Abstract
Vascular stiffening of the large arteries is a common feature of human aging. Increased aortic stiffness with age may contribute to pathological changes in the left ventricle and this can induce ventricular stiffening. Vascular-ventricular stiffening combined with abnormal arterial-cardiac interaction is considered an important pathophysiology of heart failure with a preserved ejection fraction. Here, I briefly review the concept and implications of arterial-cardiac interaction and this will pave the way to understanding and controlling heart failure with a preserved ejection fraction, which is more prevalent in the elderly.
Keywords: Aortic stiffness, Left ventricle, Heart failure
Introduction
The circulatory system is a closed system. The heart and the vascular system interact to provide adequate blood flow throughout the body. This interaction of properties of the systolic ventricle, as a pump, and the vascular system, as a load, plays a critical role in determining cardiac output. Therefore, arterial-cardiac interaction, also known as ventricular-vascular interaction or ventricular-vascular coupling, is considered a central deteminant of net cardiovascular performance.1-3) There has been much interest in the relationship among vascular stiffness, cardiac function, and cardiovascular diseases. The concept of arterial-cardiac interaction is important to understanding the pathophysiologies of heart failure, especially in patients with preserved ejection fraction. This review will focus on the concept and implications of arterial-cardiac interaction and the non-invasive methods available for its clinical assessment.
Vascular Aging and Stiffening
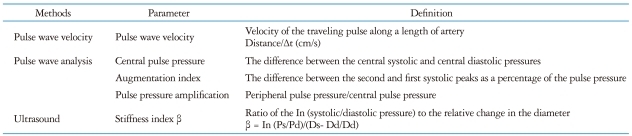
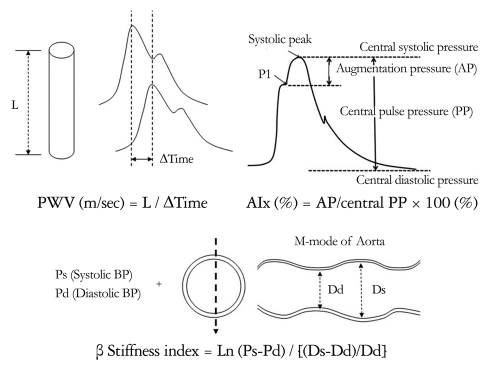
Vascular stiffening of the large arteries is a common feature of human aging and is exacerbated by many common disorders such as hypertension, diabetes mellitus, and renal disease.4-6) The normal aorta delivers blood from the heart to the capillaries and cushions pulsations.5) The arterial system in youth is a very effective conduit and a very efficient cushion.5),6) In young subjects, the wave travels slowly in the distensible tube so that the reflected wave from the resistance artery boosts pressure during diastole. As the aorta ages and stiffens, blood travels faster, returns earlier, and boosts pressure in late systole. Therefore, vascular stiffening results in widening of the arterial pulse pressure (PP), high augmentation pressure, high augmentation index (AIx) and high pulse wave velocities (PWV). Because young subjects have good pressure amplification from the central to the peripheral, and elderly subjects do not, their central blood pressures (BP) differ even when they have the same peripheral BPs.7-9) Elderly subjects have higher central BPs in similar peripheral BPs with younger sujects, that can cause pulsatile stress on the left ventricle (LV). There are several different methods of assessing arterial stiffness, some of which are more widely applicable than others.10) The representative indices and surrogates of arterial stiffness are summerized in Table 1 and shown on Fig. 1. PWV is the speed at which the forward pressure wave is transmitted from the aorta through the vascular tree.11) It is calculated by measuring the time taken for the arterial waveform to pass between two points a measured distance apart. The PWV has been validated and is reproducible, and has been widely applied as the gold standard of arterial stiffness measurement.11) Pulse waveform analysis permits measurement of central systolic BP, central PP and AIx.11),12) The arterial pressure waveform is a composite of the forward wave created by LV contraction and a reflected wave generated in the periphery, returning towards the heart. AIx is calculated as the ratio between augmentation pressure and central PP and is expressed as a percentage.11) It is a complex composite measurement, derived from many important dynamic variables. It is influenced by PWV, the reflectance point, and LV ejection characteristics.10) The age-related changes in central AIx and aortic PWV follow different patterns.9) AIx might provide a more sensitive marker of arterial aging in younger individuals, whereas aortic PWV might be a more sensitive marker in older individuals.9) Recently, PP amplification has been suggested as a mechanical biomarker of cardiovascular diesase and risk, together with global arterial properties.13) Normally, differences in vessel stiffness and reflection of pressure waves within the arterial tree result in considerable amplification of PP between the aorta and brachial artery. This so-called PP amplification is a well-established hemodynamic phenomenon and reduced PP amplification is related to increased cardiovascular risks and poor outcomes superior to the values of brachial or central arteries alone.13),14)
Table 1.
Indices and surrogates of arterial stiffness
In: natural logarithm
Fig. 1.
Indices and surrogates of arterial stiffness. PWV: pulse wave velocity, AIx: augmentation index, BP: blood pressure.
Ultrasound-based measurements of arterial stiffness are also in use. It is necessary to simultaneously measure BP. The stiffness index β is less affected by arterial pressure changes and could be more useful than other parameters, being easily determined using ultrasound.10) Carotid stiffness index β has been used to assess arterial stiffness in various cardiovascular diseases.10),15)
Influence of Vascular Stiffening on Left Ventricular Function
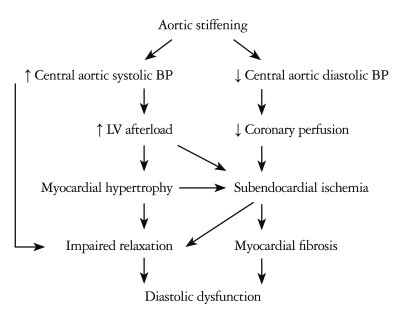
The pathophysiological and clinical implications of arterial stiffness should be considered together with LV function. Several possible pathways exist whereby aortic stiffening may contribute to pathological changes in the LV, which can be the substrate of diastolic dysfunction.16) Aortic stiffening leads to augmentation of the central aortic systolic BP, thus increasing LV afterload. Increased afterload may promote myocyte hypertrophy and slow LV relaxation. Concomitant reduction in central aortic diastolic BP may compromise coronary perfusion and aggravate subendocardial ischemia. This can further impair myocardial relaxation and promote myocardial fibrosis leading to diastolic dysfunction. Fig. 2 illustrates the mechanisms that predispose the LV to ischemia and to dysfunction with aortic stiffening.16),17) A vicious cycle becomes relevant in the development of heart failure with preserved ejection fraction, probably the most common form of heart failure in the elderly.5) In a recent study, we demonstrated the gender differences in the association between the indices of arterial stiffness and LV diastolic functional parameters in age-matched men and women with preserved LV ejection fraction.18) In women, despite similar peripheral pressures to those of men, there were significant differences in central pressures and indices of wave reflections.18),19) LV diastolic function significantly correlated with parameters representing arterial stiffness in women but not in men.18) The effect of earlier wave reflection on central pressure and stronger relation to LV diastolic function could be a possible contributor of hemodynamic liability prone to heart failure in women.
Fig. 2.
Pathophysiological pathways: Relation of arterial stiffness to diastolic dysfunction in hypertensive patients.16) BP: blood pressure, LV: left ventricular.
Ventricular-Vascular Coupling
The concept and assessment of ventricular-vascular coupling
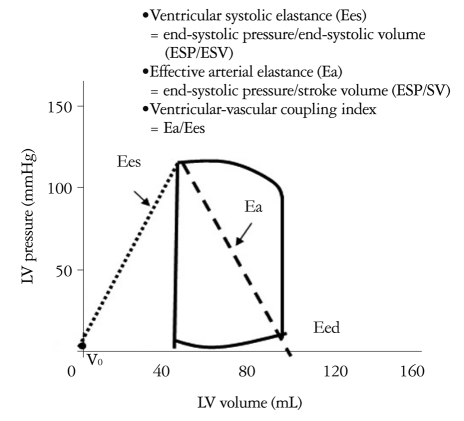
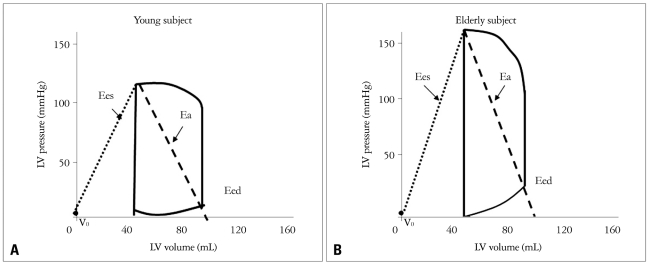
The interaction of ventricular and vascular properties, or coupling, is an important determinant of cardiac performance.1-3) Several groups of systems-physiology investigators have studied and clarified currently accepted frameworks of ventricular-vascular coupling.1),3),20),21) Many investigators have sought ways to characterize both the heart and vascular system and their interaction using common variables. Fig. 3 shows a schematic diagram of the pressure-volume loop for LV, with ventricular systolic and diastolic elastances, and effective arterial elastance. Ees defines ventricular systolic stiffness, while Eed is diastolic stiffness. Ea equals the ratio of end systolic pressure over stroke volume, and reflects arterial loading. The ratio of effective arterial elastance to LV end-systolic elastance (Ea/Ees) is used to index relative coupling between the heart and vascular systems.3),4)
Fig. 3.
Schematic diagram of the pressure-volume loop for the left ventricle.
Noninvasive assessment of ventricular-vascular coupling
Based on this concept, ventricular systolic elastance, effective arterial elastance, and the ventricular-vascular coupling index can be assessed noninvasively using echocardiography and simultaneously assessed BPs. LV end-systolic and end-diastolic volumes are measured from apical 4-chamber and 2-chamber views using the biplane method of disks (modified Simpson's rule).22) Stroke volume can be calculated by substrating the end-systolic volume from the end-diastolic volume. End-systolic pressure is approximated by [(2 × systolic BP + diastolic BP)/3]. This noninvasive assessment of end-systolic pressure accurately predicts LV pressure-volume loop measurements of end-systolic pressure.23) The Ea is estimated as the end-systolic pressure/stroke volume. The Ees of LV is calculated as end-systolic pressure/end-systolic volume. Ventricular-vascular coupling is generally assessed by the Ea/Ees ratio, termed the ventricular-vascular coupling index.3),4)
Age-related changes in ventricular-vascular coupling
Fig. 4A and B display typical pressure-volume data, along with Ees and Ea, for young and elderly subjects. In comparison with the younger subjects, the older subects display marked increases in both elastances, reflecting vascular stiffening and ventricular stiffening.24) The result is that both parameters remain relatively matched. The matched increase in arterial and cardiac stiffness with aging can maintain ventricular-vascular coupling within a normal range.3),24) However, diastolic chamber stiffness (Eed) commonly increases with age.3)
Fig. 4.
Relationship between effective arterial elastance (Ea) and ventricular systolic elastance (Ees) in young (A) versus old subjects (B).24) A: In young subject. B: A matched increase in arterial and ventricular stiffness in elderly subjects.
Dynamic changes of ventricular-vascular coupling
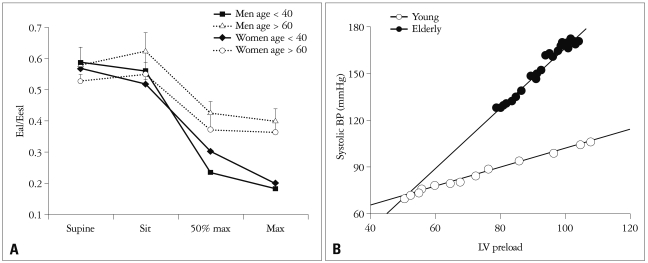
Although maintenance of ventricular-vascular coupling with age would be somewhat beneficial, the rise in both vascular and ventricular stiffening becomes apparently problematic when the system is stressed by exercise. In normal subjects, effective arterial elastance is nearly one half of LV elastance2),25) and the ventricular-vascular coupling index decreases with exercise, indicating augmented pump efficiency.26),27) Najjar et al.27) demonstrated that the ventricular-vascular coupling index during exercise decreased by a smaller degree in older subjects than in younger subjects even though there was no difference by age in resting ventricular-vascular coupling index (Fig. 5A). These findings might suggest that aging is associated with less reserve capacity, or an inability to attain maximal efficacy, manifested by a smaller reduction in the coupling index. The different responses of ventricular-vascular coupling to exercise can be related to exercise intolerance. In addition, higher ventricular and vascular stiffness has important implications regarding BP liability and loading sensitivity even though coupling is maintained with age.24) In the elderly, even a small increase in blood volume can substantially raise systolic BP24) (Fig. 5B). Therefore, enhanced BP sensitivity to circulating volume and diuretics is common in elderly subjects and the mechanism of rapid-onset pulmonary edema in elderly subjects can be explained. In summary, when the ventricular-vascular system is stressed with exercise or faced with volume overload, the coupling response may be abnormal, and it may be difficult to maintain effective cardiovascular performance.
Fig. 5.
Dynamic changes of ventricular-vascular coupling under stress caused by exercise (A)27) and volume overload (B).24)
In conclusion, abnormal arterial-cardiac interaction and stiffening of the ventricular and vascular systems may contribute to the pathophysiology of heart failure with preserved ejection fraction. Combined ventricular-vascular stiffening may have important consequences on cardiac response under stress by exertion, volume overload and abrupt changes in heart function. The major effects of ventricular-vascular stiffening can be summarized as follows: 1) enhanced liability of cardiovascular function; 2) limited reserve capacity; 3) greater pressure sensitivity to blood volume alterations; 4) greater likelihood of poorly matched ventricular-vascular system with cardiac failure; and 5) compromised coronary flow and exacerbated response to myocardial ischemia.
References
- 1.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–H780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 2.Starling MR. Left ventricular-arterial coupling relations in the normal human heart. Am Heart J. 1993;125:1659–1666. doi: 10.1016/0002-8703(93)90756-y. [DOI] [PubMed] [Google Scholar]
- 3.Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail Rev. 2002;7:51–62. doi: 10.1023/a:1013749806227. [DOI] [PubMed] [Google Scholar]
- 4.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 5.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 6.O'Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12:329–341. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 7.Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol. 2001;37:1374–1380. doi: 10.1016/s0735-1097(01)01166-4. [DOI] [PubMed] [Google Scholar]
- 8.Choi CU, Kim EJ, Kim SH, Shin SY, Choi UJ, Kim JW, Lim HE, Rha SW, Park CG, Seo HS, Oh DJ. Differing effects of aging on central and peripheral blood pressures and pulse wave velocity: a direct intraarterial study. J Hypertens. 2010;28:1252–1260. doi: 10.1097/HJH.0b013e328337dad6. [DOI] [PubMed] [Google Scholar]
- 9.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR ACCT Investigators. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Antonini-Canterin F, Carerj S, Di Bello V, Di Salvo G, La Carrubba S, Vriz O, Pavan D, Balbarini A, Nicolosi GL Research Group of the Italian Society of Cardiovascular Echography (SIEC) Arterial stiffness and ventricular stiffness: a couple of diseases or a coupling disease? A review from the cardiologists point of view. Eur J Echocardiogr. 2009;10:36–43. doi: 10.1093/ejechocard/jen236. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 12.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–1659. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 13.Benetos A, Thomas F, Joly L, Blacher J, Pannier B, Labat C, Salvi P, Smulyan H, Safar ME. Pulse pressure amplification a mechanical biomarker of cardiovascular risk. J Am Coll Cardiol. 2010;55:1032–1037. doi: 10.1016/j.jacc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 14.Nijdam ME, Plantinga Y, Hulsen HT, Bos WJ, Grobbee DE, van der Schouw YT, Bots ML. Pulse pressure amplification and risk of cardiovascular disease. Am J Hypertens. 2008;21:388–392. doi: 10.1038/ajh.2007.89. [DOI] [PubMed] [Google Scholar]
- 15.Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989;80:78–86. doi: 10.1161/01.cir.80.1.78. [DOI] [PubMed] [Google Scholar]
- 16.Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber T, Auer J, O'Rourke MF, Punzengruber C, Kvas E, Eber B. Prolonged mechanical systole and increased arterial wave reflections in diastolic dysfunction. Heart. 2006;92:1616–1622. doi: 10.1136/hrt.2005.084145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim CY, Park S, Choi D, Yang WI, Cho IJ, Choi EY, Chung N, Ha JW. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57:1226–1233. doi: 10.1016/j.jacc.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA. Sex, load, and relaxation: are women more susceptible to load-dependent diastolic dysfunction? J Am Coll Cardiol. 2011;57:1234–1236. doi: 10.1016/j.jacc.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Myhre ES, Johansen A, Piene H. Optimal matching between canine left ventricle and afterload. Am J Physiol. 1988;254:H1051–H1058. doi: 10.1152/ajpheart.1988.254.6.H1051. [DOI] [PubMed] [Google Scholar]
- 21.Elzinga G, Westerhof N. Matching between ventricle and arterial load. An evolutionary process. Circ Res. 1991;68:1495–1500. doi: 10.1161/01.res.68.6.1495. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 25.Nitenberg A, Loiseau A, Antony I. Left ventricular mechanical efficiency in hypertensive patients with and without increased myocardial mass and with normal pump function. Am J Hypertens. 2001;14:1231–1238. doi: 10.1016/s0895-7061(01)02205-1. [DOI] [PubMed] [Google Scholar]
- 26.Asanoi H, Kameyama T, Ishizaka S, Miyagi K, Sasayama S. Ventriculoarterial coupling during exercise in normal human subjects. Int J Cardiol. 1992;36:177–186. doi: 10.1016/0167-5273(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 27.Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, Becker LC, Lakatta EG. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44:611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]