Abstract
Objective
We wanted to prospectively assess the adverse events and hemodynamic effects associated with an intravenous adenosine infusion in patients with suspected or known coronary artery disease and who were undergoing cardiac MRI.
Materials and Methods
One hundred and sixty-eight patients (64 ± 9 years) received adenosine (140 µg/kg/min) during cardiac MRI. Before and during the administration, the heart rate, systemic blood pressure, and oxygen saturation were monitored using a MRI-compatible system. We documented any signs and symptoms of potential adverse events.
Results
In total, 47 out of 168 patients (28%) experienced adverse effects, which were mostly mild or moderate. In 13 patients (8%), the adenosine infusion was discontinued due to intolerable dyspnea or chest pain. No high grade atrioventricular block, bronchospasm or other life-threatening adverse events occurred. The hemodynamic measurements showed a significant increase in the heart rate during adenosine infusion (69.3 ± 11.7 versus 82.4 ± 13.0 beats/min, respectively; p < 0.001). A significant but clinically irrelevant increase in oxygen saturation occurred during adenosine infusion (96 ± 1.9% versus 97 ± 1.3%, respectively; p < 0.001). The blood pressure did not significantly change during adenosine infusion (systolic: 142.8 ± 24.0 versus 140.9 ± 25.7 mmHg; diastolic: 80.2 ± 12.5 mmHg versus 78.9 ± 15.6, respectively).
Conclusion
This study confirms the safety of adenosine infusion during cardiac MRI. A considerable proportion of all patients will experience minor adverse effects and some patients will not tolerate adenosine infusion. However, all adverse events can be successfully managed by a radiologist. The increased heart rate during adenosine infusion highlights the need to individually adjust the settings according to the patient, e.g., the number of slices of myocardial perfusion imaging.
Keywords: MRI, Adenosine, Coronary artery disease, Hemodynamics, Adverse events
INTRODUCTION
Cardiovascular magnetic resonance imaging (CMR) is becoming increasingly available for patients with suspected or known coronary artery disease. Functional imaging such as analysis of the myocardial perfusion or measurement of the coronary bypass flow reserve plays a major role in the assessment of these patients (1, 2). The mechanism and physiological consequences of pharmacological stress for inducing coronary hyperemia were described decades ago (3, 4). This now provides the basis for measuring the myocardial perfusion and coronary flow.
Dipyridamole was first used as a vasodilator, but now adenosine is most commonly applied (5). Adenosine stimulates the A2A receptor on the arterial vascular smooth muscle cells and this causes vasorelaxation. In addition, the receptor sub-types named A1, A2b and A3 are stimulated by adenosine. These receptors also account for the potential adverse effects of adenosine such as dyspnea, chest pain, atrioventricular blockage or bronchospasm (6).
Adenosine has been validated as a safe pharmacological agent for myocardial scintigraphy and stress echocardiography (7, 8). However, its use in the context of MRI has to be analysed separately since the MRI environment could induce additional effects such as claustrophobia.
The aim of this study was to investigate the tolerability of adenosine as a pharmacological agent in patients with coronary artery disease and who are undergoing CMR. We analysed the hemodynamic effects in terms of the potential significant systemic vaso-reactive reactions that could have an influence on the workflow or the CMR workflow.
MATERIALS AND METHODS
Patient Population
This study was a prospectively documented case series involving a total of 168 patients with a mean age of 64 ± 9 years (range: 31 to 83 years), a mean height of 173 ± 9 cm, a mean body weight of 82 ± 15 cm and a mean body mass index of 27.0 ± 3.8 kg/m2. The clinical indication for adenosine-based CMR was either analysis of the myocardial perfusion reserve in patients with suspected or progressive coronary artery disease or measurement of the coronary bypass flow reserve. The exclusion criteria were the typical MRI contraindications (claustrophobia, non-compatible metallic implants, pregnancy, pacemakers) or contraindications to an adenosine infusion (second or third degree atrioventricular block, a history of clinically relevant asthma or bronchospasm). All patients gave us their written informed consent prior to the CMR examination. The local ethics committee approved this study.
Cardiovascular MRI and Patient Monitoring
Cardiovascular MRI was performed using a 1.5 Tesla MRI system (Magnetom Sonata Maestro Class, Siemens AG, Germany). For signal detection, the combination of a six-channel body phased-array coil and a two-channel spine phased-array coil were used. The echocardiography (ECG)-signal was received from an external MRI-compatible system (Magnitude 3150, InVivo Research Inc., Orlando, FL). Before and during the administration of adenosine, the heart rate, blood pressure and oxygen saturation were non-invasively measured using the same system. For the documentation of rhythm disturbances, the continuous ECG monitoring was analyzed in a qualitative manner. ECG-related adverse events, such as atrioventricular blocks, were noticed online and counted. Further, any adverse events and reasons for discontinuing the examination were documented.
For the myocardial perfusion studies, after the scout imaging and standardized determination of the heart axis using the cine-sequences (7), a saturation-recovery, ECG-gated, breath-hold steady-state-free-precession (SSFP) perfusion sequence (TrueFISP, echo time [TE] = 1.0 ms, inversion time [TI] = 100 ms, a flip angle of 50°, a matrix of 120 × 192, a parallel imaging Grappa factor of 2, voxel size = 2.4 × 1.8 × 8.0 mm3, 3 slices with an acquisition time of 185 ms per slice, 40 repetitions) was positioned along the short axis at the base, centre and apex of the left ventricle and this allowed us to assess 16 of the 17 segments of the American Heart Association (AHA)/American College of Cardiology (ACC) segment model.
The contrast agent (Gadolinium-DTPA, Magnevist®, Bayer Schering AG, Germany) was administered into an antecubital vein at a flow rate of 5ml/s, and this was followed by a 30 ml saline flush using a power injector (Injektron® 82 MRT, Medtron AG, Germany).
The first contrast-enhanced perfusion study was performed using an adenosine dosage of 0.05 mmol/kg without an infusion of vasodilator. The adenosine-based series were done 10-15 minutes after the perfusion study at rest. During this time, a stack of cine-SSFP short axis slices for the left ventricular volumetric analysis was acquired.
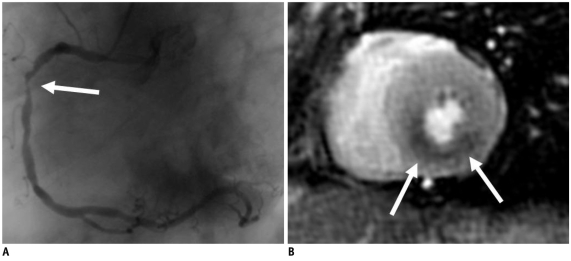
Adenosine (Adenoscan®, Sanofi-Synthelabo Ltd, Berlin, Germany) was administered at a body weight-adapted infusion rate of 140 µg/kg/min for a run-in period of 3 minutes with using an additional venous access. Subsequently, during continued adenosine administration, two myocardial perfusion series were performed with different dosages of contrast agent (a low-dose of 0.05 mmol/kg gadolimium-diethylenetriamine pentaacetic acid [Gd-DTPA] and a second high-dose of 0.1 mmol/kg Gd-DTPA). The low-dose series provided a comparison with the rest of the imaging and subsequent semi-quantification of the perfusion reserve. The later high-dose series provided the opportunity to obtain better visual detection of perfusion deficits. Consequently, the overall contrast agent dosage was 0.2 mmol/kg Gd-DTPA for the late enhancement imaging. The duration of adenosine infusion for myocardial perfusion imaging was 6-8 minutes. Figure 1 documents a typical example of adenosine-based detection of coronary artery disease using myocardial perfusion CMR, as compared to that of invasive coronary angiography.
Fig. 1.
Typical example of adenosine-based detection of coronary artery disease using myocardial perfusion cardiovascular MRI.
A. Invasive coronary angiography of 71-year-old female reveals high-grade right coronary arterial stenosis (arrow). B. Perfusion deficit in inferior wall (arrows) is documented using short axis view of cardiovascular MRI-based perfusion imaging during adenosine infusion.
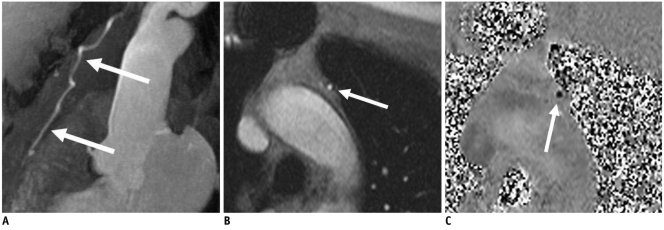
ECG-gated MR angiography and ECG-gated, breath-hold, phase-contrast flow mapping were performed to assess the flow in coronary bypass grafts (8). The typical sequence parameters were a TE of 4.2 ms, temporal resolution of 70 ms, segmentation: 7, a breath-hold of approximately 20-30 seconds, a flip angle of 30°, velocity encoding at 75 cm/s and a voxel size of 1.4 × 0.8 × 6.0 mm3. First, a flow measurement of each graft was done with the patient at rest followed by a second measurement during a continuous adenosine infusion after a run-in period of 3 minutes at a body weight-adapted infusion rate of 140 µg/kg/min. Depending on the number of bypass grafts, the overall duration of adenosine infusion for flow quantification was 5-9 minutes. Figure 2 presents a maximum intensity projection of a left internal thoracic artery bypass graft and it documents a typical example of the adenosine-based phase-contrast flow imaging.
Fig. 2.
Usefulness of adenosine-based phase-contrast flow imaging in 68-year-old man.
A. Maximum intensity projection of contrast-enhanced MR angiography shows patient with patent left internal thoracic artery bypass graft (arrows) to left anterior descending coronary artery. B, C. Using adenosine-based phase-contrast flow MR imaging (B, magnitude image; C, phase image) (arrows), high-grade stenosis of coronary bypass graft could be detected by quantification of insufficient flow reserve in comparison to flow measurement with patient at rest.
Statistics
The heart rate, blood pressure and oxygen saturation changes were documented between when the patients were at rest and during the adenosine infusion. These variables are presented as means and standard deviations. Student's paired t test was used to assess the hemodynamic differences at baseline and during stress MRI. Statistical significance was assumed for a p value < 0.05.
Any adverse events such as high-grade atrioventricular block are given as counts and percentages.
RESULTS
Adverse Events
Forty seven of 168 patients (28%) experienced an adverse event. In the majority of cases the severity of the adverse events was mild to moderate. All adverse events were fully reversible after one hour following the end of the adenosine infusion.
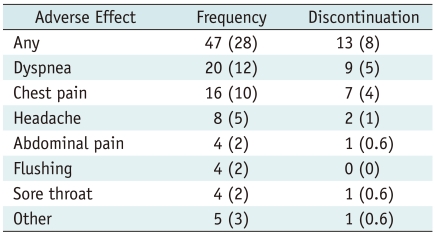
In 13 of these 47 patients, adverse events resulted in discontinuation of the adenosine-based measurement (8% of the entire study population). The most frequent reasons for discontinuation were intolerable dyspnea and chest pain. During the adenosine infusion, neither second or third degree atrioventricular block nor clinically relevant bronchospasm occurred. No life threatening adverse effects and no persistent or delayed adverse effects were observed. None of the adverse effects required treatment; in particular, it was not necessary to administer the antidote aminophylline to counteract the effects of adenosine. The total number of adverse effects, including those leading to discontinuation of the adenosine-infusion, is shown in Table 1.
Table 1.
Type and Frequency of Subjective Adverse Effects and Reasons for Interrupting Adenosine Infusion in 168 Patients
Note.- Numbers in parentheses are percentages.
Hemodynamic Parameters
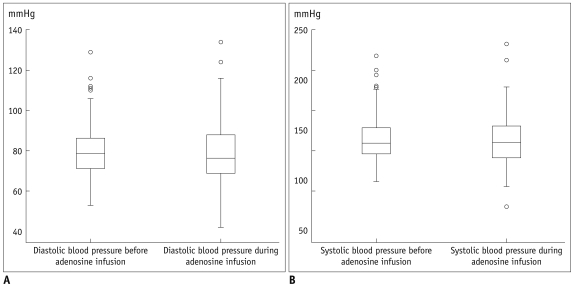
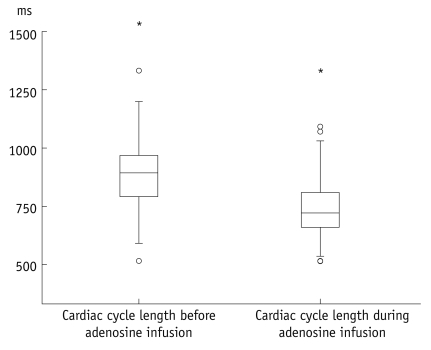
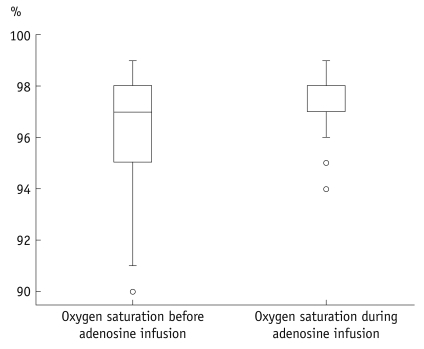
The heart rate significantly increased during adenosine infusion (69.3 ± 11.7 beats/min versus 82.4 ± 13.0 beats/min, respectively; p < 0.001). Consequently the length of the cardiac cycle was significantly reduced (890 ± 148 ms versus 748 ± 129 ms, respectively; p < 0.001). Oxygen saturation was slightly but significantly increased during adenosine infusion (96 ± 1.9% versus 97 ± 1.3%, respectively; p < 0.001). The blood pressure did not significantly change during adenosine infusion. The systolic blood pressure was 142.8 ± 24 mmHg before adenosine infusion and 140.9 ± 25.7 mmHg during the adenosine infusion; the diastolic blood pressure was 80.2 ± 12.5 mmHg before adenosine infusion and 78.9 ± 15.6 during the adenosine infusion. Figures 3-5 present boxplot diagrams of the blood pressure, the blood oxygen saturation and the length of the cardiac cycle length during adenosine-infusion.
Fig. 3.
Boxplot diagrams of diastolic (A) and systolic (B) blood pressure demonstrating no significant change before and during adenosine infusion.
Fig. 5.
Boxplot diagram demonstrates significant decrease of length of cardiac cycle during adenosine infusion.
Therefore, it could be mandatory to plan to potentially tailor examination (e.g., reduced number of slices of myocardial perfusion imaging) in advance.
DISCUSSION
This study confirms the safety of adenosine infusion during CMR. A considerable proportion of all patients (28%) will experience subjective adverse effects, but only a few patients (8%) will not tolerate adenosine infusion. However, all observed side effects in this study were not life threatening. A significant increase of the heart rate and the blood oxygen level was noticed, yet all this had no clinical relevance. There was no significant change in the blood pressure during adenosine infusion.
During the last decade, CMR has emerged as an important procedure for investigating cardiovascular diseases. The precise morphology of the heart and the great vessels can be assessed without exposing the patient to radiation. In addition, functional CMR studies that mainly measure the myocardial perfusion and flow offer new non-invasive diagnostic possibilities in patients with suspected or known coronary artery disease (7-9).
Adenosine-based myocardial perfusion CMR may be the preferred choice for patients with an intermediate pre-test probability of coronary artery disease because of its high diagnostic accuracy (10). In patients with known coronary artery disease and who have undergone bypass grafting, the analysis of phase-contrast MRI flow measurement allows for detecting high-grade stenosis in the coronary bypass grafts with high diagnostic accuracy (11, 12).
For both groups as described above, the assessment of the myocardial perfusion reserve and the bypass graft flow reserve is essential to induce maximal vasodilatation. This is usually ascertained by the administration of a pharmacological stress agent. Pharmacological stressors have a substantial advantage for MRI because physical exercise is impractical due to patient discomfort within the scanner and movement artifacts. The drugs most commonly used as pharmacological stressors are dobutamine, dipyridamole and adenosine (13).
In contrast to dobutamine, the administration of adenosine does not increase the myocardial oxygen consumption because adenosine lacks positive inotropic effects. In our study population, only a slight increase of blood oxygen saturation was observed during adenosine infusion.
Adenosine is a more potent coronary vasodilator than dipyridamole (5). Peripheral venous adenosine injection increases the coronary flow 3 to 4 times due to a drop in the coronary resistance, which results in an increase in the myocardial blood flow (13, 14). Due to its short half-life of less than 10 seconds, intravenous adenosine shows immediate effects after being started and the effects quickly wear off when the drug is discontinued (6). Moreover, the effects of adenosine can be quickly interrupted using aminophylline, which acts as an antidote. Usually, adenosine is administered as a continuous infusion for 4-6 minutes. A maximum duration of 6 minutes is recommended. In keeping with our strategy of two adenosine-based CMR perfusion series in the patients with up to four bypass grafts, we administered adenosine for a duration of up to 8 minutes without detecting additional side effects. In our study, neither second nor third degree atrioventricular node block nor bronchospasm were observed. The administration of aminophylline was also not necessary.
Due to these properties and as supported by our results, adenosine at a dosage of 140 µg/kg/min can be considered as the pharmacological agent of choice for CMR myocardial perfusion imaging. Theoretical and practical analysis of the safety of adenosine also underlines that radiologists, who usually do not deal with such medications in their daily routine, will be able to manage and supervise adenosine-based CMR examinations.
Despite the infusion of adenosine acting as a vasodilator, the blood pressure showed only a minimal and statistically non-significant tendency to decrease. This fact was confirmed by other studies and this can be regarded as being due to a compensatory effect of the increased heart rate (15). The adenosine infusion resulted in a shortening of the cardiac cycle by 16%. Adenosine induces sympathetic stimulation, which is probably caused by stimulation of chemoreceptors in the carotid body (16). Such sympathetic stimulation may explain the observed increase in the heart rate.
The potential shortening of the cardiac cycle highlights the need to individually adjust the CMR parameters. An increased heart rate could result in a reduced number of slices during the adenosine infusion since 185 ms per slice is required in our high-resolution SSFP-perfusion sequence setting.
Previous studies have been published on the adverse events of adenosine in the setting of CMR (15, 17, 18). However, those studies were only based on a small number of patients or on retrospective data. In our prospective study, 28% of the patients experienced subjective side effects and they mostly reported dyspnea, chest pain and headache. All other side effects were infrequent. This number of subjective adverse events was low when compared to that of other studies, which reported mild symptoms such as flushing, breathlessness or chest discomfort in up to 45% (15) and 63% (18) of the subjects. This is probably due to the selected patient populations with severe coronary artery disease within those previous studies.
In 8% of our study population, the side effects led to discontinuation of the adenosine infusion, which is a little more frequent than that in the previous CMR studies (termination in less than 2%). However, our results reflect the safety profile shown in the perfusion studies that used nuclear techniques (16). These studies reported that 13% of the patients required a dose reduction, and in 7% the study was terminated before the intended time. Despite the different environment, there is no evidence of an increased number of subjective or objective adverse events when performing stress CMR as compared to that of the nuclear studies.
Limitations
The reduced ECG quality within the magnetic field and during the radiofrequency pulses must be considered when comparing our data with the data from previous nuclear studies. During stress CMR, the ECG quality is sufficient to analyze the QRS duration and the heart rate, but the p-wave cannot always be reliably identified. However, the increase of the heart rate that was noted in all patients demonstrates the lack of any significant atrioventricular conduction abnormalities.
CONCLUSION
This study confirms the safety of adenosine infusion during CMR. A considerable proportion of patients will experience minor adverse effects, and some patients will not tolerate the adenosine infusion. However, a radiologist was able to successfully manage all observed adverse events. The increased heart rate during adenosine infusion highlights the need to adjust the settings to the individual, e.g., the number of slices of myocardial perfusion imaging.
Fig. 4.
Boxplot diagram shows small, but significant increase of blood oxygen saturation during adenosine infusion.
This is advantageous in contrast to dobutamine, which increases myocardial oxygen consumption due to its positive inotropic effects.
References
- 1.Budoff MJ, Cohen MC, Garcia MJ, Hodgson JM, Hundley WG, Lima JA, et al. ACCF/AHA clinical competence statement on cardiac imaging with computed tomography and magnetic resonance. Circulation. 2005;112:598–617. doi: 10.1161/CIRCULATIONAHA.105.168237. [DOI] [PubMed] [Google Scholar]
- 2.Constantine G, Shan K, Flamm SD, Sivananthan MU. Role of MRI in clinical cardiology. Lancet. 2004;363:2162–2171. doi: 10.1016/S0140-6736(04)16509-4. [DOI] [PubMed] [Google Scholar]
- 3.Gould KL. Noninvasive assessment of coronary stenoses by myocardial perfusion imaging during pharmacologic coronary vasodilatation. I. Physiologic basis and experimental validation. Am J Cardiol. 1978;41:267–278. doi: 10.1016/0002-9149(78)90165-0. [DOI] [PubMed] [Google Scholar]
- 4.Gould KL, Westcott RJ, Albro PC, Hamilton GW. Noninvasive assessment of coronary stenoses by myocardial imaging during pharmacologic coronary vasodilatation. II. Clinical methodology and feasibility. Am J Cardiol. 1978;41:279–287. doi: 10.1016/0002-9149(78)90166-2. [DOI] [PubMed] [Google Scholar]
- 5.Rossen JD, Quillen JE, Lopez AG, Stenberg RG, Talman CL, Winniford MD. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol. 1991;18:485–491. doi: 10.1016/0735-1097(91)90604-8. [DOI] [PubMed] [Google Scholar]
- 6.Belardinelli L, Linden J, Berne RM. The cardiac effects of adenosine. Prog Cardiovasc Dis. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber WG, Schmitt M, Kalden P, Horstick G, Gumbrich T, Petersen S, et al. Perfusion MR imaging of the heart with TrueFISP. Rofo. 2001;173:205–210. doi: 10.1055/s-2001-11604. [DOI] [PubMed] [Google Scholar]
- 8.Spies C, Mohrs OK, Madison JR, Fach A, Nowak B, Voigtlander T. Limited flow reserve in non-obstructed bypass grafts supplying infarcted myocardium: implications for cardiovascular magnetic resonance imaging protocols. J Cardiovasc Magn Reson. 2006;8:373–379. doi: 10.1080/10976640500452067. [DOI] [PubMed] [Google Scholar]
- 9.Wilke NM, Jerosch-Herold M, Zenovich A, Stillman AE. Magnetic resonance first-pass myocardial perfusion imaging: clinical validation and future applications. J Magn Reson Imaging. 1999;10:676–685. doi: 10.1002/(sici)1522-2586(199911)10:5<676::aid-jmri10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Schwitter J, Nanz D, Kneifel S, Bertschinger K, Buchi M, Knusel PR, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 11.Langerak SE, Vliegen HW, de Roos A, Zwinderman AH, Jukema JW, Kunz P, et al. Detection of vein graft disease using high-resolution magnetic resonance angiography. Circulation. 2002;105:328–333. doi: 10.1161/hc0302.102598. [DOI] [PubMed] [Google Scholar]
- 12.Voigtlander T, Kreitner KF, Wittlinger T, Petersen S, Horstick G, Kalden P, et al. MR angiography and flow measurement in coronary arteries and coronary bypass grafts. Z Kardiol. 2001;90:929–938. doi: 10.1007/s003920170063. [DOI] [PubMed] [Google Scholar]
- 13.Ali Raza J, Reeves WC, Movahed A. Pharmacological stress agents for evaluation of ischemic heart disease. Int J Cardiol. 2001;81:157–167. doi: 10.1016/s0167-5273(01)00536-8. [DOI] [PubMed] [Google Scholar]
- 14.Biaggioni I, Killian TJ, Mosqueda-Garcia R, Robertson RM, Robertson D. Adenosine increases sympathetic nerve traffic in humans. Circulation. 1991;83:1668–1675. doi: 10.1161/01.cir.83.5.1668. [DOI] [PubMed] [Google Scholar]
- 15.Karamitsos TD, Arnold JR, Pegg TJ, Cheng AS, van Gaal WJ, Francis JM, et al. Tolerance and safety of adenosine stress perfusion cardiovascular magnetic resonance imaging in patients with severe coronary artery disease. Int J Cardiovasc Imaging. 2009;25:277–283. doi: 10.1007/s10554-008-9392-3. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: results from the Adenoscan Multicenter Trial Registry. J Am Coll Cardiol. 1994;23:384–389. doi: 10.1016/0735-1097(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 17.Bernhardt P, Steffens M, Kleinertz K, Morell R, Budde R, Leischik R, et al. Safety of adenosine stress magnetic resonance imaging using a mobile cardiac magnetic resonance system. J Cardiovasc Magn Reson. 2006;8:475–478. doi: 10.1080/10976640600575270. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood JP, Younger JF, Ridgway JP, Sivananthan MU, Ball SG, Plein S. Safety and diagnostic accuracy of stress cardiac magnetic resonance imaging vs exercise tolerance testing early after acute ST elevation myocardial infarction. Heart. 2007;93:1363–1368. doi: 10.1136/hrt.2006.106427. [DOI] [PMC free article] [PubMed] [Google Scholar]