Abstract
Rationale
Previous studies have documented the existence of signs and symptoms of the acute tobacco abstinence syndrome; however, less attention has been paid to quantifying the magnitude of these effects.
Objective
The present study quantified the relative magnitude of subjective, cognitive, and physiological manifestations of acute tobacco abstinence.
Method
Smokers (N = 203, ≥15 cig/day) attended two counterbalanced laboratory sessions, one following 12-hr of abstinence and the other following ad-lib smoking. At both sessions, they completed an extensive battery of self-report measures (withdrawal, affect, hunger, craving, subjective attentional bias towards smoking cues), physiological assessments (heart rate, blood pressure, brain EEG), and cognitive performance tasks (psychomotor processing, sustained attention, objective attentional bias).
Results
Abstinence effects were largest for craving, subjective attentional bias, negative affect, overall withdrawal severity, concentration difficulty, hunger, and heart rate. Effects were moderate for positive affect and EEG power. Effects were small, but reliable, for psychomotor speed, sustained attention, and somatic symptoms. Effects on performance-based indices of attentional bias towards smoking-related cues were small and reliable for some indices but not others. Effects were small and inconsistent for blood pressure and EEG frequency. Variation in internal consistency accounted for 33% of the variation in abstinence effect sizes across measures.
Conclusions
There was a wide range of effect sizes both across and within domains, indicating that the acute tobacco abstinence syndrome is not a monotonic phenomenon. These findings may be indicative of the relative magnitudes of signs and symptoms that the average smoker may exhibit during acute abstinence.
Keywords: Tobacco Abstinence, Smoking Deprivation, Nicotine Withdrawal, Smoking, Tobacco Dependence
1. Introduction
The tobacco abstinence syndrome is considered an important component of tobacco addiction (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Koob & Le Moal, 2001), comprising subjective, cognitive, and physiological changes that emerge upon the cessation of tobacco use. These changes cause distress and impairment, can potentially interfere with smoking cessation, and may underlie smoking behavior in smokers not wanting to quit (Hughes, 2006; Shiffman, West, & Gilbert, 2004). Accordingly, this syndrome has attracted considerable scientific and clinical interest.
Tobacco abstinence effects include DSM-IV nicotine withdrawal symptoms (APA, 1994), such as irritability, anxiety, restlessness, dysphoria, difficulty concentrating, increased appetite and/or weight gain, sleep disturbance, and decreased heart rate. Tobacco abstinence effects may also may include changes in other subjective states (e.g., cigarette craving, diminished positive affect; al’Absi, Amunrud, & Wittmers, 2002; Sayette, Martin, Wertz, Shiffman, & Perrott, 2001). Tobacco abstinence effects have also been assessed using objective measures, such as electroencephalographic (EEG) deactivation, cognitive performance decrements, and attentional bias towards smoking-related cues (Heishman, 1999; Pickworth, Herning, & Henningfield, 1989; Waters & Sayette, 2006). The origins of abstinence effects are multifaceted and may be caused by any or all of the following: (a) nicotine withdrawal (i.e., disruptions of psychobiological homeostasis caused by the removal of nicotine); (b) nicotine offset effects (i.e., dissipation of nicotine’s acute pharmacological effects); (c) psychological factors (e.g., expectancies about the effects of abstaining, loss of sensorimotor stimulation); and (d) unmasking effects (e.g., the re-emergence of preexisting dispositions that were suppressed by tobacco use).
Understanding the tobacco abstinence syndrome is important for several reasons. First, the abstinence syndrome may potentially play an important role in the cessation process (Piasecki, 2006). Treatments that attenuate abstinence effects are among the most effective smoking cessation interventions (USDHHS, 2008) and some studies have reported that the effects of efficacious treatments on smoking cessation outcomes are partially mediated by their influence on abstinence effects (e.g., Ferguson, Shiffman, & Gwaltney, 2006; Piper et al., 2008). Second, among individuals not attempting to quit, signs and symptoms that emerge following brief periods of abstinence (e.g., overnight, tobacco use restriction during work/social events) may maintain day-to-day smoking behavior (Chandra, Shiffman, Scharf, Dang, & Shadel, 2007). Finally, the tobacco abstinence syndrome causes a degree of distress and impairment that may comparable to levels experienced by patients seeking psychiatric treatment (Hughes, 2006). Thus, the abstinence syndrome is worthy of therapeutic intervention to improve the short-term quality of life among abstinent smokers, irrespective of its influence on smoking outcome. Because it may be a primary target of clinical intervention, understanding the pattern of signs and symptoms of this syndrome is of critical importance.
Numerous studies have documented that statistically significant abstinence effects can be observed for a variety of subjective, cognitive, and physiological measures (for reviews see, Hughes, 2007a, 2007b; Shiffman et al., 2004). Investigations have less frequently characterized the magnitude of abstinence effects. This is a notable gap in the literature as understanding the relative magnitudes of specific tobacco abstinence effects may be of use to clinicians and could guide future research into the intensity of abstinence effects. Given that research resources are often limited, data on which measures demonstrate the most robust abstinence effects could inform assessment selection strategies for future studies of tobacco abstinence effects. Research of the impact of candidate treatments or individual difference characteristics (e.g., personality, genetic variation) on the acute abstinence syndrome may be more likely to detect effects when using measures that typically exhibit the largest abstinence effects. In addition, identifying the features that demonstrate the greatest changes induced by abstinence may perhaps inform clinicians as to the typical symptoms that may require the most intensive intervention. It should be noted, however, that the generalization that symptoms with larger abstinence effects require more intensive intervention may not be applicable to certain features of questionable clinical relevance (e.g., abstinence-provoked reductions in heart rate are likely to be the result of nicotine offset effects not requiring treatment). Nonetheless, particular features within a common clinically-relevant domain that are most dramatically exacerbated during abstinence (e.g., anxiety vs. sadness) are likely to require more intensive treatment (to improve short-term quality of life or possibly attenuate relapse risk).
Additionally, extant studies have frequently examined abstinence effects after the first 24 hours of abstinence and over the next several weeks (e.g., Hughes & Hatsukami, 1986; McCarthy, Piasecki, Fiore, & Baker, 2006; Piasecki, Jorenby, Smith, Fiore, & Baker, 2003; Shiffman, Patten et al., 2006). Relatively few investigations have focused on the first day of abstinence, which is important because a significant number of individuals lapse on their planned quit date (Brown, Herman, Ramsey, & Stout, 1998), which could be a function of acute tobacco abstinence symptoms. In addition, acute (but not protracted) abstinence simulates experience in smokers not attempting to quit (e.g., symptoms experienced before the first cigarette of the day).
In the current study, we examined the relative magnitudes of acute smoking abstinence (≥ 12hrs) effects across an extensive battery of subjective, cognitive, and physiological measures. We used a smoking deprivation manipulation,1 which allowed us to capture the combined effect of pharmacological and non-pharmacological influences on tobacco abstinence. The relatively large sample (N = 203) provided adequate power to detect small effect sizes, and allowed us to generate relatively precise effect size estimates. Assessments were administered prospectively in both abstinent and non-abstinent states (order counterbalanced) to neutralize experimental confounds, such as order and practice effects and retrospective recall biases. The sample was comprised of individuals who were not attempting to quit smoking. This is important for understanding the processes that might maintain day-today smoking.
In two previous manuscripts, we examined individual difference factors that predict withdrawal effects (i.e., gender, temperament) in this sample (Leventhal, Waters, Boyd, et al., 2007a, 2007b). The current report is unique in that it examines effects in the entire sample to describe the relative intensity pattern of signs and symptoms of acute tobacco abstinence. Furthermore, this report describes abstinence effects on some measures not previously reported (e.g., visual dot probe task, EEG data at the electrode level) and scrutinizes internal consistency estimates of abstinence effects.
2. Method
2.1 Participants
Participants were 203 smokers from the Baltimore metropolitan area recruited via newspaper and radio advertisements. The sample was recruited to be balanced on gender (49.8% men, 50.2% women) and race (51.7% black, 48.3% white). On average, participants were 36.7 (SD = 10.1) years of age, smoked 22.2 cigarettes/day (SD = 6.61), scored 6.47 (SD = 1.70) on the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), and smoked for 19.7 years (SD = 10.3). For inclusion in the study, participants had to: be 18 years or older; be a current smoker of at least 15 cigarettes per day; smoke for at least 2 years; smoke a brand of cigarettes that delivers at least 11.0 mg tar and 0.7 mg nicotine as rated by the Federal Trade Commission method; and have a score 3 or higher on the FTND. Participants were excluded if they reported a recent history of certain diseases, including myocardial infarction, angina, heart failure, hypertension, stroke, and diabetes; if they were treated with nicotine replacement products in the past six months, or with antidepressants in the past year; if they used any smoking cessation treatments in past 6 months; or if their estimated IQ on the Shipley Institute on Living Scale was less than 78 (Shipley, 1940). Women who were pregnant or nursing were also excluded. The study was approved by the Institutional Review Board of the NIDA-Intramural Research Program.
A total of 858 participants completed the medical screening visit, 337 of whom met inclusion/exclusion criteria. Of these, 209 completed the orientation and two experimental sessions (i.e., abstinent and non-abstinent sessions); however, as noted below, 6 did not meet criteria for biochemical confirmation of smoking.
2.2 Procedure
Following a preliminary telephone screen, eligible participants were invited to attend an in-person medical screening session conducted at NIDA-Intramural Research Program [details on the screening procedure can be found in Leventhal et al. (2007a)]. Eligible participants attended a 90-minute orientation session followed by two experimental sessions (one while abstinent and one while non-abstinent), lasting 60-minutes each. The three sessions occurred on different days. Participants were instructed to smoke normally before the first (orientation) session. During the orientation session, participants practiced the cognitive performance tasks for approximately one hour, which included two ten-minute runs of the Rapid Information Processing Task. Also at the orientation session, participants completed two 5-min EEG recordings (one with eyes open and one with eyes closed) to allow them to get acclimated to the device. Participants also filled out questionnaires assessing demographics, smoking history, and tobacco dependence (including the FTND) at orientation. During both experimental sessions, participants completed a battery of subjective, physiological, and cognitive measures (detailed below). For the non-abstinent session, participants were instructed to smoke freely before the session, and to smoke a cigarette within 20 minutes of the beginning of the session. Participants reported that they had smoked their last cigarette on average 15.7 minutes (SD = 10.7) before the non-abstinent session. For the abstinent session, participants were instructed to refrain from smoking for at least 12 hours before the session. The order of completion of the abstinent and non-abstinent sessions was counterbalanced. The average number of days between the two experimental sessions was 2.88 (SD = 3.93). Experimental sessions were scheduled to occur in the afternoons, and we attempted to schedule both experimental sessions at the same time of day for each participant.
At the abstinent session, participants were considered non-abstinent if they reported having smoked on that day or had high breath carbon monoxide (CO) levels (> 11 ppm). If this occurred, the abstinent session was re-scheduled. At the non-abstinent session, participants with low CO levels (< 10 ppm) were excluded from analyses (n = 6), as there was considerable doubt whether they were 15+ cigarettes/day smokers, or that they had complied with the instructions to smoke normally before the non-abstinent session. The final sample (N = 203) had mean CO levels of 30.0 ppm at the non-abstinent session (SD = 12.1) and mean CO levels of 6.9 ppm at the abstinent session (SD = 2.5).
2.3 Measures
During the experimental sessions, participants completed the following measures. Details on the order and timing of assessments are shown in Table 1.
Table 1.
Procedure and Timeline for Experimental Sessions
| Procedure | Assessments | Time (mins) | Questionnaire Wording |
|---|---|---|---|
| Exhaled CO | CO | 2 | - |
| Heart Rate/Blood Pressure (T1) | HR, SBP/DBP | 1 | - |
| Questionnaires (T1) | PANAS, HQ, MNWS, WSWS, SBQ | 10 | “so far today” |
| TCQ, QSU | 4 | “right now” | |
| Cognitive Performance Tasks | ST, MT, DSST, RIPT | 16 | - |
| Questionnaires (T2) | PANAS, MNWS | 4 | “in the last few minutes” |
| QSU | 2 | “right now” | |
| Attentional Bias Tasksa | Smoking Stroop Task, SSQb | 6 | - |
| Visual Probe Task | 10 | - | |
| EEG | Electrode Placement | 10 | |
| Eyes Closed | 1 | - | |
| Eyes Open | 1 | - | |
| Heart Rate/Blood Pressure (T2) | HR, SBP/DBP | 1 | - |
Note. Assessments are listed in the order they were completed. HR = Heart Rate; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; PANAS = Positive and Negative Affect Scale; HQ = Hunger Questionnaire; MNWS = Minnesota Nicotine Withdrawal Scale; WSWS = Wisconsin Smoking Withdrawal Scale; SBQ = Subjective Bias Questionnaire; TCQ = Tobacco Craving Questionnaire-Short Form; QSU = Brief Questionnaire of Smoking Urges; ST = Two-Letter Search Task; MT = Serial Math Test; DSST = Digit Symbol Substitution Task; RIPT = Rapid Information Processing Task; SSQ = Subjective Stroop Questionnaire; EEG = Electroencephalogram. See text for further details.
Order of completion of Stroop and Picture Probe was counterbalanced across subjects.
Not reported here.
2.3.1 Subjective assessments
Subjective effects were assessed using the following measures administered via paper and pencil questionnaires. Total score and subscore values are reported as mean score per item to facilitate interpretation across scales.
2.3.1.1 The Minnesota Nicotine Withdrawal Scale
(MNWS; Hughes & Hatsukami, 1986) is a widely used measure of tobacco withdrawal. The 11-item MNWS variant used in this study assessed withdrawal symptoms on 6-point Likert scales.
2.3.1.2 The Wisconsin Smoking Withdrawal Scale
(WSWS; Welsch et al., 1999) is a multi-dimensional measure of tobacco withdrawal. The WSWS contains 23 items that load onto six subscales of tobacco withdrawal (i.e., anxiety, anger, hunger, concentration problems, craving, sadness) and an overall severity scale. (We excluded the items that assess sleep disturbance because participants would not have been abstinent for a long period before going to sleep).
2.3.1.3 The Brief Questionnaire of Smoking Urges
(QSU; Cox, Tiffany, & Christen, 2001) assesses desire for the positive effects of smoking and intention to smoke (Factor 1; 5 items), and desire for relief of negative affect and an urgent need to smoke (Factor 2; 5 items).
2.3.1.4 The Tobacco Craving Questionnaire-Short Form
(TCQ; Heishman, Singleton, & Pickworth, 2008) consists of 12 items that assess four dimensions of craving: (1) Anticipation of relief from withdrawal and negative mood by smoking (Emotionality); (2) Anticipation of positive outcomes from smoking (Expectancy); (3) Inability to control smoking (Compulsivity); and (4) Intention to smoke for positive outcomes (Purposefulness).
2.3.1.5 The Positive and Negative Affect Schedule
(PANAS; Watson, Clark, & Tellegen, 1988) assesses Positive Affect (PA; 10 items, e.g., enthusiastic, strong) and Negative Affect (NA; 10 items, e.g., distressed, upset). These scales have been shown to represent orthogonal dimensions of affect (Watson et al., 1988).
2.3.1.6
Hunger was measured using the methodology of Hill and Blundell (1982). Participants rated their feelings of hunger, fullness, and desire to eat on 8 separate 10-point Likert scales.
2.3.1.7 The Subjective Attentional Bias Questionnaire
(SBQ; Leventhal et al., 2007a) is an 8-item questionnaire that assesses the extent to which participants feel or notice that their attention is captured by cigarettes and smoking cues (e.g., “So far today, how often have you found yourself staring at cigarettes and cigarette smoke?”). Each item is rated on a 5-point Likert scale.
2.3.2 Cognitive assessments: general information processing
Four cognitive performance tasks were used to assess various domains of general information processing ability (e.g., attention, concentration, psychomotor speed).
2.3.2.1
The Two-Letter Search Task (ST) and Serial Math Test (MT), drawn from the Walter Reed Performance Assessment Battery (Thorne, Genser, Sing, & Hegge, 1985). The ST assesses visual scanning, recognition, and attentional abilities. In the ST, two target letters appear at the top of a computer screen, and a string of 20 letters appears below. The 20-letter series can contain neither, one, or both of the target letters. Participants respond by pressing one key if the both target letters were in the 20-letter string or a second key if one or neither target were in the string. The task ends after 20 trials, or after 120 seconds, whichever comes sooner. The MT assesses mathematical reasoning. In the MT, two random digits are rapidly presented on the screen for 250 milliseconds, followed by a “+” or a “−” sign, and a “?” prompt. Participants are required to perform the mathematical operation and to enter the final digit of the response into the keyboard as quickly as possible by pressing a numerical key. The task ends after 50 trials, or after 240 seconds, whichever comes sooner. The mean reaction time (RT) on correct responses, and the percentage error rate, were obtained for the ST and MT.
2.3.2.2
The Digit Symbol Substitution Task (DSST) was used to assess psychomotor performance. A randomly-generated digit appears on a computer monitor, and the participant is required to reproduce the symbol as quickly as possible by pressing the corresponding keys on the number-pad of the computer keyboard. The task lasts for 90 seconds. The total number of correct symbols reproduced within the 90-second period, and the percentage of incorrect responses (error rate), were determined.
2.3.2.3
The Rapid Information Processing Task (RIPT) was used to assess sustained attention (Foulds et al., 1996). A series of single digits is presented on the computer screen at a rate of 100 digits per minute, for 10 minutes. Targets are defined as three consecutive even or odd digits. There are 8 targets per minute, making a total of 80 targets in 10 minutes, with 5–30 digits between each target. Participants are required to press the spacebar as quickly as possible after detecting a target. There is a response window of 1500 milliseconds. The percentage of targets correctly detected (hit rate), mean RT on targets (in milliseconds), and number of false positives per minute (i.e., depressing the spacebar when a target is not present) are computed.
2.3.3 Cognitive assessments: Attentional bias
Attentional bias reflects the extent to which drug-related stimuli capture attention. Numerous studies have reported that drug users exhibit attentional bias to drug-related stimuli whereas non-users do not (Cox, Fadardi, & Pothos, 2006). Two tasks were used to assess attentional bias towards smoking-related stimuli. Order of completion for the two tasks at both sessions was counterbalanced across participants.
2.3.3.1
The Smoking Stroop Task assesses the extent to which smoking cues automatically capture and hold participants’ attention, thereby interfering with their ability to execute other actions. We used the same task and methods as Waters et al. (2003). Participants are told that words written in different colors will be presented on a computer monitor, one after the other, and their task is to indicate as quickly and as accurately as possible the color the word was written in, by pressing one of three colored buttons on a keyboard. They are instructed to ignore the meaning of the word itself and just to respond to the color. Participants responded to a block of neutral words (33 trials) followed by a block of smoking words (33 trials). The smoking Stroop effect (the degree to which smoking words capture, hold, and interfere with attention) is the difference in responses on the smoking words and the neutral words. Three attentional bias outcomes were computed: (1) the standard RT Stroop effect (i.e., mean RT on 33 smoking trials - 33 neutral trials); (2) the acute RT Stroop effect (i.e., mean RT on first 11 smoking trials - 33 neutral trials); and (3) the error Stroop effect (i.e., number of errors on 33 smoking trials - 33 neutral trials).
2.3.3.2
The Visual Probe Task assesses the degree to which smoking stimuli recruit visuospatial attention. We used the same task and methods as Bradley et al. (2003). On each trial, a picture pair consisting of a smoking-related scene (e.g., person holding a cigarette to mouth) and a matched control scene is displayed on the screen. One picture is positioned on the left and the other is positioned on the right. The pictures are then removed and a visual probe (a dot) is presented at a location formerly occupied by one of the pictures. Participants are told to press one of two keys as quickly and as accurately as possible to indicate the location of the dot (left or right). On half the trials the probe replaces the smoking picture, and on the other half it replaces the control picture. Participants responded to 80 experimental smoking-control picture pairs. We computed “bias scores” (difference in performance on trials where the probe replaced the smoking picture vs. trials where the probe replaced the control picture). This indicates the degree of visuospatial attention directed toward the smoking picture. Bias scores were computed for RTs and errors.
Participants were randomly assigned to complete one of two versions of this task. In one version the picture pairs were presented for 200ms on each trial (n = 100) and in the other the picture pairs were presented for 2000ms (n = 91). The former task is thought to index the orienting of attention, whereas the latter is thought to index the maintenance of attention (Bradley, Field, Mogg, & De Houwer, 2004). Participants completed the same version at both experimental sessions.
2.3.4 Physiological assessments
Heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured using an electronic Datascope machine.
Brain Electroencephalogram (EEG) recordings were collected from Cz, Fz, and Pz electrodes (monopolar, linked ear reference) using an automated EEG collection and analysis system, the Pathfinder (Biologic Instruments, Chicago, IL) (described in Pickworth, O’Hare, Fant, & Moolchan, 2003). Participants were monitored for one minute with their eyes closed, and for one minute with their eyes open. Two-second epochs were continuously acquired from each of the EEG electrodes. The EEG was digitized at 128 Hz, and samples with artifacts were automatically rejected. The digitized EEG was converted to the frequency domain using a Fast Fourier Transform. For each epoch, the computer printed the power (μV2). EEG frequency was computed as the frequency (Hz) at which 80% of the power of the band had accumulated (resolution, 0.5 Hz) in each of the usual clinical frequency bands: delta, 0.5–3.5 Hz; theta, 4.5–7.5 Hz; alpha, 8.5–12.5 HZ; beta 1, 14.5–23.5 Hz, and beta 2, 25–31.5 Hz. Following previous studies (Pickworth et al., 2003), a percent change score [(Abstinent – Non-abstinent)/Non-abstinent] was used to index abstinence-induced changes for power values in order to control for between-person variability in baseline EEG power.
2.3.5 Re-administration of assessments
As noted in Table 1, HR, SBP, DBP, and some of the questionnaire assessments (MNWS, PANAS, QSU) were assessed at two time points. With the exception of the TCQ and QSU (which can only be used to assess craving “right now”), questionnaires completed at T1 required participants to report on experiences “so far today” in order to capture the totality of experience during the course of the day up to that point. Questionnaires completed at T2 required participants to report on experiences “in the last few minutes” in order to assess experience following the cognitive assessments.
2.4 Data Analysis
An abstinence-induced change score (score while abstinent - score while non-abstinent)2 was computed to index the abstinence effect for each T1 and T2 measure.3 Cohen’s d (M divided by SD) was also calculated for each abstinence effect. Cohen’s (1977) guidelines (small d = .20, medium d = .50, large d = .80) were used to interpret effect magnitude. Given N = 203, the error of each effect size estimate was small (standard error = 0.07; 95% Confidence Interval: d ± 0.14), indicating that two measures with standardized abstinence effects that are at least 0.15 Cohen’s d units different from each other are significantly different in magnitude. For descriptive purposes, a one-sample t-test examined whether each abstinence effect was significantly different from zero. Significance was set at .05 and all tests were 2-tailed.
We also estimated the internal consistency of the abstinence-induced change scores. For subjective multi-item measures, Cronbach’s αs were calculated. For cognitive and EEG measures, split-half reliability coefficients were calculated by examining correlations between odd and even trials or epochs (Waters et al., 2003).
To isolate the source of variation in the magnitudes of abstinence effects across measures, we conducted a regression analysis in which each measure constituted a single observation, effect size4 served as the dependent variable, and estimated internal consistency and was the predictor variable. Proportion of variance accounted for is reported.
3 Results
Summary statistics and estimates of internal consistency are reported in Tables 2–5.
Table 2.
Effects of Acute Tobacco Abstinence on Subjective Measures (Time 1)
| Variable (range) | Non-Abstinent |
Abstinent |
Abstinence-Induced Changeb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | αa | M | SD | αa | M | SD | αa | d | |
| Withdrawal | ||||||||||
| MNWS (0 – 5) | ||||||||||
| Total | 0.74 | 0.64 | .83 | 1.85 | 0.91 | .82 | 1.12 | 0.94 | .83 | 1.18† |
| Craving | 2.26 | 1.28 | - | 4.06 | 1.12 | - | 1.80 | 1.50 | - | 1.20† |
| Irritable/angry | 0.54 | 1.01 | - | 2.33 | 1.59 | - | 1.79 | 1.69 | - | 1.06† |
| Anxious/tense | 0.76 | 1.10 | - | 2.41 | 1.56 | - | 1.65 | 1.68 | - | 0.98† |
| Concentration | 0.59 | 1.00 | - | 1.71 | 1.57 | - | 1.12 | 1.63 | - | 0.69† |
| Restlessness | 0.73 | 1.09 | - | 1.94 | 1.62 | - | 1.20 | 1.76 | - | 0.68† |
| Impatient | 0.79 | 1.17 | - | 2.34 | 1.66 | - | 1.55 | 1.77 | - | 0.88† |
| Hunger | 0.92 | 1.31 | - | 2.08 | 1.67 | - | 1.16 | 1.79 | - | 0.65† |
| Autonomicc | 0.13 | 0.61 | - | 0.41 | 0.94 | - | 0.28 | 1.01 | - | 0.27† |
| Eating | 0.57 | 1.05 | - | 1.71 | 1.70 | - | 1.14 | 1.63 | - | 0.70† |
| Drowsiness | 0.45 | 0.97 | - | 0.71 | 1.23 | - | 0.26 | 1.37 | - | 0.19** |
| Headaches | 0.19 | 0.66 | - | 0.53 | 1.10 | - | 0.34 | 1.04 | - | 0.33† |
| WSWS (0 – 4) | ||||||||||
| Total | 1.27 | 0.63 | .90 | 2.11 | 0.69 | .90 | 0.84 | 0.74 | .90 | 1.14† |
| Anger | 0.89 | 1.03 | .90 | 1.90 | 1.31 | .91 | 1.00 | 1.38 | .88 | 0.73† |
| Anxiety | 1.20 | 0.87 | .75 | 2.07 | 0.87 | .65 | 0.87 | 1.00 | .70 | 0.87† |
| Concentration | 0.96 | 0.81 | .76 | 1.72 | 1.03 | .81 | 0.76 | 1.08 | .78 | 0.71† |
| Craving | 1.70 | 0.94 | .83 | 3.16 | 0.88 | .88 | 1.46 | 1.09 | .84 | 1.34† |
| Hunger | 1.53 | 0.88 | .78 | 2.12 | 1.05 | .85 | 0.60 | 1.07 | .78 | 0.56† |
| Sadness | 1.10 | 0.77 | .70 | 1.53 | 0.74 | .56 | 0.43 | 0.81 | .58 | 0.53† |
| Craving | ||||||||||
| QSU (0 – 5) | ||||||||||
| Total | 1.61 | 1.17 | .94 | 3.40 | 1.03 | .88 | 1.79 | 1.16 | .91 | 1.54† |
| Factor 1 | 2.23 | 1.42 | .93 | 4.34 | 0.91 | .90 | 2.12 | 1.42 | .92 | 1.49† |
| Factor 2 | 0.99 | 1.06 | .90 | 2.45 | 1.40 | .82 | 1.47 | 1.20 | .83 | 1.22† |
| TCQ (1 – 7) | ||||||||||
| Total | 3.19 | 1.34 | .92 | 5.02 | 1.18 | .86 | 1.84 | 1.38 | .87 | 1.33† |
| Emotionality | 2.54 | 1.60 | .90 | 4.42 | 1.83 | .83 | 1.89 | 1.84 | .78 | 1.03† |
| Expectancy | 4.05 | 1.68 | .88 | 6.29 | 1.11 | .81 | 2.25 | 1.78 | .85 | 1.26† |
| Compulsivity | 2.42 | 1.51 | .77 | 3.91 | 1.82 | .77 | 1.50 | 1.69 | .69 | 0.89† |
| Purposefulness | 3.74 | 1.52 | .69 | 5.44 | 1.22 | .54 | 1.71 | 1.66 | .57 | 1.03† |
| Attentional Bias Towards Smoking | ||||||||||
| SBQ (0 – 4) | 1.26 | 0.74 | .89 | 2.47 | 0.98 | .92 | 1.21 | 0.99 | .87 | 1.22† |
| Affect | ||||||||||
| PANAS (1 – 5) | ||||||||||
| PA | 3.11 | 0.92 | .93 | 2.89 | 0.90 | .91 | −0.22 | 0.67 | .79 | −0.34† |
| NA | 1.23 | 0.36 | .84 | 1.76 | 0.59 | .80 | 0.52 | 0.54 | .73 | 0.97† |
| Hunger | ||||||||||
| HQ (1 – 10) | 2.95 | 1.64 | .86 | 4.00 | 1.99 | .87 | 1.05 | 1.98 | .82 | 0.53† |
Note. Mean scores per item displayed. Only Time 1 assessments are shown. Due to missing data, sample sizes vary across analyses (Ns = 186 – 203).
Cronbach’s alpha internal consistency estimate.
Abstinence-Induced Change = Abstinent – Non-Abstinent.
Assessed as any one of the following: tremor, heart racing, sweating or dizzy stomach or bowel problems. MNWS = Minnesota Nicotine Withdrawal Scale (scale: 0–5); WSWS = Wisconsin Smoking Withdrawal Scale (scale: 0–4); QSU = Brief Questionnaire of Smoking Urges (scale: 0–5); TCQ = Tobacco Craving Questionnaire-Short Form (scale: 1–7); SBQ = Subjective Bias Questionnaire (scale: 0 – 4); SSQ = Subjective Stroop Questionnaire (scale: −4 – 4); PANAS = Positive and Negative Affect Scale (scale: 1–5); HQ = Hunger Questionnaire (scale: 1–10); d = Cohen’s d effect size statistic.
p < .05;
p < .01;
p < .0001
Table 5.
Effects of Acute Tobacco Abstinence on Physiological Measures
| Variable | Non-Abstinent |
Abstinent |
Abstinence-Induced Changec |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | ra | M | SD | ra | M | SD | ra | d | |
| Cardiovascular Response | ||||||||||
| T1-HR (bpm) | 80.04 | 11.37 | 70.50 | 11.31 | −9.55 | 12.07 | −0.79† | |||
| T1-SBP (mmHg) | 123.69 | 13.39 | 123.36 | 14.19 | −0.32 | 12.00 | −0.03 | |||
| T1-DBP (mmHg) | 75.05 | 9.61 | 73.76 | 9.84 | −1.29 | 8.87 | −0.15* | |||
| T2-HR (bpm) | 70.78 | 10.23 | 64.42 | 9.48 | −6.36 | 8.77 | −0.72† | |||
| T2-SBP (mmHg) | 121.64 | 14.07 | 119.90 | 14.91 | −1.74 | 11.86 | −0.15* | |||
| T2-DBP (mmHg) | 74.32 | 9.97 | 73.08 | 9.72 | −1.24 | 8.51 | −0.15 | |||
| EEG eyes closed (μV2) | ||||||||||
| Δ power-Fz | 138.26 | 130.41 | .87 | 180.39 | 146.67 | .76 | 0.72b | 1.62 | .67 | 0.44† |
| Δ power-Cz | 96.45 | 66.56 | .89 | 120.00 | 76.10 | .87 | 0.46b | 1.08 | .81 | 0.42† |
| Δ power-Pz | 77.20 | 60.53 | .89 | 92.60 | 65.88 | .87 | 0.44b | 1.13 | .84 | 0.39† |
| θ power-Fz | 73.22 | 65.28 | .92 | 93.64 | 78.89 | .93 | 0.41b | 0.67 | .66 | 0.60† |
| θ power-Cz | 72.52 | 63.08 | .94 | 86.45 | 68.41 | .91 | 0.37b | 0.79 | .71 | 0.47† |
| θ power-Pz | 59.45 | 61.02 | .94 | 74.67 | 73.89 | .94 | 0.39b | 0.76 | .71 | 0.51† |
| α power-Fz | 147.65 | 132.30 | .96 | 147.28 | 130.72 | .96 | 0.14b | 0.67 | .81 | 0.21** |
| α power-Cz | 166.41 | 139.30 | .96 | 158.57 | 129.74 | .96 | 0.11b | 0.61 | .75 | 0.19* |
| α power-Pz | 216.77 | 200.97 | .96 | 201.48 | 181.77 | .96 | 0.15b | 1.00 | .74 | 0.15* |
| β1 power-Fz | 40.61 | 26.41 | .96 | 46.56 | 31.88 | .96 | 0.20b | 0.54 | .85 | 0.38† |
| β1 power-Cz | 43.67 | 25.82 | .95 | 49.14 | 30.54 | .96 | 0.19b | 0.52 | .83 | 0.36† |
| β1 power-Pz | 42.35 | 28.98 | .96 | 46.30 | 32.50 | .97 | 0.15b | 0.52 | .85 | 0.29† |
| β2 power-Fz | 7.65 | 5.84 | .93 | 8.79 | 7.22 | .86 | 0.37b | 1.19 | .79 | 0.31† |
| β2 power-Cz | 8.43 | 6.11 | .93 | 8.79 | 5.87 | .92 | 0.27b | 1.06 | .86 | 0.26*** |
| β2 power-Pz | 5.94 | 4.32 | .95 | 6.61 | 4.70 | .94 | 0.33b | 1.30 | .93 | 0.25*** |
| Δ frequency-Fz | 2.64 | 0.26 | .60 | 2.61 | 0.30 | .64 | −0.03 | 0.33 | .50 | −0.09 |
| Δ frequency-Cz | 2.70 | 0.25 | .57 | 2.73 | 0.26 | .59 | 0.03 | 0.33 | .41 | 0.09 |
| Δ frequency-Pz | 2.71 | 0.24 | .57 | 2.73 | 0.28 | .65 | 0.02 | 0.34 | .59 | 0.05 |
| θ frequency-Fz | 6.93 | 0.33 | .78 | 6.94 | 0.34 | .78 | 0.01 | 0.30 | .51 | 0.02 |
| θ frequency-Cz | 6.95 | 0.30 | .72 | 6.97 | 0.32 | .74 | 0.01 | 0.30 | .45 | 0.04 |
| θ frequency-Pz | 7.00 | 0.29 | .74 | 7.03 | 0.32 | .73 | 0.03 | 0.28 | .43 | 0.10 |
| α frequency-Fz | 10.58 | 0.67 | .92 | 10.52 | 0.68 | .92 | −0.06 | 0.39 | .54 | −0.17* |
| α frequency-Cz | 10.66 | 0.67 | .91 | 10.58 | 0.69 | .92 | −0.08 | 0.49 | .68 | −0.17* |
| α frequency-Pz | 10.70 | 0.67 | .93 | 10.58 | 0.70 | .93 | −0.11 | 0.38 | .52 | −0.30† |
| β1 frequency-Fz | 19.97 | 0.90 | .86 | 19.89 | 0.87 | .86 | −0.08 | 0.73 | .56 | −0.11 |
| β1 frequency-Cz | 20.01 | 0.82 | .84 | 19.87 | 0.88 | .85 | −0.14 | 0.76 | .58 | −0.18* |
| β1 frequency-Pz | 19.86 | 0.85 | .86 | 19.69 | 0.90 | .87 | −0.17 | 0.70 | .59 | −0.25*** |
| β2 frequency-Fz | 29.13 | 0.54 | .78 | 29.27 | 0.52 | .64 | 0.14 | 0.55 | .48 | 0.25*** |
| β2 frequency-Cz | 29.15 | 0.57 | .76 | 29.20 | 0.50 | .69 | 0.05 | 0.58 | .54 | 0.09 |
| β2 frequency-Pz | 29.15 | 0.48 | .65 | 29.21 | 0.45 | .67 | 0.07 | 0.50 | .43 | 0.13 |
| EEG eyes open (μV2) | ||||||||||
| Δ power-Fz | 240.39 | 208.82 | .84 | 256.48 | 227.09 | .82 | 0.52b | 1.48 | .50 | 0.35† |
| Δ power-Cz | 104.63 | 62.15 | .79 | 117.23 | 68.89 | .84 | 0.27b | 0.70 | .61 | 0.38† |
| Δ power-Pz | 71.03 | 46.57 | .71 | 80.70 | 54.31 | .91 | 0.27b | 0.70 | .60 | 0.39† |
| θ power-Fz | 71.16 | 54.00 | .91 | 82.06 | 64.90 | .88 | 0.34b | 0.89 | .74 | 0.38† |
| θ power-Cz | 53.58 | 42.50 | .96 | 59.91 | 44.46 | .93 | 0.24b | 0.53 | .64 | 0.46† |
| θ power-Pz | 38.95 | 38.87 | .95 | 43.88 | 41.18 | .95 | 0.27b | 0.60 | .68 | 0.45† |
| α power-Fz | 61.42 | 61.23 | .94 | 68.85 | 61.86 | .93 | 0.30b | 0.65 | .64 | 0.45† |
| α power-Cz | 67.34 | 65.46 | .94 | 73.89 | 65.64 | .95 | 0.32b | 0.76 | .71 | 0.42† |
| α power-Pz | 86.53 | 110.61 | .94 | 89.52 | 102.19 | .94 | 0.32b | 0.79 | .61 | 0.41† |
| β1 power-Fz | 33.26 | 22.12 | .96 | 38.16 | 24.87 | .95 | 0.24b | 0.50 | .78 | 0.47† |
| β1 power-Cz | 34.53 | 22.49 | .96 | 38.40 | 24.06 | .95 | 0.19b | 0.47 | .75 | 0.41† |
| β1 power-Pz | 30.05 | 20.42 | .96 | 33.83 | 23.19 | .96 | 0.19b | 0.47 | .80 | 0.41† |
| β2 power-Fz | 9.16 | 8.22 | .95 | 9.88 | 8.25 | .92 | 0.31b | 0.84 | .79 | 0.37† |
| β2 power-Cz | 8.76 | 7.05 | .94 | 9.44 | 7.53 | .93 | 0.30b | 0.85 | .82 | 0.35† |
| β2 power-Pz | 6.25 | 5.72 | .95 | 6.81 | 6.09 | .94 | 0.32b | 0.86 | .83 | 0.37† |
| Δ frequency-Fz | 2.59 | 0.24 | .46 | 2.64 | 0.23 | .33 | 0.05 | 0.29 | .24 | 0.18* |
| Δ frequency-Cz | 2.68 | 0.20 | .35 | 2.70 | 0.23 | .58 | 0.02 | 0.28 | .39 | 0.09 |
| Δ frequency-Pz | 2.68 | 0.22 | .52 | 2.71 | 0.20 | .46 | 0.02 | 0.28 | .47 | 0.09 |
| θ frequency-Fz | 6.71 | 0.36 | .78 | 6.74 | 0.35 | .76 | 0.03 | 0.32 | .47 | 0.09 |
| θ frequency-Cz | 6.78 | 0.33 | .78 | 6.80 | 0.32 | .73 | 0.02 | 0.30 | .40 | 0.05 |
| θ frequency-Pz | 6.85 | 0.32 | .77 | 6.87 | 0.31 | .70 | 0.02 | 0.29 | .36 | 0.06 |
| α frequency-Fz | 11.03 | 0.55 | .85 | 10.92 | 0.54 | .84 | −0.12 | 0.48 | .59 | −0.24*** |
| α frequency-Cz | 11.08 | 0.56 | .85 | 10.97 | 0.53 | .85 | −0.12 | 0.46 | .59 | −0.25*** |
| α frequency-Pz | 11.09 | 0.59 | .88 | 10.97 | 0.58 | .87 | −0.12 | 0.42 | .36 | −0.29† |
| β1 frequency-Fz | 20.32 | 0.87 | .84 | 20.14 | 0.88 | .84 | −0.18 | 0.75 | .58 | −0.24*** |
| β1 frequency-Cz | 20.29 | 0.80 | .85 | 20.11 | 0.78 | .82 | −0.17 | 0.67 | .57 | −0.25*** |
| β1 frequency-Pz | 20.09 | 0.84 | .87 | 19.86 | 0.83 | .85 | −0.22 | 0.74 | .64 | −0.30† |
| β2 frequency-Fz | 29.27 | 0.52 | .71 | 29.35 | 0.51 | .75 | 0.08 | 0.53 | .45 | 0.16* |
| β2 frequency-Cz | 29.26 | 0.53 | .76 | 29.37 | 0.52 | .75 | 0.10 | 0.57 | .59 | 0.18* |
| β2 frequency-Pz | 29.28 | 0.44 | .63 | 29.34 | 0.47 | .71 | 0.06 | 0.51 | .48 | 0.12 |
Note. Due to missing data, sample sizes vary across analyses (Ns = 173 – 202).
Split-half (even vs. odd epochs) coefficient of internal consistency
Expressed as % Change [(Abstinent – Non-Abstinent)/Non-Abstinent].
Abstinence-Induced Change = Abstinent – Non-Abstinent. HR = Heart Rate; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; EEG = Electroencephalogram; T1 = Time 1; T2 = Time 2; Fz = Frontal; Cz = Central; PZ = Parietal; d = Cohen’s d effect size statistic.
p < .05;
p < .01;
p < .001;
p < .001
3.1 Subjective Measures
3.1.1 T1 assessment
As shown in Table 2, significant abstinence effects were observed on the subjective measures (ps < .01), with most being in the medium to large range. Large abstinence effects were observed on total composite scores of withdrawal (ds = 1.14 – 1.18). For specific symptoms, abstinence effects were largest on measures of craving (ds = .89 – 1.54) and subjective attentional bias towards smoking (SBQ: d = 1.22). Large abstinence effects were observed on measures tapping negative affect/distress (ds =.68 – 1.06), with the exception of the WSWS-Sadness scale (d = .53). Moderate to large abstinence effects were found for concentration problems (ds = .69 – .71) and hunger/eating (ds = .53 – .70). Abstinence effects on positive affect (PANAS-PA) and other somatic symptoms (MNWS-Autonomic, Drowsiness, Headaches) were small (|ds| = .19 – .34).
As shown in Table 2, the Cronbach αs for abstinence-induced change values were mostly in the range of .70 – .90.
3.1.2 T2 assessment
As shown in Table 3, the pattern of abstinence effects and estimated internal reliability were generally consistent with those observed at T1. However, the observed abstinence effects at T2 were typically smaller than those reported at T1.
Table 3.
Effects of Acute Tobacco Abstinence on Subjective Measures (Time 2)
| Variable (range) | Non-Abstinent |
Abstinent |
Abstinence-Induced Changec |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | αa | M | SD | αa | M | SD | αa | d | |
| Withdrawal | ||||||||||
| MNWS (0 – 5)b | ||||||||||
| Total | 0.91 | 0.81 | .84 | 1.84 | 1.02 | .85 | 0.92 | 0.93 | .81 | 0.99† |
| Craving | 2.00 | 1.68 | - | 3.85 | 1.46 | - | 1.85 | 1.63 | - | 1.13† |
| Irritable/angry | 0.79 | 1.24 | - | 2.21 | 1.77 | - | 1.41 | 1.71 | - | 0.83† |
| Anxious/tense | 1.15 | 1.26 | - | 2.37 | 1.76 | - | 1.21 | 1.71 | - | 0.71† |
| Concentration | 1.44 | 1.45 | - | 2.48 | 1.69 | - | 1.03 | 1.74 | - | 0.59† |
| Restlessness | 0.96 | 1.29 | - | 1.99 | 1.73 | - | 1.02 | 1.66 | - | 0.61† |
| Impatient | 1.14 | 1.46 | - | 2.40 | 1.74 | - | 1.25 | 1.63 | - | 0.76† |
| Hunger | 0.82 | 1.33 | - | 1.61 | 1.67 | - | 0.79 | 1.61 | - | 0.49† |
| Autonomicd | 0.14 | 0.55 | - | 0.39 | 1.04 | - | 0.25 | 1.08 | - | 0.23** |
| Drowsiness | 0.39 | 0.93 | - | 0.60 | 1.14 | - | 0.21 | 1.26 | - | 0.17* |
| Headaches | 0.24 | 0.82 | - | 0.48 | 1.18 | - | 0.24 | 1.10 | - | 0.22** |
| Craving | ||||||||||
| QSU (0 – 5) | ||||||||||
| Total | 1.94 | 1.32 | .95 | 3.38 | 1.11 | .89 | 1.44 | 1.17 | .91 | 1.23† |
| Factor 1 | 2.66 | 1.53 | .94 | 4.30 | 0.95 | .90 | 1.64 | 1.39 | .92 | 1.18† |
| Factor 2 | 1.22 | 1.31 | .93 | 2.47 | 1.50 | .85 | 1.25 | 1.26 | .85 | 0.99† |
| Affect | ||||||||||
| PANAS (1 – 5) | ||||||||||
| PA | 3.03 | 0.93 | .92 | 2.69 | 0.95 | .92 | −0.34 | 0.73 | .84 | −0.46† |
| NA | 1.44 | 0.57 | .89 | 1.75 | 0.66 | .83 | 0.31 | 0.61 | .77 | 0.50† |
Note. Mean scores per item displayed. Only Time 2 assessments are shown. Due to missing data, sample sizes vary across analyses (Ns = 198 – 203).
Cronbach’s alpha internal consistency estimate
Eating item was omitted because there was no opportunity to eat in between T1 and T2.
Abstinence-Induced Change = Abstinent – Non-Abstinent.
Assessed as any one of the following: tremor, heart racing, sweating or dizzy stomach or bowel problems. MNWS = Minnesota Nicotine Withdrawal Scale (scale: 0–5); PANAS = Positive and Negative Affect Scale (scale: 1–5); HQ = Hunger Questionnaire (scale: 1–10); d = Cohen’s d effect size statistic.
p < .05;
p < .01;
p < .0001
3.2 Cognitive Measures
As shown in Table 4, abstinence effects on cognitive performance tasks were generally of small magnitude. Tasks measuring general information processing exhibited small abstinence effects (ds ≤ .25), with the exception of the abstinence-induced response slowing on the ST, which was medium in magnitude (d = .45). On the RIPT, there was a small but significant effect of abstinence on both hit rate (d = −0.23) and reaction times (d = 0.25). The abstinence effect was small on tasks measuring attentional bias toward smoking (ds ≤ .20), with only the Acute Stroop effect and the Visual Probe Task error Bias score demonstrating statistically significant abstinence effects (see Table 4).
Table 4.
Effects of Acute Tobacco Abstinence on Cognitive Measures
| Variable | Non-Abstinent |
Abstinent |
Abstinence-Induced Changed |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | rc | M | SD | rc | M | SD | rc | d | |
| General Information Processing | ||||||||||
| RIPT | ||||||||||
| Hit rate (%) | 0.51 | 0.17 | .92 | 0.49 | 0.16 | .86 | −0.03 | 0.11 | .56 | −0.23** |
| RT (ms) | 538.48 | 104.15 | .81 | 562.80 | 110.69 | .81 | 24.32 | 95.37 | .59 | 0.25*** |
| False positives | 4.10 | 5.74 | .98 | 4.32 | 5.69 | .97 | 0.21 | 2.80 | .82 | 0.08 |
| ST | ||||||||||
| RT (s) | 5.17 | 1.68 | 5.68 | 1.90 | 0.51 | 1.12 | 0.45† | |||
| Errors (%) | 6.51 | 10.11 | 5.07 | 7.35 | −1.43 | 8.70 | −0.16* | |||
| MT | ||||||||||
| RT (s) | 1.94 | 0.83 | 2.05 | 0.84 | 0.11 | 0.61 | 0.17* | |||
| Errors (%) | 20.62 | 16.19 | 21.88 | 16.49 | 1.26 | 9.60 | 0.13 | |||
| DSST | ||||||||||
| Total correct | 23.60 | 11.15 | 22.49 | 11.21 | −1.11 | 8.78 | −0.13 | |||
| Errors (%) | 15.42 | 21.60 | 18.18 | 24.48 | 2.76 | 26.55 | 0.10 | |||
| Attentional Bias Towards Smoking | ||||||||||
| Smoking Stroop Task | ||||||||||
| Standard Stroop effect (RT) | 45.03 | 98.36 | .62 | 52.35 | 137.40 | .76 | 7.32 | 157.73 | .73 | 0.05 |
| Acute Stroop effect (RT) | 69.26 | 136.82 | .58 | 98.81 | 167.98 | .67 | 29.55 | 209.19 | .67 | 0.14* |
| Stroop effect (errors) | 0.00 | 3.05 | .01 | 0.53 | 3.68 | .29 | 0.54 | 4.83 | .15 | 0.11 |
| Visual Probe Task (200ms)a | ||||||||||
| Bias score (RT) | 5.21 | 23.16 | −.26 | 11.72 | 31.19 | .10 | 6.51 | 37.34 | −.09 | 0.17 |
| Bias Score (errors) | 0.25 | 1.71 | −.32 | 0.94 | 2.98 | .59 | 0.69 | 3.41 | .25 | 0.20* |
| Visual Probe Task (2000ms)b | ||||||||||
| Bias score (RT) | −1.15 | 35.05 | −.17 | 0.84 | 53.11 | −.02 | 1.99 | 57.44 | −.24 | 0.03 |
| Bias Score (errors) | −0.16 | 1.33 | .28 | 0.03 | 3.93 | .83 | 0.19 | 4.01 | .62 | 0.05 |
Note. Due to missing data, sample sizes vary across analyses (Ns = 195 – 202).
N= 100 because participants were randomized to version of visual probe task
N= 91 because participants were randomized to version of visual probe task
Split-half reliability coefficient of internal consistency [odd vs. even minutes (RIPT) or trials (Stroop, Visual Probe task)]. Internal reliability estimates could not be calculated for ST, MT, DSST as only the summary data were available.
Abstinence-Induced Change = Abstinent – Non-Abstinent. RIPT = Rapid Information Processing Task; ST = Two-Letter Search Task; MT = Serial Math Task; DSST = Digit Symbol Substitution Task; RT = Reaction Time; d = Cohen’s d effect size statistic.
p < .05;
p < .01;
p < .001;
p < .001
The cognitive measures exhibited moderate to good internal consistency for some measures, but poor internal consistency for others (see Table 4).
3.3 Physiological Measures
As shown in Table 5, the largest abstinence effect for a physiological measure was observed for HR (ds of −.79 and −.72). In contrast, the abstinence effect for blood pressure was small (ds of −.15 to −.03). Abstinence effects on EEG measures ranged from negligible to medium-sized (|d|s = .02 – .60). Abstinence effects were larger on measures of EEG power than frequency. Of the EEG frequency measures, abstinence effects were generally larger on the higher frequency bands (α, β1, β2) than the lower bands (Δ, θ). Overall, the pattern of abstinence effects on EEG was not markedly different across modes of assessment (i.e., eyes open vs. eyes closed).
Estimated internal consistency for EEG measures was typically greater for power (vs. frequency) readings and greater for higher (vs. lower) frequency bands. Estimated internal consistency was generally lower for abstinence-induced change values as compared to their respective assessments during abstinent and non-abstinent states (see Table 5).
3.4 Variance in Effect Size across Measures Accounted for by Internal Consistency
In an analysis in which each measure constituted a single observation, estimated internal consistency accounted for 33% of the variance in effect size estimates across measures (see Figure 1).
Figure 1.
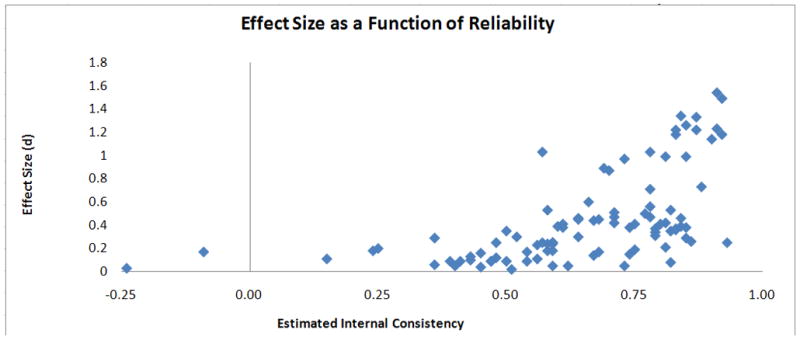
Absolute value of Cohen’s d effect size estimates for abstinence-induced change as a function of internal consistency reliability. Each measure represents a single observation. Split-half coefficients of internal consistency are illustrated for cognitive (even vs. odd trials) and EEG (even vs. odd epochs) measure. Cronbach’s alpha internal consistency estimates are illustrated for subjective measures.
3.5 Supplemental Analyses
Analyses of potential order effects showed that there were significant Order × Abstinence State interactions for the following measures: TCQ-Expectancy, T2-PANAS-NA, RIPT-Hit rate, RIPT-RT, ST-RT, MT-RT, DSST-Total Correct, Standard Stroop effect, Acute Stroop Effect, θ Frequency-Fz Eyes Closed, β2 Frequency-Fz Eyes Open. Effect sizes of adjusted for order effects were generally similar to the unadjusted estimates for these measures. Therefore, unadjusted effect sizes are presented. Effect sizes adjusted for order are available upon request to the first author (AML).
It has been proposed that CO levels of 8 – 10 ppm be used as cut-offs to verify abstinence (SRNT Subcommittee on Biochemical Verification, 2002, p. 151). Comparisons of participants who were above versus below more stringent cut-offs (< 8 ppm to < 10 ppm) on their abstinent session CO values showed no significant differences on baseline smoking variables at all cut-points. Therefore, analyses using the entire sample are presented.
4. Discussion
The aim of this study was to characterize the acute tobacco abstinence syndrome by quantifying the magnitude of acute abstinence effects on a variety of subjective, cognitive, and physiological measures. The study revealed a wide range of effect sizes, indicating that the acute tobacco abstinence syndrome is not a monotonic phenomenon. Internal consistency estimates also widely varied across measures and accounted for a third of the variance in effect size estimates across measures, suggesting that measurement error should be taken into account when interpreting the relative magnitudes of abstinence effects across measures.
4.1 Abstinence Effects on Subjective Measures
The effect sizes of subjective symptoms were as follows: Craving > Negative Affect > Concentration Problems > Hunger > Reduced Positive Affect > Other Somatic Symptoms. There are several reasons why these data are likely indicative of genuine differences in the magnitude of abstinence effects. First, multiple measures of the same construct exhibited similar effect sizes. Second, examination of raw mean scores during abstinent and non-abstinent sessions indicated that potential ceiling and/or floor effects that might have spuriously attenuated abstinence effects were not more common on measures that showed small effects. Third, most abstinence-induced change scores exhibited good reliability.
The relative magnitudes of acute subjective abstinence effects were generally consistent with those reported in prior studies using smaller samples (e.g., West, Ussher, Evans, & Rashid, 2006). Craving exhibited the largest effect size. Negative affect also exhibited large abstinence-induced changes though slightly smaller than those for craving. Given that negative affect and craving are considered to be central motivational factors underlying smoking relapse (Piasecki, 2006) and common targets of effective smoking cessation treatments (USDHHS, 2008), one might expect that these two symptoms would exhibit the most robust abstinence effects. Examination of the various types of negative affect (e.g., irritability/anger vs. anxiety) and types of craving (e.g., desire to smoke for pleasure/stimulation vs. for distress relief) revealed that each of these types exhibited moderate to large abstinence effects, although there was some variation among them. For both the QSU and TCQ, abstinence effects were larger on subscales tapping desire to smoke for pleasure/stimulation than for those tapping desire for distress relief, which is similar to previous findings (Leventhal, 2007).
Abstinence-induced increases in difficulty concentrating were smaller than increases in negative affect, but still moderate to large in magnitude. We also assessed a subjective measure of attentional bias, i.e., the tendency for smokers to report that smoking cues capture and hold their attention. This measure exhibited a large abstinence effect. Reported attention capture by smoking cues may warrant further scrutiny.
Abstinence-induced changes in sadness and positive affect were medium sized. Of note, the abstinence-induced increase in sadness was not as robust as the other types of negative affect. Perhaps because the 4-item WSWS-Sadness scale includes two items that measure positive affect (“I have felt upbeat and optimistic;” “I have felt happy and content”), it is less affected by abstinence than measures specific to negative affect. Thus, the present findings suggest that the abstinence-induced decrease in positive affect is not as substantial as the corresponding increase in negative affect. Nonetheless, abstinence-induced decreases in positive affect have been consistently demonstrated in previous studies (al’Absi et al., 2002; Gilbert et al., 1998; Leventhal et al., 2008). It therefore appears to be a valid manifestation of the abstinence syndrome.
Among the somatic effects, the abstinence-induced increase in hunger/eating was larger than increases in other somatic symptoms, such as drowsiness, headaches, and autonomic symptoms measured by an item that assessed tremor, heart racing, sweating, dizzy stomach, or bowel problems. In a recent review of studies examining the effects of ≥ 18 hr of abstinence, Hughes (2007a) found that the abstinence effect on hunger was consistent, whereas the abstinence effects on drowsiness, headaches, and some autonomic symptoms (tremor, heart racing, sweating) were equivocal or not reliable. The present data suggest that these symptoms were significantly increased by acute 12 hr abstinence, albeit to a small degree. Thus, these symptoms may only be evident in very early abstinence (12 hr), or, perhaps, previous investigations may not have had sufficiently large samples to reliably detect abstinence effects on some somatic symptoms, which may be present but relatively small in magnitude.
4.2 Abstinence Effects on Cognitive Measures
Abstinence significantly slowed response times to a small to moderate degree on the RIPT, Search Task and Serial Math Task (Cohen’s ds .17 – .45). By contrast, abstinence significantly impaired accuracy on only the RIPT. Previous studies have also reported a significant effect of abstinence on psychomotor speed, but an inconsistent effect of abstinence on accuracy (e.g., Bell, Taylor, Singleton, Henningfield, & Heishman, 1999; Domier et al., 2007; Myers, Taylor, Moolchan, & Heishman, 2008; Snyder, Davis, & Henningfield, 1989). Evidence also suggests that the effect of abstinence is most consistent on measures of sustained attention and working memory (Bell et al., 1999; Myers et al., 2008; Parrott & Garnham, 1998). This is concordant with the present results to some degree. In the current study, abstinence slowed response times but increased accuracy on the Search Task (i.e., a speed/accuracy trade-off). However, this finding should be treated with caution as no other speed/accuracy trade-offs were observed in the study.
On attentional bias tasks, the effects of abstinence were generally small. There was a small, significant effect of abstinence on reaction times on the smoking Stroop task. There was a small significant effect of abstinence for errors and a non-significant trend for reaction time (d = .17, p = .08) on the 200 ms visual probe task. In general, the findings are consistent with the literature which indicates that the effect of 12-hr abstinence on attentional bias tasks has been mixed (see Waters & Sayette, 2006).
The abstinence effects on subjective difficulty concentrating and attentional bias were much larger than the abstinence effects on the corresponding objective cognitive assessments. The meaning of this finding is not clear. Some cognitive assessments may suffer from poor reliability, and this may reduce the effect of abstinence. Larger effect sizes on cognitive measures may have been observed if more extensive practice on the cognitive tasks had been provided, particularly for the RIPT, and if catch trials involving no probe in the visual probe task were included. The effect of abstinence on the cognitive performance assessments may be also larger during more protracted abstinence.
4.3 Abstinence Effects on Physiological Measures
The average abstinence effect on physiological measures was small. However, there was substantial variability across measures. The abstinence effect was large for HR, but was small and inconsistent for blood pressure. The majority of the literature has also reported significant abstinence-induced decreases in HR, but no corresponding decreases in blood pressure during acute abstinence (al’Absi et al., 2002; Myers et al., 2008). The abstinence effect on HR may be due to the offset of nicotine’s stimulating effect (Shiffman et al., 2004), leaving its clinical relevance unclear.
For EEG responses, we found abstinence-induced increases in power across all frequency bands, which were of moderate magnitude. We also found small, but significant, abstinence-induced decreases in alpha frequency and some small decreases in beta1 and beta2 frequency. Abstinence-induced changes on frequency values for other bands were negligible.
Until recently, the primary effects of tobacco abstinence on EEG were considered to be increased theta power and reduced alpha frequency (Pickworth et al., 1989). However, recent studies of protracted abstinence have suggested a persistent increase in power occurs across more bands than previously thought (Gilbert et al., 1999; Teneggi et al., 2004). The present study is the first to report increased power across all bands and to provide a quantitative estimate of these effects. These data also suggest that examination of abstinence effects on frequency in bands outside of alpha (e.g., Beta1 and Beta2) may be worthy of future study.
4.4 Limitations and Conclusions
This study had some limitations. We did not have participants smoke at the beginning of the non-abstinent session. Thus, although participants reported smoking their last cigarette on average 15.7 minutes before that session, we did not objectively validate this. However, participants with low CO levels (< 10 ppm) during that session were excluded. Because we used an ad-lib smoking vs. smoking deprivation manipulation, we do not know whether the abstinence effects were due to nicotine offset effects, withdrawal effects, psychological effects, or the unmasking of pre-existing dispositions (Hughes, 2007b). Determining the relative magnitude of these influences should be addressed in future work. The findings only pertain to 12-hr abstinence; we do not know if the findings would generalize to 24-hr or more protracted abstinence. Indeed, the magnitude of some effects may increase over time (e.g., hunger, cognitive measures; McCarthy et al., 2006). Although we were not concerned with statistical significance per se, we conducted a large number of tests without lowering the alpha-level for each analysis, which increases the likelihood of committing a Type-I error. Lastly, we do not know if the findings would generalize to individuals attempting to quit smoking, or to smokers who were excluded from this study because of not meeting recruitment criteria.
This study also had a number of strengths. To the best of our knowledge, this is the first study to comprehensively quantify acute (12-hr) abstinence effects on subjective, cognitive, and physiological measures. The sample size was sufficiently large to yield precise abstinence effect estimates. We used a prospective, counterbalanced design to counteract experimental confounds that could bias effect size estimates. An extensive battery of measures was administered. Finally, we demonstrated that abstinence-induced change scores exhibited adequate internal consistency on many measures.
These findings may be indicative of the typical magnitudes of signs and symptoms that smokers may exhibit during acute tobacco abstinence. From a research perspective, they are relevant for studies examining the influence of individual difference characteristics or interventions on acute abstinence effects. Such investigations may be more likely to detect effects on measures that exhibit robust abstinence effects. Accordingly, if research resources are limited, assessing subjective effects rather than an extensive battery of more costly and time-consuming cognitive and physiological measures might be prudent. From a clinical standpoint, the particular signs and symptoms that exhibited the largest abstinence effects in this study may require the most intensive intervention (with certain exceptions; e.g., heart rate). Thus, treatments designed to reduce short-term distress and impairment caused by acute abstinence (and, perhaps, attenuate relapse risk) may be most effective if they target craving, negative affect, difficulty concentrating, hunger, and EEG power. Other symptoms and signs (e.g., reduced positive affect, somatic symptoms, objective decrements in attention) might also require clinical attention. However, because of their relatively smaller magnitude, the practical significance of attending to these effects is unclear and requires further study.
Acknowledgments
This research was supported by a Transdisciplinary Tobacco Use Research Center Grant from the National Cancer Institute and National Institute on Drug Abuse (P5084718), and by the Intramural Research Program of the NIH, National Institute on Drug Abuse. Dr. Leventhal’s effort was supported by NIH grant (DA025041). The authors report no competing interests related to the submission of this manuscript. The authors wish to thank Caryn Lerman, Brendan Bradley, Karin Mogg, Susan Boyd, Eun Lee, Nicole Eid, and Adrienne Heinz for their assistance on this study. Dr. Moolchan is now at Alkermes Inc., Cambridge, MA, USA. Dr. Pickworth is now at Battelle Centers for Public Health Research and Evaluation, Baltimore, MD, USA.
Footnotes
The literature defines the term deprivation to signify experimenter-initiated discontinuation of drug use, respectively (Hughes, 2007b). Thus, we use the term deprivation to refer to this study’s experimental manipulation. We use the term abstinence to refer to the psychobiological state induced by any form of tobacco use discontinuation (experimenter-initiated or subject-initiated).
Instead of using difference scores another approach would have been to compute residualized abstinence scores, covarying for values in the non-abstinent states, which could have psychometric advantages (Shiffman et al., 2004). However, we decided to use difference scores because 1) their reliability estimates were generally not much lower than corresponding scores in abstinent and non-abstinent states; and 2) they are easier to interpret.
For measures assessed twice, we conducted separate parallel analyses for each assessment point (T1 and T2). We do not evaluate Time (T1 vs. T2) effects or Time × State (Abstinent vs. Non-abstinent) interactions because it is difficult to interpret whether effects are caused by (1) stress or fatigue due to completing cognitive performance tasks in between the T1 and T2 assessments; or (2) natural increases in abstinence effects during the non-abstinent session generated by a brief period of smoking deprivation.
We use absolute value of effect size estimates in these analyses to account for the fact that some abstinence effects are expected to involve a reduction in scores (e.g., positive affect, heart rate, EEG alpha frequency). For some EEG bands frequency values, it is unclear whether abstinence should increase or decrease scores. Thus, we recognize that using absolute values could spuriously increase associations between reliability and effect sizes for some measures. Nevertheless, additional analyses using raw effect size estimates for these measures showed little change in the account of variance shared by reliability and effect size in EEG measures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacology, Biochemistry and Behavior. 2002;72(3):707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; 1994. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction Motivation Reformulated: An Affective Processing Model of Negative Reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine & Tobacco Research. 1999;1(1):45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behavioural Pharmacology. 2004;15(1):29–36. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: vigilance for cigarette-related cues in smokers. Psychology of Addictive Behaviors. 2003;17(1):66–72. doi: 10.1037/0893-164x.17.1.66. [DOI] [PubMed] [Google Scholar]
- Brown RA, Herman K, Ramsey SE, Stout R. Characteristics of smoking cessation participants who lapse on quit date. Paper presented at the First International Conference for the Society for Research on Nicotine and Tobacco; Copenhagen, Denmark. 1998. [Google Scholar]
- Chandra S, Shiffman S, Scharf DM, Dang Q, Shadel WG. Daily smoking patterns, their determinants, and implications for quitting. Experimental and Clinical Psychopharmacology. 2007;15(1):67–80. doi: 10.1037/1064-1297.15.1.67. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-Stroop test: Theoretical considerations and procedural recommendations. Psychological Bulletin. 2006;132(3):443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I--Effects on incentive motivation. Psychopharmacology (Berl) 2006;189(3):355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo-controlled experimental study of nicotine: II--Effects on response inhibition and executive functioning. Psychopharmacology (Berl) 2007;190(4):457–467. doi: 10.1007/s00213-006-0634-6. [DOI] [PubMed] [Google Scholar]
- Domier CP, Monterosso JR, Brody AL, Simon SL, Mendrek A, Olmstead R, et al. Effects of cigarette smoking and abstinence on Stroop task performance. Psychopharmacology (Berl) 2007;195(1):1–9. doi: 10.1007/s00213-007-0869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Experimental and Clinical Psychopharmacology. 2006;14(2):121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2006;74(6):1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MAH. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. 1996;127(1):31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Dibb WD, Plath LC, Hiyane S, et al. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: Relations to depressive traits, nicotine exposure, and dependence. Experimental and Clinical Psychopharmacology. 1999;7(4):427–443. doi: 10.1037//1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Jensen RA, Meliska CJ. Effects of smoking abstinence on mood and craving in men: Influences of negative-affect-related personality traits, habitual nicotine intake and repeated measurements. Personality and Individual Differences. 1998;25(3):399–423. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. Behavioral and cognitive effects of smoking: Relationship to nicotine addiction. Nicotine & Tobacco Research. 1999;1(Suppl 2):S143–147. doi: 10.1080/14622299050011971. discussion S165–146. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Pickworth WB. Reliability and validity of a Short Form of the Tobacco Craving Questionnaire. Nicotine & Tobacco Research. 2008;10(4):643–651. doi: 10.1080/14622200801908174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Blundell JE. Nutrients and behaviour: Research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. Journal of Psychiatric Research. 1982;17(2):203–212. doi: 10.1016/0022-3956(82)90023-1. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Clinical significance of tobacco withdrawal. Nicotine & Tobacco Research. 2006;8(2):153–156. doi: 10.1080/14622200500494856. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007a;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Measurement of the effects of abstinence from tobacco: A qualitative review. Psychology of Addictive Behaviors. 2007b;21(2):127–137. doi: 10.1037/0893-164X.21.2.127. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Leventhal AM. Subliminal Processes in Tobacco Dependence. University of Houston; Houston, TX: 2007. [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender Differences in Acute Tobacco Withdrawal: Effects on Subjective, Cognitive, and Physiological Measures. Experimental and Clinical Psychopharmacology. 2007a;15(1):21–36. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Heishman SJ, Lerman C, Pickworth W. Associations between Cloninger’s temperament dimensions and acute tobacco withdrawal. Addictive Behaviors. 2007b;32(12):2976–2989. doi: 10.1016/j.addbeh.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Breitmeyer BG, Tapia E, Miller EK, Li Y. Subliminal processing of smoking-related and affective stimuli in tobacco addiction. Experimental and Clinical Psychopharmacology. 2008;16(4):301–312. doi: 10.1037/a0012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: An electronic diary study. Journal of Abnormal Psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33(3):588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ. Comparative mood states and cognitive skills of cigarette smokers, deprived smokers and nonsmokers. Human Psychopharmacology: Clinical and Experimental. 1998;13(5):367–376. [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26(2):196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. Journal of Abnormal Psychology. 2003;112(1):3–13. [PubMed] [Google Scholar]
- Pickworth WB, Herning RI, Henningfield JE. Spontaneous EEG changes during tobacco abstinence and nicotine substitution in human volunteers. Journal of Pharmacology and Experimental Therapeutics. 1989;251(3):976–982. [PubMed] [Google Scholar]
- Pickworth WB, O’Hare ED, Fant RV, Moolchan ET. EEG effects of conventional and denicotinized cigarettes in a spaced smoking paradigm. Brain and Cognition. 2003;53(1):75–81. doi: 10.1016/s0278-2626(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology. 2008;117(1):94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multidimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96(10):1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. 2006;184(3):637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148(1):33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J, et al. Natural history of nicotine withdrawal. Addiction. 2006;101(12):1822–1832. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West R, Gilbert D. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research. 2004;6(4):599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. Journal of Psychology: Interdisciplinary and Applied. 1940;9:371–377. [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: Performance decrements assessed on a computerized test battery. Drug and Alcohol Dependence. 1989;23(3):259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Teneggi V, Squassante L, Milleri S, Polo A, Lanteri P, Ziviani L, et al. EEG power spectra and auditory P300 during free smoking and enforced smoking abstinence. Pharmacology, Biochemistry and Behavior. 2004;77(1):103–109. doi: 10.1016/j.pbb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thorne DR, Genser SG, Sing HC, Hegge FW. The Walter Reed performance assessment battery. Neurobehavioral Toxicology & Teratology. 1985;7(4):415–418. [PubMed] [Google Scholar]
- USDHHS. Treating Tobacco Use and Dependence: 2008 Update--Clinical Practice Guidelines. Rockville, MD: U. S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- Waters AJ, Sayette MA. Implicit Cognition and Tobacco Addiction. In: Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Sage Publications, Inc; 2006. pp. 309–338. [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22(4):378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- West R, Ussher M, Evans M, Rashid M. Assessing DSM-IV nicotine withdrawal symptoms: A comparison and evaluation of five different scales. Psychopharmacology (Berl) 2006;184(3–4):619–627. doi: 10.1007/s00213-005-0216-z. [DOI] [PubMed] [Google Scholar]


