829. doi: 10.1021/ol9910971.Burke SD, Voight EA. Org Lett. 2001;3:237–240. doi: 10.1021/ol006887l.Lambert WT, Burke SD. Org Lett. 2003;5:515–518. doi: 10.1021/ol027389a. For desymmetrization of a C2-symmetric diol using the Prins cyclization, see: Rychnovsky SD, Yang G, Hu Y, Khire UR. J Org Chem. 1997;62:3022–3023. doi: 10.1021/jo970380f.
- 7.Rychnovsky SD, Griesgraber G, Powers JP. Org Synth. 1999;77:1–11. [Google Scholar]
- 8.(a) Mislow K, Siegel J. J Am Chem Soc. 1984;106:3319–3328. [Google Scholar]; (b) Eliel EL. Stereochemistry of Carbon Compounds. McGrawHill; New York: 1962. [Google Scholar]
- 9.The Grubbs catalyst (PCy3)2(Cl)2Ru=CHPh (cat-A),10 was shown to be not as effective in this desymmetrization reaction as cat-B.11
- 10.(PCy3)2(Cl)2Ru=CHPh (cat-A) Schwab P, Grubbs RH, Ziller JW. J Am Chem Soc. 1996;118:100–110.Schwab P, France MB, Ziller JW, Grubbs RH. Angew Chem, Int Ed. 1995;34:2039–2041.
- 11.(IMesH2)(PCy3)(Cl)2Ru=CHPh (cat-B) Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q.
- 12.Grubbs and co-workers have categorized various olefins by their relative rates of homodimerization correlating with the catalyst being used for CM. These types range from Type I olefins, classified by rapid, reversible, homodimerization, to Type IV olefins which are spectators to CM. Varying product ratios can be observed when pairing different olefin types; mixing Type I olefins yields a statistical mixture of CM and homodimerization, whereas CM between Type I and TypeII olefin pairs is very selective and high yielding of CM products. Using differential reactivity of olefins allows one to design selective CM by properly pairing olefin partners. Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882.
- 13.(a) Waetzig JD, Hanson PR. Org Lett. 2006;8:1673–1676. doi: 10.1021/ol0602809. [DOI] [PubMed] [Google Scholar]; (b) Waetzig JD, Hanson PR. Org Lett. 2008;10:109–112. doi: 10.1021/ol7025944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cat-C: Hoveyda-Grubbs 2nd Generation Catalyst Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J Am Chem Soc. 2000;122:8168–8179.
- 15.Meyers AG, Zheng B, Movassaghi M. J Org Chem. 1997;62:7507. doi: 10.1021/jo9710137. (b) For recent use of NBSH as a diimide source, see Haukaas MH, O'Doherty GA. Org Lett. 2002;4:1771–1774. doi: 10.1021/ol025844x.Buszek KR, Brown N. J Org Chem. 2007;72:3125–3128. doi: 10.1021/jo0622173. (b) For a flavin-catalyzed generation of diimide, see: Imada Y, Iida H, Naota T. J Am Chem Soc. 2005;127:14544–14545. doi: 10.1021/ja053976q.
- 16.In a model study, a one-pot, sequential ring-closing, cross-metathesis and olefin reduction were also conducted to generate (R,R,RP)-5 in situ in 30% overall yield. This yield averages to 67% per synthetic step over the 3-step combined transformation from the pseudo-C2-symmetric monocyclic triene phosphate (ent-9).
- 17.For a related study on using Pd-formate reductions to form terminal olefins see: Hughes G, Lautens M, Wen C. Org Lett. 2000;2:107–110. doi: 10.1021/ol991170n.Chau A, Paquin JF, Lautens M. J Org Chem. 2006;71:1924–1933. doi: 10.1021/jo052267s.
- 18.Helmboldt H, Koehler D, Hiersemann M. Org Lett. 2006;8:1573–1576. doi: 10.1021/ol060115t. [DOI] [PubMed] [Google Scholar]
- 19.Umezawa T, Hayashi T, Sakai H, Teramoto H, Yoshikawa T, Izumida M, Tamatani Y, Hirose T, Ohfune Y, Shinada T. Org Lett. 2006;8:4971–4974. doi: 10.1021/ol062098d. [DOI] [PubMed] [Google Scholar]
- 20.(a) Chen KM, Hardtmann GE, Prasad K, Repic O, Shapiro MJ. Tetrahedron Lett. 1987;28:155–158. [Google Scholar]; (b) Evans DA, Chapman KT, Carreira EM. J Am Chem Soc. 1998;110:3560–3578. [Google Scholar]
- 21.Whitehead A, Waetzig JD, Thomas CD, Hanson PR. Org Lett. 2008;10:1421–1424. doi: 10.1021/ol8001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas BS, Luther LM, Burke SD. Org Lett. 2004;6:2965–2968. doi: 10.1021/ol0488800. [DOI] [PubMed] [Google Scholar]
- 23.Marshall JA, Eidam P. Org Lett. 2004;6:445–448. doi: 10.1021/ol0363568. [DOI] [PubMed] [Google Scholar]
- 24.Compound 11 was also synthesized through a one-pot protocol in 59% yield utilizing the same reagents shown in Scheme 3.
- 25.The low diastereoselectivity prompted additional studies where 17 was oxidized to the corresponding ketone and subjected to various reducing conditions. Initial reduction utilizing Mori's conditions (LiAlH4, LiI) generated a 1:1.5 mixture of diastereomers, see: Mori Y, Kuhara M, Takeuchi A, Suzuki M. Tetrahedron Lett. 1988;29:5419–5422.Ghosh AK, Lei H. J Org Chem. 2002;67:8783–8788. doi: 10.1021/jo020402k. Other reductants such as L-selectride (dr = 1.7:1) and the CBS (Corey EJ, Shibata S, Bakshi RK. J Org Chem. 1988;53:2861–2863.) reduction (dr = 2.2:1) gave favorable ratios as well.
- 26.(a) Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull Chem Soc Jpn. 1979;52:1989–1993. [Google Scholar]; (b) Kawanami Y, Dainobu Y, Inanaga J, Katsuki T, Yamaguchi M. Bull Chem Soc Jpn. 1981;54:943–944. [Google Scholar]
- 27.Integration of the peaks areas of each mixture component resulted in a ∼2.6:1 of 1demethyleneated product and the ratio between the title compound to the constitutional isomer as >20:1.[17] It is assumed that the constitutional isomer results from further Ru-H isomerization of dolabelide C due to a comparison of retention times with the aforementioned C14/C15 Z-configured dolabelide C analog originally separated during normal-phase flash chromatography.
- 28.Clark JS, Kettle JG. Tetrahedron Lett. 1997;38:123–126.Overmann LE, Joe D. Tetrahedron Lett. 1997;38:8635–8638. For references citing difficulty in constructing trisubstituted E-olefins using RCM, see Hoye TR, Zhao H. Org Lett. 1999;1:169–171. doi: 10.1021/ol9906514.Smith AB, Mesaros EF, Meyer EA. J Am Chem Soc. 2006;128:5292–5299. doi: 10.1021/ja060369+.Jin J, Chen Y, Li Y, Wu J, Dai WM. Org Lett. 2007;9:2585–2588. doi: 10.1021/ol0710360.Becker J, Bergander K, Frölich R, Hoppe D. Angew Chem, Int Ed. 2008;47:1654–1657. doi: 10.1002/anie.200704678.
- 29.Sequence was run simultaneously in two batches to obtain the quantities noted.
- 30.The olefin metathesis catalysts screened in Figure 2 were provided by Materia, Inc.
- 31.Rigorous purification and degassing of the solvent was achieved through distillation over CaH2 and conventional freeze/thaw technique.
- 32.The RCM was performed on 70 mg scale providing 14 mg of an analytically pure sample of the desired E isomer and 10 mg of the Z isomer.
- 33.Optical rotation was measured over several trials resulting in variation of both the value and sign of analytically pure 1 (determined by LC-MS analysis, see supporting information). This phenomenon is consistent with hydrogen-bonding systems, where inconsistency is frequently observed. Abraham E, Davies SG, Roberts PM, Russell AJ, Thomson JE. Tetrahedron:Asymmetry. 2008;19:1027–1047. and references cited within.
-
34.The major diastereomer determined to be E, due to resonances matching with listed resonances in reference 3. The E geometry is confirmed from comparison of 13C NMR, in which the C-14 methyl has a chemical shift (15.7 ppm) and the Z-isomer (23.3 ppm), which is consistent with reference 3 and the paper cited within (Carey L, Clough JM, Pattenden G. J Chem Soc Perkin Trans I. 1983:3005–3009.) where it's stated “The 13C NMR shifts of vinyl methyl and vinyl methylene carbon atoms associated with isolated trisubstituted double bonds are critically dependent on the configuration of the double bond as a result of the well-known γ-effect.” For example:

- 35.Purification of each isomer was achieved using 2-3 consecutive runs on normal phase flash chromatography (see supporting information).
- 36.(a) Spectrum integrated to 41 total H, where the unassigned H was presumed to be an O-H peak undergoing H-D exchange; (b) Spectrum integrated to 71 total H, where the unassigned H was presumed to be an O-H peak undergoing H-D exchange. Only the O-H peaks did not match exactly to the reported data; (c) Spectrum integrated to 71 total H, where the unassigned H was presumed to be an O-H peak undergoing H-D exchange; (d) Spectrum integrated to 69 total H, where the unassigned H's were presumed to be O-H peaks undergoing H-D exchange.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.