Abstract
A question of great interest in current sleep research is whether and how sleep might facilitate complex cognitive skills such as decision-making. The Iowa Gambling Task (IGT) was used to investigate effects of sleep on affect-guided decision-making. After a brief standardized preview of the IGT that was insufficient to learn its underlying rule, participants underwent a 12-hr delay containing either normal night’s sleep (Sleep-group; N=28) or continuous daytime wake (Wake-group; N=26). Following the delay, both groups performed the full IGT. To control for circadian effects, two additional groups performed both the preview and the full task either in the morning (N=17) or the evening (N=21). In the IGT, 4 decks of cards were presented. Draws from 2 “advantageous decks” yielded low play-money rewards, occasional low losses and, over multiple draws, a net gain. Draws from “disadvantageous” decks yielded high rewards, occasional high losses and, over multiple draws, a net loss. Participants were instructed to win and avoid losing as much as possible and better performance was defined as more advantageous draws. Relative to the wake group, the sleep group showed both superior behavioral outcome (more advantageous draws) and superior rule understanding (blindly judged from statements written at task completion). Neither measure differentiated the two control groups. These results illustrate a role of sleep in optimizing decision-making, a benefit that may be brought about by changes in underlying emotional or cognitive processes.
Keywords: Sleep, Decision-Making, Iowa Gambling Task, Executive Function
1. Introduction
“Sleeping on a problem” to aid in its solution is a universal tenet of folk psychology. Recent studies have shown a deleterious impact of sleep deprivation on decision-making (Venkatraman et al., 2007, Killgore et al., 2006a, Harrison and Horne, 2000). Additionally sleep loss impacts key components of effective decision-making such as executive functions (Harrison and Horne, 2000), risk proneness (Venkatraman et al., 2007), behavioral inhibition (Drummond et al., 2006), working memory (Chee et al., 2006) and moral reasoning (Killgore et al., 2007). Likewise, intervals with sleep have been shown to enhance learning (Walker and Stickgold, 2006), provide insight (Wagner et al., 2004) and facilitate creativity (Cai et al., 2009). In addition, sleep has been shown to promote memory consolidation not only for explicitly learned procedural and declarative tasks, but also for implicitly acquired information that can subsequently support new skills such as categorization (Maddox et al., 2009, Djonlagic et al., 2009) and rule discovery (Wagner et al., 2004).
To investigate if sleep modulates decision-making, we compared performance on the Iowa Gambling Task (IGT) when either overnight sleep or daytime waking followed an initial, brief exposure to the task. The IGT is an affect-guided rule-learning task that recruits many of the complex cognitive and emotional processes required for everyday judgment and decision making (Bechara et al., 1994, Guillaume et al., 2009). Many of these underlying processes have been shown to be enhanced by sleep (Cai et al., 2009, Wagner et al., 2004, Ellenbogen et al., 2007) and disrupted by sleep deprivation (Killgore et al., 2006b). Optimal performance on the IGT is believed to require emotional memories of rewards and losses that are considered possible exemplars of “somatic markers” (Bechara et al., 1997, Bechara et al., 1999). Such memories are acquired implicitly early in the task (Bechara et al., 1997, Wagar and Dixon, 2006) and contribute to explicit learning of contingencies acquired as the task proceeds (Wagar and Dixon, 2006, Guillaume et al., 2009). We hypothesized that intervening sleep would benefit performance following exposure on the IGT in much the same way that “sleeping on it” appears to facilitate decisions made in everyday life.
2. Methods
2.1 Participants
Participants were 104 adults (72 female) aged 18–29 (mean 21.2, SD 2.4) who volunteered in exchange for course credit or payment. Participants were assigned to either Sleep (N=30, 22 female) or Wake (N=31, 25 female) “experimental groups” or to Morning-Control (N=20, 12 female) or Evening-Control (N=23, 13 female) “control groups”. Advertising specified that participants must be without psychiatric, sleep or neurological disorders. A 23-item screening questionnaire, administered at Session 1, subsequently identified those individuals with potentially confounding medical or psychiatric histories, using sleep affecting or psychiatric medication, or having sleep-disrupted lifestyles. Such individuals’ data were excluded from analyses leaving a total of 92 participants: 28 Sleep (20 female), 26 Wake (21 female), 17 Morning-Control (9 female) and 21 Evening-Control (12 female). This study was approved by the University of Massachusetts, Amherst, Institutional Review Board and all participants gave written informed consent.
2.2 Procedure
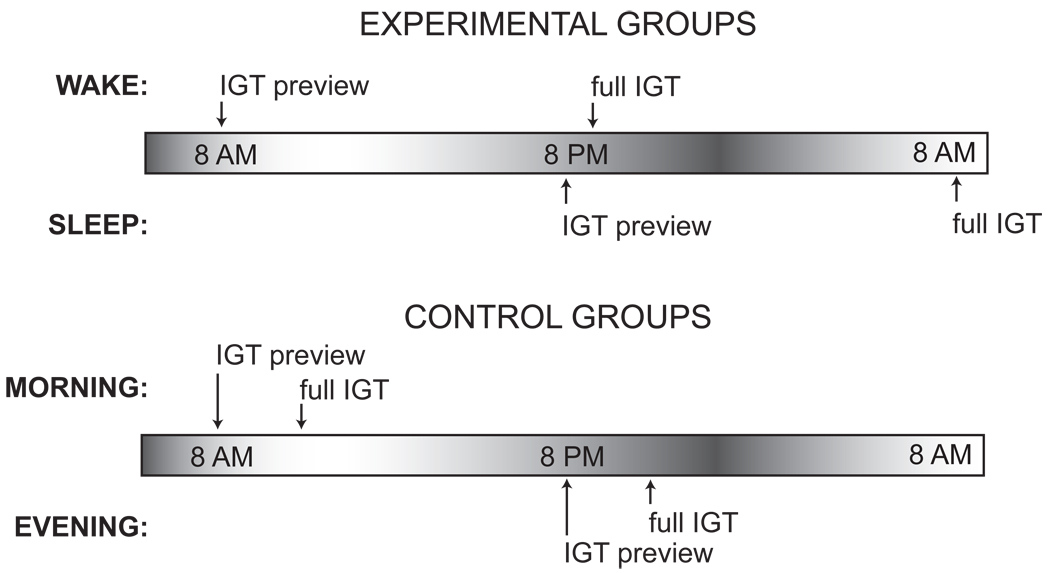
In Session 1, participants were given a preview of the IGT (“IGT Preview”) followed by a delay of 12-hrs (Sleep and Wake groups) or 30–45 min (Morning-Control and Evening-Control groups) after which they completed the IGT (“Full IGT”). The Sleep group completed Session 1 from 8:00–8:30 PM and Session 2 from 8:30–9:30 AM the following morning. The Wake group completed Session 1 from 8:00–8:30 AM and Session 2 from 8:30–9:30 PM on the same day. The Morning-Control group completed Session 1 from 8:00–8:30 AM and then, after filling out questionnaires, completed Session 2 from approximately 9:15–10:00 AM. The Evening-Control group completed these same steps between 8:00 and 10:00 PM. This protocol is illustrated in Figure 1.
Figure 1.
Experimental design showing Sleep, Wake, Morning Control and Evening Control groups.
Between the Preview and Full IGT, participants completed the Epworth Sleepiness Scale (ESS, Johns, 1994), The Pittsburgh Sleep Quality Index (PSQI, Buysse et al., 1989), the Morningness-Eveningness questionnaire (MEQ, Horne and Ostberg, 1976) and the Revised NEO Personality Inventory (NEO-PI-R, Costa and McCrae, 1992). Participants completed the Stanford Sleepiness Scale (SSS, Hoddes et al., 1973) before and after completing the IGT Preview and the Full IGT. Participants were requested to abstain from alcohol, recreational drugs and daytime napping from the day before Session 1 until completing Session 2. At Session 1, participants completed a sleep diary that retrospectively queried sleep duration and quality on the 2 preceding nights including prior daytime activity. The Sleep group also completed this diary for the night between sessions. On the night before Session 1 and between Sessions 1 and 2 (Sleep group), participants were requested to allow themselves the opportunity for at least 7 hours for sleep and to have no caffeine after arising on their first study day until the end of Session 2.
2.3 Experimental task
The IGT followed the original task described by (Bechara et al., 1994). Participants ‘drew’, via mouse clicks, from one of four decks each containing 40 cards. In two "advantageous decks", each draw yielded a constant, small play-money reward and, occasionally, small losses that, over multiple draws, resulted in a net reward. In two "disadvantageous" decks, each draw yielded a larger amount but also large losses that, over multiple draws, resulted in a net loss. Instructions were to win and avoid losing as much money as possible. A bar at the top of the screen, updated after each draw, illustrated net earnings.
In the IGT Preview, all participants made the same 24 draws (6 from each deck) following computer-guided instructions. The deck winnings matched those of the Full IGT and the losses from each deck were proportional to the average magnitude and frequency of losses during the Full IGT (see Supplementary Table 1). In the Full IGT, participants performed 100 self-selected draws but were unaware of this limit during the task. The Full IGT matched the original IGT (Bechara et al., 1994, Bechara et al., 1999) with a number of minor modifications enumerated in Supplementary Methods. Prior to both the IGT Preview and the Full IGT, instructions were read to participants verbatim from a script largely identical to that of (Bechara et al., 1999) as modified by (Wagar and Dixon, 2006) with differences noted in Supplementary Methods.
The primary dependent variable was the standard IGT “Outcome Score”: draws from advantageous decks minus draws from disadvantageous decks in each of 5 quintiles (i.e., draws 1–20, 21–40, etc.) of the total 100 draws (Bechara et al., 1994, Bechara et al., 1999). However, the IGT Preview compounded a pre-existing issue in the original IGT design whereby one or both advantageous decks could be emptied. Since the inability to draw from advantageous decks forced disadvantageous draws, Outcome Scores from draws 81–100 were artificially reduced, especially in the best-performing subjects. Specifically, by draw 100, 17 participants (4 Sleep, 5 Wake, 2 Morning-Control and 6 Evening-Control) had emptied both 40-draw advantageous decks. In contrast, by draw 80, no participant had emptied both advantageous decks. Since, additionally, 80 draws in Session 2 when added to the 24 IGT-Preview draws approximated the customary 100 draws of the standard IGT, only the first 4 of 5 quintiles of the Full IGT were analyzed.
2.4 Assessment of cognitive understanding of task
Immediately after completing the Full IGT, participants were given a sheet of paper with the following written instructions (also read verbatim): “Please write down in your own words what you believe is the rule of this game. That is, what is the strategy that will earn you the most money? Which decks should you choose and why? Please include all the details you think are important.” Three judges who were not involved in data collection and were blind to group, independently scored all responses on a scale of 0–3 using the following rules:
3 Points: Participant explicitly states the rule that one can earn more from decks C and D than decks A and B because, although you win less from decks C and D, you also lose less.
2 Points: Participant states both that decks C and/or D are “good”, “better”, “safe” (or another synonym) AND that decks A and/or B are “risky”, “bad”, “unsafe” (or another synonym).
1 point: Participant states that either deck C or D was “good”, “better”, “safe” OR that either deck A or B was “risky”, “bad” “unsafe”.
0 Points: Participant states that there is no strategy or describes an incorrect strategy.
Means of the 3 judges’ scores for each participant (“Rule Understanding”) were used in subsequent analyses. In the combined experimental and control groups’ data, the kappa statistic for inter-rater concordance was 0.49, indicating a moderate strength of agreement (Sim and Wright, 2005).
2.5 Statistical analyses
Outcome Scores for the first 4 quintiles were analyzed using mixed ANOVA with Group as a between-subjects factor and Quintile (First to Fourth) as a within-subject factor. The Group factor included 2 levels (Sleep, Wake) when comparing only experimental groups and 4 levels (Sleep, Wake, Evening-Control, Morning-Control) when all groups were compared. The Greenhouse-Geisser correction was applied to within-subject main effects and their interactions. When there was a Group×Quintile interaction, groups were compared at each quintile using unpaired t-tests when comparing Sleep vs. Wake groups or Bonferroni-Dunn post-hoc tests when comparing all 4 groups. Likewise, Rule Understanding was compared using unpaired t-tests when comparing Sleep vs. Wake groups or one-way ANOVA and Bonferroni-Dunn post-hoc tests (all 4 groups). Demographic and questionnaire data were compared using unpaired t-tests.
3. Results
3.1 Comparison of Outcome Score and Rule Understanding between Sleep and Wake groups
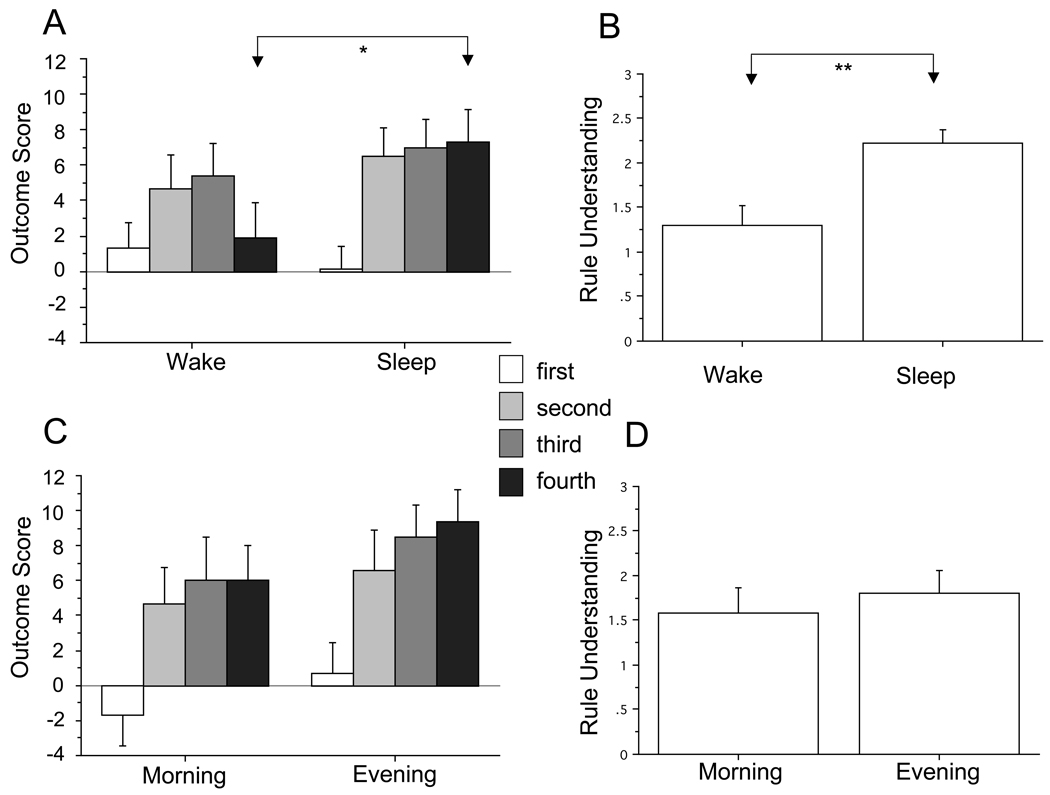
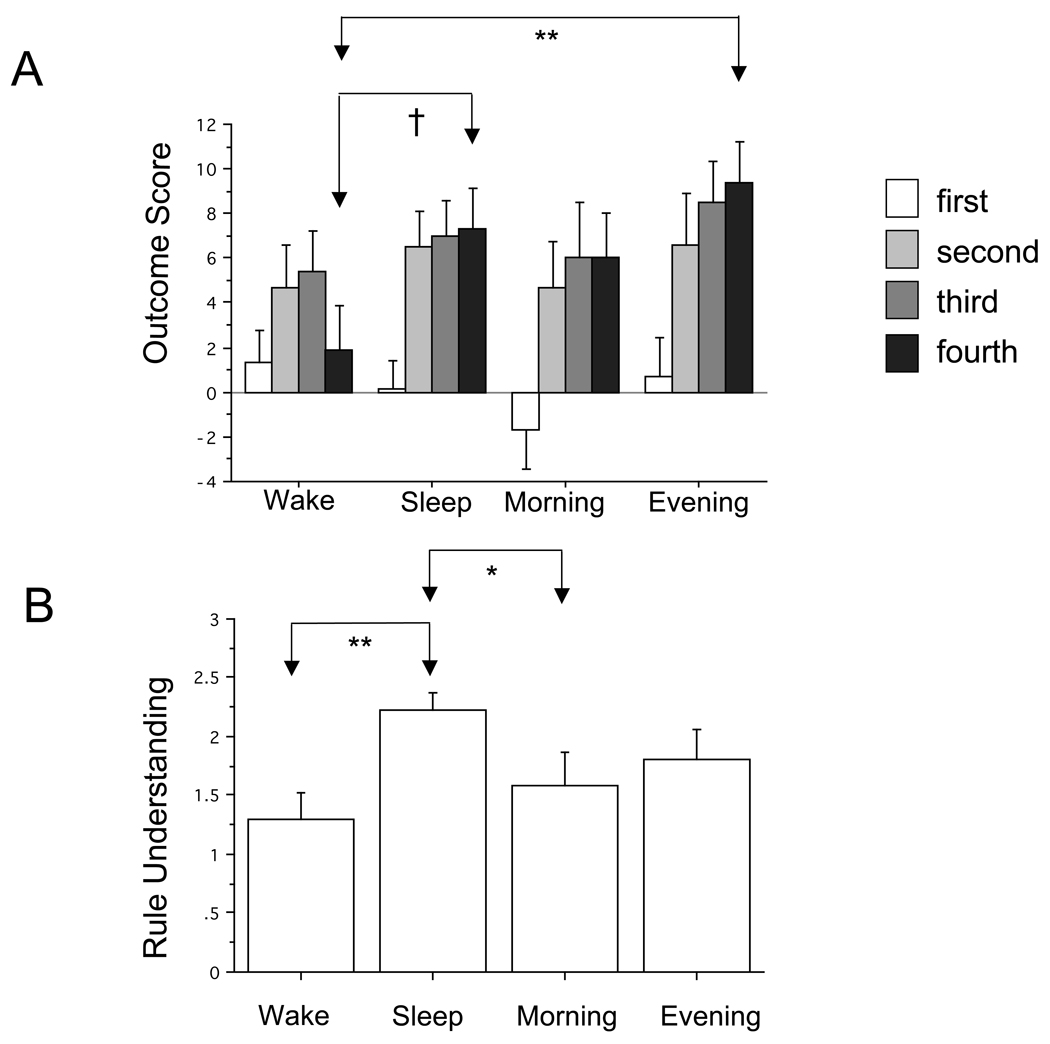
In the experimental groups, ANOVA revealed a significant Group (Sleep vs. Wake)×Quintile interaction [F(3,156)=3.28, p=.030]. Post-hoc tests showed the Quintile-4 (draws 61–80) Outcome Score to be significantly higher in the Sleep vs. Wake group [F(1,52) = 4.02, p = .050] (Figure 2A). In addition, Rule Understanding in the Sleep group was significantly higher than in the Wake group [F(1,52) = 11.92, p = .001] (Figure 2B). Moreover, Chi-squared contingency table analysis showed significant group differences for high (mean of 3 judges’ scores = 3), medium (2 > score < 3) and low (score < 2) Rule Understanding (Chi-squared = 6.31, p = .043). The overall Outcome Score for Quintiles 1–4 (i.e., draws 1–80) was closely correlated with Rule Understanding (R = .61, p < .0001) as was Outcome Scores for Quintile 4 (R = .66, p < .0001), Quintile 3 (R = .54, p < .0001) and Quintile 2 (R = .61, p < .0001).
Figure 2.
Outcome Scores and Rule Understanding in experimental (Sleep and Wake) and control (Morning and Evening) groups. A. Outcome Scores in experimental groups. B. Rule Understanding in experimental groups. C. Outcome Scores in control groups. D. Rule Understanding in control groups. Shadings indicating “First… Fourth” depict Outcome Scores (number of draws from advantageous decks minus draws from disadvantageous decks) in 20-draw quintiles (i.e., 1–20 … 61–80) of the entire 100-draw task. Because both advantageous decks were depleted by about 20% of participants during the fifth quintile (i.e., draws 81–100), this last quintile was omitted from analyses. * p < .05, ** p < .01. Bars indicate standard error.
3.2 Ruling out circadian effects on Outcome Score and Rule Understanding
Comparison of the Sleep and Wake groups suggested that a 12-hr interval with sleep following IGT exposure promoted better Outcome Score and Rule Understanding than a 12-hr interval of continuous waking. Nonetheless, the possibility remained that better performance in the Sleep group resulted from circadian effects on cognition that allowed better Full-IGT performance in the morning. Therefore, Morning-Control and Evening-Control groups were compared. Unlike in the experimental groups, the Group×Quintile interaction for Outcome Score in the control groups (Figure 2C) was not significant. There was also no difference in Rule Understanding between the Morning-Control and Evening-Control groups (Figure 2D). Therefore there was no evidence that the difference between Sleep and Wake subjects arose due to a morning performance advantage on the Full IGT.
However, the fact that the Evening-Control group (who were tested at the same time as the Wake group’s Session 2) had a non-significant tendency towards better Outcome Score than the Morning-Control group (particularly notable at Quintile 4 in Figure 2C, [F(1,36) = 1.45, p = .24], raises the possibility of another confounding circadian effect. If performance on the Full IGT was superior in the Evening-Control group at 30–45 minutes following the IGT Preview, then perhaps evening exposure allowed some in the Sleep group to discover (or guess) the task rule from the IGT Preview alone (memory for which may then have been strengthened by subsequent sleep). To explore this possibility, we eliminated from analyses the 20 individuals who had emptied one of the advantageous decks by draw 80, which left 18 in the Sleep and 16 in the Wake groups. By doing so, we most likely eliminated those who would have learned or guessed the rule in the IGT Preview at Session 1 or early in the Full IGT at Session 2. In addition, by excluding these participants, we eliminated any possibility of a single emptied advantageous deck influencing choices prior to draw 80.
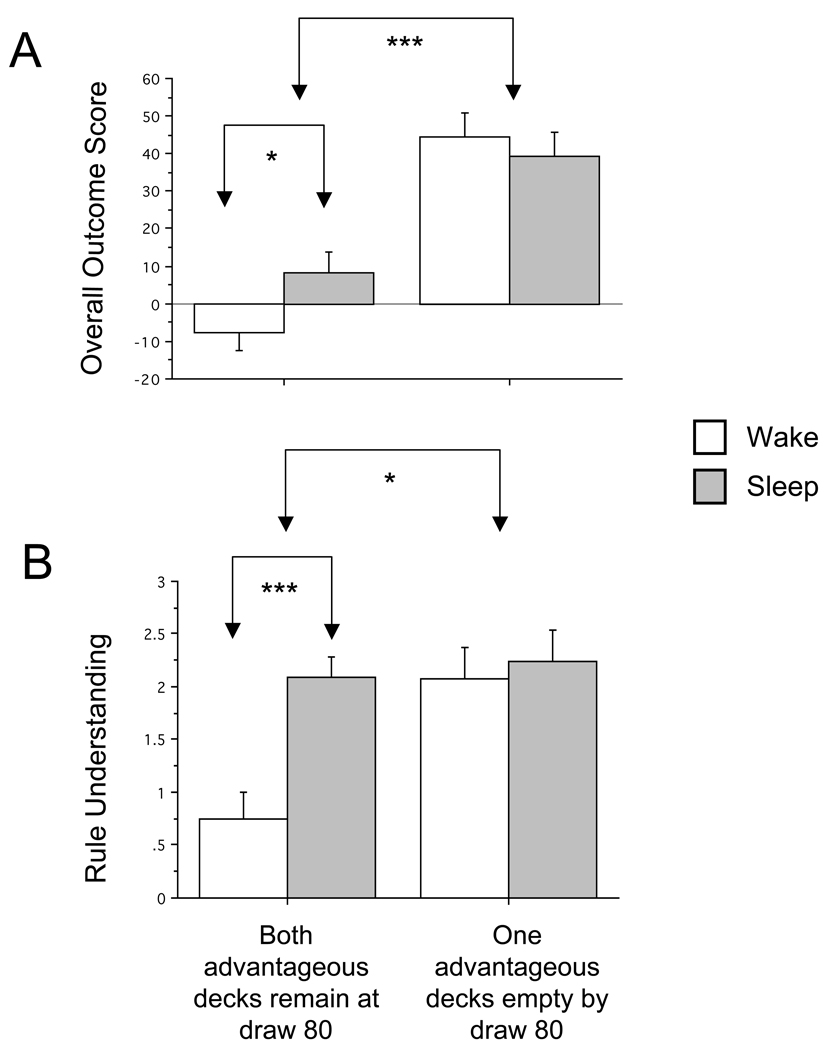
Participants with cards remaining in both advantageous decks at draw 80 were a more poorly performing subset in both overall Outcome Score (Figure 3A) and Rule Understanding (Figure 3B) compared to those who had emptied one advantageous deck by draw 80. Nonetheless, their Outcome Scores remained a meaningful measure of their grasp of the IGT as evidenced by the fact that their Rule Understanding continued to correlate with Outcome Score over Quintiles 1–4 (R = .51, p = .002), Quintile 4 (R = .57, p = .0005), Quintile 3 (R = .38, p = .025) and Quintile 2 (R = .52, p = .002).
Figure 3.
Comparison of overall Outcome Score (A) and Rule Understanding (B) among participants who emptied one advantageous deck by the end of the fourth quintile (draw 80 of the Full IGT) and those that retained cards in both advantageous decks at this point. Sleep vs. Wake group performance is compared within each of these performance categories. * p < .05, *** p < .001. Bars indicate standard error.
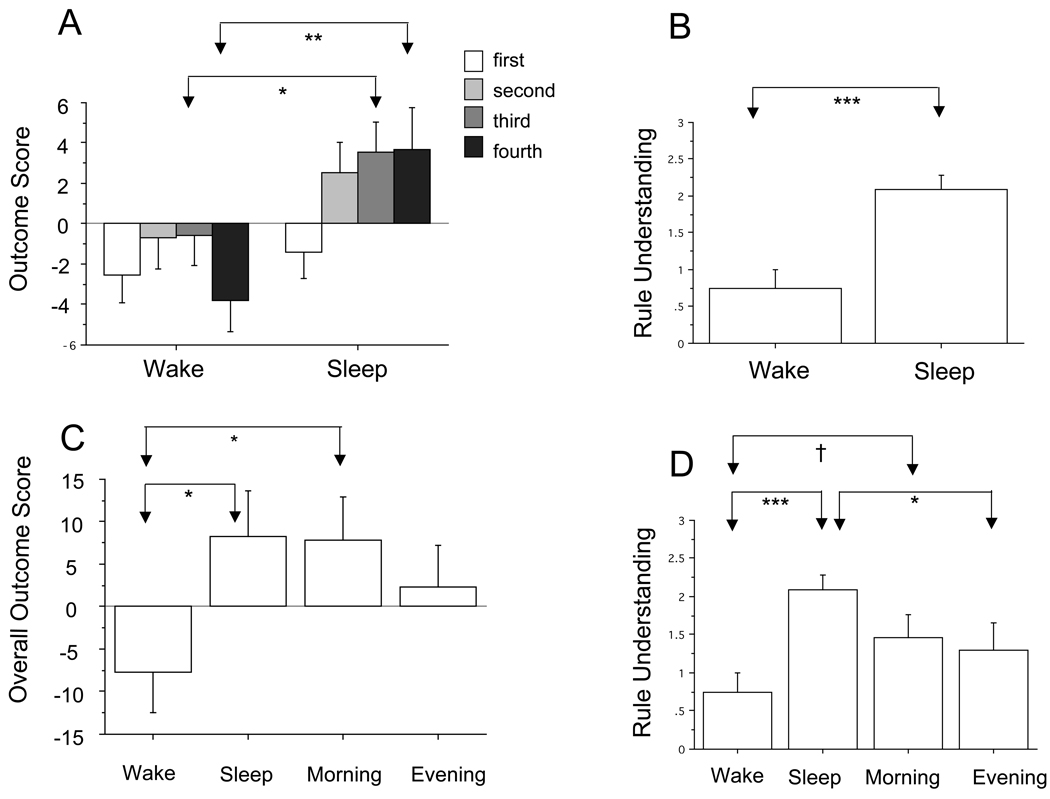
Among these 16 Wake and 18 Sleep participants, the Group×Quintile interaction [F(3,96) = 3.43, p = .024], the Sleep-group advantage in Quintile-4 Outcome Score [F(1,32) = 8.54, p = .006] (Figure 4A) and Rule Understanding [F(1,32) = 18.40, p = .0002] (Figure 4B) all remained significant and a Quintile-3 Outcome Score trend [F(1,32) = 3.99, p = .054] (Figure 4A) as well as a Group main effect [F(1,32) = 5.18, p = .03, Sleep greater] emerged. Therefore, it is also unlikely that rapid learning during an evening IGT Preview accounted for the Sleep group advantage.
Figure 4.
Outcome Scores and Rule Understanding in experimental (Sleep and Wake) and control (Morning and Evening) groups among participants who retained cards in both advantageous decks at the end of the fourth quintile (draw 80). A. Outcome Scores in experimental groups. B. Rule Understanding in experimental groups. C. Overall Outcome Scores (total advantageous draws during draws 1–80) in both experimental and control groups. D. Rule Understanding in both experimental and control groups. † p < .10, * p < .05, ** p < .01, *** p < .001. Bars indicate standard error.
3.3 Combined analysis of experimental and control groups
Among all groups (Sleep, Wake, Morning-Control Evening-Control), there was no Group main effect but a Group×Quintile interaction near trend was observed [F(9,264) = 1.6, p = .14]. When decomposed by Quintile, a significant Group effect was observed for the fourth Quintile [F(3,88) = 2.74, p = .048]. Post-hoc Bonferroni/Dunn tests (6 comparisons, critical alpha p = .0083) for Quintile 4 revealed difference trends between Wake and Sleep (p = .058) and Wake and Evening-Control groups (p = .01) (Figure 5A). Similarly, ANOVA of all four groups’ Rule Understanding was significant [F(3,88) = 3.70, p = .015] and post-hoc Bonferroni/Dunn tests revealed the above noted significantly greater (p = .004) Rule Understanding in the Sleep vs. Wake groups as well as a trend for greater Rule Understanding in the Sleep group relative to the Morning-Control group (p = .046) (Figure 5B). As in the experimental groups alone, Rule Understanding among all participants was correlated with overall Outcome Score (R = .59, p < .0001) and Outcome Scores for Quintiles 4 (R = .59, p < .0001), 3 (R = .51, p < .0001) and 2 (R = .58, p < .0001).
Figure 5.
Outcome Scores and Rule Understanding compared across both experimental (Sleep and Wake group) and control (Morning and Evening group) participants. A. Outcome Scores. Shadings depict Outcome Scores in 20-draw quintiles. B. Rule Understanding. † p < .10, * p < .05, ** p < .01. Bars indicate standard error.
To similarly eliminate effects of deck depletion, we removed from analyses the 14 control-group individuals who had emptied one of the advantageous decks by draw 80 and combined the remaining 11 Evening-Control and 13 Morning-Control individuals with the above similarly selected Sleep and Wake group participants. A Group main effect trend was present [F(3.53) = 2.37, p = .08] and post-hoc tests showed trends for higher Outcome Score in Sleep (p = .032) and Morning-Control (p = .038) compared to Wake Groups (Figure 4C). Decomposing a Group×Quintile near trend [F(9,162) = 1.64, p = .13] by Quintile showed Outcome Score for Quintile 4 in the Wake group to be significantly less than the Evening-Control (p = .007) and Morning-Control (p = .006) groups with a similar trend for Sleep (p = .009) (not shown in Figure 4). Rule understanding remained significantly greater in Sleep vs. Wake (p = .001) with a trends for Morning-Control to exceed Wake (p = .066) and for Sleep to exceed Evening-Control (p = .042) (Figure 4D).
Therefore, as was the case in separate comparisons between Sleep vs. Wake and Morning-Control vs. Evening-Control groups, when all four groups were compared, the Sleep group performed better than the Wake group whereas the Evening-Control and Morning-Control groups performed equivalently. Moreover eliminating those individuals who emptied one advantageous deck by draw 80 tended to sharpen the Sleep group superiority over Wake whereas still no differences were seen between Morning-Control and Evening-Control groups.
3.4 Potentially confounding differences between Sleep and Wake groups
In addition to circadian effects, age, sex ratio, personality characteristics (NEO-PI-R), habitual sleep (ESS, PSQI, MEQ), sleep duration on nights prior to study (diary measures) and sleepiness proximal to testing (SSS) could potentially have influenced group differences in IGT performance. Complete group comparisons for each of these factors as well as re-analyses of group differences controlling for each are detailed in Supplementary Results. The above findings of a Quintile×Group interaction as well as a Sleep-group advantage for Quintile-4 Outcome Score and for Rule Understanding survived adjustment for each of the above potentially confounding factors.
4. Discussion
After a 12-hr delay intervening between an informative IGT Preview and Full-IGT performance, participants whose delay included a normal night’s sleep (PM to AM) showed better performance than those whose delay spanned daytime waking (AM to PM). Superior performance by the Sleep group was reflected in a greater number of draws from advantageous versus disadvantageous decks during the final 20 analyzed draws of the task as well as in their judged understanding of the principle of the IGT. While these findings could be explained by circadian fluctuations in attention and emotion, this account is unlikely given that performance and Rule Understanding did not differ between Morning-Control and Evening-Control participants. Additionally, the Evening-Control group showed a trend toward better performance than the Wake experimental group and the Sleep experimental group showed a trend toward better Rule Understanding than the Morning-Control group. These findings suggest that it was intervening sleep, not circadian phase, that enhanced performance in the Sleep group.
4.1 Sleep provides greater benefit to more poorly performing participants
The benefit of sleep relative to wake for IGT performance and cognitive understanding was strongest among those individuals who had not emptied one of the advantageous decks by draw 80, a subset of participants with lower overall Outcome Score and Rule Understanding (Figure 3). This may be because achieving greater cognitive understanding overwhelms subtler sleep vs. wake effects. Alternatively, lack of a sleep-wake difference in better performing participants may result from a ceiling effect (or from a combination of both factors). In either case, results suggest that sleep benefits earlier stages of understanding the IGT rule, a period during which decision making is believed to rely to a greater extent on affective guidance than at later stages of conceptual understanding (Bechara et al., 1997, Wagar and Dixon, 2006). Therefore, processing during sleep of information encoded at pre-sleep task exposure may facilitate the later integration of affective, perceptual and cognitive feedback during what Bechara and colleagues (1997) term the “hunch” phase and Wagar and Dixon (2006) term “Level 1” understanding, when IGT performance is improving without full awareness. Notably, even at this early stage (e.g., during Quintile 2), Outcome Score closely correlated with Rule Understanding at the conclusion of the task. This, combined with the fact that Rule Understanding eventually showed an even stronger difference between Sleep and Wake groups than did Outcome Score (Figs 2,4,5), suggests that sleep not only improved early, hunch-driven behavior but also facilitated the eventual conceptual understanding of the IGT.
4.2 Sleep-dependent facilitation of rule learning in the IGT
Poorer performance in the Wake group contrasts with similar performance among the remaining three groups and raises the question as to whether daytime activities interfered with the Wake group’s evening performance and, therefore, sleep played a protective vs. facilitative role. Two observations argue against this explanation. First, the Sleep group’s Rule Understanding was the greatest of all 4 groups exceeding that of the Morning-Control (Figure 5B) and the Evening-Control (4D) groups, an unlikely outcome if sleep was solely protecting vs. augmenting the information processing that led to Rule Understanding. Second, one might predict that performance would have been better in the control groups because the Full IGT closely followed the IGT Preview. In the Evening- and Morning-Control groups, the IGT Preview remaining fresh in participants’ minds at the start of the Full IGT would effectively have resulted in an IGT of 104 draws vs. 80 draws in the experimental groups. In contrast, in the Sleep and Wake groups, a 12-hour delay would provide greater opportunity for memory of the IGT Preview to decay. As predicted by the hypothesis that a shorter preview-test interval should augment performance, the Wake group performed more poorly than the control groups whereas the Sleep group’s performance was unexpectedly similar to controls. This, in combination with the Sleep group’s superior Rule Understanding, suggests that sleep-dependent enhancement of performance conferred upon the 12-hr delay Sleep group an advantage that equalized their performance to that of the 45-min delay control groups.
Should the above prediction not be true, i.e., that a shorter delay between the IGT Preview and the Full IGT would not, in itself, improve performance, then the similar Outcome Score in the Sleep and Morning-Control groups would suggest a non-specific effect of prior sleep on performance. However, Outcome Scores in the Evening-Control group were no worse and were even non-significantly better than in the Morning-Control group (Figure 2C). Moreover, when considering only the more poorly performing participants, Rule Understanding in the Sleep group also exceeded that of the Evening-Control group (Figure 4D).
Determining the appropriate design to control for circadian effects was carefully considered and we opted for Morning/Evening Control groups as they mitigate time of day effects on performance. However, as seen in these results, the disadvantage of this strategy is that the time between exposure and testing can not be equated. Methods employed among different studies vary considerably and each has its own advantages and disadvantages. Others have used 24-hour delays that reverse the circadian phase at which conditions are tested, comparisons of nap vs. wake at a fixed time of day or inclusion of an overnight wake-delay condition. In choosing a method, one must take into account the possibility of interactions between the sleep vs. wake distinction and other specific characteristics of the test-retest interval in interpreting observed group effects on the consolidation or integration of information. These considerations merit further research and review.
4.3 Development of group differences across quintiles
The Quintile-4 score of the Wake group, unlike the other 3 groups, decreased relative to previous quintiles (Figures 2, 4, 5). Since the only 4th quintile statistically differed between Sleep and Wake groups, it is important to consider whether or not this may be an artifact. Two observations suggest it is not. First, when only the individuals retaining cards in both advantageous decks at draw 80 were analyzed, superior performance by the Sleep group appeared at the third quintile, and an overall group difference emerged. Second, when Rule Understanding was regressed against Outcome Scores for each quintile, strong correlations were revealed at all except the first quintile indicating that participant behavior was related to eventual conceptual learning even before group differences in behavior became evident. Particularly striking was the fact that, among only those individuals who retained cards in both advantageous decks by the 80th draw, relationships between Outcome Score at each quintile and Rule Understanding were almost identical to these relationships among all subjects. Therefore, the augmented group difference in Outcome Score among these more poorly performing individuals reflected meaningful Sleep vs. Wake differences in eventual Rule Understanding over and above the apparent Quintile-4 deterioration in Wake-group performance.
4.4 Learning and memory in the IGT
Learning during the IGT Preview includes both implicit and explicit components. Whereas participants are made aware that the goal in the second session will be to win and avoid losing as much money as possible, they are unaware that there will be a specific rule for doing so. It has been suggested for motor tasks that explicit learning is required for there to be preferential consolidation over sleep (Robertson et al., 2004). Nonetheless implicit learning on a number of tasks does indeed display sleep-dependent consolidation. Sleep-dependent consolidation has been observed for probabilistic category learning of the Weather Prediction Task (WPT) in its observational mode (Djonlagic et al., 2009), information-integration category learning (Maddox et al., 2009) and transitive inference learning (Ellenbogen et al., 2007), all of which are primarily implicitly learned. Hippocampal involvement may be required for sleep dependency in the consolidation of implicitly learned material since hippocampus-independent probabilistic motor sequence learning (Song et al., 2007) and motor sequence learning (Spencer et al., 2006) are not preferentially benefited by sleep. For example, striatum-based learning, that is believed to produce performance gains during the feedback version of the WPT, may not benefit from sleep in the same manner as hippocampus-based learning in the observational learning mode (Djonlagic et al., 2009).
Similar to the IGT, for the number reduction task (NRT), there is a greater likelihood of later discovering a hidden strategy (insight) at re-exposure if the preceding delay contained sleep vs. continuous wake (Wagner et al., 2004). Implicit learning of the numeric patterns, that results from following the initial NRT instructions, contributes to the relational information that is processed in the hippocampal formation during sleep to produce the greater insight seen at re-exposure (Yordanova et al., 2008). Clearly, during the delay following NRT exposure, the brain can process complex and inseparably interwoven implicitly and explicitly acquired information.
Tasks involving risk and reward, including other studies of the IGT (Killgore et al., 2006a), have proven sensitive to sleep deprivation. For example, in another gambling task, enhanced reward sensitivity and reduced loss sensitivity following sleep deprivation were suggested, respectively, by an increased nucleus accumbens response to reward and reduced insula response to loss in comparison to wake following normal sleep (Venkatraman et al., 2007). The IGT Preview provides abundant explicit relational information (deck contingencies) as well as bi-valent emotional information that has been shown to activate punishment and reward-related limbic structures during task performance (Li et al., 2010). Therefore, the IGT Preview produces several forms of learning that have been shown to preferentially benefit from sleep.
4.5 Potential physiological correlates of sleep-dependent improvement on IGT performance
Brain activity in REM during sleep following IGT exposure may contribute to preparatory, integrative activity that leads to improved IGT performance. There is striking overlap between brain regions activated in REM sleep and those shown to be involved in IGT performance by lesion and functional neuroimaging studies. Whereas lateral areas of the prefrontal cortex remain relatively deactivated in REM sleep, ventromedial prefrontal (vmPFC) regions reactivate along with subcortical limbic structures (Nofzinger et al., 2004, Braun et al., 1997). Therefore, the vmPFC, a region believed to be of primary importance in supporting IGT performance (Bechara et al., 1994, Lawrence et al., 2009), is being selectively activated during REM. Nonetheless, the preferential role of early-night, SWS-rich sleep in achieving explicit understanding following implicit learning on the NRT (Yordanova et al., 2008) suggests that NREM as well as REM may augment performance on a task such as the IGT that recruits implicit and explicit as well as emotional (reward and loss) memory systems.
4.6 Implications of findings and future studies
The current study complements findings from sleep deprivation studies showing impaired performance on the IGT (Killgore et al., 2006a) and similar tasks (Venkatraman et al., 2007) following sleep deprivation by showing a beneficial effect of sleep following an initial exposure to task contingencies on the later performance of the same task. Adding to existing evidence that sleep promotes explicit insight into implicitly learned cognitive tasks (Wagner et al., 2004, Yordanova et al., 2008), the current study provides evidence that normal sleep benefits cognitive preparedness to learn a task that simulates the contingencies of everyday, affectively guided decision making under conditions of uncertainty (Bechara et al., 1997, Wagar and Dixon, 2006). During the IGT Preview, implicit, explicit and emotional memories are formed and heterogeneous memory traces are consolidated and later retrieved when the task is reencountered. Although this complexity precludes a simple categorization of memory systems and other cognitive processes involved, it constitutes a naturalistic demonstration of how normal sleep may assist real-life decision making.
The current experiment clearly suggests specific future studies. First, greater sleep benefit after excluding the best-performing individuals suggests that integration of implicitly encoded information, such as affective responses to rewards and punishment, may play a major role in sleep’s facilitation of performance and explicit understanding of the IGT. Future studies incorporating measurement of anticipatory skin conductance responses (e.g., Bechara et al., 1999) as well as assessment of cognitive understanding following the IGT Preview (e.g., Wagar and Dixon, 2006) may prove useful in determining the relative importance of encoded affective and cognitive information in promoting improved IGT performance following sleep. Second, a larger deck size would eliminate any potential confounding effects of deck depletion as well as allow the Outcome Score in the fifth quintile to remain meaningful. Third, model-based analyses that have been effectively used to categorize decision processes and characterize sleep deprivation effects among individual participants in an information-integration category task (Maddox et al., 2009) have also been developed for the IGT (Yechiam et al., 2005) and may provide additional insight into individuals’ decision-making processes following wake and sleep.
5. Conclusion
The complexity of the IGT, a task that requires coordination of executive and mnemonic capacity with emotional experience, while posing interpretive challenges, has a unique ecological validity as it mirrors the decisions made under uncertainty that characterize everyday life. Likewise, the present design mirrors common scenarios in which information is acquired about a choice set and a decision follows when one returns to the scene or task. In the current study, sleep following exposure yielded more optimal decisions, possibly supporting the adage that “sleeping on it” is beneficial to decision-making.
Supplementary Material
Acknowledgments
Supported by NIA grant R00AG029710 to Rebecca M. C. Spencer. We thank Bengi Baran, Tobias Bennett, Stefano Daniele, Brendan Flanagan, Sara Heaton, Shannon McKeon, Enmanuelle Pardilla-Delgado, Amanda Schultz and Chris Zombik for research assistance.
Footnotes
Disclosure Statement
This was not an industry-supported study and no author has conflicts of interest.
References
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Chuah LY, Venkatraman V, Chan WY, Philip P, Dinges DF. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. Neuroimage. 2006;31:419–428. doi: 10.1016/j.neuroimage.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Costa PT, Mccrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) Professional Manual. Odessa FL: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- Djonlagic I, Rosenfeld A, Shohamy D, Myers C, Gluck M, Stickgold R. Sleep enhances category learning. Learn. Mem. 2009;16:751–755. doi: 10.1101/lm.1634509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J. Sleep Res. 2006;15:261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume S, Jollant F, Jaussent I, Lawrence N, Malafosse A, Courtet P. Somatic markers and explicit knowledge are both involved in decision-making. Neuropsychologia. 2009;47:2120–2124. doi: 10.1016/j.neuropsychologia.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–710. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J. Sleep Res. 2006a;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Day LM, Li CJ, Kamimori GH, Balkin TJ, Killgore DB. Moral reasoning is affected by sleep deprivation. Sleep. 2006b;29 [Google Scholar]
- Killgore WD, Killgore DB, Day LM, Li C, Kamimori GH, Balkin TJ. The effects of 53 hours of sleep deprivation on moral judgment. Sleep. 2007;30:345–352. doi: 10.1093/sleep/30.3.345. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O'daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cereb. Cortex. 2009;19:1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D'argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Hum. Brain Mapp. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox WT, Glass BD, Wolosin SM, Savarie ZR, Bowen C, Matthews MD, Schnyer DM. The effects of sleep deprivation on information-integration categorization performance. Sleep. 2009;32:1439–1448. doi: 10.1093/sleep/32.11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Carter C, Luna B, Price JC, Meltzer CC, Miewald JM, Reynolds CF, 3rd, Kupfer DJ. Increased activation of anterior paralimbic and executive cortex from waking to rapid eye movement sleep in depression. Arch. Gen. Psychiatry. 2004;61:695–702. doi: 10.1001/archpsyc.61.7.695. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr. Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys. Ther. 2005;85:257–268. [PubMed] [Google Scholar]
- Song S, Howard JH, Jr, Howard DV. Sleep does not benefit probabilistic motor sequence learning. J. Neurosci. 2007;27:12475–12483. doi: 10.1523/JNEUROSCI.2062-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RM, Sunm M, Ivry RB. Sleep-dependent consolidation of contextual learning. Curr. Biol. 2006;16:1001–1005. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- Wagar BM, Dixon M. Affective guidance in the Iowa gambling task. Cognitive, Affective & Behavioral Neuroscience. 2006;6:277–290. doi: 10.3758/cabn.6.4.277. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu. Rev. Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision-making deficits. Psychol Sci. 2005;16:973–978. doi: 10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Verleger R, Bataghva Z, Born J, Wagner U. Shifting from implicit to explicit knowledge: different roles of early- and late-night sleep. Learn. Mem. 2008;15:508–515. doi: 10.1101/lm.897908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.