Abstract
Histone deacetylases (HDACs) are a group of enzymes that modulate gene expression and cell state by deacetylation of both histone and non-histone proteins. A variety of HDAC inhibitors (HDACi) have already undergone clinical testing in cancer. Real-time in vivo imaging of HDACs and their inhibition would be invaluable; however, the development of appropriate imaging agents has remained a major challenge. Here, we describe the development and evaluation of 18F-suberoylanilide hydroxamic acid (18F-SAHA 1a), a close analog of the most clinically relevant HDACi, suberoylanilide hydroxamic acid (SAHA). We demonstrate that 1a has near identical biochemical activity profiles to SAHA, and report findings from pharmacokinetic studies. Using a murine ovarian cancer model, we likewise show that HDACi target binding efficacy can be quantitated within 24 hours of administration. 1a thus represents the first 18F-positron emission tomography (PET) HDAC imaging agent, which also exhibits low nanomolar potency and is pharmacologically analogous to a clinically relevant HDACi.
Keywords: Histone Deacetylase, Epigenetics, PET, imaging, in vivo, inhibitor
Introduction
Histone deacetylases (HDACs) are enzymatic components of chromatin modifying complexes and modulate gene expression via interactions with transcriptional regulatory proteins, and via modification of the amino-terminal histone tail lysine residues.1, 2 More recently a considerable number of non-histone protein substrates have also been identified, indicating that lysine acetylation represents a post-translational modification with broad regulatory significance.3 To date, 18 different HDACs have been characterized and, based on their phylogeny, grouped into 4 major classes.4 Class I (HDAC1–3, 8), class II (HDAC4–7,9) and class IV (HDAC11) are zinc-dependent hydrolases that are formally referred to as HDACs. In contrast, class III (Sirt1–7) enzymes, the sirtuins, function by a catalytically distinct, NAD+-dependent mechanism. While the enzymatic activities of HDAC isoforms overlap in vitro, their biological activity and substrate selectivities in vivo are typically regulated by specific multi-protein binding partners, as well as by their unique tissue and cellular distribution.
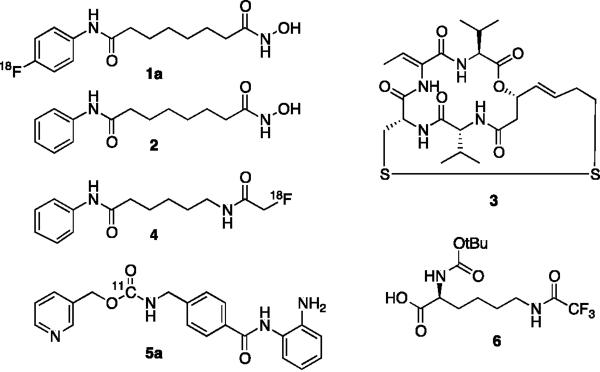
The first HDAC inhibitors (HDACi) were developed for cancer applications following findings that they induce apoptosis and differentiation in malignant cell lines.5, 6 Thus far, two chemically distinct HDACi, suberoylanilide hydroxamic acid (SAHA) 2 (Vorinostat, Merck Research Laboratories) and the more recently developed microbial natural product, FK228 3 (Romidepsin, Celgene),2 have received FDA approval for the treatment of specific T-cell malignancies (Fig. 1). In addition, increasing information regarding HDAC function from genetic studies have now elucidated specific roles for different HDAC isoforms in the regulation of fundamental biological processes.3 Notably, they have been demonstrated to be master regulators of mitosis, cell differentiation, apoptosis,6 chromatin organization,7 metabolism,8 learning and memory,9 as well as of the immune response10. Given the relevance that most of these critical cellular functions have to disease, small molecule modulators of chromatin modifying enzymes are now being developed, not only for oncological applications, but more broadly for the treatment of psychiatric, immunological, neuromuscular, inflammatory, metabolic, genetic as well as infectious diseases.2, 11, 12
Figure 1.
Structures of HDAC inhibitors and HDAC imaging agents.
SAHA is a broad spectrum inhibitor that potently targets class I and class II HDAC enzymes.13 At present, SAHA as well as other HDACi, are being explored as potential drug therapies for a number of different malignancies, both as single agents and in combination with other agents.2, 12, 14 A critical bottleneck in the clinical development and use of HDACi has been the inability to rapidly quantitate target inhibition in the clinic. Acetylation of peripheral mononuclear cells initially showed early promise as a potential biomarker, but ultimately failed to correlate with clinical response in patients.15 Conversely, conventional anatomic imaging modalities used to monitor clinical response are only capable of reporting on tumor size or on rudimentary physiologic changes such as alterations in perfusion, both of which occur relatively late after treatment onset. In vivo imaging of HDAC proteins (and to some degree their activity state) has likewise been rudimentary at best and, as such, detailed analyses have been limited to readily accessible tissue specimens, which can only be analyzed using ex vivo techniques. Thus, in order to investigate the efficiency of existing and novel HDACi, there is a clear need for imaging probes. Moreover, imaging of HDACs may provide important basic scientific insights, which could lead to novel clinical applications.
To date, only a few compounds for in vivo HDAC imaging have been reported. Reid et al., for example, developed 6-([18F]-fluoroacetamide)-1-hexanoicanilide (FAHA) 4 for cerebral imaging. 4 is an 18F-functionalized hybrid of the HDACi SAHA an acetyl-lysine mimic that lacks the hydroxamate chelator, which is required for potent target binding.16 Furthermore, 4, which features a reactive fluoro-ketone and resembles an HDAC substrate rather than an inhibitor, is rapidly metabolized in vivo, thus complicating imaging and analysis. More recently the development of [11C]MS-275 5a a carbon11-labeled version of the benzamide-class HDACi MS-275 (Entinostat, Syndax Pharmaceuticals) 5b has been reported for cerebral imaging.175b, as most benzamide based HDAC inhibitors, is selective for HDAC1–3, albeit 20–70 fold less potent than SAHA.13 Unfortunately, the blood brain barrier penetration of 5a was found to be unsuitable for the intended studies and competition with cold 5b failed to demonstrate specific binding.17 Further, the short half-life of carbon-11 (~20 min) compared to fluorine-18 (~110 min) imposes technical challenges and limits potential applications. Another study by Ronen and co-workers, investigated the use of Boc-lysine trifluoroacetic acid 6 (BLT) as a substrate for HDACs, using 19F magnetic resonance spectroscopy.18 Trifluoroacetyl-lysine-based substrates, however, are not suitable for all HDACs as they exhibit large variations in their specificity for individual HDAC isoforms. For example, the principal enzymes that metabolize BLT are HDAC 8 and (to a lesser degree) class IIa HDACs. In contrast, current clinical HDACi candidates primarily target HDACs 1, 2, 3 and 6.13, 19
Results/Discussion
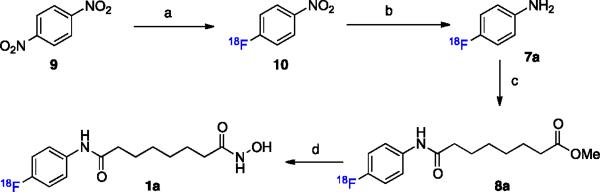
Here we describe the development of an in vivo PET imaging agent with strong similarities in pharmacology, potency and isoform selectivity to SAHA, the most clinically relevant HDACi. Introduction of an 18F label to the 4-position of the aromatic ring of SAHA (18F-SAHA 1a) appeared to be the most practical approach. To first determine that this functionalization would not negatively impact the compound's biological activity, we synthesized and profiled 19F-SAHA 1b against HDACs 1–9. The synthesis was readily accomplished by following reported strategies, with minor modification (Scheme 1).20 Synthesis began with acylation of the commercially available 4-fluoro aniline 7b, with suberic chloride methyl ester. Subsequent treatment with basic hydroxylamine resulted in the conversion of the methyl ester 8b to the hydroxamic acid.
Scheme 1.
Synthesis of 1b
(a) Methyl 8-chloro-8-oxooctanoate, Et3N, CH2Cl2, rt, 1hr, 54%; b) 50% NH2OH(aq), 1 N NaOH(MeOH), rt, 12hr, 65%.
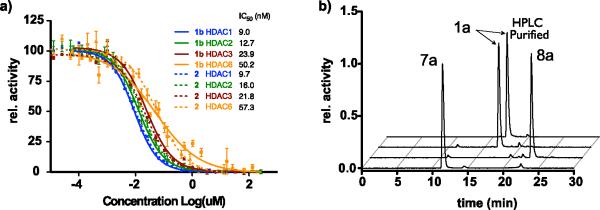
The biochemical profiling of 1b against purified HDAC isoforms demonstrated that 1b, as expected, inhibited only HDACs 1–3 and 6.13 Moreover, inhibition of these primary HDAC targets by 1b exhibited a potency and selectivity virtually indistinguishable from SAHA itself. This finding thus identified 1b as a promising candidate for the development of a PET imaging probe (Fig. 2a).
Figure 2.
a) Dose-response profile for 1b and 2 against HDACs1-3 and 6; b) Analytical Radio-HPLC traces.
Our next goal was to develop a reliable synthetic strategy for the routine synthesis of 1a, which would enable generation of 1a in quantities sufficient even for large animal studies. Direct fluorination of 1,4-dinitrobenzene 9 with 18F- (n.c.a.) under phase transfer conditions and microwave heating, afforded 1-[18F]-fluoro-4-nitrobenze 10 after 5 minutes at 120 °C. This intermediate was consecutively reduced by catalytic hydrogenation with sodium borohydride and Pd/C to yield 4-[18F]-fluoro-aniline 7a, which was isolated on a LiChrolut EN column.21 The 18F-labeled aniline was subsequently converted to the desired product via compound 1a, following the same strategy employed for synthesis of cold 1b using optimized conditions for radiochemical synthesis (Fig. 2b). Overall, this 4-step synthesis is remarkably clean, can be carried out in less than 2 hours and affords 1a with a decay corrected yield of 40%. Following HPLC purification, the radiochemical purity was greater 98% (Scheme 2). Several alternative routes attempting to introduce the 18F later in the synthesis were explored unsuccessfully.
Scheme 2.
Synthesis of 1a
(a) 1. K2CO, K222,18F (n.c.a.), DMSO, 120 °C microwave, 5 min; 2. C18 isolation, MeOH elution. 78% (b) 1. NaBH4, 10% Pd/C,rt., 10 min; 2. LiChrolut EN isolation, THF elution, Na2SO4/Celite drying. 58% (c) Methyl 8-chloro-8-oxooctanoate, rt, 5 min. (d) 1. 50% NH2OH(aq), 1 N NaOH(MeOH), rt, 3min min; 2. HPLC Purification, concentration; 3. Reconstitution into vehicle (88% two steps).
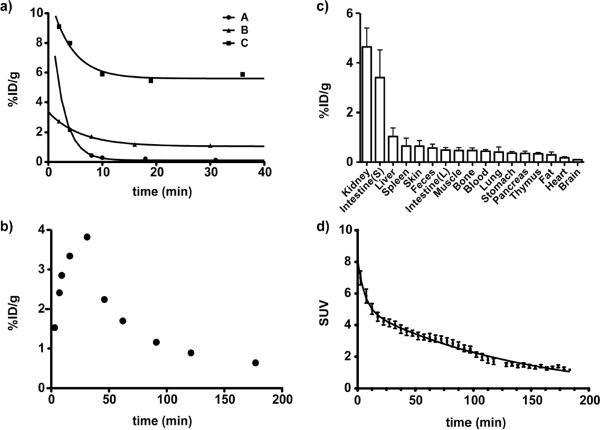
To date, very little information exists in the published scientific literature on the pharmacokinetic and tissue distribution of SAHA in murine models. Such knowledge however, is critical for the development of therapeutic applications in central nervous system disorders or spinal muscular atrophy. Thus, following scale-up synthesis and characterization of the radiotracer, we next tested the compound in vivo. Initially, we determined the plasma half-life of 1a and its tissue distribution. The blood half-life, following a single bolus intravenous injection, was then measured for three commonly utilized pharmacological formulations (A: 60% PEG, 40% water; B: 10% DMSO in water; and C: 10% DMAC, 10% Solutol HS15, 80% PBS). All formulations exhibited a biphasic pharmacokinetic profile, with rapid elimination during the first 10 minutes. The initial t1/2 was estimated as <1–4 minutes, mostly through renal clearance, and this was followed by a terminal half-life that was approximately 10 times longer. Notably, the initial half-lives appeared to be strongly dependent on the formulation, but these early time points are generally not captured by traditional liquid chromatography/mass spectrometry (LC/MS)-based approaches. Less than 1% of the dosed compound was still present in whole blood when using formulation A (60% PEG400, 40% water), while formulation B (10%DMSO, 90% water) and C (10% dimethylacetamide, 10% Solutol HS15, 80% PBS) resulted in retention of approximate 3% and 6% of the compound, respectively (Fig. 3a). Following this initial “burst” phase, elimination half-lives appeared to be equivalent, and correlated well with the blood-half life, as determined by serial in vivo PET-imaging (Fig. 3d). We also tested oral administration of the compound (formulation C, Fig. 3b) and observed similar kinetics as determined by conventional HPLC mass spectrometry.
Figure 3.
(a) Blood half-life of 1a following i.v. and (b) p.o. administration; c) biodistribution; d) blood half-life determined by dynamic PET-scan (%ID/g - Injected Dose per Gram of Tissue; SUV - standardized uptake value).
We subsequently determined the tissue distribution of 1a. For each experiment, cohorts of mice were sacrificed at 1 hour and 3 hours following intravenous injection of the probe. Tissues were then excised, weighed and 18F-activity measured. As shown in Fig. 3c, 1a had the highest concentration in the kidney, liver and blood, with relatively low distribution in other tissues (Fig. 3c,4a). Interestingly, cardiac tissue also appeared to have comparatively low concentrations of 1a, which may in part explain the relatively low cardiotoxicity of SAHA compared to other HDACi. Of all organs investigated, the thymus and brain exhibited the lowest concentration of 1a, implying that if used to study brain function, SAHA would likely require intrathecal administration.
Figure 4.
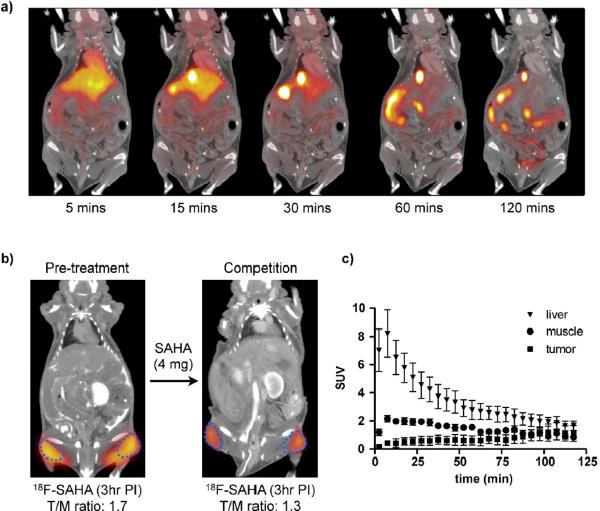
a) Dynamic PET-CT following i.v. administration of 1a in C57BL/6 mouse; b) PET-CT scan of tumor xenograft before and after competition with `cold' SAHA, blue dotted line indicates tumor perimeter; c) Time dependent distribution of 1a.
Upon demonstrating that 1a exhibits a suitable pharmacological profile, we next assessed whether administration of `cold' SAHA could be used to measure HDAC inhibition in a mouse model of cancer. Since SAHA is currently undergoing clinical trials for the treatment of ovarian cancer, we selected the A2780 ovarian cancer model for this study. Consistent with class I HDAC expression levels in both malignant and benign tissues (Fig. 4c), 1a was observed to accumulate in tumors in a time-dependent manner, following its systemic administration. To subsequently determine whether 1a imaging could be used to measure HDAC inhibition in vivo, we performed repeat 1a PET imaging experiments on a cohort of mice both before and after cold SAHA treatment (2 × 2 mg injection; Fig. 4b). As expected for selective competition, the tumor/muscle ratio decreased from 1.7 ± 0.12 to 1.3 ± 0.01 (23%), following two single doses of cold SAHA treatment in a 24 hour period (Fig. 4b). These studies thus show proof-of principle that 1a imaging can be used to measure target engagement within 24 hours of drug administration. Overall, these studies describe the development of a versatile HDAC PET imaging agent, which not only exhibits low nanomolar potency but is also pharmacologically similar to a clinically relevant HDACi. It is likely that this compound will be useful for testing SAHA as well as other HDACi in the clinic.
Experimental Methods
Chemistry
Reagents were purchase from Acros, Alfa-Aesar and Sigma-Aldrich Co. and used without further purification unless otherwise noted. Silica gel used for purification was Silica Gel 60, 40–63 μm. Proton and carbon nuclear magnetic resonance (1H & 13C NMR) spectra were recorded on a Varian AS-400 (400MHz) spectrometer. Chemical shifts for protons are reported in parts per million (ppm) and are referenced against the dimethylsulfoxide lock signal (1H, 2.50ppm; 13C, 39.52 ppm). Data is reported as follows: chemical shift, integration, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet) and coupling constants (Hz). Preparatory and analytical LCMS were performed on mass directed Autopurification systems from Waters Co. (Milford, MA), operated by Fractionlynx 4.0 or Masslynx software with Waters Xterra columns (C18, 10 μm, 19 × 50 mm for preparatory; C18, 5 μm, 4.6 × 50 mm for analytical). High-resolution mass spectra were acquired on a Bruker Daltonics APEXIV 4.7 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer (FT-ICR-MS), with ESI (Electro Spray Ion) source. The chemical purity of the target compounds was determined by LC/MS using the following conditions: a Waters SFO/3100 with Waters Xterra C18 column using a binary solvent system (0.1% TFA in water and acetonitrile) monitoring between 200–400nm. The purity of each compound was ≥95%.
7-(4-Fluoro-benzoylamino)-heptanoic acid methyl ester 8b
4-Fluoroaniline 7b (259 μL, 2.7 mmols) and triethylamine (376 μL, 2.7 mmols) were dissolved in DCM (2 mL) and cooled to 0 °C. To this solution was added methyl suberyl chloride (350 μL, 2.5 mmols). After 1 hour the reaction was diluted with DCM (10 mL) and then washed with H2O (10 mL), brine (10 mL) and then dried with magnesium sulfate. Purification via column chromatography (silica gel, DCM/EtOAc 40%) gave product 8b (383 mg, 54%). 1H NMR (400 MHz, DMSO)-d6) δ 9.91 (s, 1H), 7.59 (dd, J = 9.2, 5.1 Hz, 2H), 7.12 (t, J = 9.0 Hz, 2H), 3.57 (s, 3H), 2.28 (q, J = 7.5 Hz, 4H), 1.63 – 1.44 (m, 4H), 1.29 (dd, J = 7.3, 3.6 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) δ 173.37, 171.09, 157.778 (d, JC–F = 240.4 Hz), 135.77, 120.69 (d, JC–F = 8.1 Hz), 115.20 (d, JC–F = 22.2 Hz), 51.21, 36.24, 33.24, 28.25, 24.94, 24.34. HRMS (ESI) for C15H20FNO3: Calculated 282.1500; Observed 282.1506 [M+H]+, 304.1332 [M+Na]+
N-Hydroxy-N'-(4-fluorophenyl)octanediamide 1b
Methyl ester 8b (180 mg, 0.640 mmols) was suspended in a 1:1 (v/v) mixture of methanol and 50% hydroxyl amine (aq). To this suspension was added 1N NaOH(aq) (1.5 mL). After 12 hours the reaction had become a homogeneous mixture. 1N HCl was added bringing the solution back to a neutral pH, upon which the product precipitated from solution. Filtration of the white solid afforded the product 6 (84 mg, 65%). 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 9.92 (s, 1H), 8.67 (s, 1H), 7.59 (dd, J = 9.2, 5.1 Hz, 2H), 7.12 (t, J = 8.9 Hz, 2H), 2.27 (t, J = 7.4 Hz, 2H), 1.93 (t, J = 7.4 Hz, 2H), 1.55 (t, J = 14.5, 7.2 Hz, 2H), 1.47 (t, J = 14.2, 7.2 Hz, 2H), 1.31 – 1.20 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ 171.11, 169.09, 157.78 (d, JC–F = 240.4 Hz), 135.77, 120.70 (d, JC–F = 8.1 Hz), 115.21 (d, JC–F = 22.2 Hz), 36.29, 32.26, 28.43, 25.06, 25.03. HRMS (ESI) for C14H19FN2O3: Calculated 283.1452; Observed 283.1444 [M+H]+, 305.11258 [M+Na]+
Radiochemistry
[18F]-Fluoride (n.c.a.) in 18O enriched water was purchased from PETNET (Woburn, MA). Solid phase extraction cartridges used were purchased from Thermo (HYPERSEP C18, 500 mg, 3 mL) and Merck (LiChrolut EN, 200 mg, 3 mL). Preparative high performance liquid chromatography (HPLC) purification (Method A) was achieved using a Machery-Nagel Nucleodur C18 Pyramid 250 × 10 mm Vario-Prep column (80:20 (v/v) in water:acetonitrile (MeCN) with 0.1 % trifluoroacetic acid at 5.5 ml˙min−1) and analytical HPLC (Method B) was performed employing a Grace VYDAC (218TP510) C18 reversed-phase column (eluents: A = 0.1% TFA (v/v) in water and B = MeCN; gradient: 0–17 min, 5–60% B; 17–21 min, 60–95% B; 21–24 min, 95% B; 24–25 min, 95-5% B; 25–30 min, 5% B; 2 mL min−1) with a dual-wavelength UVVis detector followed by a flow-through gamma detector connected in series. Preparative and analytical HPLC analyses of 18F-labeled compounds were calibrated with the corresponding 19F analogs. Microwave synthesis was conducted in a CEM (Matthews, North Carolina) Microwave Synthesizer operated with Discover® software package. Radioactive samples injected into mice were measure using a Capintec 127R dose calibrator. Radioactivity in blood half-life and biodistribution studies was quantified using a Perkin Elmer Wallac Wizard 3” 1480 Automatic Gamma Counter.
Synthesis of 1a
A 10-mL microwave test tube was charged with 26.9 ± 2.7 mCi (1.0 GBq) of [18F]-fluoride (n.c.a.), K2CO3 (1.38 mg, 10 μmol) in water 135 μL, Kryptofix 222 (6.8 mg, 18 μmol), and 1 mL MeCN. Water was removed azeotropically by microwave heating to 98 °C and a flow of argon. Azeotropic drying is repeated by the addition of anhydrous MeCN (4×1 mL) at 3 min intervals. After cooling to rt, 1,4-dinitrobenzene 9 (4 mg, 23.8 μmol) in DMSO (500 μL) was added. The vessel was sealed and heated to 120 °C for 5 min then cooled to rt. The reaction mixture is diluted with water (8 mL) and passed through a C18 cartridge (Thermo, 500 mg, conditioned with 10 mL EtOH and 2×10 mL water) trapping the 4-[18F]-fluoronitrobenzene (10). The C18 cartridge was washed with water (5 mL) and 16.1 ± 0.9 mCi (596 MBq) of 10 was eluted from the cartridge with MeOH (1 mL) in 78.3 ± 6.4% dcRCY. A 10 μL aliquot was removed for HPLC analysis (Method B), 10 tR = 21.4 min. After addition of 3 mg Pd/C and 28 mg (740 μmol) NaBH4, the reaction mixture was stirred at rt for 5 min at which time unreacted NaBH4 was quenched by the addition of 300 μL 6 N HCl. The mixture was diluted with 1 N NaOH(aq) (8 mL) and passed through a Lichrolut EN cartridge (Merck, 500 mg, conditioned with 10 mL EtOH and 2×10 mL water). 4-[18F]-Fluoroanaline 7a was eluted from the Lichrolut EN cartridge and through 500 mg of Na2SO4 and Celite (3:2 w/w) with THF (1 mL) to give 10.1 ± 1.3 mCi (370 MBq) in 57.5 ± 5.0% dcRCY. A 10 μL aliquot was removed for HPLC analysis (Method B), 7a tR = 11.4 min. To the 7a /THF solution was added methyl 8-chloro-8-oxooctanoate (34 μL, 242 μmol) and was stirred for 5 min at rt. A 10 μL aliquot was removed for HPLC analysis (Method B), 8a tR = 22.8 min. The addition of 250 μL 50% NH2OH(aq) and 750 μL 1 N NaOH(MeOH) and stirring for 3 min provided the desired hydroxamic acid 1a. A 10 μL aliquot was removed for HPLC analysis (Method B), 1a tR = 17.0 min. THF and MeOH were evaporated off (50 °C microwave heating under a stream of argon) and the reaction mixture purified by preparative HPLC (Method A) providing 5.1 ± 0.7 mCi (189 MBq) of 1a in 120 ± 12 min, a 39.5 ± 6.0% dcRCY in 97.0 ± 4.7% radiochemical purity over 4 steps and HPLC purification.
Mice
Five Nu/Nu mice received subcutaneous injections with 106 A2780 human ovarian cancer cells in matrigel into each flank and were imaged 10 days later. Mice were anesthetized (Isoflurane 1.5%, O2 2L/min) during PET-CT imaging. Therapeutic doses of SAHA (2 mg in 100 uL 60%PEG400:40%water) were administered i.p. following initial PET-CT scan and repeated 24 hours later. After 48 hr from initial therapeutic dose, PET-CT scans were performed again following the i.v. administration of 1a. Ten C57BL/6 mice were used for blood half-life studies. Mice were administered 243 ± 25 μCi of 1a by tail vein i.v. injection formulated in 60% PEG400 in water (n=3), 10% DMSO in water (n=3), 20% DMAC:Solutol (1:1) in saline (n=3). In a separate experiment a mouse (n=1) was administered 265 μCi of 1a by p.o. Blood sampling was performed by retro-orbital bleed using tared capillary tubes. Samples were weighed and activity measured by gamma counter. Six C57BL/6 mice were injected with 241 ± 18 μCi of 1a by tail vein i.v. injection and used to determine biodistribution. Animals were sacrificed at 1 h (n=3) and 3 h (n=3) post injection. Tissues were excised, weighed and activity measured. Graphpad Prism 4.0c (GraphPad Software Inc, San Diego, CA) was used for regression and statistical analysis. All animal experiments were approved by the Massachusetts General Hospital Institutional Review Committee.
PET-CT
All images were acquired on a Siemens Inveon PET-CT. Each PET acquisition was ~80 minutes in duration. PET was reconstructed from 600 million coincidental, 511 keV photon counts on a series of LSO (lutetium oxyorthosilicate) scintillating crystal rings. Counts were rebinned in 3D by registering photons spanning no more than 3 consecutive rings, then reconstructed into sinograms by utilizing a high resolution Fourier Rebin algorithm. A reconstruction of sinograms yielded a 3D mapping of positron signal using a 2D filtered back-projection algorithm, with a Ramp filter at a Nyquist cut-off of 0.5. Image pixel size was anisotropic, with dimensions of 0.796 mm in the z direction and 0.861 mm in the x and y directions, for a total of 128 × 128 × 159 pixels. Calibration of PET signal preceded all scans and was done by scanning an 8.0 cm cylindrical phantom containing a known amount of 18F isotope.
CT was reconstructed from 360 projections of X-rays with a cone beam angle of 9.3 degrees over 360 degrees perpendicular to the animal bed. 80 keV X-rays were transmitted from a 500 μA anode source, 347 mm from the center of rotation and recorded on a CCD detector, containing 2048 transaxial and 3072 axial pixels. Projections were calibrated using 70 dark and 70 light images, interpolated bilinearly, processed through a Shepp-Logan filter, then reconstructed using a filtered back projection algorithm. Isotropic CT pixel size was 110.6 μm, with a total of 512 × 512 × 768 pixels. Scaling to Hounsfield Units, calibration was done using a 8.0 cm cylindrical phantom containing water prior to CT acquisition.
HDAC Assay
The activity of HDAC inhibitors against individual HDAC isoforms was performed as reported previously.13
Supplementary Material
ACKNOWLEDGMENT
Financial support from the NCI (P50CA086355 and U24CA092782) is gratefully acknowledged. T.R. received a stipend from the Deutschen Akademie der Wissenschaften Leopoldina (LPDS 2009-24)
Footnotes
SUPPORTING INFORMATION PARAGRAPH. NMR data for compounds 1b and 8b. This material is available free of charge via the Internet at http://pubs.acs.org.
ABBREVIATIONS: BLT - Boc-lysine trifluoroacetic acid, DCM - Dichloromethane, DMSO - Dimethyl sulfoxide, DMAC - Dimethylacetamide, FAHA - 6-([18F]-fluoroacetamide)-1-hexanoicanilide, HDAC -Histone deacetylases, HDACi - Histone deacetylases inhibitor, HPLC - High-performance liquid chromatography, LC/MS - Liquid chromatography–mass spectrometry, PEG - Polyethylene glycol, PBS -Phosphate buffered saline, PET - Positron emission tomography, PET-CT - Positron emission tomography - computed tomography, SAHA - Suberoylanilide hydroxamic acid, SUV - standardized uptake value, THF -Tetrahydrofuran
REFERENCES
- (1).Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- (3).Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- (4).Gregoretti I, Lee Y-M, Goodson HV. Molecular Evolution of the Histone Deacetylase Family: Functional Implications of Phylogenetic Analysis. J. Mol. Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- (5).Haigis MC, Sinclair DA. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol.: Mech. Dis. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Richon VM, Webb Y, Merger R, Sheppard T, Jursic B, Ngo L, Civoli F, Breslow R, Rifkind RA, Marks PA. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc. Natl. Acad. Sci. U S A. 1996;93:5705–5708. doi: 10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- (8).Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc. Natl. Acad. Sci. U S A. 2004;101:18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- (10).Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zhong L, Urso AD, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The Histone Deacetylase Sirt6 Regulates Glucose Homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Guan J-S, Haggarty SJ, Giacometti E, Dannenberg J-H, Joseph N, Gao J, Nieland TJF, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai L-H. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kim D, Frank CL, Dobbin MM, Tsunemoto RK, Tu W, Peng PL, Guan J-S, Lee B-H, Moy LY, Giusti P, Broodie N, Mazitschek R, Delalle I, Haggarty SJ, Neve RL, Lu Y, Tsai L-H. Deregulation of HDAC1 by p25/Cdk5 in Neurotoxicity. Neuron. 2008;60:803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, Hancock WW. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol. Cell. Biol. 2011;31:2066–2078. doi: 10.1128/MCB.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nat. Rev. Drug. Discov. 2009;8:969–981. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Patel V, Mazitschek R, Coleman B, Nguyen C, Urgaonkar S, Cortese JF, Barker RH, Greenberg E, Tang W, Bradner JE, Schreiber SL, Duraisingh MT, Wirth DF, Clardy J. Identification and Characterization of Small Molecule Inhibitors of a Class I Histone Deacetylase from Plasmodium falciparum. J. Med. Chem. 2009;52:2185–2187. doi: 10.1021/jm801654y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wiech NL, Fisher JF, Helquist P, Wiest O. Inhibition of histone deacetylases: a pharmacological approach to the treatment of non-cancer disorders. Curr. Top. Med. Chem. 2009;9:257–271. doi: 10.2174/156802609788085241. [DOI] [PubMed] [Google Scholar]
- (18).Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, Mazitschek R. Chemical phylogenetics of histone deacetylases. Nat. Chem. Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280:168–176. doi: 10.1016/j.canlet.2008.10.047. [DOI] [PubMed] [Google Scholar]
- (20).O'Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, MacGregor-Cortelli B, Curly T, Moskowitz C, Portlock C, Horwitz S, Zelenetz AD, Frankel S, Richon V, Marks P, Kelly WK. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J. Clin. Oncol. 2006;24:166–173. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- (21).Reid AE, Hooker J, Shumay E, Logan J, Shea C, Kim SW, Collins S, Xu Y, Volkow N, Fowler JS. Evaluation of 6-([(18)F]fluoroacetamido)-1-hexanoicanilide for PET imaging of histone deacetylase in the baboon brain. Nucl. Med. Biol. 2009;36:247–258. doi: 10.1016/j.nucmedbio.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hooker JM, Kim SW, Alexoff D, Xu Y, Shea C, Reid A, Volkow N, Fowler JS. Histone Deacetylase Inhibitor MS-275 Exhibits Poor Brain Penetration: Pharmacokinetic Studies of [11C]MS-275 using Positron Emission Tomography. ACS Chem. Neurosci. 2010;1:65–73. doi: 10.1021/cn9000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Sankaranarayanapillai M, Tong WP, Yuan Q, Bankson JA, Dafni H, Bornmann WG, Soghomonyan S, Pal A, Ramirez MS, Webb D, Kaluarachchi K, Gelovani JG, Ronen SM. Monitoring histone deacetylase inhibition in vivo: noninvasive magnetic resonance spectroscopy method. Mol. imaging. 2008;7:92–100. [PubMed] [Google Scholar]
- (24).Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones PA, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, Koch U, De Francesco R, Steinkuhler C, Gallinari P. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc. Natl. Acad. Sci. U S A. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Gediya LK, Chopra P, Purushottamachar P, Maheshwari N, Njar VCO. A New Simple and High-Yield Synthesis of Suberoylanilide Hydroxamic Acid and Its Inhibitory Effect Alone or in Combination with Retinoids on Proliferation of Human Prostate Cancer Cells. J. Med. Chem. 2005;48:5047–5051. doi: 10.1021/jm058214k. [DOI] [PubMed] [Google Scholar]
- (26).Olma S, Ermert J, Coenen HH. 4-[18F]fluorophenyl ureas via carbamate-4-nitrophenyl esters and 4-[18F]fluoroaniline. J. Labelled Compd. Radiopharm. 2006;49:1037–1050. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.