Abstract
Dominantly inherited mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common cause of familial Parkinson’s disease (PD). Understanding LRRK2 biology and pathophysiology is central to the elucidation of PD etiology and development of disease intervention. Recently a number of genetic mouse models of LRRK2 have been reported utilizing different genetic approaches. Some similarities in PD-related pathology emerge in these genetic models, despite lack of substantial neuropathology and clinical syndromes of PD. The systematic characterization of these models has begun to shed light on LRRK2 biology and pathophysiology and is expected to offer the identification and validation of drug targets. In this review, we summarize the progress of genetic LRRK2 mouse models and discuss their utility in understanding much needed knowledge regarding early stage (pre-symptomatic) disease progression, identifying drug targets, and exploring the potential in aiding compound screening focused on inhibitors of kinase activity of LRRK2.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease and is characterized pathologically by the loss of dopaminergic neurons of the substantia nigra pars compacta (SNpc) and the presence of intraneuronal proteinacious cytoplasmic inclusions, termed Lewy bodies. Clinically PD is primarily a movement disorder; it is also associated with a series of non-motor symptoms. Although most PD cases are sporadic, approximately 5 – 10 % of cases are inherited. The major breakthrough in recent years in PD research has been the mapping of 16 genetic loci, named PARK1–16 and the subsequent cloning of several genes involved in familial PD. LRRK2 was first reported in 2004 as a gene corresponding to PARK8 1, 2. LRRK2 encodes a complex protein (285 kD) containing kinase and GTPase activities, which are apparently altered by several familial LRRK2 mutations 3–8. G2019S mutation, located in kinase domain of LRRK2, is the most prevalent mutation, present in more than 85% of PD patients carrying LRRK2 mutations, with R1441C/G (within GTPase domain) as the next most prevalent, at approximately 10% 9. In addition, the G2019S was observed in 4% of all familial PD cases. Strikingly, the G2019S was also identified in 1% of sporadic PD cases, suggesting that the mutation is linked to the etiology of PD 9.
The majority of LRRK2 pathogenic mutations cause clinically typical PD, but remarkable variability in neuropathological features exists 2. Noticeable genetic feature of the G2019S–linked PD is the incomplete penetrance 10–12. The risk of PD with an LRRK2 G2019S mutation increases with age, and it is 28% at 59 years, 51% at 69 years, and 74% at 79 years 9. The reduced penetrance is also noted in R1441G/C individuals 13. Since the identification of LRRK2 in PD, there has been extensive investigation of LRRK2 function and pathophysiology of the mutants in cultured cells and invertebrate models. Recent in vitro studies suggest that some, but not all, PD-linked mutations in LRRK2 cause increased kinase activity 7, 8, which is correlated with enhanced neuronal toxicity 5, 7, 14. The previous study in invertebrate models implicates LRRK2 in diverse cellular functions in vesicular trafficking, neurite outgrowth, cytoskeletal regulation and translational control 15–19.
Modeling PD in genetically modified rodent is an essential step toward understanding of the pathogenesis of PD and developing therapeutic strategy. A recent review has provided an excellent overview of current progress of genetic animal models of PD 20. As noted, however, none of the existing genetic mouse models, including overexperessing autosomal-dominant PD mutations and targeted deletion of autosomal recessive PD genes as well as their genetic combination, fully recapitulates key clinical and neuropathological features of PD, highlighting the great challenge of generating an “ideal” genetic PD model for mechanistic study and application in drug development. The lack of visible success also underscores the complexity of PD pathogenic pathways in human that are not immediately accessible in rodents. With increasing number of PD-related genes identified and perhaps linked to diverse functions, a question is raised whether PD can be considered as one or more disease entity 21. Therefore, manifestation of a complete spectrum of PD features in mouse models may require multiple factors that are yet to be defined. This notion contrasts to the success in generating familial ALS model carrying SOD1 mutation and Huntington’s disease (HD) model containing polyQ expansion in huntingtin 20. In each case the pathogenic process of the diseases is determined by single genetic cause, and mouse models harboring the pathogenic mutation resembled closely the clinical and neuropatholgocial features of human diseases.
Recently several groups have reported the generation of LRRK2 genetic mouse models (including transgenic expression and targeted deletion). Without exception all LRRK2 genetic models are viable, fertile, and no gross abnormality in neural development and differentiation stage have been reported. As with other genetic mouse models of PD, there is lack of substantial neurodegenerative process and clinical syndromes of PD. However, common PD-related pathologies were observed in these genetic models, and study of these models has begun to shed light on LRRK2 biology and pathophysiology. In this review, we summarize the characterization of available LRRK2 models and discuss their potential in understanding much needed knowledge regarding early stage (pre-symptomatic) disease progression, identifying drug targets, and exploring the potential in aiding compound screening focused on inhibitors of kinase activity of LRRK2.
BAC mediated transgenic mouse models
Bacterial artificial chromosome (BAC)-mediated transgenesis is a proven genetic tool for studying gene function in vivo. Carrying intact genomic regulatory sequence, the BAC confers precise temporal and spatial expression pattern of transgene under the control of endogenous promoter 22. BAC can be modified by genetic engineering in host bacterial cells to carry disease-related mutations. BAC transgenesis has been successfully applied to the modeling of neurodegenerative diseases with dominant disease transmission such as Huntington’s disease (HD) 23, 24. At least three groups so far have reported BAC transgenic mice expressing the common PD mutations of LRRK2 G2019S and R1441G.
Li et al reported the first BAC transgenic mice expressing human LRRK2 R1441G mutant 25. The BAC model develops an age-dependent and levodopa-responsive movement abnormality that is characterized with periods of immobility. Although total content of striatal dopamine is unaltered, the extracellular dopamine levels measured by microdialysis are reduced in the mutant mice. Furthermore, examination of the nigrostriatal projection by tyrosine hydroxylase immunostaining in the mutant mice reveals sign of axonal dystrophy and degeneration. However, the prevalence of the axonal pathology, as well as whether the aberrant dopamine efflux is caused by the impaired presynaptic terminals in this model remains unclear. Strikingly, the axonal pathology in these mice can also be demonstrated by immunostaining for phospho-tau with AT8 antibody. However, there is no aggregation of α-synuclein and the number of midbrain dopaminergic neurons is unaltered.
In a parallel study, our group developed two BAC transgenic models expressing murine form LRRK2 wildtype (LRRK2-Wt) and G2019S mutant 26. Analysis of the two BAC models showed similar expression patterns and levels in the CNS, offering a unique opportunity to “tease out” G2019S–specific pathogenic effect from that resulted from LRRK2-Wt overexpression. Close comparison of the two BAC models revealed distinct effect of LRRK2 variants on striatal dopamine transmission (measured through cyclic voltametry) and consequent motor function: LRRK2-Wt mice have elevated striatal dopamine release with unaltered DA uptake or tissue content, and accordingly, they are hyperactive and show enhanced performance in motor function tests; by contrast, LRRK2-G2019S mice show an age-dependent decrease in striatal DA content (~25%), as well as decreased striatal DA release and uptake. However, LRRK2-G2019S mice do not display significant motor function deficits up to 12 months 26. Interestingly, a small portion of LRRK2-G2019S mice (10%-15% of total cohort) developed abnormal motor behavior after age of 20 months (Wang and Yue, unpublished data). Furthermore, the study shows that LRRK2-Wt and G2019S overexpression have distinct effects on regional phospho-tau levels, suggesting that G2019S impairs the normal function of LRRK2 that protects against the accumulation of phospho-tau.
The third report of BAC transgenic models of LRRK2 was recently shown by Melrose, Farrer and colleagues 27. They examined two BAC models expressing human LRRK2 wildtype (hWT) and G2019S mutant. While no motor function deficits were observed in these BAC models, they develop abnormal dopamine transmission, evidenced by the reduction of extracellular dopamine levels at striatum via microdialysis. Remarkably, overexpression of G2019S mutant, but not hWT LRRK2, causes age-dependent changes in localization and increased phosphorylation tau in various brain regions. The study also suggests that G2019S mutation causes an increase in tau posttranslational modification, leading to the enhanced phosphorylation levels of tau. Interestingly, although motor function is unaltered, G2019S mice exhibit anxiety-like behavior. More recently, G2019S mice were shown to have impaired adult neurogenesis and neurite outgrowth 28.
What do we learn from these BAC models? First, despite the lack of degeneration of dopamine neurons and α-synuclein inclusions in the above models, the BAC mice expressing G2019S or R1441G mutant share similar pathological features that are highly relevant to PD: impairment of striatal dopamine transmission and aberrant levels of phospho-tau. In addition, our G2019S BAC mice show 25% reduction of striatal dopamine content, in contrast to LRRK2-Wt BAC mice or control mice 26. Considering the enhancement of dopamine transmission and motor performance caused by overexpression of wildtype LRRK2, we propose that at system level LRRK2 plays an important role in regulating striatal dopamine transmission and consequent motor function control; the G2019S or R1441G pathogenic mutation disrupts LRRK2-mediated dopamine transmission, which is linked to motor function deficits. Second, the collective results clearly demonstrate a physiological connection between LRRK2 and tau, and they are compatible with the observation in a subset of PD patients carrying LRRK2 mutations that are associated only with tauopathies but not α-synuclein Lewy bodies 29–31. Therefore, these models are useful for investigating cellular pathway that MAPT/tau becomes involved in the pathogenesis of PD 32, as well as potential functional interaction of LRRK2 with tau in vivo. Third, not surprisingly, some discrepancies of certain phenotypes exist in the different BAC models. For example, the prevalent motor impairment associated with BAC R1441G mice (e.g. periodic immobility) was not observed in the two BAC G2019S models, although it is unlikely or at least no evidence thus far has suggested that R1441G is more neuropathogenic than G2019S in human PD. Moreover, what is also puzzling is that BAC R1441G mice do not show obvious change in striatal dopamine content, whereas our BAC G2019S mice exhibit reduced striatal dopamine levels but in the absence of apparent motor dysfunction as observed in BAC R1441G mice. Other factors, such as different BAC constructs, host strain genetic background, expression levels of transgenes, can each or combined account for the variability of the phenotypes in these BAC models 33. It is worth mentioning that, despite ~90% overall sequence homology between human and mouse LRRK2 proteins, the genomic sequence difference between human BAC 25, 27 and murine BAC 26 including coding regions and regulatory elements may contribute significantly to the phenotype variability as previously noted for various HD animal models 34.
Knockin and knockout models
Several groups have reported the generation of targeted deletion of the LRRK2 gene (knockout) or insertion of PD pathogenic mutation (knockin) 35–39. The R1441C knockin mice shown by Tong et al are grossly normal even at 2 years of age 36. For example, PD-related neuropathology was not detected in homozygous knockin mice under normal condition. However, they show attenuated amphetamine (AMPH)-induced locomotor activity and reduction of evoked catecholamine release in cultured chromaffin cells. Furthermore, the sensitivity to the suppression of the firing of nigral neurons by quinpirole, dopamine and AMPH is markedly reduced in mutant mice than the control. Therefore, consistent with the studies of aforementioned BAC mice, the result of R1441C knockin model provides a further support to the notion that LRRK2 pathogenic mutations (G2019S and R1441C/G) cause disruption of dopamine transmission. This study may also validate the dopamine transmission phenotype observed in the BAC models. Therefore, the dopamine transmission impairment in the BAC models expressing LRRK2 mutants is unlikely a result of transgene overexpression effect, given the physiological expression level of R1441C protein in knockin model.
Dawson, Shen and Cai groups have independently reported the characterization of LRRK2 knockout mice. The results from at least four independent LRRK2 knockout lines indicate that LRRK2 is not an essential gene for mouse survival or associated with significant role in early neural development, differentiation or viability. All knockout lines are viable, fertile and exhibit no detectable motor function abnormality 35, 37, 39, in an agreement with the lack of PD-related neuropathology in these mutant mice. A simple explanation for the above observation is that loss of LRRK2 function is compensated for by LRRK2 homologue LRRK1 33. On another note, disruption of several known PD-related genes, such as α-synuclein, Parkin, DJ-1, and PINK-1, has not been associated with obvious loss of dopaminergic neurons or accumulation of α-synuclein in the CNS. It is intriguing to note that none of these PD-related genes are essential for neural development and differentiation, which is consistent with the hypothesis that the mutations of these PD genes only lead to disruption of neuronal functions mostly at adult stage. Moreover, the above studies collectively suggest that LRRK2-mediated PD is not due to a loss of function in LRRK2. Interestingly, Andres-Mateos et al showed that lack of LRRK2 unexpectedly has no impact on MPTP-induced selective toxicity of dopamine neurons in mice 39.
Although no obvious pathology was observed in the CNS, the LRRK2 knockout mice reported by Tong et al suffer renal abnormality at old age evidenced by cell type-dependent impairment of protein degradation pathways, accumulation of α-synuclein, and enhanced apoptotic cell death 31. Whether this observation is mechanistically connected to the PD pathogenesis in the CNS is unclear, as the CNS in the absence of LRRK2 is largely intact. Furthermore, the knockout kidney showed inflammatory response and oxidative damage in old mice. This result may be related to a recent report linking LRRK2 to cellular pathway of immune response 40. Therefore, the study by Tong et al may suggest that normal function of LRRK2 is involved in suppressing age-related inflammation or oxidative damage, which is an important contributing factor to PD pathophysiology 35, 41. Consistent with the protective role of LRRK2, overexpression of LRRK2 wildtype resulted in a decrease of toxic phospho-tau levels in regional brain of the previous BAC model. In contrast, G2019S mutant is stripped of the protection 26.
Inducible transgenic model
Lin et al previously described transgenic models containing tetracycline-regulated expression of human LRRK2 wildtype, G2019S and mutant with kinase domain deletion 37. The driver line CamKIIa-tTA was used to direct transgene expression 42, which shows a similarity to endogenous LRRK2 expression pattern in regional brain 43. All inducible lines are viable and developed normally, and no PD-related neuropathology was noticed, despite the 8–16 fold overexpression of transgene. However, overexpression of LRRK2 variants markedly accelerated the progression of neuropathological abnormalities developed in PD-related A53T α-synuclein transgenic mice under the similar inducible control system. Furthermore, the excessive LRRK2 expression promotes somatic accumulation of α-synuclein, accompanied with impaired microtubule dynamics, Golgi organization and the UPS pathways. Interestingly, deletion of endogenous LRRK2 expression seems to reduce some of the abnormalities and alleviate the progression of neuropathology observed in A53T mice. This study suggests the genetic interaction of LRRK2 with α-synuclein, and therefore, the two PD-related genes may be placed in the same pathogenic pathways. However, the exact mechanism whereby the two are functionally connected is unclear. In addition, the lack of difference among LRRK2 wildtype, G2019S and kinase-dead (KD) mutant in altering the progression of neuropathology in α-synuclein A53T mice calls for caution in the interpretation of the results, as the possibility of disease-unrelated toxicity resulted from overexpression of the two genes cannot be completely excluded, although the result may implicate a less critical role of LRRK2 kinase activity in aggravating α-synuclein-mediated neuropathology. Furthermore, the study concludes that, while overexepression of LRRK2 worsens, ablation of LRRK2 protects against α-synuclein-mediated neuropathology. The results, however, seemed at odds with a previously suggested, protective role of LRRK2 in the suppression of age-dependent renal accumulation of α-synuclein, inflammation, oxidative damage 35 and brain phospho-tau levels 27. Future experiments are apparently needed to resolve the conflict and clarify the exact cellular function of LRRK2.
Cellular functions and potential pathogenic pathways of LRRK2 in PD
a. LRRK2, dopamine neurotransmission, and regulation of synaptic vesicles
A consensus of the pathophysiology emerging from multiple LRRK2 models is that the common mutations G2019S and R1441C/G cause an impairment of dopamine transmission that is likely associated with a deficit in striatal dopamine content 26 and motor function abnormality 25, 36, 37 (Wang and Yue, unpublished), the clinical hallmarks of PD. Whether LRRK2 mutations affect other neurotransmitter system is unclear and merits future investigation. Furthermore, the impaired neurotransmission may not be necessarily secondary to degenerating presynaptic terminals. Although the BAC R1441G mice displayed axonopathy and aberrant dopamine efflux 25, no obvious axonal abnormalities were noticed in the knockin R1441C mice 36 or the two BAC G2019S mice 26, 27. Therefore, the PD mutations can trigger an initial defect in neuronal/axonal functions in the absence of physical denervation of presynaptic terminals. Previous evidence that LRRK2 function is linked to the regulation of synaptic vesicle protein localization or synaptic vesicle trafficking is compatible with this notion 19, 44. Therefore, impairment of synaptic vesicle function caused by G2019S or R1441C/G could be the underlying mechanism for presynaptic dysfunction. Whether the dysfunction of presynaptic terminals results in cell autonomous, age-dependent axonal and neural degeneration remains to be established.
b. LRRK2, Tau, and regulation of cytoskeletal (such as microtubule and actin) dynamics
The second common PD-related pathology arising from the genetic models is the aberrant levels phospho-tau and/or tauopathies induced by G2019S and R1441G mutants 25–27. The observation not only confirms the pathology in a subgroup of LRRK2 PD patients that are associated with tauopathies 29–31, but also provides strong support for the tie of LRRK2 and Tau in the same pathway. Indeed, recent genome-wide association studies (GWAS) showed surprisingly that MAPT (genetic term for tau) is a risk factor for sporadic PD 45, 46, therefore placing tau in the pathogenic cascade of PD in addition to other neurological disorders with tau pathology such as Alzheimer’s disease and frontotemporal dementia. It also suggests that these diseases may in part share the pathogenic mechanism involved in tau dysfunction.
Tau binds tubulin to stabilize microtubules and promote tubulin assembly into microtubules. Phosphorylation of tau causes disruption of microtubule organization. The increase of phospho-tau levels in LRRK2 mutant transgenic mice, therefore, may result in impairment of microtubule network. In fact, Lin et al showed that overexpression of LRRK2 wildtype or G2019S perturbs the β-tubulin assembly, significantly altering microtubule dynamics 37. Moreover, a previous work by Gillardon shows that LRRK2 G2019S preferentially phosphorylates β-tubulin and enhances the assembly of microtubules 38. While the levels of free tubulin are increased in LRRK2 knockout brain 38, the soluble tubulin levels (in Reassembly-High-salt Buffer) are decreased (accompanied with an increase of pellet tubulin) in LRRK2 transgenic mice 37. In a fly model, LRRK2 was shown to phosphorylate a microtubule associated protein Futsch (fly homologue of MAP1B) 47. Therefore, LRRK2 can directly act on tubulin via phosphorylation, a signaling event perhaps corroborating with phosphorylation of the MAPs including tau and MAP1B and together regulating tubulin assembly and stability of microtubules (although no evidence has indicated that LRRK2 directly phosphorylates tau). Thus, LRRK2 levels and activity seem to play a critical role in controlling the dynamics of microtubule network. Furthermore, it was previously noted that LRRK2 also interacts with actin and modulates the actin assembly 48. By phosphorylating ezrin/radixin/moesin (ERM) proteins, LRRK2 may promote the re-arrangement of actin cytoskeleton in neuronal morphogenesis 48. Taken together, the data from the genetic models shows an important role of LRRK2 in regulating the cytoskeletal dynamics. Finally, since tau and MAP1B are abundant MAP proteins in axons, this may explain the axonal pathology and presynaptic abnormality in several LRRK2 genetic models 25–27.
c. Potential link of LRRK2 and α-synuclein
A body of evidence suggests that LRRK2 and α-synuclein, the only two genes associated with a dominant disease inheritance, are central to the disease pathogenesis 21. However, the overall in vivo evidence for the link of LRRK2 and α-synuclein based on LRRK2 mouse models is less clear. The study described by Lin et al showed that LRRK2 expression levels are critical for the development of α-synuclein-induced neuropathology in mice, suggesting that LRRK2 acts upstream of and regulates α-synuclein in disease pathway. The excessive LRRK2 expression seems to enhance the accumulation of α-synuclein protein and neuropathology when α-synuclein is also overexpressed in neurons; loss of LRRK2 seems to alleviate the α-synuclein evoked neuropathology. However, unlike tau, α-synuclein produced at physiological level is not affected by LRRK2 (wildtype or PD mutants) overexpression in multiple transgenic models. Therefore, the result suggests that the pathogenic effect of LRRK2 via α-synuclein action requires additional factors conferring α-synuclein accumulations or related changes. However, a relevant and seemingly conflicting result from LRRK2 knockout mice suggests that endogenous LRRK2 appears to be important to suppress abnormal accumulation of α-synuclein as well as accompanied inflammatory response in kidney 35. But the argument may be tentative, as the knockout brain is largely intact, and it is likely that the effect of loss-of-function is tissue-specific. Nonetheless, given the current knowledge that α-synuclein is associated with multiple cellular functions 49, 50, one can speculate that in the CNS LRRK2 and α-synuclein are functionally related and cause neuropathology perhaps through the following pathways: (1) interfere with synaptic vesicle functions by directly engaging vesicle-associated proteins and membrane trafficking; and/or (2) impair microtubule and actin dynamics by modulating cytoskeletal protein biochemical properties at presynaptical terminals.
d. Protein degradation, mitochondrial, and other pathways in LRRK2 models?
Previous characterization of several known PD gene functions strongly suggests that mitochondria and protein degradation pathways including ubiquitin proteasome system (UPS) and autophagy-lysosome are the primary cellular pathways relevant to the pathogenesis of PD. However, the significant role for LRRK2 in these pathways has yet to be demonstrated in the reported LRRK2 models, except that Tong et al described the reduced autophagic activity or possibly UPS function, as evidenced with enhanced ubiquitin levels and altered autophagic markers in knockout kidney. But the molecular evidence that LRRK2 directly participates in protein degradation pathways is lacking 35. In addition, overexpression of LRRK2 seems to exacerbate the impairment of UPS and mitochondrial injury triggered by α-synuclein overexpression 37. However, overexpression of LRRK2 wildtype or mutants alone did not seem to cause obvious UPS or mitochondrial abnormalities in available LRRK2 models. Finally, the study of fly models suggests that protein translation pathway is involved in LRRK2-mediated PD pathology 16, 17. But related observation has yet to be shown in the LRRK2 mouse models.
The absence of overt neurodegeneration in LRRK2 genetic models
Until now the LRRK2 G2019S or R1441C models have shown reportedly no sign of cell loss or accumulation of α-synuclein in the CNS. Nor do they exhibit substantial motor function abnormalities (with the exception of R1441C BAC models, but concern of overexpression effects cannot be excluded) (Table 1). The results suggest that PD-related mutations of LRRK2 do not directly participate in cell death signaling cascade or at least do not activate cell death pathways at early disease stage. Rather, the mutants of LRRK2 may impair cellular functions that are important for adult nervous system function as described above.
Table 1.
A summary of reported LRRK2 genetic mouse models and viral delivery rodent models of LRRK2. N/D, not described.
| Mutation | Genetic approach (strain) |
Neuropathology | Abnormal motor Behaviour |
Dopamine system dysfunction |
other abnormalities |
Reference |
|---|---|---|---|---|---|---|
| R1441G |
BAC transgenics (FVB) |
tau pathology and axonal dystrophy |
progressive and L- dopa-responsive abnormal motor activity |
impaired dopamine release by microdialysis |
N/D | Li Y et al.25 |
| G2019S |
BAC transgenics (C57/BL6) |
enhanced striatal phospho-tau staining compared to only LRRK2-Wt overexpression line |
not up to 18–20 months (but abnormal >20 month with low penetrance) |
Decrease in striatal DA content; reduced striatal DA release and uptake by voltametry analysis |
enhanced kinase activity in brain G2019S proteins |
Li X et al.26 and unpublished results |
| G2019S |
BAC transgenics (FVB and ICR) |
phospho-tau accumulation in brain of aged mice |
N/D | reduction of extracellular dopamine levels at striatum |
anxiety-like behaviour; abnormal adult neurogenesis |
Melrose HL et al.27 Winner B et al.28 |
| G2019S |
Tet inducible transgenics (C57/BL6) |
No Changes | increased ambulatory activities at 12 months of age |
N/D | N/D | Lin X et al.37 |
|
α-syn- A53T/LRRK2- G2019S |
Tet inducible transgenics (C57/BL6) |
microglial activation; accelerated neurodegeneration and enhanced α-syn accumulation compared to A53T single transgenic mice. |
N/D | N/D | impaired microtubule dynamics, Golgi organization and the UPS pathways |
Lin X et al.37 |
| R1441C |
knockin (129 and C57/BL6) |
No Changes | Reduced response to AMPH in locomotor activity |
Reduced catecholamine release in cultured mutant chromaffin cells |
N/D | Tong Y et al.36 |
|
LRRK2−/− (2 lines) |
knockout (129 and C57/BL6) |
No Changes | N/D | No Changes | Impaired autophagy– lysosomal pathway and increased apoptosis and inflammation in aged mice; age-dependent accumulation of α- synuclein and ubiquitinated proteins in the kidneys |
Tong Y et al.35 |
| LRRK2−/− |
knockout (C57/BL6) |
No Changes | no major progressive behavioral deficits |
No Changes | Lack of Hypersensitivity to MPTP |
Andres-Mateos et al.39 |
| LRRK2−/− | knockout | moderate increase of activated microglia |
No Changes | N/D | N/D | Lin X et al.37 |
| G2019S |
HSV-delivery in mice (C57/BL6) |
loss of TH–positive neurons |
N/D | N/D | N/D | Lee BD et al.52 |
| G2019S |
Adenovirus vector in rat (Wistar) |
progressive loss of TH- and VMAT2-positive dopaminergic neurons; abnormal hyperphosphorylation of tau in dystrophic nigral neuritic processes (WT and G2019S LRRK2 overexpression) |
N/D | N/D | N/D | Dusonchet J et al.53 |
The lack of robust PD-related pathology and clinical syndrome in LRRK2 models is not surprising, considering the previous studies in other PD mouse models that alteration of PD-related genes, regardless of autosomal dominant (α-synuclein) or autosomal recessive (DJ-1, Parkin, and Pink1), has not captured the key neuropathological features or clinical spectrum of PD. Although the evidence does not help gain any insight, we argue that the results of LRRK2 models may well reflect the intrinsic features of LRRK2 pathogenic mutations that are only partially penetrable in disease transmission. In fact, unlike SNCA (α-synuclein) PD that is fully penetrant, LRRK2-G2019S and R1441G mutations have reduced penetrance that is age-dependent 9–13. Ageing is apparently the single most important risk factor for the majority of PD cases. Interestingly, a recent report indicated that a significant subset of non-manifesting carriers of G2019S (with average age of 82) is not on the motor trajectory to disease 51. The evidence from human study suggests that the LRRK2-mediated pathogenic pathway is particularly susceptible to modifications by other genetic or environmental mechanisms, and the pathogenic effect can be significantly attenuated under certain circumstances. The LRRK2 pathogenic pathway perhaps interacts with various cellular pathways that are likely to play a significant role in modulating disease onset and progression. This hypothesis can also explain the variability of the neuropathology among different LRRK2 patients. Alternatively, the environmental factors may contribute to the LRRK2 disease pathogenesis. Andres-Mateos et al treated LRRK2 knockout mice with neurotoxin MPTP; however, they found that LRRK2 plays little role in MPTP-mediated toxicity to the SNpc dopamine neurons 39. It is likely that LRRK2 may collaborate with completely different sets of neurotoxin to cause the neurodegeneration. Therefore, additional genetic or environment factors are required for the LRRK2-mediated full penetrance of the disease, and apparently these factors, yet to be identified, are missing in available LRRK2 models.
Neurodegeneration in rodent models with viral delivery of LRRK2: a role of inflammatory pathway in glia?
More recently, Lee et al described a herpes simplex virus (HSV) amplicon-based mouse model of LRRK2 dopaminergic neurotoxicity. In this model, delivery of viral LRRK2 G2019S expression causes significant loss of dopamine neurons accompanied with local inflammatory response; in contrast, LRRK2-wildtype, KD mutant, or GFP control expression has no effect in the neuron viability or inflammation levels 52. In addition, a most recent report by Dusonchet and colleagues showed that expression of LRRK2-G2019S assisted with adenovirus vector delivery induced progressive degeneration of nigral dopamine neurons in a rat model 53. The results from the viral expression system supports the notion that inflammatory pathway activated in glial cells, which is largely absent in the genetic LRRK2 models, is likely to play an important role in driving the phenotype of neuronal loss in vivo. Therefore, the non-cell autonomous effects elicited by glial inflammation pathway may provide a promising mechanism that is critically required for LRRK2-mediated neurodegeneration in PD.
Understanding the disease with the “imperfect” LRRK2 models
The lack of the substantial PD neuropathology and clinical features of the PD syndrome indicate that the reported genetic models of LRRK2 are hardly robust PD models, thereby limiting their use with respect to understanding the exact mechanism whereby pathogenic mutations of LRRK2 leads to dopamine neuron degeneration, α-synuclein associated Lewy Bodies, and progression of motor deficits associated with PD. It raises the questions for how we evaluate application of the “imperfect” models for basic and clinical research of PD.
PD is a complex neurological disorder, and with increasing number of PD loci identified, it may be viewed as a spectrum of highly related disease rather than a single entity 21. The neuropathological features of the identified PD genes are not completely identical, therefore, it is plausible to speculate that the molecular function of each familial gene is actually diverse but converges at system levels. Thus, for modeling PD carrying different gene mutations one may not apply the same criteria especially at early disease stage. In fact, available evidence does not support a strong functional connection between α-synuclein and other recessive genes such as DJ-1, PINK1 or Parkin. Furthermore, there has been no convincing evidence that LRRK2 is linked to any other PD-related proteins at molecular levels.
The available large cohort of human G2019S not only provides a unique and promising opportunity for systematic examination of LRRK2 neuropathology and clinical syndrome in PD, but also offers critical clinical experience for validation of the available LRRK2 models. The late onset and age-dependent penetrance of the G2019S presents a long window time for tracking disease progression, and it may also explain the lack of neurodegeneration in LRRK2 genetic mouse models with maximal 2–3 year in life span. A number of functional imaging studies show abnormal striatal dopamine system in both symptomatic G2019S patients, which is indistinguishable from idiopathogenic PD cases 54, 55, and asymptomatic G2019S individuals, who might be in the course of developing PD symptom 56–58. This result demonstrates that the striatal dopamine abnormality occurs preceding the frank motor function deficits in G2019S PD patients and perhaps loss of the dopamine neurons. Therefore, the impairment of striatal dopamine transmission observed in several LRRK2 models that have not been associated with motor abnormalities recapitulates the specific disease stage, and may represent an early pathological alteration before the loss of dopamine neurons.
Moreover, the specific tau pathology that is associated with a subset of G2019S patients appears common in LRRK2 transgenic models. The result offers further validation, to some degree, of the LRRK2 models in capturing LRRK2-associated neuropathology. Therefore, along these lines LRRK2 mouse models set a platform for dissecting specific pathophysiology of LRRK2 PD and particularly at early stage of the disease. We propose that these models can be best used to identify the molecular events that trigger signaling cascade and can be causative to the ultimate manifestation of motor deficits and neurodegeneration. In addition, they should be valuable for delineating the sequence of pathological events that are responsible for the slow progression of the disease. The results will provide critical information for the discovery of effective drug targets and biomarkers that will guide the clinical research in diagnostics and drug screening.
Finally, PD is a motor as well as non-motor disease. LRRK2 is expressed in broad brain regions that are in charge of more than just motor functions. In addition, LRRK2 patients also exhibit non-motor symptoms similar to sporadic PD 9, 59–61. It is worth mentioning that G2019S models reported by Melrose et al 27 and from our group (Wang and Yue, presented in SFN 2010) showed abnormal psychiatric-related behavior, which was shown to be associated with pre-motor episode in PD 62. Future experiments should investigate the molecular and cellular basis of this observation and may shed light on the pathogenic mechanism for the non-motor components of PD.
Utility of LRRK2 models in clinical research
a. kinase activity as the drug target
LRRK2 is considered a promising drug target for PD, however, given the current knowledge, we do not know exactly how to tame it with available tools. The toxic gain-of-function associated with LRRK2 kinase activity has been a dominant hypothesis, and therefore, inhibition of kinase activity is being pursued as the primary therapeutic strategy for LRRK2-mediated PD 8, 52. A number of in vitro assays based on primary neuronal cultures supports a pathogenic function of LRRK2 kinase in retarding neuritic outgrowth or extension 7, 18, 63–65. Numerous genetic models of invertebrates including nematode worms and fruit flies show in vivo evidence for the neurotoxic hyperactivity of LRRK2 kinase 17, 66, 67. The study of LRRK2 kinase activity in genetic mouse models is limited, with only one report by Lin et al that seems to argue against a significant role of LRRK2 kinase activity in the neuropathology induced by α-synuclein 37. While the results may need further validation in other mouse models, Dawson’s group has recently shown that LRRK2 kinase activity is required to cause the degeneration of dopaminergic neurons in HSV-LRRK2 mouse models. More importantly, they identified LRRK2 inhibitors through screening a pool of available industrial kinase inhibitors that potently block LRRK2 kinase activity in vitro, and further showed that the inhibitors are neuroprotective in their models 52. Therefore, this study provides the first in vivo evidence in a mammalian model with a proof of principle that pharmacological inhibition of LRRK2 kinase activity is a potentially promising strategy for the treatment of PD. However, a caveat is that, as the authors also cautioned, the effective inhibition of LRRK2 kinase activity has yet to be confirmed in vivo because no physiological substrates of LRRK2 are currently available for the evaluation. Nonetheless, this report is expected to facilitate the campaign in rodent and other animal models to ultimately validate/invalidate LRRK2 kinase activity as a drug target. Until then, the gain-of-function of LRRK2 kinase activity remains as the most attractive hypothesis and deserves a thorough assessment, particularly given that no other hypotheses proposed to date are equally as competitive. However, one that is worth mentioning is the GTPase activity, another major enzymatic activity in LRRK2 that was also implicated in LRRK2-mediated pathogenesis 6, 14, 68. Future study should also explore LRRK2 GTPase activity as a potential drug target.
b. How can the available genetic LRRK2 models aid the target validation and inhibitor screening?
With increasing number of LRRK2 inhibitors identified, a suitable mouse model(s) is highly desirable for the functional validation of their efficacy in vivo. Although the rodent models mediated by HSV or adenovirus delivery of LRRK2 may provide promising systems, the expansion of the study for thorough screening and validation of inhibitors may be difficult due to overall high variability of the results in this type of study. For available LRRK2 genetic models, despite the lack of the prominent PD neuropathological features and clinical syndrome, the previous evidence that some PD relevant pathological features shared by multiple lines of LRRK2 transgenics may assist to establish potential endpoints in assessing the efficacy of inhibitors in these models.
First, it is necessary to demonstrate the inhibition of LRRK2 activity in the CNS caused by the administration of inhibitors in vivo. This can be assessed by evaluation of autophosphorylation of LRRK2 and phosphorylation of LRRK2 substrates via phospho-specific antibody detection. Using a pair of BAC lines producing FLAG-tagged LRRK2-Wt and G2019S, we showed that the kinase activity of the brain G2019S protein is 2–3 fold higher than that of wildtype 26. These models are potentially useful to test the idea that suppression of enhanced kinase activity in G2019S (without a complete block of wildtype LRRK2 kinase activity) is correlated with the reversal of the observed neuropathology in G2019S mice. Second, we propose the premise of impaired striatal dopamine transmission (and levels) and tau pathology, the two common disease-related features in LRRK2 genetic models, as primary neuropathology endpoints, and the motor function deficits in R1441G BAC mice as potential behavioral endpoint. Third, more than one models is necessary for the study in order to draw a meaningful conclusion. However, it would increase the cost significantly by recruiting multiple models. Systematic characterization of the available LRRK2 models through consortium effort will facilitate the identification and further validation of the endpoints.
While an “ideal” LRRK2 PD model is currently unavailable and perhaps we will never have one with only LRRK2 mutation due to the specific nature of LRRK2-associated PD, e.g. reduced penetrance and high variability of neuropathology (see above discussion), generation of compound LRRK2 models in combination with additional PD-linked mutations or with environmental toxin may facilitate development of PD models with prominent neuropathology. However, generation of such compound models and follow-up validation is expected to be a long process and would take for years. Until then, probe of the specific disease feature or stage in available LRRK2 models should be highly feasible, and most likely will advance our knowledge and move the field forward.
c. Potential use of available LRRK2 models in developing pharmacodynamic biomarkers
LRRK2 signaling pathway mediated by its kinase/GTPase activity is largely unclear. The lack of understanding LRRK2-regulated function is a major roadblock to the effective evaluation of therapeutic strategy targeting LRRK2 activity. Comparably high levels of LRRK2 are observed in multiple tissues/organs including kidney, spleen, lung and brain, suggesting that LRRK2 function is also important outside the CNS. In support of this notion, recent reports have linked LRRK2 abnormal function to the pathological conditions associated with Crohn’s disease 69, cancer 70 and leprosy 71. It is likely that LRRK2-mediated signaling pathway is conserved between the CNS and other tissues, and elucidation of signaling pathway in peripheral tissues will facilitate the development of pharmacodynamic biomarkers of LRRK2 activity and can assist to predict the outcome of clinical trials of promising therapeutic strategies. For this purpose, we propose that the LRRK2 models, particularly BACs and knockin/knockout mice that use endogenous promotors for LRRK2 expression, are suitable to study the gene expression profiling impacted by the overexpression, loss of function, and pathogenic expression of PD mutations of LRRK2 in the peripheral tissues. This would allow us to quickly establish the fingerprints of gene expression patterns that caused by LRRK2 kinase/GTPase activity. The information will guide the investigation and verification of the effects of small compounds targeting LRRK2 activity in a dynamic fashion. Therefore, these LRRK2 models provide a platform for elucidating the mechanism of drug action and yielding critical information for the future clinical trials.
Conclusion and Future Perspective
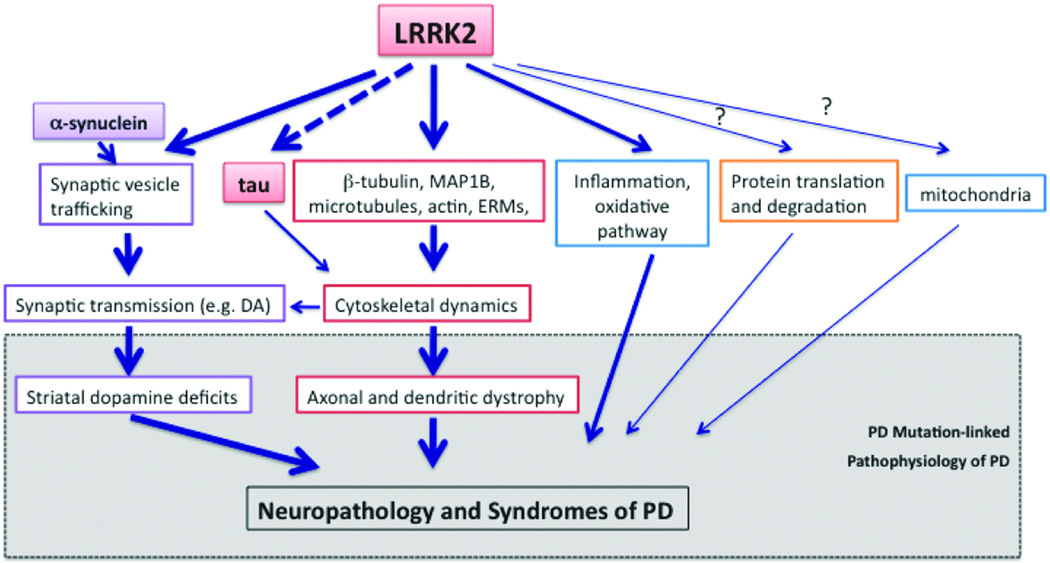
Understanding LRRK2 biology and pathophysiology is central for the ultimate elucidation of pathogenesis of inherited and perhaps idiopathic PD. As LRRK2 kinase/GTPase activity is a highly “druggable” target, therapeutic strategies targeting aberrant LRRK2 activity hold a great promise for the treatment of PD. The development of the genetic mouse models of LRRK2 has made one step forward toward this goal and the emerging evidence has provided insightful information (Figure 1), but many pressing questions remain unanswered, such as what is the molecular and genetic basis of reduced penetrance of LRRK2 common mutations (e.g. G2019S and R1441C)? What is the underlying mechanism contributing to drastic variability of LRRK2 neuropathology/clinical syndromes and how does LRRK2-associated PD differ from other inherited and sporadic types of PD? What is the exact cellular function and dysfunction of LRRK2 in PD and how is LRRK2 connected to other PD-related gene pathways, especially tau and α-synuclein? Whether or not aberrant LRRK2 kinase/GTPase activity is the culprit of the disease and how can they be harnessed for the purpose of therapeutics? Finding clues to these questions requires rigorous characterization of numerous LRRK2 genetic models, and validations of the outcome should be closely inspected by corroborating with the analysis of disease process in the available cohorts of human LRRK2 G2019S. This can be achieved only through large consortium efforts. Continuous exploration of the existing and incoming new LRRK2 models will provide critical information for drug target validation and novel endpoint identification in the development of therapeutic strategies.
Figure 1.
An illustration of LRRK2-regulated cellular functions and pathophysiology associated with mutant LRRK2 based on primarily the in vivo studies of LRRK2 genetic mouse models.
Acknowledgement
We thank Drs Robert E. Burke, Ted M. Dawson, Valina Dawson, Jie Shen, Matthew Farrer, Chenjian Li, Huaibin Cai and members of Yue laboratory for critical reading and comments; and this work is supported by NIH/NINDS grants NS060809-01, NS072359-01 and Michael J Fox Foundation for Parkinson’s Research.
Footnotes
Authors’ Roles:
Contributions of Zhenyu Yue: conception and design, literature review, manuscript drafting and editing and revising of the text. Contributions of M. Lenard Lachenmayer: literature review, data acquisition, manuscript drafting and editing and revising of the text.
Financial Disclosures:
Zhenyu Yue received grants from the National Institutes of Health (NIH), the Bachmann-Strauss Dystonia and Parkinson foundation and the Michael J. Fox Foundation for Parkinson’s Research. M. Lenard Lachenmayer was supported by the Deutsche Forschungsgemeinschaft (DFG).
References
- 1.Paisan-Ruiz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44(4):595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Gloeckner CJ, Kinkl N, Schumacher A, et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15(2):223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 4.Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL, Chen SG. The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res. 2007;313(16):3658–3670. doi: 10.1016/j.yexcr.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito G, Okai T, Fujino G, et al. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson's disease. Biochemistry. 2007;46(5):1380–1388. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants. J Neurochem. 2007;103(1):238–247. doi: 10.1111/j.1471-4159.2007.04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9(10):1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 8.West AB, Moore DJ, Biskup S, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102(46):16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark LN, Wang Y, Karlins E, et al. Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology. 2006;67(10):1786–1791. doi: 10.1212/01.wnl.0000244345.49809.36. [DOI] [PubMed] [Google Scholar]
- 11.Goldwurm S, Zini M, Mariani L, et al. Evaluation of LRRK2 G2019S penetrance: relevance for genetic counseling in Parkinson disease. Neurology. 2007;68(14):1141–1143. doi: 10.1212/01.wnl.0000254483.19854.ef. [DOI] [PubMed] [Google Scholar]
- 12.Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2006;354(4):424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Martinez J, Gorostidi A, Ibanez B, et al. Penetrance in Parkinson's disease related to the LRRK2 R1441G mutation in the Basque country (Spain) Mov Disord. 2010;25(14):2340–2345. doi: 10.1002/mds.23278. [DOI] [PubMed] [Google Scholar]
- 14.West AB, Moore DJ, Choi C, et al. Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16(2):223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 15.Biskup S, Moore DJ, Celsi F, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60(5):557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 16.Gehrke S, Imai Y, Sokol N, Lu B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature. 2010;466(7306):637–641. doi: 10.1038/nature09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai Y, Gehrke S, Wang HQ, et al. Phosphorylation of 4E–BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. Embo J. 2008;27(18):2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52(4):587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Shin N, Jeong H, Kwon J, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314(10):2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010;66(5):646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy J. Genetic analysis of pathways to Parkinson disease. Neuron. 2010;68(2):201–206. doi: 10.1016/j.neuron.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heintz N. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;2(12):861–870. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- 23.Gray M, Shirasaki DI, Cepeda C, et al. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008;28(24):6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spampanato J, Gu X, Yang XW, Mody I. Progressive synaptic pathology of motor cortical neurons in a BAC transgenic mouse model of Huntington's disease. Neuroscience. 2008;157(3):606–620. doi: 10.1016/j.neuroscience.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Liu W, Oo TF, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci. 2009;12(7):826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Patel JC, Wang J, et al. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson's disease mutation G2019S. J Neurosci. 2010;30(5):1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melrose HL, Dachsel JC, Behrouz B, et al. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis. 2010;40(3):503–517. doi: 10.1016/j.nbd.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winner B, Melrose HL, Zhao C, et al. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol Dis. 2011;41(3):706–716. doi: 10.1016/j.nbd.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajput A, Dickson DW, Robinson CA, et al. Parkinsonism, Lrrk2 G2019S, and tau neuropathology. Neurology. 2006;67(8):1506–1508. doi: 10.1212/01.wnl.0000240220.33950.0c. [DOI] [PubMed] [Google Scholar]
- 30.Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson's disease. Nat Rev Neurosci. 2010;11(12):791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wszolek ZK, Pfeiffer RF, Tsuboi Y, et al. Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology. 2004;62(9):1619–1622. doi: 10.1212/01.wnl.0000125015.06989.db. [DOI] [PubMed] [Google Scholar]
- 32.Devine MJ, Lewis PA. Emerging pathways in genetic Parkinson's disease: tangles, Lewy bodies and LRRK2. Febs J. 2008;275(23):5748–5757. doi: 10.1111/j.1742-4658.2008.06707.x. [DOI] [PubMed] [Google Scholar]
- 33.Yue Z. LRRK2 in Parkinson's disease: in vivo models and approaches for understanding pathogenic roles. Febs J. 2009;276(22):6445–6454. doi: 10.1111/j.1742-4658.2009.07343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrnhoefer DE, Butland SL, Pouladi MA, Hayden MR. Mouse models of Huntington disease: variations on a theme. Dis Model Mech. 2009;2(3–4):123–129. doi: 10.1242/dmm.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong Y, Yamaguchi H, Giaime E, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107(21):9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong Y, Pisani A, Martella G, et al. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106(34):14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin X, Parisiadou L, Gu XL, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron. 2009;64(6):807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability--a point of convergence in parkinsonian neurodegeneration? J Neurochem. 2009;110(5):1514–1522. doi: 10.1111/j.1471-4159.2009.06235.x. [DOI] [PubMed] [Google Scholar]
- 39.Andres-Mateos E, Mejias R, Sasaki M, et al. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) J Neurosci. 2009;29(50):15846–15850. doi: 10.1523/JNEUROSCI.4357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardet A, Benita Y, Li C, et al. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J Immunol. 2010;185(9):5577–5585. doi: 10.4049/jimmunol.1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23(4):474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 42.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274(5293):1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 43.Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann Neurol. 2006;59(4):714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- 44.Sakaguchi-Nakashima A, Meir JY, Jin Y, Matsumoto K, Hisamoto N. LRK-1, a C. elegans PARK8-related kinase, regulates axonal-dendritic polarity of SV proteins. Curr Biol. 2007;17(7):592–598. doi: 10.1016/j.cub.2007.01.074. [DOI] [PubMed] [Google Scholar]
- 45.Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards TL, Scott WK, Almonte C, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74(2):97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Liu HP, Lin WY, Guo H, Lu B. LRRK2 kinase regulates synaptic morphology through distinct substrates at the presynaptic and postsynaptic compartments of the Drosophila neuromuscular junction. J Neurosci. 2010;30(50):16959–16969. doi: 10.1523/JNEUROSCI.1807-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parisiadou L, Xie C, Cho HJ, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29(44):13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng F, Vivacqua G, Yu S. The role of alpha-synuclein in neurotransmission and synaptic plasticity. J Chem Neuroanat. 2010 doi: 10.1016/j.jchemneu.2010.12.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Abeliovich A, Schmitz Y, Farinas I, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 51.San Luciano M, Lipton RB, Wang C, et al. Clinical expression of LRRK2 G2019S mutations in the elderly. Mov Disord. 2010;25(15):2571–2576. doi: 10.1002/mds.23330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee BD, Shin JH, VanKampen J, et al. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson's disease. Nat Med. 2010;16(9):998–1000. doi: 10.1038/nm.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dusonchet J, Kochubey O, Stafa K, et al. A Rat Model of Progressive Nigral Neurodegeneration Induced by the Parkinson's Disease-Associated G2019S Mutation in LRRK2. J Neurosci. 2011;31(3):907–912. doi: 10.1523/JNEUROSCI.5092-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isaias IU, Benti R, Goldwurm S, et al. Striatal dopamine transporter binding in Parkinson's disease associated with the LRRK2 Gly2019Ser mutation. Mov Disord. 2006;21(8):1144–1147. doi: 10.1002/mds.20909. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein DS, Imrich R, Peckham E, et al. Neurocirculatory and nigrostriatal abnormalities in Parkinson disease from LRRK2 mutation. Neurology. 2007;69(16):1580–1584. doi: 10.1212/01.wnl.0000268696.57912.64. [DOI] [PubMed] [Google Scholar]
- 56.Sossi V, de la Fuente-Fernandez R, Nandhagopal R, et al. Dopamine turnover increases in asymptomatic LRRK2 mutations carriers. Mov Disord. 2010;25(16):2717–2723. doi: 10.1002/mds.23356. [DOI] [PubMed] [Google Scholar]
- 57.Nandhagopal R, Mak E, Schulzer M, et al. Progression of dopaminergic dysfunction in a LRRK2 kindred: a multitracer PET study. Neurology. 2008;71(22):1790–1795. doi: 10.1212/01.wnl.0000335973.66333.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams JR, van Netten H, Schulzer M, et al. PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation. Brain. 2005;128(Pt 12):2777–2785. doi: 10.1093/brain/awh607. [DOI] [PubMed] [Google Scholar]
- 59.Kasten M, Kertelge L, Bruggemann N, et al. Nonmotor symptoms in genetic Parkinson disease. Arch Neurol. 2010;67(6):670–676. doi: 10.1001/archneurol.67.6.670. [DOI] [PubMed] [Google Scholar]
- 60.Silveira-Moriyama L, Guedes LC, Kingsbury A, et al. Hyposmia in G2019S LRRK2-related parkinsonism: clinical and pathologic data. Neurology. 2008;71(13):1021–1026. doi: 10.1212/01.wnl.0000326575.20829.45. [DOI] [PubMed] [Google Scholar]
- 61.Haugarvoll K, Rademakers R, Kachergus JM, et al. Lrrk2 R1441C parkinsonism is clinically similar to sporadic Parkinson disease. Neurology. 2008;70(16 Pt 2):1456–1460. doi: 10.1212/01.wnl.0000304044.22253.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolters EC, Francot CM. Mental dysfunction in Parkinson's disease. Parkinsonism Relat Disord. 1998;4(3):107–112. doi: 10.1016/s1353-8020(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 63.Smith WW, Pei Z, Jiang H, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A. 2005;102(51):18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greggio E, Jain S, Kingsbury A, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23(2):329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Dachsel JC, Behrouz B, Yue M, Beevers JE, Melrose HL, Farrer MJ. A comparative study of Lrrk2 function in primary neuronal cultures. Parkinsonism Relat Disord. 2010;16(10):650–655. doi: 10.1016/j.parkreldis.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao C, El Khoury R, Wang W, et al. LRRK2-mediated neurodegeneration and dysfunction of dopaminergic neurons in a Caenorhabditis elegans model of Parkinson's disease. Neurobiol Dis. 2010;40(1):73–81. doi: 10.1016/j.nbd.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z, Wang X, Yu Y, et al. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A. 2008;105(7):2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong Y, Coombes CE, Kilaru A, et al. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 2010;6(4):e1000902. doi: 10.1371/journal.pgen.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40(8):955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saunders-Pullman R, Barrett MJ, Stanley KM, et al. LRRK2 G2019S mutations are associated with an increased cancer risk in Parkinson disease. Mov Disord. 2010;25(15):2536–2541. doi: 10.1002/mds.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang FR, Huang W, Chen SM, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361(27):2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]