Abstract
Background
Immune responses to Pneumocystis jirovecii are not well understood in HIV infection, but antibody responses to proteins may be useful as a marker of Pneumocystis risk or presence of Pneumocystis pneumonia (PcP).
Design
Retrospective analysis of a prospective cohort
Methods
Enzyme-linked immunosorbent assays of antibodies to recombinant Pneumocystis proteins of major surface glycoprotein fragments (MsgC1, C3, C8, and C9) and of antibody titers to recombinant kexin protein (KEX1) were performed on three sequential serum samples up to 18 months prior to and three samples after first AIDS-defining illness from Multicenter AIDS Cohort Study participants and compared between those who had PcP or a non-PcP AIDS-defining illness.
Results
Fifty-four participants had PcP and 47 had a non-PcP AIDS-defining illness. IgG levels to MsgC fragments were similar between groups prior to first AIDS-defining illness, but the PcP group had higher levels of IgG to MsgC9 (median units/ml 50.2 vs. 22.2, p=0.047) post-illness. Participants with PcP were more likely to have an increase in MsgC3 (OR 3.9, p=0.02), MsgC8 (OR 5.5, p=0.001), and MsgC9 (OR 4.0, p=0.007). The PcP group was more likely to have low KEX1 IgG prior to development of PcP (OR 3.6, p=0.048) independent of CD4 cell count and to have an increase in high IgG titers to KEX1 after PcP.
Conclusion
HIV-infected individuals develop immune responses to both Msg and kexin proteins after PcP. Low KEX1 IgG titers may be a novel marker of future PcP risk before CD4 cell count has declined below 200 cells/μl.
Keywords: HIV, Acquired Immunodeficiency Syndrome, Pneumocystis, serology
INTRODUCTION
In HIV-infected individuals, Pneumocystis pneumonia (PcP) remains the most common acquired immune deficiency syndrome (AIDS)-defining illness despite advances in antiretroviral therapy and prophylactic treatment.1,2 PcP is also an unusual, serious complication in non-HIV-infected individuals who are immunosuppressed due to hematologic malignancies, organ transplants, congenital immunodeficiencies, and those receiving specific immunosuppressive drugs such as high dose corticosteroids.3 PcP can also be a complication of treatment with tumor necrosis factor inhibitors.4–8 Although the greatest risk factor for development of PcP is a lack of T-cell related immunity9,10, humoral responses are also of considerable importance, but their role has been less well-studied.11,12
The Pneumocystis (Pc) surface proteins, major surface glycoprotein (Msg) and kexin (KEX1) are important antigens in the immune response to Pc.12–22 Msgs are products from a family of genes encoding surface glycoproteins, which are multiply repeated in the species where they have been studied. Msgs are used to evade host defense and adhere to host cells as well as other sibling organisms in different life cycle stages.23 Various recombinant Msg segments (MsgA, MsgB, and MsgC) have been used to study humoral responses to Pc.12–15,19 MsgC, the carboxyl terminus, and variants of this fragment (MsgC1, MsgC3, MsgC8, MsgC9) are most strongly recognized by serum antibodies of patients with HIV infection who recovered from PcP. In addition, HIV-infected individuals who died from PcP had higher levels of antibody to MsgC8 than those dying from other causes.12,14,24–26 Whether responses to Msg peptides have any protective or predictive value in PcP infection is currently unclear.
KEX1 is a serine protease with an antigenically stable active site peptide sequence.18,27,28 Immune responses to active site peptide sequence of KEX1 in mice have been found to be protective in both passive and active immunity.16,20–22 In a simian model of HIV infection, antibody responses to the KEX1 peptide were detected with the onset of Pc colonization of the macaque airways17, and low kexin titers prior to immunosuppression predicted development of Pc colonization after monkeys were immunosuppressed.29 These studies suggest humoral responses to KEX1 may be important in the pathogenesis and epidemiology of PcP, but humoral immunity or responses to KEX1 have not been evaluated in humans at risk for PcP.
In the current study, we examine the IgG levels to 4 MsgC fragments (MsgC1, MsgC3, MsgC8, MsgC9) and IgG and IgM levels to KEX1 in HIV-infected individuals at serial time points. We compare the levels between those with PcP and those with another AIDS-defining illness, both before and after their first AIDS-defining illness, to determine if antibody levels up to 18 months prior to PcP predicted risk for developing PcP and to identify factors associated with changes in antibody levels after infection.
METHODS
Study population and data collection
Participants were selected from the Multicenter AIDS Cohort Study (MACS). Details on the design of the MACS study have been previously reported.30 Participants were selected for the following characteristics: occurrence of PcP as first AIDS-defining illness during this period; no participation in a previous study of Msg serology26, and having a total of 6 serum specimens available for analysis at 6 month intervals from 18 months before to 18 months after the first episode of PcP. Controls were MACS participants who developed a non-PcP AIDS-defining illness during this period and with three available specimens both before and after the AIDS-defining illness. All participants meeting these criteria were included in the study.
Participants attended MACS study visits every 6 months. Clinical and laboratory data were entered into a central database, and blood specimens were stored at −70°C in a national repository. For each study participant, we studied 3 sequential time points prior to the AIDS-defining illness (visits 1, 2, and 3) and 3 sequential time points subsequent to AIDS-defining illness (visits 4, 5, and 6). Clinical data included age, race/ethnicity, smoking history, intravenous drug use history, use of PcP prophylaxis, and antiretroviral treatment as defined by the use of ≥1 antiretroviral drugs. Laboratory data included CD4+ cell counts and serum HIV viral levels (although these were not available for all individuals). PcP and other AIDS-defining illnesses were assessed by MACS study administration review of medical records and determined based on predefined criteria.30 For PcP, a definite diagnosis was defined as sputum, bronchoalveolar lavage, or biopsy specimen positive for Pneumocystis and either a new infiltrate on chest x-ray or new respiratory symptom such as cough or shortness of breath. We did not include participants whose first AIDS-defining illness was not PcP, but who later developed PcP during the period in which serum samples were collected. All participants signed written informed consent, and the study was approved by the Institutional Review Boards of the University of Cincinnati and the University of Pittsburgh.
Recombinant antigens
Recombinant MsgC fragments were prepared via polymerase chain reaction (PCR) using DNA isolated from P. jirovecii infected lung or cloned msg genes as templates. MsgA and MsgB fragments were not used in this study because they elicit less robust antibody responses in USA patients.12,14,19,24–26 MsgC1, 3, 8, and 9 fragments, which code for approximately 425 amino acids, exhibit 80–90% homology at the nucleotide level and 77–90% at the amino acid level 25. Yet, these fragments generate unique antibody responses. Amplitaq enzyme (Applied Biosystems, Foster City, CA) was used to generate msg gene segments.12,14,24,25 PCR products were cloned into the pET30 vector (Novagen, Madison WI) and the recombinant MsgC proteins expressed in Escherichia coli and purified as previously described.25 A partial fragment of the macaque-derived Pneumocystis kexin gene in the pBAD expression vector (gift from C. G. Haidaris, University of Rochester) was used to produce recombinant KEX1 as described previously.31
ELISA
Msg ELISA was performed as previously described.14,24–26 Serum specimens from participants and the standard reference serum were tested against recombinant MsgC fragments, and the results were quantified using the method of Bishop and Kovacs.13 A standard curve of reactivity for each antigen was used to quantify antibody reactivity in each test serum. Samples whose values were below the standard curve were assigned the lowest possible value of 1U. The ELISA for the kexin antibodies was performed as described previously.31 Pooled normal human serum samples (Atlanta Biologicals, Georgia, USA) were used as negative controls. The reciprocal end point titer was calculated as the highest dilution at which the optical density was the same or less than that of the control.
Statistical analysis
Participant characteristics at visit 1 were compared between those with first AIDS-defining illness due to PcP (PcP group) or those with the first AIDS-defining illness due to another cause (non-PcP group) by t-tests, Wilcoxon rank sum test, and chi-square test where appropriate. The distribution of Msg C1, 3, 8, and 9 antibody levels were non-normally distributed, and there was no suitable transformation that achieved normality; therefore the Msg C1, 3, 8, and 9 levels at each visit were determined and compared between groups by Wilcoxon rank sum test. Based on the distribution of KEX1 reciprocal endpoint antibody titers and prior data from a separate cohort that showed a significant association of KEX1 antibody levels above a reciprocal endpoint titer 1:3200 with exposure to P. jirovecii32, the KEX1 antibody level was dichotomized into low ( 1:3200 reciprocal endpoint titer) and high (>1:3200 reciprocal endpoint titer). Proportions of participants with low and high titers were compared between the PcP group and non-PcP group at each visit and between visit 1 and visit 6 in each group by the chi-square test. Based on previous literature that demonstrate exposure to Pc cause an increase of MsgC antibody levels to greater than or equal o 1.5 times baseline level 24,26,33, proportions with an increase in titers to Msg C1, 3, 8, and 9 of greater than or equal to 1.5 times from visit 3 to visit 4 were compared by chi-square. Based on literature of KEX1 antibody responses to Pc in macaques29, proportions with a 4-fold or greater increase in titers to kexin from the lowest titer of the pre-AIDS-defining illness visits (visits 1–3) to the highest post-AIDS-defining illness visit titer (visit 4–6), were compared using the chi-square test. Multivariable logistic regression was used to determine independent associations between potential predictors (age, race, study site, smoking history (as the product of the average packs of cigarettes smoked per day and years smoked), history of intravenous drug use, CD4+ T-lymphocyte count, use of PcP prophylaxis) and antibody levels at visits prior to the first AIDS-defining illness. Models were developed by considering all variables with univariable associations of significance <0.2 and using forward selection to keep all variables with a likelihood ratio test yielding significance <0.05. We tested for interactions between our main predictor (antibody levels) and CD4 counts, and the models were assessed for colinearity. Hosmer-Lemshow test was used to assess the models for goodness of fit.
RESULTS
One hundred and one HIV-infected MACS participants met criteria and were included; 54 had PcP and 47 had a disease other than PcP as their first AIDS-defining illness. Three participants were placed in the PcP group for analyses as they had PcP shortly after their first non-PcP AIDS-defining illness (2 participants within two days of the first AIDS-defining illness and one within 120 days). While the time between visits 1, 2, and 3 and visits 4, 5, and 6 were 6 months on average, there were some participants who had longer periods between visit 3 and 4, the visits before and after the first AIDS-defining illness, respectively. The difference in time between visit 3 and when the participant developed AIDS and the time between when the participant developed AIDS and visit 4 was similar in the two groups (4.3 months vs. 4.8 months between visit 3 and AIDS and 5.5 months vs. 4.6 months between AIDS and visit 4 in the PcP group vs. non PcP group, respectively). Clinical characteristics were similar between the groups at the baseline visit (Table 1). At the visit just prior to the AIDS-defining illness (visit 3), the PcP group was less likely to be on antiretroviral therapy (13.0% vs. 46.8%, p<0.001) and had a lower CD4 cell count (median cells/μL 137 vs. 311, p<0.001). The majority of AIDS-defining illnesses in the non-PcP group were Kaposi sarcoma (31.9%), wasting syndrome (21.3%), and Candida esophagitis (10.6%).
Table 1.
Clinical and demographic characteristics of participants according to AIDS-defining illness type and low vs. high KEX1 IgG titer.
| Non-PcP (n=47) | PcP (n=54) | Low KEX1 IgG titer (n=87) | High KEX1 IgG titer (n=14) | |
|---|---|---|---|---|
| Age, median years (range) | 40 (25–52) | 38 (28–57) | 38 (26–57) | 38 (25–52) |
|
| ||||
| African-American race, n (%) | 2 (4.3) | 6 (11.1) | 8 (9.2) | 0 |
|
| ||||
| Hispanic ethnicity, n (%) | 2 (4.3) | 5 (9.3) | 7 (8.0) | 0 |
|
| ||||
| Site, n (%) | ||||
| Baltimore | 8 (17.0) | 7 (13.0) | 14 (16.1) | 1 (7.1) |
| Chicago | 7 (14.9) | 11 (20.4) | 17 (19.5) | 1 (7.1) |
| Pittsburgh | 9 (19.2) | 11 (20.4) | 16 (18.4) | 4 (28.6) |
| Los Angeles | 23 (48.9) | 25 (46.3) | 40 (46.0) | 8 (57.1) |
|
| ||||
| Current smokers, n (%) | 13 (27.7) | 11 (20.4) | 22 (25.3) | 2 (14.3) |
|
| ||||
| Pack-years, median (range) | 2.6 (0–54.3) | 0.7 (0–83.3) | 2.4 (0–83.3) | 0 (0–42.7) |
|
| ||||
| IV drug use, n (%) | 5 (10.6) | 6 (11.1) | 11 (13.1) | 0 |
|
| ||||
| PcP prophylaxis, n (%) | 13 (27.7) | 9 (16.7) | 20 (23.0) | 2 (14.3) |
|
| ||||
| ART, n (%) | 9 (19.1) | 7 (13.0) | 14 (16.1) | 2 (14.3) |
|
| ||||
| CD4 cell count, median cells/μL(IQR) | 331.5 (201–468) | 282 (173–418) | 282 (173–420) | 402.5 (336–484)* |
p = 0.02
ART = Antiretroviral therapy, IQR = interquartile range, IV = intravenous, n = number, PcP = Pneumocystis pneumonia, % = percentage; Low KEX1 IgG titer = less than or equal to a titer of 1:3200, High KEX1 IgG titer = greater than a titer of 1:3200.
Antibody levels to each of the 4 MsgC fragments were similar between the two groups prior to the AIDS-defining illness (Table 2). The PcP group had higher levels of IgG to MsgC9 at the visit immediately after AIDS-defining illness (median [interquartile range] units/ml 50.2 [4.9–123.2] vs. 22.2 [2.6–50.9], p=0.047), and antibody levels to the MsgC3 and MsgC8 fragments tended to be higher in the PcP group at that time as well (median [interquartile range] units/ml 11.6 [1–86.0] vs. 6.9 [1–41.8], p=0.06 for MsgC3 and 43.8 [5.8–96.7] vs. 15.3 [1–84.4], p=0.1 for MsgC8) (Figure 1). The PcP group were more likely to have an increase in antibody level greater than or equal to 1.5 times for antibodies to MsgC3 (odds ratio [OR] 3.9, 95% confidence interval [CI] 1.3–11.5, p=0.01), MsgC8 (OR 5.5, 95% CI 2.0–15.0, p=0.001), and MsgC9 (OR 4.0, 95% CI 1.5–11.1, p=0.005) (Figure 2).
Table 2.
Median [interquartile range] IgG levels to four antigens at each visit grouped by PcP status as first AIDS-defining illness (between visit 3 and 4).
| Antigen | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 |
|---|---|---|---|---|---|---|
| MsgC1 | ||||||
| PcP | 13.0 [2.3–46.2] | 8.3 [2.9–41.8] | 11.2 [1.7–41.2] | 25.3 [2.3–56.3] | 21.4 [1.7–41.7] | 19.6 [1.7–42.0] |
| No PcP | 21.1 [3.2–43.1] | 18.2 [3.8–40.7] | 15.2 [3.6–39.0] | 12.1 [4.3–31.2] | 10.6 [3.3–39.6] | 12.7 [2.2–35.1] |
|
| ||||||
| MsgC3 | ||||||
| PcP | 13.1 [1–56.4] | 18.3 [1–55.3] | 16.4 [2.2–47.7] | 11.6 [1–86.0] | 14.8 [1–70.1] | 17.6 [2.5–72.4] |
| No PcP | 3.2 [1–41.7] | 3.7 [1–50.1] | 5.7 [1–49.0] | 6.9 [1–41.8] | 8.1 [1–39.7] | 8.8 [1–46.4] |
|
| ||||||
| MsgC8 | ||||||
| PcP | 18.6 [4.1–67.0] | 18.9 [2.1–58.1] | 17.2 [4.5–79.2] | 43.8 [5.8–96.7] | 31.5 [1–76.2] | 26.1 [3.4–74.7] |
| No PcP | 20.6 [1–63.4] | 21.3 [2.5–67.3] | 14.9 [1–64.3] | 15.3 [1–84.4] | 15.8 [1–76.1] | 12.1 [1–81.8] |
|
| ||||||
| MsgC9 | ||||||
| PcP | 29.7 [7.8–80.5] | 32.8 [7.9–80.0] | 36.7 [11.1–93.6] | 50.2 [4.9–123.2] | 37.6 [4.7–112.1] | 48.4 [10.1–105.7] |
| No PcP | 18.9 [1–61.1] | 28.5 [2.3–72.4] | 26.5 [2.0–64.2] | 22.2 [2.6–50.9]* | 14.4 [2.8–52.4] | 22.1 [3.5–63.1] |
Wilcoxon rank-sum test – p = 0.05
PcP = Pneumocystis pneumonia
Figure 1.
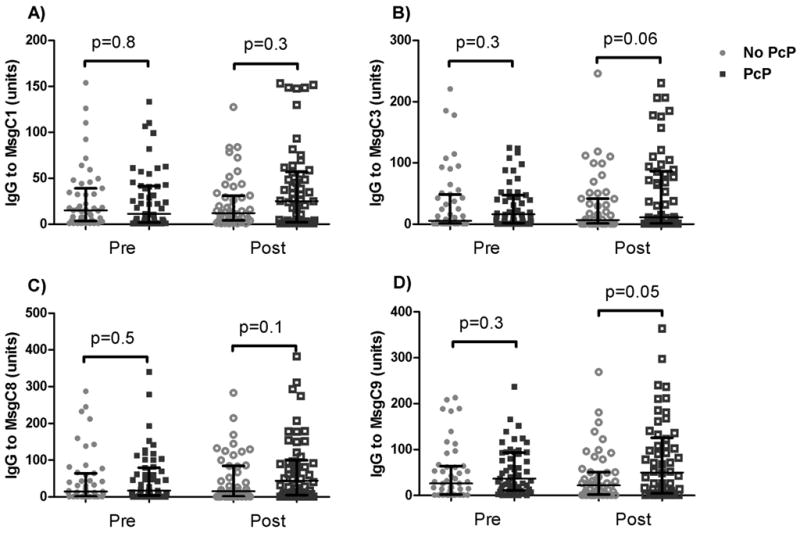
Level of antibodies to MsgC segments at visit 3 (Pre) and visit 4 (Post), before and after the first AIDS-defining illness, respectively. Lines and whiskers represent the median value and interquartile range. No PcP = group that had an illness other than PcP for their first AIDS-defining illness. PcP = group that had PcP as their first AIDS-defining illness.
Figure 2.
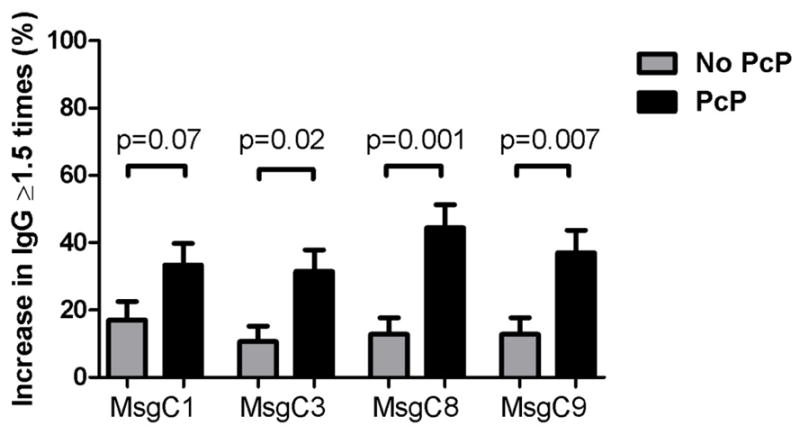
Proportion of subjects in each group who had an increase in antibody level to each MsgC segment after their first AIDS-defining illness (visit 4) of ≥1.5 times the level prior to the first AIDS-defining illness (visit 3). No PcP = group that had an illness other than PcP for their first AIDS-defining illness. PcP = group that had PcP as their first AIDS-defining illness.
Several factors other than PcP were associated with an increase in antibody levels to MsgC fragments of greater than or equal to 1.5 times that prior to first AIDS-defining illness in univariable analyses. Factors independently associated with an increase in antibody levels for MsgC8 were PcP (OR 6.6, 95% CI 2.3–19.3, p=0.001) and being in Chicago (OR compared to being in Baltimore 0.14, 95% CI 0.02–0.79, p=0.03); and for MsgC9 was PcP (OR 4.0, 95% CI 1.4 (or 1.5)-11.1, p=0.007). No factors were significantly associated with a rise for MsgC1, and only PcP was associated with an increase in MsgC3 antibodies as above.
We examined KEX1 IgG at 12–18 months prior to PcP or other AIDS-defining infection in order to determine if KEX1 IgG might be an early marker of PcP risk. The PcP group was more likely to have low pre-AIDS KEX1 IgG titers than those who developed another AIDS-defining illness (at visit 1, OR 3.4, 95% CI 1.0–11.6, p=0.053; at visit 2, OR 3.9, 95% CI 1.0–15.6, p=0.047) (Figure 3A). A low KEX1 IgG level at 18 months prior to AIDS-defining illness (visit 1) had 92% sensitivity, 21% specificity, 57% positive predictive value, and 71% negative predictive value in predicting future development of PcP. A low KEX1 IgG level at 12 months pre-AIDS-defining illness (visit 2) had a slightly better sensitivity (94%) and negative predictive value (75%) while the specificity was slightly less (19%) in predicting future development of PcP. Because the KEX1 IgG titer at visit 1 was predictive of development of PcP, we determined factors associated with having a high versus low IgG titer to KEX1 at visit 1 (Table 1). The CD4 cell count was lower in those with a low IgG titer to KEX1 compared to a high IgG titer to KEX1 (median cells/μl 282 vs. 402.5, p=0.02). No other factors were associated with the KEX1 IgG titer. There was no association between visit 1 CD4 cell count and developing PcP, and a low KEX1 IgG at visit 1 remained an independent predictor of PcP when controlling for CD4 cell count (OR 3.6, 95% CI 1.02–12.6, p=0.048). KEX1 IgM levels were similar between the PcP and non-PcP groups at each visit with similar proportions having low and high titers.
Figure 3.
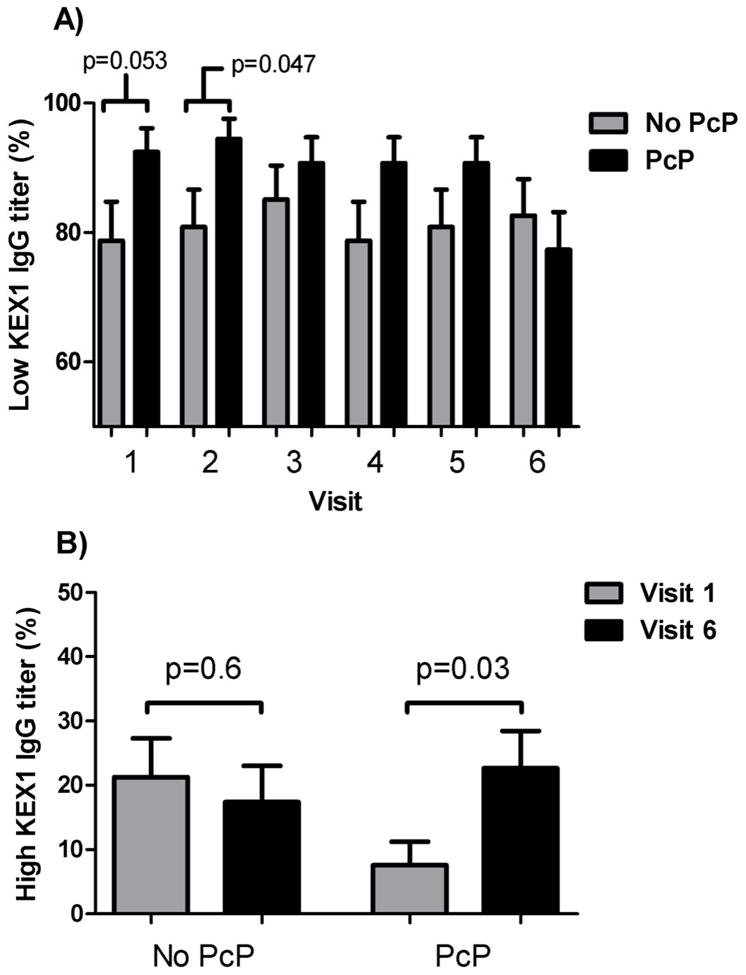
A) Proportion of subjects in each group who had a low (≤1:3200) IgG reciprocal endpoint titer to KEX1 at each visit. Visits 1, 2, and 3 are prior to the first AIDS-defining illness, and visits 4, 5, and 6 are after the first AIDS-defining illness. B) Proportion of subjects in each group who had a high (>1:3200) IgG antibody titer to KEX1 before (Visit 1) and after (Visit 6) AIDS-defining illness. No PcP = group that had an illness other than PcP for their first AIDS-defining illness. PcP = group that had PcP as their first AIDS-defining illness.
We examined other baseline factors associated with development of PcP. No clinical factors at visit 1 except low KEX1 IgG were predictive of subsequent PcP. The majority of subjects had CD4 cell counts greater than 200 cells/μl at the time of their first visit, and visit 1 CD4 cell count did not predict future PcP risk. Factors present at visit 3 (the visit just prior to developing the first AIDS-defining illness) independently associated with PcP were lower CD4 cell count (OR per 100 cells/μL decrease 1.50, 95% CI 1.16–1.93, p=0.002) and not using antiretroviral medications (OR 7.2, 95% CI 2.7–19.4, p<0.001).
The proportion of participants with high IgG titers to KEX1 increased significantly in the PcP group after PcP, but there was no increase in proportion with high IgG titer to KEX1 in the non-PcP group (7.4% pre vs. 22.6% with high titers post, p=0.03 for the PcP group, and 21.3% pre vs. 17.4% post, p=0.6 for the non-PcP group) (Figure 3B). The proportion who had a high IgM titer to KEX1 decreased in each group after AIDS-defining illness, but there was a significant decrease only for the PcP group (48.1% pre vs. 29.4% post, p=0.052 for the PcP group, and 45.7% pre vs. 36.4% post, p=0.4 for the non-PcP group). No factors were related to an increase in IgG or IgM titer to KEX1 of 4 fold or greater after first AIDS-defining illness in multivariable or univariable analyses including whether participants were started on any form of antiretroviral therapy.
DISCUSSION
We investigated humoral immune responses in HIV-infected individuals to two different Pneumocystis surface proteins with different properties and found that measuring antibody titers to each are likely useful in different situations. There were higher levels of IgG antibodies to MsgC9 after an episode of PcP than after other AIDS-defining illnesses, and those who had PcP had a greater proportion of participants with increases in IgG level to MsgC3, MsgC8, and MsgC9. Subjects in Chicago were less likely to have a rise in MsgC9 IgG level, suggesting a regional difference in serotypes. Low levels of KEX1 IgG at 12 to 18 months prior were predictive of subsequent PcP episodes. This relationship of low KEX1 to future PcP risk was seen before participants had decreases in CD4 cell counts to levels generally considered at risk for PcP. KEX1 IgG levels were more likely to rise after PcP than after another AIDS-defining illness.
The current study supports previous work suggesting humoral responses to the MsgC fragment may be useful in identifying acute or recent PcP infection. Prior studies have found that serum from HIV-infected individuals with past PcP recognize the MsgC fragment significantly better than those who have not had PcP, even more than other Msg fragments (MsgA and MsgB).12–14,24,25 While we showed that HIV-infected individuals with PcP as their first AIDS-defining illness have higher levels of IgG to only MsgC9, prior studies have shown differences in MsgC1, Msg C3, and MsgC8.25,26 In the current study, participants with PcP were also more likely to have a rise in titer of 1.5 times or greater to MsgC3, MsgC8, and MsgC9, while prior work detected a difference in all four MsgC fragments.26 These discrepancies may be related to studying a separate set of subjects who might be exposed to a different Pc serotype and evaluating somewhat different time points in relationship to PcP. We also found that there was a geographic difference in the likelihood to respond to the MsgC8 fragment, supporting previous findings.26
In contrast to the relationship of Msg to prior infection, KEX1 levels seem to be more useful as an indicator of subsequent PcP risk earlier than decreases in CD4 cell counts. The current study demonstrated that a low IgG level to KEX1 as early as 12 to 18 months prior to PcP predicted the development of PcP with 92% sensitivity, but only modest specificity. This high sensitivity for subsequent development of PcP could be useful in predicting who is at risk for PcP and who may need prophylactic therapy. In addition, this finding is not explained by a generally suppressed ability to produce antibodies as Msg levels did not demonstrate this pattern. Immediately prior to PcP diagnosis, KEX1 levels were no longer predictive of PcP potentially because levels had started to rise in response to pre-clinical infection. At this time point, CD4 cell count was a much stronger marker of PcP risk. These findings suggest that low IgG levels to kexin may be a novel, early marker of future PcP risk, and its clinical utility in predicting need for PcP prophylaxis should be evaluated in larger clinical studies.
The association of low KEX1 antibody levels with subsequent development of PcP also supports prior studies documenting a preventive role of KEX1 antibodies in animal models. Both passive and active immunization to KEX1 protein of Pneumocystis protects animals from development of PcP in rodent models of PcP, even in T-cell independent vaccination.16,20–22 In a non-human primate model of HIV-infection and Pc colonization, high KEX1 antibody titers protected against colonization with Pc, and memory B-cells were maintained while CD4+ cells declined.29 The findings of these studies suggest that vaccination for the prevention of PcP may be potentially useful. Although PcP prophylaxis is available, a vaccine has several features that make it an attractive option for disease prophylaxis. First, antibiotic prophylaxis is not uniformly effective even when taken correctly and may not be available in areas where resources are limited.34,35 In addition, prophylactic medications are associated with a variety of adverse effects, including allergic reactions, renal dysfunction, methemoglobinemia and hemolytic anemia, and bone marrow suppression.36 Continued use of antibiotics for prophylaxis may lead to mutations at the dihydropteroate synthase locus which have been linked to drug resistance in other organisms, although clinical relevance in Pneumocystis is uncertain.37–39 Vaccination for the prevention of PcP could allow patients to avoid these negative aspects of antibiotic prophylaxis.
There are several limitations to our study. First, it is difficult to compare Msg and KEX1 levels directly as different laboratory methods are used to detect each of these antibodies. The sample size for this study was also small which may have limited our ability to detect differences between the clinical groups. It is possible that participants were not selected because there were not serum samples available around the time of first AIDS diagnosis which may cause a selection bias and reduce the applicability to the entire AIDS population. In addition, participants developed AIDS mostly in the pre-combination antiretroviral therapy era, and thus our results may not be generalizable to the current era of antiretroviral therapy. However, it would be difficult to perform a similar study in the current era given the large number of people who would need to be followed to detect sufficient cases of PcP. It is possible that low IgG levels to KEX1 may be a sign of generalized immunodeficiency from HIV or other causes leading to PcP, but we did not see differences in the levels of antibodies to the MsgC fragments between those who developed PcP or another AIDS-defining illness, making generalized immunodeficiency less likely and suggesting specificity of the KEX1 response. In addition, participants were still able to generate an increase in antibody levels after PcP. Although CD4 cell counts were slightly lower in those with low KEX1 titers, most were still above 200 cells/μl, so these subjects would not have been thought to be at substantial risk for PcP. In addition, KEX1 remained an independent risk factor when adjusted for CD4 cell count. We also lack information on adherence to prophylaxis in these subjects as data were collected by participant self-report. Finally, antibody levels were measured at intervals of 6 months for most participants, but some participants had antibody levels measured at greater time periods from their first AIDS-defining illness. These measures could have been less relevant than measures nearer AIDS, but this limitation was similar between the groups.
In conclusion, we have found that humoral responses to the Pc surface proteins MsgC and KEX1 have utility in identifying different aspects of PcP infection and may help in understanding the epidemiology of PcP. Both Msg and KEX1 increase after acute PcP, but low baseline KEX1 levels are associated with subsequent development of PcP even in subjects not yet at risk for PcP by CD4 cell count criteria. Low KEX1 IgG titers may therefore be a novel marker of future PcP risk.
Acknowledgments
Sources of Financial Support: Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at The Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick, Lisa P. Jacobson), Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair, Steven M. Wolinsky), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles R. Rinaldo). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute. UO1-AI-35042, 5-MO1-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041. Website located at http://www.statepi.jhsph.edu/macs/macs.html.
M.R.G. receives support from National Institutes of Health grant T32 HL007563.
M.W. receives support through the National Institutes of Health GCRC grant M01 RR00425.
P.D.W. is supported by the Medical Research Service, Department of Veterans Affairs and National Institutes of Health grants R01AI-06492 and F33 AI-065207.
A.M. is supported by National Institutes of Health grant R01 HL083461 and HL090339.
Footnotes
This work is original and has not been published elsewhere. Some data contained in this manuscript were presented at the 11th International Workshop in Opportunistic Protists, Hilo, Hawaii, August 1 - 5, 2010.
Conflicts of Interest: The authors have no conflicts of interest.
References
- 1.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350(24):2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 3.Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34(8):1098–1107. doi: 10.1086/339548. [DOI] [PubMed] [Google Scholar]
- 4.Harigai M, Koike R, Miyasaka N. Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med. 2007;357(18):1874–1876. doi: 10.1056/NEJMc070728. [DOI] [PubMed] [Google Scholar]
- 5.Imaizumi K, Sugishita M, Usui M, Kawabe T, Hashimoto N, Hasegawa Y. Pulmonary infectious complications associated with anti-TNF-alpha therapy (infliximab) for rheumatoid arthritis. Intern Med. 2006;45(10):685–688. doi: 10.2169/internalmedicine.45.1623. [DOI] [PubMed] [Google Scholar]
- 6.Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis. 2007;39(5):475–478. doi: 10.1080/00365540601071867. [DOI] [PubMed] [Google Scholar]
- 7.Lahiff C, Khiaron OB, Nolan N, Chadwick GA. Pneumocystis carinii pneumonia in a patient on etanercept for psoriatic arthritis. Ir J Med Sci. 2007;176(4):309–311. doi: 10.1007/s11845-007-0087-x. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Imamura F, Kiyofuji C, et al. Pneumocystis jiroveci pneumonia in a patient with rheumatoid arthritis as a complication of treatment with infliximab, anti-tumor necrosis factor alpha neutralizing antibody. Mod Rheumatol. 2006;16(1):58–62. doi: 10.1007/s10165-005-0454-2. [DOI] [PubMed] [Google Scholar]
- 9.Mansharamani NG, Balachandran D, Vernovsky I, Garland R, Koziel H. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118(3):712–720. doi: 10.1378/chest.118.3.712. [DOI] [PubMed] [Google Scholar]
- 10.Phair J, Munoz A, Detels R, Kaslow R, Rinaldo C, Saah A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. Multicenter AIDS Cohort Study group. N Engl J Med. 1990;322(3):161–165. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- 11.Roths JB, Sidman CL. Single and combined humoral and cell-mediated immunotherapy of Pneumocystis carinii pneumonia in immunodeficient scid mice. Infect Immun. 1993;61(5):1641–1649. doi: 10.1128/iai.61.5.1641-1649.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly KR, Fichtenbaum CJ, Tanaka R, et al. Serologic responses to epitopes of the major surface glycoprotein of Pneumocystis jiroveci differ in human immunodeficiency virus-infected and uninfected persons. J Infect Dis. 2002;186(5):644–651. doi: 10.1086/341565. [DOI] [PubMed] [Google Scholar]
- 13.Bishop LR, Kovacs JA. Quantitation of anti-Pneumocystis jiroveci antibodies in healthy persons and immunocompromised patients. J Infect Dis. 2003;187(12):1844–1848. doi: 10.1086/375354. [DOI] [PubMed] [Google Scholar]
- 14.Daly KR, Koch J, Levin L, Walzer PD. Enzyme-linked immunosorbent assay and serologic responses to Pneumocystis jiroveci. Emerg Infect Dis. 2004;10(5):848–854. doi: 10.3201/eid1005.030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garbe TR, Stringer JR. Molecular characterization of clustered variants of genes encoding major surface antigens of human Pneumocystis carinii. Infect Immun. 1994;62(8):3092–3101. doi: 10.1128/iai.62.8.3092-3101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gigliotti F, Haidaris CG, Wright TW, Harmsen AG. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infect Immun. 2002;70(3):1069–1074. doi: 10.1128/IAI.70.3.1069-1074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kling HM, Shipley TW, Patil S, Morris A, Norris KA. Pneumocystis colonization in immunocompetent and simian immunodeficiency virus-infected cynomolgus macaques. J Infect Dis. 2009;199(1):89–96. doi: 10.1086/595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutty G, Kovacs JA. A single-copy gene encodes KEX1, a serine endoprotease of Pneumocystis jiroveci. Infect Immun. 2003;71(1):571–574. doi: 10.1128/IAI.71.1.571-574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei Q, Turner RE, Sorial V, Klivington D, Angus CW, Kovacs JA. Characterization of major surface glycoprotein genes of human Pneumocystis carinii and high-level expression of a conserved region. Infect Immun. 1998;66(9):4268–4273. doi: 10.1128/iai.66.9.4268-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells J, Haidaris CG, Wright TW, Gigliotti F. Active immunization against Pneumocystis carinii with a recombinant P. carinii antigen. Infect Immun. 2006;74(4):2446–2448. doi: 10.1128/IAI.74.4.2446-2448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng M, Ramsay AJ, Robichaux MB, et al. CD4+ T cell-independent DNA vaccination against opportunistic infections. J Clin Invest. 2005;115(12):3536–3544. doi: 10.1172/JCI26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng M, Shellito JE, Marrero L, et al. CD4+ T cell-independent vaccination against Pneumocystis carinii in mice. J Clin Invest. 2001;108(10):1469–1474. doi: 10.1172/JCI13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stringer JR. Antigenic variation in Pneumocystis. J Eukaryot Microbiol. 2007;54(1):8–13. doi: 10.1111/j.1550-7408.2006.00225.x. [DOI] [PubMed] [Google Scholar]
- 24.Daly KR, Huang L, Morris A, et al. Antibody response to Pneumocystis jirovecii major surface glycoprotein. Emerg Infect Dis. 2006;12(8):1231–1237. doi: 10.3201/eid1208.060230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daly KR, Koch JV, Shire NJ, Levin L, Walzer PD. Human immunodeficiency virus-infected patients with prior Pneumocystis pneumonia exhibit increased serologic reactivity to several major surface glycoprotein clones. Clin Vaccine Immunol. 2006;13(10):1071–1078. doi: 10.1128/CVI.00140-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walzer PD, Djawe K, Levin L, et al. Long-term serologic responses to the Pneumocystis jirovecii major surface glycoprotein in HIV-positive individuals with and without P. jirovecii infection. J Infect Dis. 2009;199(9):1335–1344. doi: 10.1086/597803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee LH, Gigliotti F, Wright TW, Simpson-Haidaris PJ, Weinberg GA, Haidaris CG. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene. 2000;242(1–2):141–150. doi: 10.1016/s0378-1119(99)00533-8. [DOI] [PubMed] [Google Scholar]
- 28.Russian DA, Andrawis-Sorial V, Goheen MP, et al. Characterization of a multicopy family of genes encoding a surface-expressed serine endoprotease in rat Pneumocystis carinii. Proc Assoc Am Physicians. 1999;111(4):347–356. doi: 10.1046/j.1525-1381.1999.99118.x. [DOI] [PubMed] [Google Scholar]
- 29.Kling HM, Shipley TW, Patil SP, et al. Relationship of Pneumocystis jiroveci humoral immunity to prevention of colonization and chronic obstructive pulmonary disease in a primate model of HIV infection. Infect Immun. 2010;78(10):4320–4330. doi: 10.1128/IAI.00507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 31.Morris A, Netravali M, Kling HM, et al. Relationship of Pneumocystis antibody response to severity of chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47(7):e64–68. doi: 10.1086/591701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristoff J, Morris A, Tipirneni R, et al. Health care worker occupation is associated with Pneumocystis KEX1 antibody responses. Am J Respir Crit Care Med. 2009;179:A5539. [Google Scholar]
- 33.Djawe K, Huang L, Daly KR, et al. Serum antibody levels to the Pneumocystis jirovecii major surface glycoprotein in the diagnosis of P. jirovecii pneumonia in HIV+ patients. PLoS One. 2010;5(12):e14259. doi: 10.1371/journal.pone.0014259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30 (Suppl 1):S5–14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan JE, Hanson DL, Navin TR, Jones JL. Risk factors for primary Pneumocystis carinii pneumonia in human immunodeficiency virus-infected adolescents and adults in the United States: reassessment of indications for chemoprophylaxis. J Infect Dis. 1998;178(4):1126–1132. doi: 10.1086/515658. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Ssociety of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. [PubMed] [Google Scholar]
- 37.Helweg-Larsen J, Benfield TL, Eugen-Olsen J, Lundgren JD, Lundgren B. Effects of mutations in Pneumocystis carinii dihydropteroate synthase gene on outcome of AIDS-associated P. carinii pneumonia. Lancet. 1999;354(9187):1347–1351. doi: 10.1016/S0140-6736(99)03320-6. [DOI] [PubMed] [Google Scholar]
- 38.Iliades P, Meshnick SR, Macreadie IG. Mutations in the Pneumocystis jirovecii DHPS gene confer cross-resistance to sulfa drugs. Antimicrob Agents Chemother. 2005;49(2):741–748. doi: 10.1128/AAC.49.2.741-748.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazanjian P, Armstrong W, Hossler PA, et al. Pneumocystis carinii mutations are associated with duration of sulfa or sulfone prophylaxis exposure in AIDS patients. J Infect Dis. 2000;182(2):551–557. doi: 10.1086/315719. [DOI] [PubMed] [Google Scholar]


