Abstract
Previous studies have shown that both bipolar disorder (BPD) and psychomotor agitation (PMA) are associated with substance dependence. These two findings have yet to be integrated, despite evidence that PMA is closely linked with the bipolar spectrum. Accordingly, the current study examined whether BPD and PMA had unique or overlapping associations with substance dependence disorders. Participants were 2300 individuals seeking outpatient psychiatric treatment. Before treatment, participants were assessed using structured clinical interviews, which yielded DSM-IV psychiatric diagnoses and clinical ratings of mood symptoms. Current PMA and lifetime BPD were present in 483 and 172 (Bipolar I, n = 71; Bipolar II, n = 101) participants, respectively. Current PMA and lifetime BPD each were associated with increased prevalence of lifetime nicotine, alcohol, and drug dependence (ORs ≥ 1.52, ps ≤ .0004). These associations remained significant when controlling for demographic characteristics and comorbid psychiatric disorders, except the link between agitation and alcohol dependence, which was reduced to a trend (p=.058). Although BPD and PMA were associated with each other, these two factors demonstrated unique, non-overlapping relationships to nicotine, alcohol, and drug dependence. Individuals with both PMA and BPD exhibited especially high rates of comorbid substance dependence. The present results replicate and extend previous findings documenting the relations of BPD and PMA to substance dependence. BPD and PMA may represent independent psychopathological correlates of substance dependence. Future research should explore the theoretical and clinical significance of these potentially distinct relations to substance dependence.
Bipolar disorder (BPD) is robustly associated with smoking and substance use disorders (Compton, Thomas, Stinson, & Grant, 2007; Grant, Hasin, Chou, Stinson, & Dawson, 2004; Hasin, Stinson, Ogburn, & Grant, 2007; Merikangas et al., 2007; Sbrana et al., 2005; Vanable, Carey, Carey, & Maisto, 2003; Wilens et al., 2008). These relations have been shown for both bipolar I and II subtypes and are particularly strong for lifetime substance dependence diagnoses, including nicotine, alcohol, and drug dependence (Compton et al., 2007; Grant et al., 2004; Hasin et al., 2007; Merikangas et al., 2007). This relation is not accounted for by demographics or comorbid psychiatric disorders and is stronger than the corresponding association between unipolar depression and substance dependence (Compton et al., 2007; Hasin et al., 2007).
Other research indicates that psychomotor agitation (PMA; a mood disorder symptom involving unintentional motor activity, manifested as fidgeting, pacing, and hand-wringing) is also associated with substance dependence (Leventhal, Francione Witt, & Zimmerman, 2008; Leventhal, Pettit, & Lewinsohn, 2008). Specifically, depression with PMA is associated with increased rates of substance dependence, alcohol abuse/dependence, nicotine dependence, and polysubstance use (Leventhal, Francione Witt et al., 2008;Leventhal, Pettit et al., 2008; Maremmani et al., 2007). This relation remains after controlling for demographics, depressive severity, chronicity, and other psychiatric disorders, suggesting that this link is specific to the PMA phenotype (Leventhal, Francione Witt et al., 2008; Leventhal, Pettit et al., 2008).
These lines of research have been developed independently with no previous investigations attempting to integrate BPD and PMA as factors that jointly relate to substance dependence. Nonetheless, research suggests that PMA is closely associated with BPD (Akiskal, Benazzi, Berugi, & Rihmer, 2005; Benazzi, 2004; Benazzi, Helmi, & Bland, 2002). In fact, some researchers have proposed that depression with PMA may be closer to the bipolar than the unipolar spectrum (Benazzi et al., 2002) and is a key psychopathologic marker of a depressive mixed state (Akiskal et al., 2005; Benazzi, Koukopoulos, & Akiskal, 2004).
Given that there is overlap between BPD and PMA, the relative roles of these two factors in substance dependence are unclear. One possibility is that the relationship with substance dependence attributed to BPD and PMA may be shared with one another and accounted for by a single pathway. That is, individuals with BPD who are likely to have comorbid substance dependence may be the same individuals as those with PMA, which would suggest that assessing PMA among BPD patients would not have additional utility for identifying those who may also have substance dependence. Alternatively, their relations to substance dependence may be distinct from one another and accounted for by two independent pathways (i.e., patients with PMA but not BPD may nonetheless have disproportionally high rates of substance dependence). Disentangling the relative roles of BPD and PMA in substance dependence could: a) clarify the psychopathologic mechanisms underlying the link between mood-related psychiatric phenotypes and substance dependence; and b) have clinical implications for the assessment of mood-related correlates of substance dependence. Investigating these relations in psychiatric patients is particularly significant because rates of substance dependence, BPD, and PMA are especially high this population (Akiskal et al., 2005; Benazzi, 2004; Compton et al., 2007)
Accordingly, the current study aimed to evaluate: 1) the association between lifetime BPD and lifetime substance dependence; 2) the association between current PMA and lifetime substance dependence; and 3) whether BPD and PMA exhibit unique or overlapping associations with substance dependence disorders. We examined these aims in 2300 psychiatric outpatients who underwent in-depth structured psychiatric interviews before beginning treatment as part of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project (Zimmerman, 2003; Zimmerman & Mattia, 1999). Analyses were conducted both unadjusted and adjusted for demographics and comorbid psychiatric disorders (including unipolar major depression) to elucidate the specific associations of BPD and PMA to substance dependence.
In replication and extension of prior findings, we hypothesized that BPD and PMA would be associated with nicotine, alcohol, and drug dependence above and beyond the influence of demographics and psychiatric comorbidity. We did not predict whether BPD and PMA would have unique or overlapping relations to dependence due to the paucity of research on this topic.
Method
Participants
Participants were recruited as part of the MIDAS project (Zimmerman, 2003; Zimmerman & Mattia, 1999). During their initial telephone screen, patients from the Rhode Island Hospital Department of Psychiatry’s outpatient department were invited to participate in an in-depth face-to-face diagnostic evaluation prior to meeting with their treating clinician.
To date, 2300 participants have been evaluated and comprise the present sample. Participants had an average age of 38.2 (SD = 12.8, range 14 – 85). Sixty-five percent were female and 88% were white. Thirty-one percent were single, 46.2% were married or cohabitating, 20.8% were divorced or separated, and 1.8% were widowed. In patients aged over 18 (n = 2238), 9.3% did not receive a high school diploma, 22.4% graduated high school, 31.4% attended some college, and 36.8% had a college degree or higher. Rates of lifetime psychiatric disorders were as follows: anxiety (63.8%), psychotic (3.0%), eating (12.7%), impulse control (14.8%), attention deficit/disruptive behavior (8.9%), and unipolar mood (67.3%) disorder.
Assessment
Patients were interviewed using the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Williams, & Gibbon, 1997) to diagnose current and past psychiatric disorders (APA, 1994). All participants were administered the entire SCID current depressive episode module, which rated the presence (vs. absence) of all individual current depressive symptoms, including PMA. PMA was rated present if fidgeting, pacing, handwringing, and/or other purposeless movements were evident nearly every day for at least a 2-week period leading up to the assessment. PMA-related behaviors had to be noticed by others and/or directly observable during the interview. Interviewers probed to distinguish PMA from mere subjective feelings of restlessness and to ensure that PMA and other psychiatric symptoms were not directly due to substance use, medication, or physical illness. Thus, all diagnoses and symptom ratings presented herein were classified “independent” rather than “substance-induced.” Lifetime substance dependence and BPD diagnoses were classified according to DSM-IV definitions. Nicotine dependence evaluations were included in the protocol for only the first 1800 patients. Thus, nicotine dependence diagnoses were available only for that portion of the sample, and analyses involving nicotine dependence were conducted using only those patients.1
Prior analyses of joint-interview data collected on a portion of the sample indicated adequate interrater reliability: BPD [Kappa (K) = 1.0], substance dependence diagnoses (K =.64 – 1.0), and PMA (K = .83) ratings (Leventhal, Kahler, Ray, & Zimmerman, 2009; Zimmerman, Chelminski, & McDermut, 2002; Zimmerman, Chelminski, & Posternak, 2004). Interviewers underwent extensive training, as described previously (Zimmerman & Mattia, 1999).
Data Analysis
Considerations for classification
Given that substance dependence has been associated with both bipolar I and II (Compton et al., 2007; Grant et al., 2004; Hasin et al., 2007; Merikangas et al., 2007), both variants were combined into a general BPD category as in previous research (Wilens et al., 2008). In concordance with extant reports (Compton et al., 2007; Grant et al., 2004; Hasin et al., 2007; Merikangas et al., 2007), all illicit and prescription drug dependences were analyzed as a combined drug dependence category to increase prevalence and reduce the number of comparisons. Substance abuse was not analyzed, given that BPD and PMA have been more strongly and consistently linked with dependence than abuse (Compton et al., 2007; Grant et al., 2004; Hasin et al., 2007; Merikangas et al., 2007). Because the link between BPD and drug dependence when controlling for comorbid psychiatric disorders is more consistent over lifetime than 12-month periods (Compton et al., 2007), analyses focused on lifetime rates of these disorders. Current rates of PMA were analyzed because evaluation of PMA relies on direct behavioral observation (APA, 1994; First et al., 1997).
Analytic plan
Logistic regression models were conducted to examine whether the presence (vs. absence) of BPD and PMA were associated with the presence (vs. absence) of substance dependence. For each substance dependence diagnosis outcome variable (nicotine, alcohol, drug), two “individual models” were tested: 1) a baseline unadjusted model in which either BPD or PMA was the sole predictor; and 2) an adjusted model which included the primary predictor and controlled for demographics (gender, age, race, marital status, education; included as separate variables and coded in the fashion described in the sample characteristics section) and comorbid psychiatric conditions (lifetime history of any anxiety, psychotic, eating, impulse control, unipolar major depressive, and attention deficit/disruptive behavior disorder; included as separate variables and coded as present vs. absent). These models were re-tested in “combined models” in which BPD and PMA were simultaneously entered as predictors (to account for individuals who were positive for both BPD and PMA) in order to elucidate whether their associations with dependence were unique or overlapping. A substantial reduction in the effect sizes of BPD and PMA from individual to combined models would indicate that their relations to dependence were due to shared variance (i.e., accounted for by the same individuals). Little or no reduction in effect sizes would indicate that their associations with dependence were unique.
Results are reported as odds ratios (ORs), with 95% confidence intervals (CIs). For all models, statistical significance was set at p < 0.05, and all tests were 2-tailed.
Results
Rates of BPD, PMA, and their co-occurrence
Of the 2300 patients in the overall sample, 172 (7.5%) were diagnosed with lifetime BPD (bipolar I, n = 71; bipolar II, n = 101) and 483 (21.0%) exhibited PMA. BPD and PMA co-occurred in 56 patients and were associated (PMA was present in 33% of patients with BPD vs. 21% of patients without, p = .0001).
Rates of substance dependence
Rates of lifetime substance dependence were as follows: nicotine dependence (n = 682/1800, 37.9%), alcohol dependence (n = 501/2300, 21.8%), and drug dependence (n = 368/2300, 16.0%). Those in the drug category had one or more of the following dependence diagnoses: amphetamine (n = 29), cannabis (n = 159), cocaine (n = 139), sedative (n = 33), opioid (n = 54), hallucinogen (n = 2), polysubstance (n = 51), other (n = 9).
Independent relations of BPD and PMA to substance dependence
As reported in Table 1, results of individual logistic regression models indicated that presence (vs. absence) of lifetime BPD was associated with significant increases in rates of lifetime nicotine, alcohol, and drug dependence. These associations remained significant when controlling for demographic characteristics and comorbid psychiatric disorders (Table 1).2
Table 1.
Associations between Lifetime Bipolar Disorder and Lifetime Substance Dependence (Individual Models)
| Patients without Lifetime BPD (n = 2128) |
Patients with Lifetime BDP (n = 172) |
Baseline Modela |
Adjusted Modelb |
|||
|---|---|---|---|---|---|---|
| % | % | OR (95% CI) | p | OR (95% CI) | p | |
| Nicotine dependencec | 36.7 | 52.7 | 1.92 (1.34-2.75) | .0004 | 2.26 (1.49-3.45) | .0001 |
| Alcohol dependence | 21.0 | 32.0 | 1.77 (1.27-2.48) | .0009 | 1.97 (1.31-2.96) | .001 |
| Drug dependence | 15.2 | 26.2 | 1.98 (1.38-2.84) | .0002 | 2.20 (1.40-3.44) | .0006 |
Note. Percentages indicate rates of respective substance dependence diagnosis by lifetime bipolar disorder status. ORs indicate association of lifetime bipolar disorder with respective substance dependence diagnosis outcome in logistic regression model. BPD = Bipolar Disorder; OR = Odds Ratio; CI = Confidence Interval
Baseline model including lifetime bipolar disorder as the sole predictor
Adjusted for gender, age, race, marital status, education, and history of any anxiety, psychotic, eating, impulse control, unipolar major depressive, and attention deficit/disruptive behavior disorder
Analyses based on subset of participants who underwent nicotine dependence evaluations (n = 1800)
The presence (vs. absence) of PMA was also associated with significant increases in nicotine, alcohol, and drug dependence in individual logistic regression models (see Table 2). These relationships were reduced in strength but remained significant in adjusted models, except for the association with alcohol dependence (p = .058), which was reduced to a trend (Table 2).
Table 2.
Associations between Current Psychomotor Agitation and Lifetime Substance Dependence (Individual Models)
| Patients without PMA (n = 1817) |
Patients with PMA (n = 483) |
Baseline Modela |
Adjusted Modelb |
|||
|---|---|---|---|---|---|---|
| % | % | OR (95% CI) | p | OR (95% CI) | p | |
| Nicotine dependencec | 34.8 | 48.3 | 1.75 (1.40-2.19) | <.0001 | 1.51 (1.19-1.90) | .0006 |
| Alcohol dependence | 20.2 | 27.7 | 1.52 (1.21-1.90) | .0004 | 1.27 (0.99-1.62) | .058 |
| Drug dependence | 14.1 | 23.0 | 1.81 (1.41-2.33) | <.0001 | 1.52 (1.17-1.99) | .002 |
Note. Percentages indicate rates of respective substance dependence diagnosis by psychomotor agitation status. ORs indicate association of psychomotor agitation with respective substance dependence diagnosis outcome in logistic regression model. PMA = Psychomotor agitation; OR = Odds Ratio; CI = Confidence Interval.
Baseline model including psychomotor agitation as the sole predictor
Adjusted for gender, age, race, marital status, education, and history of any anxiety, psychotic, eating, impulse control, unipolar major depressive, and attention deficit/disruptive behavior disorder
Analyses based on subset of participants who underwent nicotine dependence evaluations (n = 1800)
Concomitant relations of BPD and PMA to substance dependence
Results of combined logistic regression models which included both BPD and PMA as simultaneous predictors are presented in Table 3. For the baseline models that did not control for demographics and psychiatric disorders, the effect size of BPD was slightly smaller in the combined than in the individual models. This was the case for nicotine (combined OR = 1.78 vs. individual OR = 1.92), alcohol (1.69 vs. 1.77), and drug (1.85 vs. 1.98) dependence. A similar pattern arose in the comparison of the effect sizes of PMA in combined versus independent baseline models (nicotine: 1.70 vs. 1.75; alcohol: 1.48 vs. 1.52; drug: 1.75 vs. 1.81).
Table 3.
Concomitant Relations of Psychomotor Agitation and Bipolar Disorder to Substance Dependence (Combined Models)
| Baseline Modela |
Adjusted Modelb |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Predictor: bipolar disorder | ||||
| Nicotine dependencec | 1.78 (1.24-2.56) | .002 | 2.07 (1.35-3.17) | .0008 |
| Alcohol dependence | 1.69 (1.20-2.37) | .002 | 1.88 (1.25-2.84) | .002 |
| Drug dependence | 1.85 (1.29-2.66) | .0009 | 2.02 (1.28-3.17) | .002 |
| Predictor: psychomotor agitation | ||||
| Nicotine dependencec | 1.70 (1.36-2.12) | <.0001 | 1.42 (1.12-1.80) | .004 |
| Alcohol dependence | 1.48 (1.17-1.86) | .001 | 1.20 (0.94-1.54) | .15 |
| Drug dependence | 1.75 (1.36-2.25) | <.0001 | 1.43 (1.09-1.88) | .009 |
Note. ORs indicate association of predictor with respective substance dependence diagnosis outcome in logistic regression model. OR = Odds Ratio; CI = Confidence Interval
Baseline model including both psychomotor agitation and lifetime bipolar disorder as the sole predictors
Model including both psychomotor agitation and lifetime bipolar disorder as predictors, adjusted for gender, age, race, marital status, education, and history of any anxiety, psychotic, eating, impulse control, unipolar major depressive, and attention deficit/disruptive behavior disorder
Analyses based on subset of participants who underwent nicotine dependence evaluations (n = 1800)
For the models that adjusted for demographics and comorbid psychiatric disorders, the differences across combined and individual models were also small for the effect sizes of BPD (nicotine: 2.07 vs. 2.26; alcohol: 1.88 vs. 1.97; drug: 2.02 vs. 2.20) and PMA (nicotine: 1.42 vs. 1.51; alcohol: 1.20 vs. 1.27; drug: 1.43 vs. 1.52). The significance of all relations was consistent in individual and combined models. Given the lack of differences in effect sizes in individual and combined models, the pattern of results indicate that the relations of BPD and PMA to substance dependence were largely unique and not accounted for by overlapping variance.
Given the pattern of findings, we conducted additional unplanned analyses of substance dependence prevalence among those with both BPD and PMA. Figure 1 reports the rates of substance dependence separately among those with: a) neither BPD nor PMA, b) only one factor, and c) both BPD and PMA. In comparison to patients with neither BPD nor PMA, those with both factors had substantially higher rates of lifetime dependence in unadjusted logistic regression models (nicotine: OR = 3.21, p = .0004; alcohol: OR = 2.33, p = .008; drug: OR = 3.91, p < .0001) and models that adjusted for demographics and psychiatric characteristics (nicotine: OR = 2.66, p = .002; alcohol: OR = 1.86, p = .04; drug: OR = 2.59, p = .002). As compared to those with only one factor (either BPD or PMA), patients with both BPD and PMA had higher rates of nicotine, alcohol, and drug dependence (ORs from 1.17 to 1.85 in unadjusted and adjusted models; see Figure 1). However, these comparisons were nonsignificant, except for the unadjusted model predicting lifetime drug dependence (OR = 1.85, p = .04).
Figure 1.
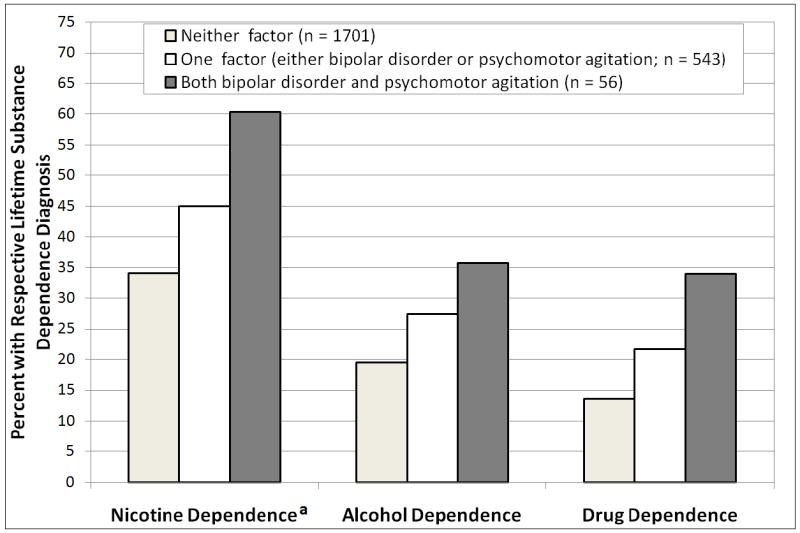
Prevalence of Lifetime Nicotine, Alcohol, and Drug dependence across Patients with Neither, One, or Both Psychomotor Agitation and Lifetime Bipolar Disorder
aRates based on subset of participants who underwent nicotine dependence evaluations (n = 1800)
Exploratory analyses of the interaction of BPD and PMA were not significant for any outcome in baseline or adjusted models (ps ≥ .67).3
Discussion
This study evaluated the relative roles of BPD and PMA in substance dependence in 2300 psychiatric outpatients. Consistent with prior reports (Compton et al., 2007; Hasin et al., 2007; Merikangas et al., 2007), lifetime BPD was associated with lifetime nicotine, alcohol, and drug dependence. This investigation extends previous epidemiologic findings (Compton et al., 2007; Hasin et al., 2007) by documenting the link between BPD and substance dependence (above and beyond demographics and psychiatric comorbidity) in a treatment-seeking psychiatric sample.
Current PMA was also significantly associated with lifetime nicotine, alcohol, and drug dependence. Relations with nicotine and drug dependence remained significant when controlling for demographics and psychiatric comorbidity; the association with alcohol dependence in the adjusted model was reduced to a trend (p = .058). Notably, the association between PMA and drug and nicotine dependence remained after adjusting for unipolar depression, suggesting that the relationship was independent of unipolar depression. A previous analysis of a subset of the current sample who met criteria for current major depression found that severely marked PMA was associated with concurrent nicotine dependence and drug abuse/dependence (Leventhal, Francione Witt et al., 2008). A separate investigation of young adults with lifetime major depression found that those who exhibited PMA (vs. psychomotor retardation) in their most severe depressive episode had higher rates of lifetime substance dependence (Leventhal, Pettit et al., 2008). The current study extends past findings to a broader sample of psychiatric outpatients with and without mood disorders (25% of the current sample had no lifetime mood disorder).
As in prior studies (Benazzi, 2004; Benazzi et al., 2002), PMA and BPD were associated with each other. Despite their association, relations of BPD and PMA to substance dependence were not substantially reduced in size when controlling for each other. The reduction of ORs from individual to combined models ranged from 0.04 to 0.19. This pattern was observable for both PMA and BPD and was consistent across all three dependence diagnoses. Taken together, these findings indicate that BPD and PMA had unique relations to substance dependence, above and beyond any relationship explained by their covariance. Additional analyses indicated that patients with both BPD and PMA exhibited especially high rates of comorbid substance dependence.
The results of this study should be interpreted within the context of its limitations. Because the design was cross-sectional and included retrospective reports, these findings cannot clarify the temporal aspects of the relationships demonstrated herein. Thus, any interpretation of these findings as indicative of BPD and PMA influencing risk of substance dependence is speculative and should be made with caution. Indeed, there are several other plausible explanations of the findings (e.g., substance dependence increases risk of PMA or BPD, or there are unmeasured third variables that account for the relationships). PMA ratings were based on current presentation and report during the past two weeks. Although this approach had the advantage of more accurate diagnosis because patients’ behavior could be directly observed, ratings do not capture PMA over the lifespan, which may be relatively unstable (Leventhal, Pettit et al., 2008). Thus, some patients with past (but not present) PMA were not identified, and patients with current PMA may represent chronic, severe cases of agitation. The influence of these potential biases on the PMA-dependence relationships presented herein is unknown. Aggregating drug dependence diagnoses and bipolar I and II into combined categories is a notable limitation, as different forms of drug dependence and BPD may exhibit disparate relationships with each other and PMA. No other information on the extent of substance use beyond DSM-IV diagnoses was collected. Thus, it would be useful for future research to explore whether these findings extend to alternate self-report and biochemical measures of substance use. Finally, these results are most applicable to treatment-seeking psychiatric outpatients and may not entirely generalize to individuals uninterested in treatment.
To the best of our knowledge, this was the first study to examine the concomitant roles of BPD and PMA in substance dependence. These results replicate and extend previous findings indicating relations of BPD and PMA to substance dependence. Although risk cannot be determined by the present study, one provisional interpretation is that BPD and PMA may influence substance dependence vulnerability and their influence may involve two separate risk pathways with distinct psychopathological underpinnings. Given the independence of BPD and PMA’s relations to dependence, clinicians should be aware that patients with both BPD and PMA may be especially likely to have comorbid substance dependence. Likewise, psychiatric patients with substance dependence may have higher rates of PMA and BPD, which is important to consider for treatment planning. Finally, because PMA (irrespective of BPD and other psychiatric disorders) is associated with substance dependence, clinicians should potentially assess this often overlooked symptom in the context of substance use or psychiatric treatment.
Acknowledgments
This research was supported, in part, by grant DA025041, from the National Institute on Drug Abuse.
Footnotes
There were no significant differences between patients with versus without nicotine dependence data on demographics, BPD, alcohol dependence, or drug dependence; there was a higher rate of PMA in the first 1800 patients (23% vs. 14%). Alcohol and drug dependence analyses using the 1800 of patients with nicotine dependence data available generated equivalent results to analyses using the entire 2300.
Although controlling for unipolar depression in adjusted analyses examining associations between bipolar disorder and substance dependence permits interpretation of relations that are independent of this factor, unipolar depression diagnoses are mutually exclusive from bipolar disorder. Accordingly, models that simultaneously include these two variables demonstrate quasicomplete separation (all unipolar depression cases are negative for bipolar disorder), which potentially inflates odds ratios for associations with bipolar disorder. Additional analyses of individual adjusted models that did not control for unipolar depression yielded a similar pattern of significant results for bipolar disorder, although the odds ratios were smaller (ORs 1.57 – 1.89, ps < .01).
Due to the comorbidity across substance dependence diagnoses, many individuals were represented in more than one dependence category. Of the three dependence diagnoses, 891/1800 (49.5%) had no dependence diagnoses, 540/1800 (30.0%) had only one, 269/1800 (14.9%) had two, and 100/1800 (5.6%) had all three. Consistent with the primary analyses, individual multinomial logistic regression analyses predicting the number of dependence diagnoses (0 vs. 1 vs. 2 vs. 3) after adjusting for demographics and psychiatric variables showed significant associations for both BPD and PMA and only minor reductions in effect sizes from individual to combined models for BPD (ORs 2.63 vs. 2.40) and PMA (1.50 vs. 1.39). Similarly, additional analyses of nicotine dependence diagnosis after eliminating cases with comorbid drug and/or alcohol dependence diagnoses demonstrated similar patterns of ORs as the primary analyses. However, p-values were generally higher but remained significant (even in combined models). There were too few cases of alcohol or drug dependence without nicotine dependence to analyze as non-comorbid outcomes.
The authors report no competing interests.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/adb
References
- Akiskal HS, Benazzi F, Berugi G, Rihmer Z. Agitated ‘unipolar’ depression re-conceptualized as a depressive mixed state: Implications for the antidepressant-suicide controversy. Journal of Affective Disorders. 2005;85(3):245–258. doi: 10.1016/j.jad.2004.12.004. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. fourth edition. American Psychiatric Association; 1994. [Google Scholar]
- Benazzi F. Agitated depression: A valid depression subtype? Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28(8):1279–1285. doi: 10.1016/j.pnpbp.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Benazzi F, Helmi S, Bland L. Agitated depression: Unipolar? bipolar? or both? Annals of Clinical Psychiatry. 2002;14(2):97–104. doi: 10.1023/a:1016854904941. [DOI] [PubMed] [Google Scholar]
- Benazzi F, Koukopoulos A, Akiskal HS. Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression) European Psychiatry. 2004;19(2):85–90. doi: 10.1016/j.eurpsy.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Francione Witt C, Zimmerman M. Associations between depression subtypes and substance use disorders. Psychiatry Research. 2008;161(1):43–50. doi: 10.1016/j.psychres.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray L, Zimmerman M. Refining the depression-nicotine dependence link: Patterns of depressive symptoms in psychiatric outpatients with current, past, and no history of nicotine dependence. Addictive Behaviors. 2009;34(3):297–303. doi: 10.1016/j.addbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Pettit JW, Lewinsohn PM. Characterizing major depression phenotypes by presence and type of psychomotor disturbance in adolescents and young adults. Depression & Anxiety. 2008;25(7):575–592. doi: 10.1002/da.20328. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Rehm LP. The empirical status of melancholia: Implications for psychology. Clinical Psychology Review. 2005;25(1):25–44. doi: 10.1016/j.cpr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Pacini M, Pani PP, Perugi G, Deltito J, Akiskal H. The mental status of 1090 heroin addicts at entry into treatment: should depression be considered a ‘dual diagnosis’? Annals of General Psychiatry. 2007;6:31. doi: 10.1186/1744-859X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Hadzi-Pavlovic D, Brodaty H, Boyce P, Mitchell P, Wilhelm K, et al. Psychomotor disturbance in depression: defining the constructs. Journal of Affective Disorders. 1993;27(4):255–265. doi: 10.1016/0165-0327(93)90049-p. [DOI] [PubMed] [Google Scholar]
- Sbrana A, Bizzarri JV, Rucci P, Gonnelli C, Doria MR, Spagnolli S, et al. The spectrum of substance use in mood and anxiety disorders. Comprehensive Psychiatry. 2005;46(1):6–13. doi: 10.1016/j.comppsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Carey MP, Carey KB, Maisto SA. Smoking among psychiatric outpatients: relationship to substance use, diagnosis, and illness severity. Psychology of Addictive Behaviors. 2003;17(4):259–265. doi: 10.1037/0893-164X.17.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Adamson JJ, Henin A, Sgambati S, Gignac M, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug & Alcohol Dependence. 2008;95(3):188–198. doi: 10.1016/j.drugalcdep.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M. Integrating the assessment methods of researchers in routine clinical practice: The Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project. In: First M, editor. Standardized Evaluation in Clinical Practice. 22. 2003. pp. 29–74. [Google Scholar]
- Zimmerman M, Chelminski I, McDermut W. Major depressive disorder and axis I diagnostic comorbidity. Journal of Clinical Psychiatry. 2002;63(3):187–193. doi: 10.4088/jcp.v63n0303. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Posternak M. An illustration of how a self-report diagnostic screening scale could improve the internal validity of antidepressant efficacy trials. Journal of Affective Disorders. 2004;80(1):79–85. doi: 10.1016/S0165-0327(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI. Psychiatric diagnosis in clinical practice: Is comorbidity being missed? Comprehensive Psychiatry. 1999;40:182–191. doi: 10.1016/s0010-440x(99)90001-9. [DOI] [PubMed] [Google Scholar]


