Abstract
Shallow reefs (reef flats <1.5 m) in the northern Red Sea are impacted by growing tourism that includes swimmers, snorkellers and reef walkers but have largely been neglected in past studies. We selected a fringing reef along the lagoon of Dahab (Sinai, Egypt) as a model for a management strategy. Point-intercept line transects were used to determine substrate composition, coral community and condition, and the coral damage index (CDI) was applied. Approximately 84% of the coral colonies showed signs of damage such as breakage, partial mortality or algal overgrowth, especially affecting the most frequent coral genus Acropora. Questionnaires were used to determine the visitors’ socio-economic background and personal attitudes regarding snorkelling, SCUBA-diving and interest in visiting a prospective snorkelling trail. Experiencing nature (97%) was by far the strongest motivation, and interest in further education about reef ecology and skill training was high. Less experienced snorkellers and divers – the target group for further education and skill training – were those most prepared to financially support such a trail. We therefore recommend a guided underwater snorkelling trail and restricting recreational use to a less sensitive ‘ecotourism zone’ while protecting the shallow reef flat. Artificial structures can complete the trail and offer the opportunity to snorkel over deeper areas at unfavourable tide or wind conditions. This approach provides a strategy for the management and conservation of shallow-water reefs, which are facing increasing human impact here and elsewhere.
Keywords: Shallow-water corals, Reef management, Tourism impact, Snorkelling trail, Northern Red Sea
Highlights
► We assess the condition of a highly threatened shallow-water reef in the Red Sea. ► The reef is used by fishermen and tourists and shows much coral rubble and breakage. ► A high proportion of branching corals makes the model reef particularly vulnerable. ► Questionnaires revealed that tourists are interested in nature and education. ► We suggest a snorkelling trail to reduce tourism-based damage and increase awareness.
1. Introduction
The decline of coral reefs worldwide calls for quantifying the damage, identifying the causes and suggesting solutions. Reefs supply millions of people with economic and environmental services (Moberg and Folke, 1999). The net benefits are estimated to be US$ 30 billion per year to world economies including tourism, fisheries and coastal protection (Cesar, 2003). Yet precisely such recreational and commercial human activities, coupled with growing human coastal populations, pose key threats to reefs (Pandolfi et al., 2003; Schuttenberg, 2001; Wilkinson, 2004).
The Egyptian coast of the Red Sea combines this complex of issues. Tourism, along with destructive fishing methods and coastal development, pose the greatest threat (Cesar, 2003; Saila et al., 1993). According to a report on biodiversity conservation capacity building in Egypt (Egyptian Biodiversity CHM, 2006), more than 8 million tourists visit Egypt annually, whereby coastal tourism is the largest sub-sector within the market. This nature-based industry contributes significantly to national income and depends largely on intact reefs. Market-demand studies for the South Sinai region show that development has concentrated on Sharm El-Sheikh followed by Dahab and Nuweiba: foreign tourism has grown 42% per year (PERSGA/GEF, 2001): snorkellers make up 20–40% of recreational water sport tourists (Cesar, 2003).
The status of reefs in the Red Sea and Gulf of Aqaba is generally good, with an average live coral cover of 20–50%, a positive ratio of live to dead coral cover, and high species diversity (Kotb et al., 2004). Yet coral cover in the Egyptian Red Sea has declined by over 30% in some places during recent decades, coupled with a significant increase of broken and damaged colonies (Jameson et al., 1999). This trend is closely related to the rapid growth of tourism (Ibrahim and Ibrahim, 2006; Elrefaie unpublished).
Riegl and Velimirov (1991) determined that, in the Red Sea (Hurghada, Egypt and Eilat, Israel), coral breakage was the most frequent damage category. Partial mortality along with a “coral damage index” (Jameson et al., 1999) are good indicators for reef condition and help estimate the severity of damage due to natural or anthropogenic stressors. Such breakage was significantly higher at reefs with high visitor frequency. Moreover, damage was worst within the first 10 m depth (Leujak and Ormond, 2008; Riegl and Velimirov, 1991). Tourists (divers or snorkellers) posed the greatest threat (Loya, 2004; Zakai and Chadwick-Furman, 2002), but fishers also broke corals through trampling (Leujak and Ormond, 2008) and net fishing.
Direct diver or snorkeller impacts include trampling, fin contact, standing on corals and resuspension of sediment (Chabanet et al., 2005). The level of physical damage corresponds with visitor numbers (Hawkins and Roberts, 1993; Riegl and Velimirov, 1991; Rodgers and Cox, 2003). Snorkeller damage is mostly limited to shallow areas where visitors can stand on or kick coral (Plathong et al., 2000; Rogers, 1990). Much fewer studies have been conducted on snorkellers versus SCUBA-divers, though the former are known to deteriorate shallow-water reefs (Allison, 1996; Harriott, 2002; Riegl and Velimirov, 1994). Depending on growth form (Riegl et al., 1996; Rogers, 1990) and species composition, coral communities differ in their susceptibility to recreational activities. Those dominated by branched forms (e.g. the genus Acropora) are the most fragile (Riegl and Cook, 1995).
Diver carrying capacities therefore play an important role in managing physical damage (Jameson et al., 1999). Recent studies report capacities between 4000 and 15,000 dives per site per year (Cesar, 2003; Davis and Tisdell, 1995; Jameson et al., 1999). Leujak and Ormond, 2007 showed that the social carrying capacity – e.g. a level of visitors that avoids a decline in attractiveness of the environment or avoids crowding – is another useful tool for sustainably managing coastal tourism. Based on research conducted in Ras Mohammed National Park, Red Sea, an “environmental briefing” for divers also reduces damage (Medio et al., 1997). Environmentally responsible behaviour can further be promoted by improving diver and snorkeller skills and their knowledge about potential and actual threats to the reef (Rouphael and Inglis, 2001). Finally, snorkelling trails can give tourists an understanding of the marine environment and help restrict impacts to defined areas (Harriott, 2002). This can further be refined by managing snorkel impacts, for example by protecting more sensitive areas dominated by fragile branching corals (Egyptian Biodiversity CHM, 2006; Harriott, 2002).
The present study focuses on Napoleon Reef, a very shallow (0.5–1.5 m) fringing reef flat along the lagoon of Dahab, Egypt (Fig. 1). The high abundance and diversity of corals and fishes is attractive to snorkellers and fishers; these users therefore pose a risk for their own resource. We begin by assessing the overall level of damage and determining the source and level of deterioration. We then evaluate the socio-economic parameters of tourists here and suggest a management strategy that involves a snorkelling trail on a subsection of this reef.
Fig. 1.
Location of the study area. Left: Gulf of Aqaba, northern Red Sea, and the Sinai Peninsula, Egypt. Bottom: study area at the Napoleon Reef (Dahab, Gulf of Aqaba); dotted line: course of reef crest; rectangles: area 1 and 2, where transects were deployed. Top right: Ten plots (each 50 × 50 m or 40 × 50 m) subdividing the study area.
2. Study area
The main study site (area 1, Fig. 1) was surveyed between July and October 2007 at Dahab (28°28′ N, 34°30′ E), in the southern Gulf of Aqaba, northern Red Sea (Sinai, Egypt). The study site is a fringing reef flat (termed “Napoleon Reef” here, although slightly to the north of that reef) (28°28′00″ N, 34°30′21″ E) outside of the lagoon of Dahab (Fig. 1). This reef is very shallow (∼0.5–1 m deep (mid-water level); tidal range 0.4–1 m) and is frequently used as a day trip destination for snorkellers and as a fishing area for locals. Most tourists come from Dahab or Sharm el Sheikh, the latter usually visiting the reef for a single day for swimming and snorkelling. Tourist numbers are estimated at a maximum of 100 visitors a day. Wind surfers are numerous but only occasionally cross the very shallow reef flat to surf offshore. Fishing activities include net fishing by Bedouin men and collecting organisms such as clams with long sticks by Bedouin women, both usually walking along and across the reef flat. Reef condition was determined by examining an area of 23,500 m2.
An additional survey in March 2008 focused on the proposed snorkelling trail area (area 2, Fig. 1) along the inner reef edge at the southern end of the investigation area, which is popular for snorkellers, wind surfers and local fishers. The reef here is up to 200 m wide and, on the reef flat, coral cover increases towards the wind- and wave-exposed outer reef edge. The inner reef edge (back reef) bordering the lagoon is sheltered from waves and lined with small coral pinnacles, followed by a smooth sandy slope downwards towards the lagoon. The area was divided into back reef (coral pinnacles) and reef flat (horizontal coral cover). This more than 200-m-long back reef enables snorkelling without crossing over the reef flat.
3. Materials and methods
3.1. Sampling design
Based on a satellite image from Google Earth, the main investigation site (area 1) was divided into ten 50 m × 50 m (or 50 m × 40 m at the southern end) quadrat plots (Fig. 1). The vertices of the grid pattern were fixed by GPS-coordinates (Garmin Geko 201) during a ground truthing procedure for potential follow-up long-term monitoring – the most common approach to examine long-term trends in the status of coral reefs. Nineteen numbered markers were placed at the GPS locations. Water depths were measured with a diving computer (0.1 m accuracy). Measured depth and tidal fluctuations were gauged using WX-Tide32 version 4.7.
Sampling was conducted by visual census and photo documentation while snorkelling on the reef flat. In area 1, transects (50-m-long measuring tape) were laid perpendicular to the shoreline and parallel to each other every 10 m (white lines in Fig. 1). Transect lengths varied from 100 to 150 m (subdivided into two or three 50 m transects) depending on reef flat width. A total of 51 transects, each 50 m long, were surveyed using a photo point intercept method. At each meter the substrate point was fathomed, recorded on an underwater datasheet and photographed, yielding a total of 2550 data points.
In area 2, five line transects (each 50 m long) were positioned in the area of the back reef facing the lagoon, and an additional one was placed on the reef flat next to the back reef. Benthic communities were sampled as above.
3.2. Substrate determination
Substrate composition was determined using the photo point intercept transect method. A digital photograph (Panasonic Lumix DMC-TZ1) of the substrate beneath the plumb bob was taken at every transect meter. The substrate was classified in the field into eleven categories: algae, broken coral colony, broken coral fragment, coral rock, dead coral, dead coral with algae, hard coral, rubble, rubbish, soft coral and sand (Table 1). These eleven categories were then pooled in five new categories: sand (SD), live substrate (LS: algae, hard coral, soft coral), dead substrate (DS: coral rock, dead coral, dead coral with algae, rubble), broken corals (BC: broken coral colony and broken coral fragment) and rubbish (RU) to increase sample number within each category. In the field, four classes (1: <25%, 2: 26%–50%, 3: 51%–75%, 4: 76%–99%) of partial mortality (PM) and breakage (BC) were determined for live corals (dead coral = 100% PM). Live corals were mostly identified at genus level. The genus Acropora (colonies > 10 cm maximum diameter) was identified to species level based on digital images.
Table 1.
Shannon-Index (H′) and Evenness (E) of the coral genera in the different subdivisions of the investigation area 1. The subdivisions are based on the 3-cluster-model of 51 line transects (ns = nearshore, int = intermediate, off = offshore), and on ten plots (I to X).
| 3 Clusters |
|||
|---|---|---|---|
| ns | int | off | |
| H′ | 1.61 | 1.31 | 1.64 |
| E | 0.65 | 0.53 | 0.66 |
| Plots |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | |
| H′ | 1.52 | 1.21 | 0.93 | 1.41 | 1.51 | 1.62 | 1.60 | 1.53 | 1.38 | 1.73 |
| E | 0.69 | 0.58 | 0.45 | 0.64 | 0.73 | 0.70 | 0.77 | 0.79 | 0.60 | 0.69 |
3.3. Socio-economic survey
Questionnaires were printed in 7 languages (English, German, Dutch, Italian, Spanish, French, Russian) and distributed among the visitors of 29 randomly chosen dive centers in Dahab. A total of 318 visitors responded to 64 questions subdivided into four sections (Heinisch, 2006; Huxham and Tett, 2005): The first section consisted of 10 general questions about demographic characteristics (age, sex and nationality), snorkel proficiency (beginner or advanced) and diver-certification level: Open Water Diver (OWD), Advanced/Rescue Diver (AOWD) or Dive Master/Instructor. The second and third sections contained 28 Likert-scaled questions with first-person statements depicting differing viewpoints on a four-point scale (totally agree, agree, disagree, fully disagree). Respondents indicated which number best represented their own viewpoint. Questions of the second section helped to distinguish the respondent’s motivation for snorkelling/diving (sporting experience, natural experience, recreation, adventure, social contact, i.e. family and friends or meeting people), perceptions about further environmental education, skill training and briefings in proper behaviour related to visiting a snorkelling trail. Respondents were also asked to depict the role of environmental education during their diver education on a three point-scale (major, minor, irrelevant). In the third section, respondents indicated their desire for each of 10 different facilities at a snorkel site (sanitary, sunshades, snack bar, equipment rental, snorkelling lessons, bar, children’s playground, sunbathing, lifeguard and first aid). Respondents were then asked to choose one out of five price categories they would consider paying for a guided trip on a snorkelling trail (<10, 11–20, 21–30, 31–40, >40 €; US$ equivalent (averaged conversion rate for 2007): <13, 14–27, 28–41, 42–54, >55). The fourth and last part queried the respondent’s source of knowledge about reef ecology (university, diver education, technical literature, conversations with other snorkellers/divers) or whether they had no previous knowledge. Finally, 24 statements about reef ecology with a bi-polar answer scale (true, false) were put forward. Statements were randomly repeated to avoid response-set bias.
3.4. Statistical analysis
3.4.1. Ecological survey
To test for potential influence of wave exposure on substrate composition along transects, hierarchical cluster analyses were performed using PC-ORD v4.25. This test for exposure-related similarities involved a hierarchical cluster analysis (AHCA) based on Sorensen (Bray–Curtis) distance and flexible beta (β = −0.25) linkage function. “Flexible beta with β = −0.25 is a space-conserving method that avoids distortion and has less propensity to chain” (Reese et al., 2005). To determine the optimum number of cluster groups, a significance test and effect size (A = within group agreement) of groups using a multi-response permutation procedure (MRPP, Sorensen distance) was done (Reese et al., 2005).
As a measure of the information entropy of substratum diversity along the transect, the Shannon-index (H′) was calculated:
H′ gives a measure of diversity in categorical data where ni = the number of individuals in each genus (the abundance of each genus), S = the number of genera, N = the total number of all individuals and pi = the relative abundance of each genus. pi was calculated as the proportion of individuals of a given genus to the total number of individuals in the community ni.N−1. This index considers the number of genera and the Evenness (E, measure of diversity) of the genus.
Two forms of physically damaged corals that best incorporate past (rubble) and more recent (broken coral) degradation are used for the coral damage index (CDI) (Jameson et al., 1999). It is designed to determine whether a reef is threatened and to estimate the intensity of physical damage. Sites with a percentage of broken coral colonies (BCC) ≥ 4% and/or a percent cover of coral rubble (CR) ≥ 3% are characterized as “hot spots” (Jameson et al., 1999).
3.4.2. Socio-economic survey
All statistical analyses were processed using SPSS for Windows version 15.0 (Eckstein, 2006). Frequencies included median and range for ordinal-scaled data and mode for nominal-scaled data. For interval-scaled data, mean scores, median, range and standard error were calculated for each question (age, snorkel- and SCUBA-dives, number of correct ecology answers). Normal distribution was tested using the Kolmogorov-Smirnov-Test. Ordinal-scaled data were compared using the Kruskal–Wallis test. Associations between variables were investigated using Spearman rank-order correlations. Differences of nominal-scaled variables were determined using non-parametric Chi2-Goodness-of-fit, and associations were calculated using Chi2-Independence related to crosstabs.
4. Results
4.1. Hierarchical cluster analysis
Hierarchical cluster analysis of 51 line point intercept transects from the main investigation site (area 1) yielded a dendrogram containing two main clusters with two subclusters each. The multi-response permutation procedure (MRPP analysis) revealed high within-group agreement (A, change corrected) within three clusters (A = 0.26; 20% information remaining). The more negative the test statistic T is, the stronger the separation between the cluster groups, which means three clusters (one main cluster and two subclusters) have a stronger (T3 = −13.77) and 2 clusters (main clusters; A = 0.17; 12% information remaining) a lower (T2 = −14.41) coherence. Accordingly, the 3 cluster model was selected. The clusters follow a west-east distribution, agreeing well with increasing wave exposure, and were therefore termed “nearshore”, “intermediate” and “offshore”.
Analyses were based on the four categories sand (SD; n = 790), live substrate (LS: algae, hard coral, soft coral; n = 919), dead substrate (DS: coral rock, dead coral, dead coral with algae, rubble; n = 748) and broken corals (BC: broken coral colony and broken coral fragment; n = 89). Rubbish (RU; n = 4) was omitted because of its very low frequency. Across the three exposure regimes, sand decreased strongly from “nearshore” to “offshore” (45.9–18.4%), whereas live (27.6–41.8%) and dead substrate (22.9–37%) increased considerably (Fig. 2).
Fig. 2.
Frequency [%] of pooled substrate categories (sand; live substrate: algae, hard coral, soft coral; dead substrate: coral rock, dead coral, dead coral with algae, rubble; broken corals: broken coral colony and fragments) in the “nearshore”, “intermediate” and the “offshore” areas (according to the three clusters of transects as revealed by the cluster analysis) at Napoleon Reef (Dahab, Gulf of Aqaba).
4.2. Coral community
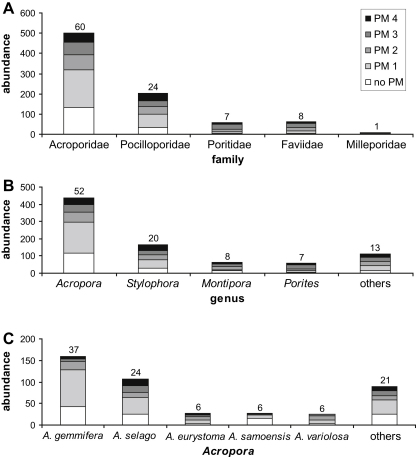
Five different families of hard corals (Fig. 3A) were recorded in the main investigation site (area 1). The dominant family was the Acroporidae, whereby the genus Acropora had the highest relative abundance in every plot and in all three exposure regimes. Another representative, Montipora, was also common (Fig. 3B). Among the Pocilloporidae, the second most common family, Stylophora was highly abundant in most plots and Pocillopora (4.4% of all corals) was also common. The Faviidae were mainly represented by Favites (2.4%), Cyphastrea (2.1%) and others (3.1%; namely the four genera Favia, Goniastrea, Leptastrea, Platygyra). The Poritidae were exclusively represented by Porites, and the Milleporidae by Millepora. Acropora colonies (n = 438) were identified at species level (Fig. 3C). The most common species was Acropora gemmifera, followed by Acropora selago, Acropora eurystoma, Acropora samoensis and Acropora variolosa. Acropora loripes, Acropora subulata, Acropora hyacinthus, Acropora secale, Acropora acuminata, Acropora digitifera and unidentified species (each less than 5%) contributed a total of about 21%. The species evenness was lowest in plot III and highest in plots VII and VIII (Table 1). When moving from “nearshore” to “offshore” (west to east), the more genera that are present in the sample, the more even their representation, and diversity increases accordingly.
Fig. 3.
Abundance (number of colonies) of partial mortality (PM) categories (no PM = 0%, PM 1 = < 25%, PM 2 = < 50%; PM 3 = < 75% and PM 4 = > 75%) across A: families, B: genera (others, each with n < 40: Astreopora, Cyphastrea, Favia, Favites, Goniastrea, Leptastrea, Millepora, Platygyra, Pocillopora and unidentified genera) and C: Acropora species (others, each with n < 20: A. acuminata, A. digitifera, A. hyacinthus, A. loripes, A. secale, A. subulata and unidentified Acropora). Values above bars indicate proportions (in %) of the respective taxon among all taxa.
The survey at the proposed snorkelling trail site (area 2) showed that sand dominated over hard coral in the back reef area and vice-versa on the reef flat. The third most common substrate was coral rock. The six point intercept transects (300 data points) yielded 122 (40.7%) hard coral colonies. The genus Acropora clearly dominated (49.1% of all corals). Millepora was the second most abundant genus (13.2%), followed by Pocillopora and Stylophora (each, 8.5%). A total of 106 hard coral colonies were identified into 6 families and 10 genera (Montipora, Porites, Favites, Platygyra, Galaxea and Favia in addition to the four mentioned above and arranged by decreasing abundance). Nine species of Acropora were determined. The total live hard coral cover was 33.6% (101 colonies); the remaining 21 (7.1%) colonies were dead. Of the latter, 16 could not be identified due to algal overgrowth. Most hard corals were branched forms (93 of 122 colonies = 76.2%). The rest were encrusting (13), massive (12) and foliaceous (4). All living broken coral colonies, broken coral fragments, recently killed corals and nine of ten dead, algae-overgrown corals were also branching forms. The genera categorized as branching were Acropora, Pocillopora and Stylophora. Millepora showed branching and foliaceous growth forms.
4.3. Partial mortality
In area 1, the relative frequency of coral families shows that Acroporidae yielded 44% of all corals with some amount of PM (Fig. 3A). This was followed by the Pocilloporidae with 20.1%, the Faviidae with 6.9%, the Poritidae with 6.2% and the Milleporidae with 0.7%. Among all coral genera (Fig. 3B), Porites (n = 58) was most affected (PM rate: 89.7%), followed by Stylophora (n = 167; 82.6%), Montipora (n = 63; 77.8%) and Acropora (n = 438; 73.1%). Genera with n < 40 colonies were pooled as “others” (Astreopora, Cyphastrea, Favia, Favites, Goniastrea, Leptastrea, Millepora, Platygyra, Pocillopora and unidentified genera; n = 114) and yielded an average PM rate of 78.2%. Within Acropora (Fig. 3C), A. eurystoma (n = 28) was the most affected (85.7% of all colonies with some level of PM), followed by A. variolosa (n = 26; 84.6%), A. selago (n = 107; 75.7%) and A. gemmifera (n = 160; 73.1%). A. samoensis (n = 27) had the lowest value (40.7%). Species with n < 20 were pooled (A. acuminata, A. digitifera, A. hyacinthus, A. loripes, A. secale, A. subulata and unidentified Acropora) and had a value of 72.2%. The amount of PM per live coral cover (LCC) ranged between 68.3% (plot I: LCC of 20%) and 85.1% (plot X: LCC of 38%).
In area 2, PM rates were evaluated in 81 colonies. The rate was 58% across all genera and was therefore much lower than in area 1. In particular, Acropora colonies were less affected; 50% of colonies had no PM and about 40% had a PM <25%. In contrast, values of Stylophora, Millepora and Pocillopora were similar as in area 1.
4.4. Coral damage index (CDI)
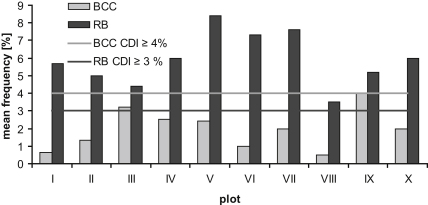
To relate data to areas, to define hot spots and to improve the graphical presentation, transects of area 1 were pooled into plots (Fig. 1). Plot IX had the highest amount of broken coral colonies (4% BCC; Table 2), followed by plots III (3.2%), IV (2.5%) and V (2.4%). All other plots had values ≤2% (Fig. 4). In terms of coral rubble (RB) frequency, plot V was the most affected (8.4%), followed by plots VII (7.6%) and VI (7.3%). All other plots had values between 3.5% and 6%. The most affected plots regarding CDI (BCC and RB) were V, VII and IX; plot VIII was least affected. Accordingly, 23.5% of all transects were “hot spots” in regard to broken coral colonies (BCC) and 76.5% of all transects in regard to RB. Only 19.5% of the transects had a CDI between 0% and 2% and were thus not “hot spots”. Overall, this area of Napoleon Reef had a CDI of 1.9% BCC and 6% RB.
Table 2.
Percentage of different corals/substrate types per plot (I to X) at the Napoleon Reef, Dahab, Egypt (Gulf of Aqaba).
| Substrate |
Plots |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I |
II |
III |
IV |
V |
VI |
VII |
VIII |
IX |
X |
|
| Hard coral | 19.3 | 32.0 | 32.4 | 34.0 | 26.4 | 26.3 | 32.0 | 40.0 | 37.6 | 35.6 |
| Broken coral | 0.7 | 1.3 | 3.2 | 2.5 | 2.4 | 1.0 | 2.0 | 0.5 | 4.0 | 1.6 |
| Broken coral fragment | – | 0.3 | 1.6 | 4.5 | 1.2 | 2.3 | 1.6 | 3.5 | 1.6 | 0.4 |
| Dead coral with algae | 3.0 | 7.0 | 8.0 | 4.0 | 6.0 | 12.3 | 10.4 | 8.0 | 8.0 | 15.2 |
| Dead coral | 6.3 | 4.0 | 8.0 | 4.0 | 10.8 | 4.3 | 4.4 | 9.0 | 10.0 | 4.8 |
| Soft coral | 3.0 | 2.0 | 1.2 | 2.0 | 0.4 | 3.7 | 2.4 | 3.0 | 1.6 | 4.4 |
| Rubble | 5.7 | 5.0 | 4.4 | 6.0 | 8.4 | 7.3 | 7.6 | 3.5 | 5.2 | 6.0 |
| Sand | 58.7 | 37.0 | 27.6 | 38.0 | 41.6 | 20.3 | 19.6 | 19.5 | 28.4 | 12.8 |
| Coral rock | 2.7 | 10.0 | 9.6 | 1.5 | 2.0 | 20.0 | 14.4 | 7.5 | 1.2 | 16.0 |
| Algae | 0.7 | 1.3 | 3.6 | 2.5 | 0.4 | 2.3 | 5.6 | 5.5 | 2.4 | 3.2 |
| Rubbish | – | – | 0.4 | 1.0 | 0.4 | – | – | – | – | – |
Fig. 4.
Coral damage index (CDI) of ten reef plots at Napoleon Reef (Dahab, Gulf of Aqaba). CDI represents the average frequency (%) of broken coral colonies (BCC) or coral rubble (RB) of all transects within each plot. The grey and the black lines indicate the threshold value for a “hot spot” within the BCC and the RB category, respectively.
Acropora was the most frequently broken coral genus (87.8% of all broken corals). 10.2% of broken colonies were Stylophora corals, 2% Pocillopora. This index was also applied to species of Acropora, whereby A. selago was the most affected (44.9%), followed by A. gemmifera (16.3%), A. eurystoma (6.1%), A. acuminata, A. secale and A. variolosa (4.1% each), as well as A. loripes, A. samoensis, A. subulata and unidentified Acropora (2% each).
In area 2, live broken coral colonies made up 1.3% on the back reef and 2.0% on the reef flat, similar to area 1. Coral rubble was less common here and showed a higher frequency on the back reef (4.7%) versus reef flat (3.3%). The mean frequency of living unbroken hard coral colonies was significantly higher (Chi2 = 5.14; p < 0.05) in reef flat transects (mean ± S.E. = 20.3 ± 3.9: 40.6% of 50 data points) versus the back reef (13.3 ± 0.3: 26.6%).
4.5. Socio-economic survey
4.5.1. Demographic characteristics
Of the 318 respondents, 57% were male and 43% female. Age ranged from 14 to 65 years (median: 30). Half of the respondents were 25–36 years old. Most participants were from Western Europe (80.5%). The majority was German (36%), followed by Dutch (20%), Austrian (10%), British (9%), Swiss (6%) and Hungarian (4%). Furthermore, 52 respondents of 20 nationalities, each contributing < 3%, were categorised as ‘others’.
4.5.2. Snorkel- and SCUBA-dive frequencies and experience
In total, 71.1% (226) were snorkellers, whereof almost half (48.7%) rated themselves as beginners (51.3% as advanced). However, the stated numbers of snorkel dives in tropical seas showed a median of 20 (range: 0–1000). Amongst the 226 snorkellers, 44.0% had 0 to 10 dives in tropical seas, and 81.9% were also SCUBA-divers with a median of 20 dives in tropical seas. Overall, 84.3% were SCUBA-divers, of whom 41.5% had an advanced- or rescue-diver certification level, followed by 27.7% divers with an Open Water brevet (the lowest certification level). A further 15.7% were novice divers taking their first dive lessons. The remaining 15.1% were dive-leaders or dive-instructors. The number of SCUBA dives in tropical seas per person ranged from 0 to 3000 (median 31.5). Amongst the 268 SCUBA-divers, 26.9% had dived 0–10 times in tropical seas and 69.0% rated themselves also as snorkellers (median: 10 snorkel dives in tropical seas). There was a moderate positive correlation between snorkel proficiency and diver certification, i.e. highly certified SCUBA-divers tended to rate themselves as advanced snorkellers (Spearman’s Rho = 0.29; p < 0.001).
4.5.3. Motivation for snorkelling/diving
Nature (96.5% agreeing) and recreation (86.2%) were by far the highest motivations for snorkelling or diving. In comparison, other motivations – sport (65.1%), adventure (61.0%), social contacts, i.e. friends or family (61.6%), and meeting people (57.5%) – were ticked by more than half of the respondents (categories ‘agree’ and ‘fully agree’; Fig. 5A). Men tended to rate ‘adventure’ higher than women did (Chi2 = 12.4; df = 1; p < 0.01).
Fig. 5.
Motivation for snorkelling and diving and interest in environmental education. A: Motivations for snorkel- and SCUBA-dives (n = 318). advent. = adventure, meet.ppl. = meeting people, soc.cont. = social contacts. B: Interest in further education and training relating to a snorkelling trail (n = 318). Posed questions (a–g): (a) Offering environmental education courses for snorkellers/divers is sensible; (b) A briefing in snorkel skills before visiting a snorkelling trail is sensible; (c) A briefing in proper behaviour before snorkel/dive trips for reef protection is sensible; (d) A snorkelling trail is one reason for me to choose a snorkel site; (e) Offering environmental education courses for snorkellers/divers is one reason for me to choose a dive centre; (f) Offering environmental education courses for snorkellers/divers promotes business for dive centres; (g) A well-trained guide leading a snorkel trip on a snorkelling trail is sensible.
4.5.4. Knowledge and perception about reef ecology and threats to reefs
Of 318 respondents, 10.4% had no previous knowledge about reef ecology. Amongst the SCUBA-divers, 59.4% considered that environmental protection played a major role in their diver-education; 31.1% rated the role as minor, 9.4% as irrelevant. Related to the variable ‘diver-certification’, respondents with a lower certification level (Open Water Diver) tended to rate the role of environmental protection during their diver-education as minor, while better certified dive masters or instructors tended to rate it as major (Spearman’s Rho = −2.1; p < 0.01; Chi2 = 14.0; df = 2; p < 0.01). Most respondents were aware of potential reef threats, e.g. global warming (87.1%), slow coral recovery after breakage (94.3%), physical contact with corals (96.9%), feeding of marine animals (92.1%), sedimentation (89.0%), and nutrient contamination (88.4%). They further agreed that snorkelling affects reef condition (94.0%) and can impact reef quality (82.1%). Finally, 95.6% agreed that protected areas, i.e. excluding tourism and fishing from threatened areas, would be effective (n = 318).
4.5.5. Interest in further education and training relating to a snorkelling trail
Most respondents agreed that sensible approaches included offering environmental education courses for snorkellers/divers (89.9%; Fig. 5B), briefings in snorkel skills (89.6%), briefings in proper behaviour (95.6%) and a well-trained guide leading a snorkel trip on a snorkelling trail (85.8%). Furthermore, most stated that such a trail would be one reason for them to choose a snorkel site (71.7%) or a dive centre (66.4%). Finally, 78.3% agreed that offering environmental education courses would promote business for dive centres. There was a highly significant correlation (Spearman’s Rho = 0.2; p < 0.01) between age-class and the perceptions about a briefing in snorkel skills before visiting a snorkelling trail. The younger the respondents, the more they agreed that such prior briefings would be sensible. We also found highly significant differences between the mean ranks of groups, in particular relating to the age-class (Chi2 = 12.6; df = 3; p < 0.01) and relating to the variable ‘gender’: women tended to agree more on skill-briefings than men (Chi2 = 7.2; df = 1; p < 0.01).
4.5.6. Willingness to pay for visiting a snorkelling trail and desired facilities
Most of the 318 respondents were willing to pay for a guided trip or for renting a guide book when visiting a snorkelling trail. For a guided trip, 44.2% were prepared to pay 11 to 20 € (14–27 US$), followed by 37.2% who would pay up to 10 €. Moreover, 16.0% were ready to pay 21 to 30 € and a minority of 2.2% even 31 to 40 €. One visitor would have spent more than 40 € (six respondents did not answer the question). The responses regarding renting a guide book showed a similar ratio. There was a highly significant negative relationship between snorkel proficiency and the willingness to pay for visiting a snorkelling trail, guided or with a guide book (Spearman’s Rho = −0.24; p < 0.01). Thus, beginners were willing to pay more than advanced snorkellers. Within SCUBA-divers, the same trend was found related to diver-certification level (Spearman’s Rho = −0.33; p < 0.01; Chi2 = 11.0; df = 2; p < 0.01).
Concerning the desired infrastructure, first aid, sunshade and sanitary facilities topped the list, followed by floating aids, a life guard and rental snorkelling equipment.
5. Discussion
The coral community at the study site was dominated by the branching coral genera Acropora and Stylophora. The coral coverage values of 33% agree with those found elsewhere in the Gulf of Aqaba by Kotb et al. (2004) and Dirnwöber and Herler (2007), i.e. a mean of 35% and 37%, respectively. Live coral cover here is lower than in the Red Sea main basin (45%), but still within the range considered good (20–50%; Kotb et al., 2004). Coral diversity generally increases from the sandy inner reef part towards the outer part. This probably reflects the greater disturbance due to re-suspended sand along the shore, a sublethal stress that reduces coral cover, alters coral communities (Nyström et al., 2000) and increases partial mortality (Rogers, 1990). Such sedimentation damages the Acropora/Stylophora community in the shallow northern Red Sea and is a key disturbance factor for healthy reefs (Riegl and Piller, 2000). The investigation area is therefore a representative northern Red Sea shallow-water reef.
In contrast to other regions, where reefs suffer most from hurricanes and global effects such as climate change and coral bleaching, the northern Red Sea is mainly affected by coastal development, Acanthaster outbreaks, destructive fishing methods, water sport tourism and reef walking (Kotb et al., 2004; Loya, 2004). These threats no doubt affect many other shallow-water reefs, but are rarely investigated. This is because most studies on reef damage focus on deeper areas (i.e. Reef Check) and on diver damage, although impacts may be high in very shallow reefs (<2 m water depth) due to swimming, snorkelling, reef walking and net fishing. Despite the high probability of physical contact in very shallow-water, there are no or few regulations or educational programs for snorkellers and swimmers. In contrast, divers learn or face regulations through their certification, their abilities are commonly tested in so-called check dives prior to first reef dives, and national diving rules often apply (e.g. guided diving in small groups). Among recreationally used reefs, fringing reef flats are the most severely impacted zones (Red Sea: Riegl and Piller, 2000). Thus, the reef investigated here is a good model because it is frequently used for recreation and fishing, which together cause most of the coral breakage. Natural physical causes of breakage seem unlikely: the semi-enclosed Gulf of Aqaba shows no coral damage due to currents or storms (Zakai and Chadwick-Furman, 2002), although non-athropogenic and man-made damage is often difficult to differentiate (Riegl and Velimirov, 1991).
5.1. Condition of the model reef
Over 84% of the coral colonies showed signs of damage (breakage, partial mortality and/or algal overgrowth). Several factors can cause the high partial mortality (PM) rate (78% of live coral community). Extreme springtide ebbs at noon on days with low wind can completely desiccate the reef flat (Anthony and Kerswell, 2007; Herler personal observation), damaging coral tissue and promoting mortality (Loya, 2004). The combination of non-anthropogenic stressors (e.g. spring tide ebbs, Drupella outbreaks) and anthropogenic pressures, as found here, counteract ecosystem stability (Birkeland, 2004; Done, 1992; Moberg and Folke, 1999; Nyström et al., 2000). Although corals adapted to low-energy environments are most vulnerable (Rodgers and Cox, 2003), in situ experiments show that no corals are entirely resistant to trampling. Riegl and Velimirov (1991) recorded the highest damage frequency (breakage, tissue loss, algal overgrowth) within the first 10 m depth. In the Red Sea, this mainly reflects underwater sports (Loya, 2004; Zakai and Chadwick-Furman, 2002), but also fishers through trampling (Leujak and Ormond, 2008) and net fishing (personal observations). All these impacts are frequent at the study site: particularly in calm weather conditions, we observed fishers walking over the reef flat in groups of two to five, pulling nets (ca. 3 × 20 m and larger) over the reef flat, severely damaging coral colonies. Trampling is a recognized threat (Dizon and Yap, 2006; Plathong et al., 2000; Richmond and Hunter, 1990), and algal overgrowth is one follow-up effect of that damage (Riegl and Velimirov, 1991). This, in turn, can impact reef- and coral-associated fishes (Jones et al., 2004; Schiemer et al., 2009) along with commensal species such as crabs and shrimps (Caley et al., 2001).
Riegl and Cook (1995) rank Acropora spp. communities of shallow-water reefs, especially on the outer reef flat, as the most fragile. Riegl and Velimirov (1991) also reported Acropora spp. to be the most trampling-affected genus and Millepora dichotoma to be the most affected species. Our results corroborate that damage strongly reflects growth form (Anthony and Kerswell, 2007; Loya, 1972; Riegl and Velimirov, 1991): species from the families Acroporidae and Pocilloporidae (Acropora, Pocillopora and Stylophora) are most affected. In the main investigation area (area 1), Acropora was the dominant genus, whereas in the heavily used proposed snorkelling trail area (area 2), Acropora and Millepora dominated in the back reef and reef flat. The study site is clearly vulnerable to breakage. The most abundant Acropora species here, A. gemmifera, showed the poorest health condition (high PM). Acropora selago showed the highest breakage rate (20.8%) followed by A. acuminata (18.2%) and A. secale (16.7%). Among all broken coral colonies (BCC), 54% were branching forms (Acropora, Pocillopora and Stylophora), 46% perforated plates or tables (Acropora). Reef walkers (Hawkins and Roberts, 1993; Liddle, 1991; Neil, 1990) and water sport activities (Loya, 2004; Zakai and Chadwick-Furman, 2002) are known physical causes. This is reflected in the coral damage index (CDI) based on rubble (RB) and broken coral colonies (BCC). The high percentage of RB (76.5% of all transects exceeded “hot spot”-levels) indicates that breakage has been occurring for a long time on the reef flat. Although the frequency of BCC was lower, it reached “hot spot”-levels in some parts, implying ongoing degradation. Recovery requires protecting such reefs from additional future damage and protecting undamaged areas (Edinger et al., 1998; Zakai and Chadwick-Furman, 2002). Destructive activity by visitors and fishers can often be ascribed to a lack of understanding and ignorance (Leujak and Ormond, 2008). This calls for a management plan involving visitor education, as proposed below.
5.2. Tourist impacts and attitudes
The back reef zone here is visited daily by snorkellers and swimmers from Dahab and Sharm El Sheikh. Visitor numbers peak in the afternoon, and snorkellers swimming over the reef flat re-suspend sediment as well as kick and stand on corals. Coral breakage frequency here was about 2% but coral rubble clearly exceeded 3%. According to the coral damage index (Jameson et al., 1999), this is a “hot spot” of deterioration. This confirms a trampling effect (Liddle and Kay, 1987; Riegl and Cook, 1995; Riegl and Riegl, 1996). The combination of less than a half meter water depth at low tide, abundant branching colonies, and many daily visitors (up to 100) from Sharm El-Sheikh point to a strong relation between visitor frequency and coral breakage (Marshall and Baird, 2000; Riegl and Velimirov, 1991; Rodgers and Cox, 2003). In addition, wind-surfers, kite-surfers (Dahab lagoon is a famed surf spot) and kayaks pass over the reef flat.
We assume that the demographic characteristics (nationality, age, proportion of SCUBA-divers and snorkellers) of our questionnaire respondents are representative for the general profile of visitors interested in underwater sports activities. Note, however, that the omission of all-inclusive clubs may be a potential source of bias. Most respondents were from Western Europe (80.5%), although more than 20 other nationalities were recorded. Leujak and Ormond (2007) reported somewhat different results for Ras Mohammed National Park and Sharm El-Sheikh, South Sinai: 60% of the visitors came from Italy, 15% from other Western European countries, 12% from Russia and 11% from Egypt. They also noted a shift in nationality over the last two decades from German and British, to Italian and Russian. One interpretation of Leujak & Ormond’s (2007) findings is that the Western European visitors that formerly came to Sharm El-Sheikh have switched to Dahab, a fast-growing but still smaller and less frequented location. Russian and Italian visitors to Egypt have rapidly increased since 2000 (Ibrahim and Ibrahim, 2006). The changes in proportions of nationalities could affect touristic hot spots like Sharm El-Sheikh earlier than elsewhere (Dahab).
Nature and recreation were by far the highest motivations for snorkelling or diving (versus sport, adventure or meeting people). Importantly, the perception about reef health is connected to knowledge and experience (Leujak and Ormond, 2007). Our survey rates the experience and proficiency of snorkellers and divers as low in Dahab (despite not including certain nationalities with the lowest knowledge and skill levels as reported by the above study). The problem would be compounded by a reported shift from SCUBA-divers to snorkellers and to less experienced visitors with poorer knowledge of reef ecology in Sharm el Sheikh, South Sinai (Leujak and Ormond, 2007), who also represent a major group of the current visitors to the model reef in Dahab.
It is precisely this group of current and future inexperienced users, corresponding to a stagnation stage in the concept of a “tourism area cycle” (Butler, 1980), who a management strategy must address (Leujak and Ormond, 2007). Our results suggest that, beyond considering tourist numbers and traditional typologies based on the psychology of travel (motivation, desires, etc.), management strategies in coral reef destinations should incorporate the state of biological knowledge of visitors and thus the degree of threat they pose to the marine ecosystem. This may well be related to nationality, and would be an important factor in avoiding the final stage in the evolution of tourist areas, namely decline (Butler, 1980). Further education and information have a two-fold effect: they influence behaviour (Medio et al., 1997) and also put pressure on the industry to meet the demand for healthy reefs. Skill training, briefings on proper behaviour and information about reef ecology are therefore sensible tools to reduce physical damage (Rouphael and Inglis, 2001; Tratalos and Austin, 2001; Zakai and Chadwick-Furman, 2002).
Although 10.4% of those surveyed had no previous knowledge about reef ecology, the vast majority were aware of the potential threats, i.e. global warming, physical contact with corals, feeding of marine animals, and sedimentation. Almost half of the divers rated the role of environmental protection during their certification as minor or irrelevant. Accordingly, diver (and snorkeller) education should pay more attention to environmentally relevant skill training before sending people into the reef. Guides can play a crucial role in informing visitors about the relevant national park rules (Leujak and Ormond, 2007), although in some cases they inadequately perform this task: 37.5% of respondents on guided tours to the Ras Mohammed National Park did not know that corals were animals (Leujak and Ormond, 2007). The tour leaders bringing groups to Dahab from Sharm El-Sheikh need to combine such information with skill checks and training. The many snorkellers entering the water aside of dive sites, without any briefing, skill training or guidance, must be included in management actions. This calls for further education and training for both guides and visitors.
5.3. Proposed sustainable solution
In the Great Barrier Reef Marine Park, environmental interpretation programs were very effective: participants gained more knowledge and understanding about the reef environment and anthropogenic impacts than non-participants (Madin and Fenton, 2004). The effect was positively correlated with the amount of interpretive activities. Heinisch (2006) reported similar results for diver-related environmental education in Hurgada, Egypt. In our study, most respondents recognized the value of such courses, briefings in proper behaviour and a well-trained snorkel guide. A trail was a clear reason for them to choose a snorkelling site, and they were willing to pay for a guide or a guide book. This supports results obtained elsewhere. A main attraction at the U.S. Virgin Islands (Caribbean), for example, is a snorkelling trail established in 1958: nearly 90% of the 50,000 annual visitors use it (Thorsell and Wells, 1990). Several other studies recommend managing snorkel impacts by protecting sensitive areas dominated by fragile branching corals (Egyptian Biodiversity CHM, 2006; Harriott, 2002). In Australia, coral damage around interpretive signs increased during an initial one-month period, but then stabilised (Plathong et al., 2000). The authors suggested short briefings, careful site selection, floating stations to hold on and rest, life vests, and periodic rotation of trails as useful management strategies. Additional trail stations based on artificial structures open the way for such a rotating system, enable recovery periods for impacted parts, and provide an environmental enhancement (three-dimensional habitat for benthic and pelagic organisms) (Armono, unpublished; Cabaitan et al., 2008; Harris, unpublished; Plathong et al., 2000).
Importantly, less experienced snorkellers and divers were prepared to pay more than experienced ones. This is a crucial insight: they are the target group for further education and training because they pose the greatest threat.
Better information and training could also help increase carrying capacity (Davis and Tisdell, 1995; Hawkins and Roberts, 1992, 1993), with a snorkel trail further promoting this goal. A report on biodiversity conservation capacity building in Egypt (Egyptian Biodiversity CHM, 2006) pointed out that the challenge for reef-associated tourism in Egypt is to generate economic benefits while maintaining the ecosystem on which it depends. The estimated annual recreational value of Egypt’s reefs – using the ‘zonal travel cost method’ (ZTCM) and including a vector for the socio-economic characteristics of the visitors’ home countries – is US$ 142 million. The ‘individual travel cost method’ (ITCM), including a vector for the individual characteristics of the visitor, yielded US$ 191 million per year. The willingness of visitors to pay (WTP) for coral reef conservation was estimated to be about US$ 1.5 million per year (Egyptian Biodiversity CHM, 2006). This valuation highlights the importance of proper management in Egypt from both an economic and ecological point of view. The economic benefits must go beyond the people already working in tourism-related businesses. In Dahab, the Bedouins use the same reef resource for subsistence fishing yet have only minimal contact with tourists. Their situation parallels that of tourists: they also rely on an intact environment, but trampling and snagged nets impact the reef. At the same time, conflicts with tourism are pre-programmed, calling for incorporating the local community in future management plans.
5.4. Recommendations for the model reef
The evaluation of reef condition, of the ongoing threats, and of visitor attitudes all point to proceeding with a multi-pronged effort involving improved information, creating a snorkelling trail and incorporating the local population. Establishing no-use and multi-use areas, protecting “hot spot” areas and developing sustainable use would reduce threats, improve carrying capacity and provide economic benefits.
Specifically:
-
(1)
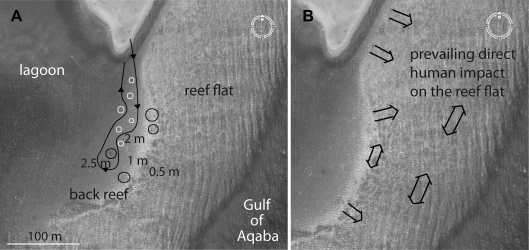
Divide the area into zones (terminology after Roman et al., 2007): Close the reef flat (shallow ‘sensitive zone’ dominated by branching Acropora communities) for recreational activities and fishing. Declare the back reef (‘relatively sensitive’ habitat dominated by shallow, hard coral communities) as an ‘ecotourism zone’. Restrict access here to small groups of snorkellers guided by certified tour guides. Minimize impacts by requiring that all guides provide prior briefings and skill training. Create a snorkelling trail (Fig. 6). Restrict snorkelling to clearly defined pathways that are deep enough (>2 m) to avoid contact (standing, fin kicking) (Allison, 1996). The trail in the shallow and vulnerable reef flat (‘sensitive zone’) should be limited to a small strip along the back reef. Here, create additional trail stations based on artificial structures to enable a rotating trail system and promote recovery of frequented parts. Define the extensive beach area bordering the western margin of the ‘ecotourism zone’ as a ‘very resistant zone’: depths below 3 m with sandy substrate and sporadic coral and sea grass patches reduce snorkeller contacts with corals.
-
(2)
Keep surfers in the adjacent lagoon at a safe distance from the swimming and snorkelling area.
-
(3)
Submerge purpose-built, nature-orientated artificial structures in different sizes and shapes. Concrete structures, for example, combine longevity in salt water with low cost and suitability for attaching coral fragments (see below; Omori and Fujiwara, 2004). The proposed trail (Fig. 6) extends over a 150-m-long strip in a minimum depth of 2 m to minimize fin contact with the bottom and sediment resuspension (Harriott, 2002; Rogers, 1990). Beyond the known benefits (rotating snorkelling trail system, recovery of impacted parts, environmental enhancement), such structures enable viewing benthic and fish communities in deeper areas when snorkelling along the shallow back reef at low tide is inadvisable. Such artificial pinnacles can be incorporated into environmental interpretation programmes for tourists, school-classes and biology students; they also play a role in reef restoration efforts and scientific research (Madin and Fenton, 2004).
-
(4)
Transplant the abundant broken but living coral fragments on the sand to artificial structures to increase their chances of survival (Dizon and Yap, 2006). The procedures for such efforts – suitable material, transportation, fixation, location characteristics, seasonal considerations – are fast improving (Edwards and Gomez, 2007; Omori and Fujiwara, 2004).
-
(5)
Provide professionally trained guides and water-proof guide books on reef biology and ecology (Hasler and Ott, 2008; Leujak and Ormond, 2007; Medio et al., 1997).
-
(6)
Provide infrastructure for snorkelling lessons, rental snorkel equipment, sanitary facilities, sunshades, a life guard and first aid.
-
(7)
Introduce entrance fees and fees for rental equipment and guided tours to help fund coral reef conservation (Arin and Kramer, 2002). Providing advertising surfaces for tour operators and other companies promotes a self-sustaining system. Note that less experienced visitors (“target group”) were most prepared to help fund such a management project.
-
(8)
Give the local community responsibilities in management to develop a sense of ownership for the reef and to promote adherence to the regulations that they helped establish (Crosby et al., 2002). This would include professional training of local fishers in equipment maintenance, provisions to restrict destructive fishing, and the substitution of inappropriate gear. Use the traditional knowledge for a better understanding of reef ecosystems, e.g., fish behaviour, habitat and migration patterns (Bunce et al., 2000). Interested local Bedouins could be offered training as snorkel guides, creating alternative incomes.
-
(9)
Open the reef for further research. Regular monitoring on the snorkelling trail and the adjoining natural reef yields the necessary information about coral damage, community status and ecosystem health (Wilkinson et al., 2003; Hawthorne, unpublished). Closed research areas would provide an opportunity to conduct undisturbed long-term studies in shallow reef areas.
Fig. 6.
Proposed course for a snorkelling trail (A) and prevailing direct human impact (B) at Napoleon Reef next to the lagoon of Dahab (Gulf of Aqaba, Egypt). A: natural pinnacles (black circles), proposed artificial structures (white circles) and an example for a snorkel trip along the underwater snorkelling trail (black line with arrows). Trail length measures about 150 m one way; depths refer to mid-water level; tidal range ± 0.4m (nip tide) ± 1.0m (spring tide). B: directions and areas of frequent direct human impact caused mainly by snorkellers, fishers and surfers (black double arrows).
6. Conclusion
This study uses a fringing coral reef in the Red Sea as a model for integrating scientific research on reef status with specific management suggestions. The coral damage index indicates abundant coral rubble (past damage) and broken colonies (recent damage), in particular of fragile, branched colonies. The cause is a combination of uncontrolled recreational activities (swimming, snorkelling) and reef trampling by tourists and local net fishers. We propose a zonation for different use categories, including implementing a snorkelling trail at a back reef site (‘ecotourism zone’) already heavily used by tourists.
This approach was supported by a questionnaire-based socio-economic survey that showed interest in nature to be the greatest motivation factor for diving and/or snorkelling activities here. Beginners – who tend to cause the most damage – were prepared the most to pay to visit such an underwater nature trail, including the costs for a guide and/or guide book. Briefings, training and guided tours would help protect the reef, increase its carrying capacity, create jobs and involve the local community. This approach has broad applicability wherever tourism and fishing rely heavily on intact, extremely shallow reefs.
Acknowledgements
We are grateful to Moustafa Fouda (Nature Conservation Section NCS of the Egyptian Environmental Affairs Agency EEAA) and Ayman Mabrouk (Nabq Managed Resource Protected Area MRPA) for granting research permission and supporting the project. Andreas Tischer and Hans Lange (DiveIn Dahab and DMRC) provided support for field work and laboratories. Jörg Ott supervised the Master’s theses of the first two authors. Thanks to Stefan Heinisch for providing advice in preparing the questionnaires, to those who helped translate the questionnaires, and to the dive centres involved in Dahab. This study was financially supported by the International Office of the University of Vienna (to J. Hannak) and by the APART-programme of the Austrian Academy of Sciences and the Austrian Science Fund (FWF-grant number P 21616-B12) (to J. Herler).
Contributor Information
Judith S. Hannak, Email: judithsunsun@yahoo.com.
Sarah Kompatscher, Email: sarah_kompatscher@hotmail.com.
Michael Stachowitsch, Email: Michael.Stachowitsch@unvie.ac.at.
Jürgen Herler, Email: Juergen.Herler@univie.ac.at.
References
- Allison W.R. Snorkeler damage to reef corals in the Maldive islands. Coral Reefs. 1996;15:215–218. [Google Scholar]
- Anthony K.R.N., Kerswell A.P. Coral mortality following extreme low tides and high solar radiation. Marine Biology. 2007;151:1623–1631. [Google Scholar]
- Arin T., Kramer R.A. Diver’s willingness to pay to visit marine sanctuaries: an exploratory study. Ocean & Coastal Management. 2002;45:171–183. [Google Scholar]
- Birkeland C. Ratcheting down the coral reefs. Bioscience. 2004;54:1021–1027. [Google Scholar]
- Bunce L., Townsley P., Pomeroy R., Pollnac R. Australian Institute of Marine Science; 2000. Socioeconomic manual for coral reef management. [Google Scholar]
- Butler R.W. The concept of a tourist area cycle of evolution: implications for management of resources. Canadian Geographer. 1980;1:5–12. [Google Scholar]
- Cabaitan P.C., Gomez E.D., Alono P.M. Effects of coral transplantation and giant clam restocking on the structure of fish communities on degraded patch reefs. Environmental Marine Biology and Ecology. 2008;357:85–98. [Google Scholar]
- Caley M.J., Buckley K.A., Jones G.P. Separating ecological effects of habitat fragmentation, degradation, and loss on coral commensals. Ecology. 2001;82:3435–3448. [Google Scholar]
- Cesar H. Unit of the Egyptian Environmental Policy Program; 2003. Report on the Economic Valuation of the Egyptian Red Sea Coral Reef. Monitoring, Verification, and Evaluation (MVE) [Google Scholar]
- Chabanet P., Adjeroud M., Andréfouët S., Bozec Y.M., Jocelyne Ferraris J., Garcìa-Charton J.A., Schrimm M. Human-induced physical disturbances and their indicators on coral reef habitats: a multi-scale approach. Aquatic Living Resource. 2005;18:215–230. [Google Scholar]
- Crosby M.P., Brighouse G., Pichon M. Priorities and strategies for addressing natural and anthropogenic threats to coral reefs in Pacific Island Nations. Ocean & Coastal Management. 2002;45:121–137. [Google Scholar]
- Davis D., Tisdell C. Recreational SCUBA diving and carrying capacity in marine protected areas. Ocean & Coastal Management. 1995;26:19–40. [Google Scholar]
- Dirnwöber M., Herler J. Microhabitat specialization and ecological consequences for coral gobies of the genus Gobiodon in the Gulf of Aqaba, northern Red Sea. Marine Ecology Progress Series. 2007;342:265–275. [Google Scholar]
- Dizon R.M., Yap H.T. Effects of multiple perturbations on the survivorship of fragments of three coral species. Marine Pollution Bulletin. 2006;52:928–934. doi: 10.1016/j.marpolbul.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Done T.J. Effects of tropical cyclone waves on ecological and geomorphological structures on the Great Barrier Reef. Continental Shelf Research. 1992;12:859–872. [Google Scholar]
- Eckstein P.P. 5 ed. 2006. Angewandte Statistik mit SPSS - Praktische Einführung für Wirtschaftswissenschaftler. (Gabler, Germany) [Google Scholar]
- Edinger E.N., Jompa J., Limmon G.V., Widjatmoko W., Risk M.J. Reef degradation and coral biodiversity in Indonesia: effects of land-based pollution, destructive fishing practices and changes over time. Marine Pollution Bulletin. 1998;36:617–630. [Google Scholar]
- Edwards A., Gomez E.D. Coral Reef Targeted Research & Capacity Building for Management Programme; St. Lucia, Australia: 2007. Reef Restoration - Concepts and Guide Lines. [Google Scholar]
- Egyptian Biodiversity CHM . 2006. Report on Biodiversity Conservation Capacity Building in Egypt.http://www.egyptchm.org/chm/biodiversity/doc/Biodiversity1.pdf August 2009. [Google Scholar]
- Harriott V.J. CRC Reef Research Centre & James Cook University; Townsville, Australia: 2002. Marine Tourism Impacts and Their Management on the Great Barrier Reef. [Google Scholar]
- Hasler H., Ott J.A. Diving down the reefs? Intensive diving tourism threatens the reefs of the northern Red Sea. Marine Pollution Bulletin. 2008;56:1788–1794. doi: 10.1016/j.marpolbul.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Hawkins J.P., Roberts C.M. Effects of recreational SCUBA diving on fore-reef slope communities of coral reefs. Biological Conservation. 1992;62:171–178. [Google Scholar]
- Hawkins J.P., Roberts C.M. Effects of recreational SCUBA diving on coral reefs: trampling on reef flat communities. Journal of Applied Ecology. 1993;30:25–30. [Google Scholar]
- Heinisch S. University of Vienna; Austria: 2006. Umweltbildung im Sporttauchen. PhD in Natural Sciences. [Google Scholar]
- Huxham M., Tett P. School of Life Sciences, Napier University; 2005. Scientific Methods: Introduction to Questionnaires - Learning Objectives and Study Guide.http://www.lifesciences.napier.ac.uk/teaching/SM/Quest.htm#TOP August 2009. [Google Scholar]
- Ibrahim F.N., Ibrahim B. Wissenschaftliche Buchgesellschaft; Darmstadt: 2006. Ägypten - Geographie, Geschichte, Wirtschaft, Politik. [Google Scholar]
- Jameson S.C., Ammar M.S.A., Saadalla E., Mostafa H.M., Riegl B. A coral damage index and its application to diving sites in the Egyptian Red Sea. Coral Reefs. 1999;18:333–339. [Google Scholar]
- Jones G.P., McCormick M.I., Srinivasan M., Eagle J.V. Coral decline threatens fish biodiversity in marine reserves. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8251–8253. doi: 10.1073/pnas.0401277101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb M., Abdulaziz M., Al-Agwan Z., Al-Shaikh K., Al-Yami H., Banajah A., DeVantier L., Eisinger M., Eltayeb M., Hassan M., Heiss G., Howe S., Kemp J., Klaus R., Krupp F., Mohamed N., Rouphael T., Turner J., Zajonz U. 4. Status of coral reefs in the Red Sea and Gulf of Aden in 2004. In: Wilkinson C., editor. Status of Coral Reefs of the World. fourth ed. Australian Institut of Marine Science, Queensland; Townsville, Australia: 2004. pp. 137–154. [Google Scholar]
- Leujak W., Ormond R.F.G. Visitor perceptions and the shifting social carrying capacity of South Sinai’s coral reefs. Environmental Management. 2007;39:472–489. doi: 10.1007/s00267-006-0040-1. [DOI] [PubMed] [Google Scholar]
- Leujak W., Ormond R.F.G. Reef walking on Red Sea reef flats - quantifying impacts and identifying motives. Ocean & Coastal Management. 2008;51:755–762. [Google Scholar]
- Liddle M.J. Recreational ecology: effects of trampling on plants and corals. Trends in Ecology and Evolution. 1991;6:13–17. doi: 10.1016/0169-5347(91)90141-J. [DOI] [PubMed] [Google Scholar]
- Liddle M.J., Kay A.M. Resistance survival and recovery of trampled corals on the Great Barrier Reef. Biological Conservation. 1987;42:1–18. [Google Scholar]
- Loya Y. Community structure and species diversity of hermatypic corals at Eilat, Red Sea. Marine Biology. 1972;13:100–123. [Google Scholar]
- Loya Y. The coral reefs of Eilat – past, present and future: three decades of coral community structure studies. In: Rosenberg E., Loya Y., editors. Coral Health and Disease. Springer-Verlag; Berlin, Germany: 2004. pp. 1–34. [Google Scholar]
- Madin E.M.P., Fenton M.D. Environmental interpretation in the Great Barrier reef marine park: an assessment of programme effectiveness. Journal of Sustainable Tourism. 2004;12:121–137. [Google Scholar]
- Marshall P.A., Baird A.H. Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs. 2000;19:155–163. [Google Scholar]
- Medio D., Ormond R.F.G., Pearson M. Effect of briefings on rates of damage to corals by SCUBA divers. Biological Conservation. 1997;79:91–95. [Google Scholar]
- Moberg F., Folke C. Ecological goods and services of coral reef ecosystems. Ecological Economics. 1999;29:215–233. [Google Scholar]
- Neil D. Potential for coral stress due to sediment resuspension and Deposition by reef walkers. Biological Conservation. 1990;52:221–227. [Google Scholar]
- Nyström M., Folke C., Moberg F. Coral reef disturbance and resilience in a human-dominated environment. Trends in Ecology and Evolution. 2000;15:317–413. doi: 10.1016/s0169-5347(00)01948-0. [DOI] [PubMed] [Google Scholar]
- Omori M., Fujiwara S. Nature Conservation Bureau, Ministry of the Environment; Japan: 2004. Manual for Restoration and Remediation of Coral Reefs. [Google Scholar]
- Pandolfi J.M., Bradbury R.H., Sala E., Hughes T.P., Bjorndal K.A., Cooke R.G., McArdle D., McClenachan L., Newman M.J.H., Paredes G., Warner R.R., Jackson J.B.C. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- PERSGA/GEF . 2001. Strategic Action Programme (SAP) for the Red Sea and Gulf of Aden: Country Reports, Jeddah. [Google Scholar]
- Plathong S., Inglis G.J., Huber M.E. Effects of self-guided snorkelling trails in a tropical marine park. Conservation Biology. 2000;14:1821–1830. doi: 10.1111/j.1523-1739.2000.99301.x. [DOI] [PubMed] [Google Scholar]
- Reese D.C., Miller T.W., Brodeur R.D. Community structure of near-surface zooplankton in the northern California Current in relation to oceanographic conditions. Deep-Sea Research Part II: Topical Studies in Oceanography. 2005;52:29–50. [Google Scholar]
- Richmond R.H., Hunter C.L. Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Marine Ecology. 1990;60:185–203. [Google Scholar]
- Riegl B., Cook P.A. Is damage susceptibility linked to coral community structure? A case study from South Africa. Beitraege zur Palaeontologie. 1995;20:65–73. [Google Scholar]
- Riegl B., Heine C., Branch G.M. Function of funnel-shaped coral growth in a high-sedimentation environment. Marine Ecology Progress Series. 1996;145:87–93. [Google Scholar]
- Riegl B., Piller W.E. Mapping of benthic habitats in northern Safaga Bay (Red Sea, Egypt): a tool for proactive management. Aquatic Conservation: Marine and Freshwater Ecosystems. 2000;10:127–140. [Google Scholar]
- Riegl B., Riegl A. Studies on coral community structure and damage as a basis for zoning marine reserves. Biological Conservation. 1996;77:269–277. [Google Scholar]
- Riegl B., Velimirov B. How many damaged corals in Red Sea reef systems? A quantitative survey. Hydrobiologia. 1991;216/217:249–256. [Google Scholar]
- Riegl B., Velimirov B. The structure of coral communities at Hurgada in the Northern Red Sea. Marine Ecology. 1994;15:213–231. [Google Scholar]
- Rodgers K.U.S., Cox E.F. The effects of trampling on Hawaiian corals along a gradient of human use. Biological Conservation. 2003;112:383–389. [Google Scholar]
- Rogers C. Responses of coral reefs and reef organisms to sedimentation. Marine Ecology Progress Series. 1990;62:185–202. [Google Scholar]
- Roman G., Dearden P., Rollins R. Application of zoning and “limits of acceptable change” to manage snorkeling tourism. Environmental Management. 2007;39:819–830. doi: 10.1007/s00267-006-0145-6. [DOI] [PubMed] [Google Scholar]
- Rouphael A.B., Inglis G.J. "Take only photographs and leave only footprints?": an experimental study of the impacts of underwater photographers on coral reef dive sites. Biological Conservation. 2001;100:281–287. [Google Scholar]
- Saila S.B., Kocic V.L., McManus J.W. Modelling the effects of destructing fishing practices on tropical coral reefs. Marine Ecology Progress Series. 1993;94:51–60. [Google Scholar]
- Schiemer L., Niedermüller S., Herler J. The influence of colony size and coral health on the occupation of coral-associated gobies (Pisces: Gobiidae) Coral Reefs. 2009;28:137–142. [Google Scholar]
- Schuttenberg H.Z. Selected Papers Presented at the 9th International Coral Reef Symposium. 2001. Coral Bleaching: Causes, Consequences and Response.http://hdl.handle.net/1834/872 August 2009. [Google Scholar]
- Thorsell J., Wells S. National Coastal Resources Research and Development Institute Newport; Oregon: 1990. A Global Overview of Tourism Activities in Coastal and Marine Parks, Proceedings of the 1990 Congress on Coastal and Marine Tourism. 221–224. [Google Scholar]
- Tratalos J.A., Austin T.J. Impacts of recreational SCUBA diving on coral communities of the Caribbean island of Grand Cayman. Biological Conservation. 2001;102:67–75. [Google Scholar]
- Wilkinson C. fourth ed. Australian Institute of Marine Science; 2004. Status of Coral Reefs of the World: 2004. [Google Scholar]
- Wilkinson C., Green A., Almany J., Dionne S. Australian Institute of Marine Science (AIMS); 2003. Monitoring Coral Reef Marine Protected Areas. A Practical Guide on How Monitoring Can Support Effective Management of MPAs. [Google Scholar]
- Zakai D., Chadwick-Furman N.E. Impacts of intensive recreational diving on reef corals at Eilat, Northern Red Sea. Biological Conservation. 2002;105:179–187. [Google Scholar]