Abstract
Introduction:
Research indicates that exercise may be helpful for smoking cessation; however, the majority of studies have focused only on women and only on aerobic exercise. This pilot study explored the use of resistance training (RT) (i.e., weight lifting) as an adjunctive strategy for quitting smoking for both men and women.
Methods:
A sample of 25 smokers received a brief smoking cessation counseling session and the nicotine patch prior to being randomized into a 12-week RT or contact control (CC) group. Assessments were conducted at baseline, 3-month, and at a 6-month follow-up.
Results:
Participants (52% female) averaged 36.5 years (SD = 12.0) of age and 19.1 years (SD = 12.0) of smoking. At the 3-month assessment, objectively verified 7-day point prevalence abstinence (PPA) rates were 46% for the RT group and 17% for CC; prolonged abstinence rates were 16% and 8%, respectively. At the 6-month assessment, objectively verified 7-day PPA rates were 38% for the RT group and 17% for CC; prolonged abstinence rates were 15% and 8%, respectively. Mean body weight decreased 0.6 kg (SD = 1.7) in the RT group and increased 0.6 kg (SD = 2.8) in the CC group. Mean body fat decreased 0.5% (SD = 1.8) in the RT group and increased 0.6% (SD = 0.7) in the CC.
Conclusions:
This is the first study reporting on the use of a RT program as an aid to smoking cessation treatment. The findings suggest that such a program is feasible as an adjunctive treatment for smoking cessation. An adequately powered trial is warranted.
Introduction
There is evidence to suggest that exercise may be useful for smoking cessation (Ussher, Taylor, & Faulkner, 2008). Exercise has been shown to reduce many of the negative experiences that accompany quitting, such as cigarette cravings, withdrawal symptoms, negative mood states, and weight gain (Parsons, Shraim, Inglis, Aveyard, & Hajek, 2009; Taylor, Ussher, & Faulkner, 2007). The vast majority of studies, however, have focused on female smokers and aerobic exercise (Taylor et al., 2007; Ussher et al., 2008). Resistance training (i.e., weight lifting), a form of exercise that increases muscular strength, may also offer smokers a useful strategy for quitting. Like other types of exercise, resistance training (RT) has the potential to moderate postcessation weight gain (American College of Sports Medicine[ACSM], 2009b) and negative mood states (Arent, Landers, Matt, & Etnier, 2005). Resistance training is typically facility based but can be done effectively at home, using equipment or body weight, and research indicates that some individuals prefer RT over other types of exercise (Centers for Disease Control and Prevention, 2006). Currently, no studies have assessed RT for smoking cessation. However, studies have shown that a single resistance exercise, isometric contraction (i.e., static muscle contraction), is effective for reducing tobacco cravings and is perceived as helpful during a quit attempt (Al-Chalabi et al., 2008; Ussher, Cropley, Playle, Mohidin, & West, 2009; Ussher, West, Doshi, & Sampuran, 2006). As such, a RT program could offer smokers a new aid to quitting. The purpose of this pilot study was to (a) obtain initial estimates of the effects of RT as an adjunct to smoking cessation counseling on smoking abstinence and body weight/composition and (b) test the feasibility of our research methods for use in a future, adequately powered efficacy trial.
Methods
Participants
Male and female smokers (≥5 cigarettes/day ≥1 year) aged 18–65 years were recruited via newspaper, Internet, and television advertisements. Volunteers were excluded for participating in regular exercise (>60 min/week), presence of a chronic health condition, pregnancy, smokeless tobacco use, or current smoking cessation treatment. For safety reasons, all were required to obtain a physician’s consent to participate prior to providing informed consent.
Measures
At each session, participants were asked: “Are you currently smoking?” (yes/no) and (if not smoking) “Have you smoked even one puff in the past 24 hr?” and “in the past 7 days?” Participants reporting no smoking in the past 7 days were asked to indicate the last day they smoked “even a puff of a cigarette.” Carbon monoxide (CO) concentration was assessed via the Micro 4 Smokerlyzer (Bedfont Scientific).
At baseline, nicotine dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991), nicotine withdrawal symptoms (West & Hajek, 2004), and perceived ability to quit (Etter, Bergman, Humair, & Perneger, 2000) were assessed. Baseline and 3-month assessments included body composition, determined from dual-energy x-ray absorptiometry technology; and upper and lower body strength (i.e., chest press, leg press) using the ACSM’s 5-Repetition Maximum test to estimate maximal strength (ACSM, 2009a). Baseline, 3-month, and 6-month assessments included body weight and a 3-month physical activity recall questionnaire (Kohl, Blair, Paffenbarger, Macera, & Kronenfeld, 1997).
Procedures
Enrollment
Participants attended four 30-min pre-randomization sessions (including baseline assessments) over a 2-week run-in period. Participants were asked not to quit smoking or begin exercising during the run-in. This strategy was used to exclude individuals unlikely to adhere to the study requirements (Cipriani & Geddes, 2010). Prior to randomization, all participants received a 15- to 20-min smoking cessation counseling session (American Lung Association’s “Freedom From Smoking” manual). Additionally, all were given the first box of an 8-week supply of nicotine patches, with further patches supplied as needed. Nicotine patch dose was tailored according to cigarette consumption. Participants were told to apply the first patch the morning of quit day. Randomization, via a computer generated list of numbers, into RT or contact control (CC) conditions occurred immediately following the counseling.
Resistance Training Condition
Participants engaged in two 60-min RT sessions/week for 12 weeks. The full-body routine (ACSM, 2009b), involved 10 exercises, with set intensity and volume adjusted every 3 weeks. For the first 3 weeks, participants completed one set (10 repetitions) of each exercise at 65%–75% of their estimated maximal strength. From weeks 4–12, participants completed two sets per exercise. Weight was systematically increased by a researcher to match gains in strength and maintain intensity at weeks 7–10. Researchers monitored exercise for safety, interactions were minimized, and smoking was not discussed. Participants exercised alone and could attend up to three sessions/week to make up for one missed session in the prior week, with no more than one session/day. All were asked not to engage in RT beyond the supervised sessions or change their other exercise.
Contact Control Condition
Participants watched one 25-min video, twice/week, in a room alone. The films covered various health-related issues (e.g., nutrition) shown to be acceptable in similar research (Marcus et al., 2005). Assessments and frequency of sessions were identical to the RT condition, and interactions with staff were minimized. Participants were asked not to change their current exercise.
Smoking Assessment and Incentives
Smoking status was assessed twice/week during the 12-week intervention and at follow-up. Participants achieved 7-day point prevalence abstinence (PPA) at posttreatment or follow-up if they (a) reported no smoking in the past 7 days, (b) obtained a CO reading <10 ppm, and (c) had no self-reports of smoking or CO ratings >9 ppm at any of the assessments in the previous seven days (West, Hajek, Stead, & Stapleton, 2005). Participants achieved prolonged abstinence at posttreatment/follow-up if they (a) reported no smoking since the beginning of Week 3 (allowing a two-week grace period after quitting), and (b) obtained a CO rating <10 ppm at each session. Participants were paid $15 for completing weeks 1–6, $25 for weeks 7–12, and $10 for the 6-month follow-up.
Data Analysis
Between-group differences in baseline variables, number of sessions attended, and study attrition rates at follow-up (3 month and 6 month) were assessed using analysis of variance and chi-square tests. Using a logistic regression model, we examined the effect of treatment assignment on the odds of being quit at 3-month and the 6-month follow-up. Effect sizes are presented as odds ratios (odds ratio [OR], 95% CIs), with unadjusted quit rates presented by treatment arm at each timepoint (3 month and 6 month). All analyses were intent-to-treat, excluding one participant diagnosed with lung cancer. Participants with missing smoking outcomes were considered not quit. Finally, we assessed the differences in mean change in body weight (baseline to 3 months, 3 month to 6 months), body composition (baseline to 3 months), chest and leg strength (baseline to 3 months), and total physical activity (baseline to 3 months, baseline to 6 months).
Results
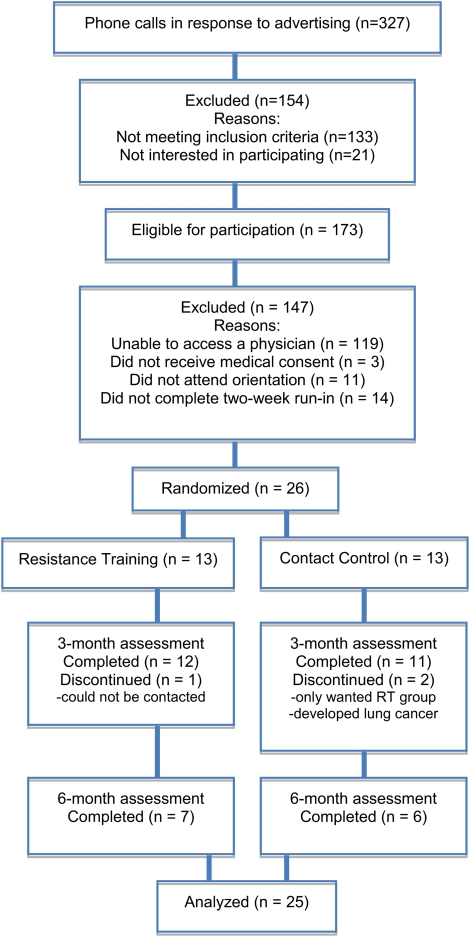
A total of 306 individuals were screened by telephone. Of these, 173 were eligible and 51 were able to gain access to a physician for medical consent. Forty volunteers remained interested in the study and consented to participate. After the 2-week run-in period, 26 were randomized into RT or CC. This included 13 women and 12 men, excluding the participant who developed lung cancer. Obtaining physician’s consent was the only factor related to drop out prior to randomization. Figure 1 summarizes participant flow. The mean age was 36.5 (SD = 12.0), with 53% Caucasian, 31% Hispanic, 12% Black, and 4% Asian. There were no significant between-group differences in any of the baseline measurements. Average duration of smoking was 19.1 years (SD = 12.0), with an average Fagerström Test for Nicotine Dependence score of 4.0 (SD = 2.6) and a mean of 18.0 (SD = 10.1) cigarettes smoked daily. Average body weight at baseline was 81.8 kg (SD = 16.1) and average body fat was 35.1% (SD = 7.0).
Figure 1.
CONSORT flow diagram.
RT participants attended an average of 18.8 (SD = 6.1) sessions and CC attended 18.2 (SD = 5.7). The attrition rate for both RT and CC was 8% at 3 months and 38% and 50% at 6 months, respectively. Nicotine patch use was similar for both groups; RT participants reported use on 73.1% (SD = 21.9) of the days during the 8-week period and CC reported 71.1% (SD = 21.0). At 3 months, objectively verified 7-day PPA rates were 46% for RT and 17% for CC (OR 4.3, 95% CI = 0.7–27.8); prolonged abstinence was 16% and 8%, respectively (OR 2.0, 95% CI = 0.2–25.4). At 6 months, objectively verified 7-day PPA was 38% for RT and 17% for CC (OR 3.1, 95% CI = 0.5–20.1); prolonged abstinence was 15% and 8%, respectively (OR 2.0, 95% CI = 0.2–25.4). At 3 months, RT participants showed a mean reduction in body weight (0.6 kg; SD = 1.7) and fat (0.5%; SD = 1.8), while CC participants showed a mean increase in body weight (0.6 kg; SD = 2.8) and fat (0.6%; SD = 0.7). From 3–6 months, RT participants had a mean reduction in body weight (0.1 kg; SD = 1.8), while CC had a mean increase (0.5 kg; SD = 2.0). See Table 1 for a summary of effect sizes. At 3 months, the RT group increased average chest (9.7 kg; SD = 12.0) and leg (67.8 kg; SD = 50.6) strength, and the CC decreased chest (0.2 kg; SD = 4.4) and increased leg (3.5kg; SD = 6.5) strength. Average physical activity completed increased for RT at 3 months (9.1 Metabolic Equivalent [MET] h/wk; SD = 3.0) and decreased for CC (0.02 MET h/wk; SD = 0.6) but increased for both RT (5.2 MET-h/wk; SD = 7.8) and CC (3.8 MET h/wk; SD = 3.3) at 6 months.
Table 1.
Summary of Effect Size Differences Between Groups
| Resistance training (n = 13) | Contact control (n = 12) | Effect size | |
| 7-Day point prevalence abstinence (%) | |||
| 3 Months | 46 | 17 | 4.3a |
| 6 Months | 38 | 17 | 3.1a |
| Prolonged abstinence (%) | |||
| 3 Months | 16 | 8 | 2.0a |
| 6 Months | 15 | 8 | 2.0a |
| Mean change in body weight | |||
| Baseline to 3 months | −0.6 (1.7) | 0.6 (1.7) | −0.7b |
| 3 Months to 6 months | −0.1 (1.8) | 0.5 (2.0) | −0.3b |
| Mean change in body fat | |||
| Baseline to 3 months | −0.5 (1.8) | 0.6 (0.7) | −0.8b |
Effect sizes are summarized as odds ratios.
Effect sizes are summarized as Cohen’s d.
Discussion
This is the first study to explore RT as an aid for smoking cessation. Results show that participants attended approximately 75% of the intervention sessions, and retention was 92% in both groups at 3 months and 62% (RT) and 50% (CC) at 6 months. At the 3-month assessment, 16% of the RT participants achieved prolonged abstinence versus 8% of the CC, and 15% in the RT condition sustained prolonged abstinence at the 6-month no-treatment follow-up versus 8% of the CC. Overall, these results suggest a RT program could be a viable adjunct to brief smoking cessation treatment.
There are several strengths to this pilot investigation. First, both men and women were recruited. Previously, the overwhelming majority of exercise-based smoking cessation studies have focused on women (Ussher et al., 2008). Second, the smoking cessation treatment used was a single, 15- to 20-min counseling session. In comparison to other treatments (e.g., cognitive behavioral therapy), such brief counseling is potentially more generalizable and has a lower cost. Third, there was a large effect size difference between the groups at the 3-month and 6-month follow-up, despite the small amount of time participants were asked to engage in the intervention. Each RT participant was required to attend only two 60-min sessions/week. Comparatively, this is a much less time-intensive program than other exercise-based smoking cessation interventions producing similar results (e.g., Williams et al., 2010). Fourth, this study is one of the few to report on body composition among those trying to quit. At 3 months, there was a medium effect size difference in body weight and body composition between the conditions, suggesting that RT could not only prevent weight gain but more importantly preserve muscle mass when trying to quit. Last, the recruited sample was diverse, with almost half of the participants identifying as a racial or ethnic minority.
There were also limitations to this investigation. Although similar to other exercise-based smoking cessation research (Ussher et al., 2008), recruitment was approximately 10%, reducing external validity. A barrier to enrollment was access to a physician and medical consent; however, this important component ensured participant safety. The 2-week run-in period also reduced enrollment. It was used to increase the likelihood of enrolling participants that would be compliant, in an attempt to increase internal validity (Cipriani & Geddes 2010). The differential time requirement between groups was also limiting; however, the number of contacts and assessments were identical between the groups, and there was no differential drop-out. Finally, carbon monoxide was the only biological measure of smoking, and a more sensitive indicator (e.g., cotinine) would have yielded more definitive results. Despite these limitations, the large effect size difference between groups for abstinence, the medium effect size difference for body weight/composition, and the successful adherence rate, together suggest that RT is a feasible smoking cessation intervention. Adequately powered trials of RT for smoking cessation, including comparisons to other types of physical activity (e.g. aerobic exercise) and cost-effectiveness analyses are now required.
Funding
National Cancer Institute (R03 A132475 to J.T.C.).
Declaration of Interest
None declared.
Acknowledgments
We would like to thank Phil Stanforth and the staff of the Fitness Institute of Texas, Dixie Stanforth, Patty Coffman, Tammy Metzger, Murtaza Aziz, Nina DiBenedetto, Anna Hughes, Maegan Williams, MattDavis, and Dr. John Ivy for support and research assistance. Additional thanks to all the research participants for devoting their time and effort to this study.
References
- Al-Chalabi L, Prasad N, Steed L, Stenner S, Aveyard P, Beach J, et al. A pilot randomised controlled trial of the feasibility of using body scan and isometric exercises for reducing urge to smoke in a smoking cessation clinic. BMC Public Health. 2008;6:349. doi: 10.1186/1471-2458-8-349. doi:10.1186/1471-2458-8-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009a. [Google Scholar]
- American College of Sports Medicine. American college of sports medicine position stand. Progression models in resistance training for healthy adults. Medicine and Science in Sports & Exercise. 2009b;41:687–708. doi: 10.1249/MSS.0b013e3181915670. doi:10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- Arent SM, Landers DM, Matt KS, Etnier JL. Dose-response and mechanistic issues in the resistance training and affect relationship. Journal of Sport and Exercise Psychology. 2005;27:92–110. [Google Scholar]
- Centers for Disease Control and Prevention. Trends in strength training—United States, 1998–2004. Morbidity and Mortality Weekly Report. 2006;55:769–772. doi:10.1001/jama.296.12.1459. [PubMed] [Google Scholar]
- Cipriani A, Geddes JR. What is a run-in phase? Epidemiologia e Psichiatria Sociale. 2010;19:21–22. [PubMed] [Google Scholar]
- Etter JF, Bergman MM, Humair J, Perneger TV. Development and validation of a scale measuring self-efficacy of current and former smokers. Addiction. 2000;95:901–913. doi: 10.1046/j.1360-0443.2000.9569017.x. doi:10.1046/j.1360-0443.2000.9569017.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kohl HW, Blair SN, Paffenbarger RS, Macera CA, Kronenfeld JJ. The aerobics center longitudinal study physical activity questionnaire. Medicine & Science in Sports & Exercise. 1997;29:10–14. [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate–intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine & Tobacco Research. 2005;7:871–880. doi: 10.1080/14622200500266056. doi:10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews. 2009:CD006219. doi: 10.1002/14651858.CD006219.pub2. 1. doi:10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: A systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. doi:10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Ussher M, Cropley M, Playle S, Mohidin R, West R. Effect of isometric exercise and body scanning on cigarette cravings and withdrawal symptoms. Addiction. 2009;104:1251–1257. doi: 10.1111/j.1360-0443.2009.02605.x. doi:10.1111/j.1360-0443.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Doshi R, Sampuran AK. Acute effect of isometric exercise on desire to smoke and tobacco withdrawal symptoms. Human Psychopharmacology. 2006;21:39–46. doi: 10.1002/hup.744. doi:10.1002/hup.744. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor AH, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2008:CD002295. doi: 10.1002/14651858.CD002295.pub3. 4. doi:10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology. 2004;177:195–199. doi: 10.1007/s00213-004-1923-6. doi:10.1007/s00213-004-1923-6. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction. 2005;100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x. doi:10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- Williams DM, Whiteley JA, Jennings EG, Albrecht AE, Ussher M, Dunsiger S, et al. Moderate intensity exercise as an adjunct to standard smoking cessation treatment for women: A pilot study. Psychology of Addictive Behaviors. 2010;24:349–354. doi: 10.1037/a0018332. doi:10.1037/a0018332. [DOI] [PMC free article] [PubMed] [Google Scholar]