Abstract
Reward-motivated behavior is strongly influenced by the learned significance of contextual stimuli in the environment. However, the neural pathways that mediate context-reward relations are not well understood. We have identified a circuit from area CA3 of dorsal hippocampus to ventral tegmental area (VTA) that uses lateral septum (LS) as a relay. Theta frequency stimulation of CA3 excited VTA dopamine (DA) neurons and inhibited non-DA neurons. DA neuron excitation was likely mediated by disinhibition because local antagonism of γ-aminobutyric acid receptors blocked responses to CA3 stimulation. Inactivating components of the CA3-LS-VTA pathway blocked evoked responses in VTA and also reinstatement of cocaine-seeking by contextual stimuli. This transsynaptic link between hippocampus and VTA appears to be an important substrate by which environmental context regulates goal-directed behavior.
Efficient reward-seeking requires that environmental stimuli be properly interpreted, to predict when and where reward can be expected. The ventral tegmental area (VTA) and its dopaminergic projections are critical components of a reward circuit. Although the influence of temporal cues on this system has been well studied (1), it is unknown how the VTA system relates to other contextual information, such as where reward can be expected. The hippocampus organizes aspects of context into a relational memory network (2). Interactions between hippocampus and VTA are important for context-reward associations (3). However, the circuitry by which the two interact remains to be elucidated.
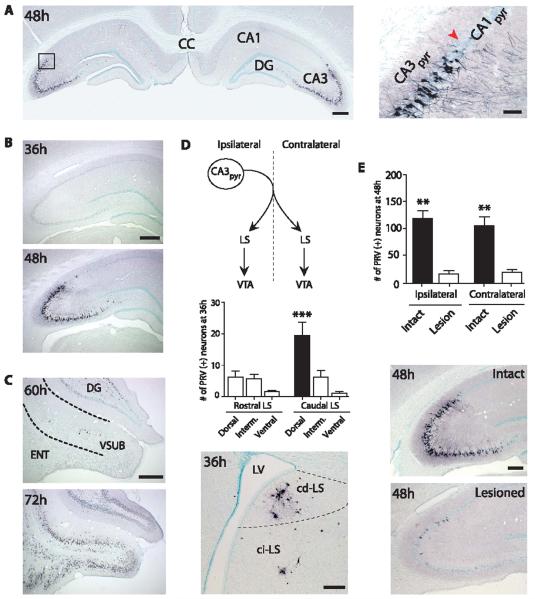
We used pseudo-rabies virus (PRV), a retrograde, transsynaptic tracer (4), to delineate circuit projections to VTA. Beginning 48 hours after unilateral injection into VTA, PRV (+) neurons were found bilaterally in dorsal (Fig. 1A), intermediate, and ventral hippocampus (fig. S4 and SOM). PRV labeling was highly restricted to the CA3 and CA2 pyramidal cell layers (5). This time course of PRV labeling in dorsal hippocampus is consistent with an indirect projection, because no labeling was observed in hippocampus 36 hours postinjection (Fig. 1B), when known direct VTA afferents (e.g., nucleus accumbens) were labeled (6).
Fig. 1.
A dorsal CA3–cd-LS–VTA pathway. (A) Photomicrographs of PRV immunoreactivity in dorsal hippocampus 48 hours after PRV injection in VTA. Area delineated by rectangle is magnified at right. Red arrowhead indicates approximate boundary between CA1 and CA3. (B) Photomicrographs of PRV labeling in dorsal hippocampus and (C) vSUB at increasing postinjection times. (D) (Top) Schematic of projections from CA3 to LS (9) and LS to VTA (19); (middle) PRV (+) cell counts (Dunn’s post hoc test, ***P ≤ 0.0001); (bottom) photomicrograph of lateral septum subregions at 36 hours postinjection. (E) Cell counts and photomicrographs of dorsal CA3 PRV (+) neurons from intact and cd-LS–lesioned animals (Newman-Keuls post hoc test, **P = 0.0005). Hemisphere ipsilateral to PRV injection is shown in all photomicrographs with one hemisphere and left in (A). Dorsal is up for all panels. Tissue sections are coronal. CA1pyr, CA1 pyramidal layer; CA3pyr, CA3 pyramidal layer; CC, corpus callosum; ci-LS, caudointermediate-lateral septum; DG, dentate gyrus; ENT, entorhinal cortex; Interm, intermediate; LV, lateral ventricle; VSUB, ventral subiculum. Scale bars: (A) (left), (B), and (C), 500 μm; (D) and (E), 250 μm; (A) (right), 100 μm.
PRV injections in VTA also labeled the ventral subiculum (vSUB), a hippocampal region previously reported to project multisynaptically to VTA (7, 8), but at a longer delay than CA3. Very few PRV (+) neurons were detected in vSUB, even at 60 hours postinjection (Fig. 1C), a time point with abundant labeling in CA3 (fig. S5 and SOM). Substantial PRV labeling was not observed in vSUB until 72 hours postinjection (Fig. 1C).
We next sought to identify intermediary brain nuclei in this circuit. A single CA3 pyramidal cell projects bilaterally to lateral septum (LS) (Fig. 1D), the only major nonhippocampal target of CA3 (9). The caudodorsal-LS (cd-LS) showed the largest number of PRV (+) neurons 36 hours postinjection (Kruskal-Wallis, P ≤ 0.0001) (Fig. 1D).
To test whether cd-LS is a relay from CA3 to VTA, we bilaterally lesioned cd-LS cell bodies with ibotenic acid, 5 to 7 days before unilateral PRV injections in VTA. These lesions resulted in a significant reduction in PRV (+) neurons in dorsal CA3 (Fig. 1E), but not intermediate or ventral CA3 (fig. S4 and SOM). In dorsal CA3, cd-LS lesions produced an 86.6% decrease in PRV-labeled ipsilateral CA3 pyramidal neurons and an 81.8% reduction in contralateral CA3 pyramidal neurons [factorial analysis of variance (ANOVA) P = 0.0007].
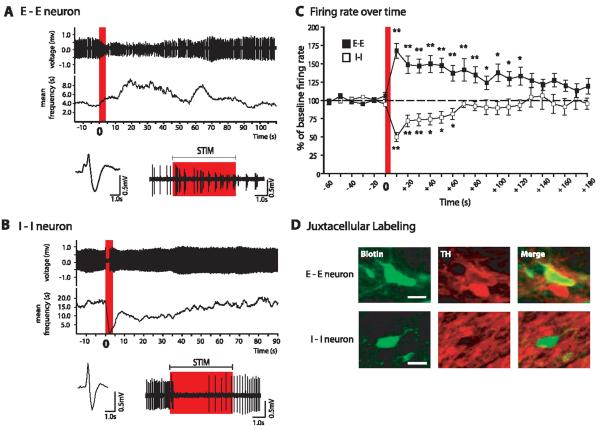
We next examined the dorsal CA3–cd-LS–VTA pathway using electrophysiology in urethane-anesthetized rats. We recorded single-unit VTA neuron impulse activity while electrically stimulating dorsal CA3 with single pulses (table S2 and SOM) or with a stimulus that mimics endogenous hippocampal theta frequency rhythm (SOM), because these rhythms are associated with learning and memory (10). Post-theta responses were divided into early and late epochs, and each neuron was assigned to a category on the basis of the direction of its responses (11). Of all VTA neurons tested, 86.0% (98 of 114) responded to theta stimulation of dorsal hippocampus (fig. S6 and SOM). The two most common responses observed were sustained excitation (E-E; excited during early and late epochs) or sustained inhibition (I-I). We further studied E-E and I-I neurons because their pronounced responses suggested that they were particularly engaged components of the dorsal CA3–cd-LS–VTA pathway.
E-E neurons were confirmed VTA dopamine (DA) neurons with five of six juxtacellularly labeled cells positive for tyrosine hydroxylase. (Fig. 2D; see also table S1). In response to theta frequency stimulation, E-E neurons showed substantial response scores during the early epoch, and increases in firing activity during the late epoch (Fig. 2, A and C, and table S1). Firing rates remained elevated for 2 min after stimulation (10-s bins, repeated ANOVA, P = 0.0001) (Fig. 2C). The percentage of spikes in burst (% SIB) also increased, peaking 10 s poststimulation to 327.5% (5.1 to 21.8% SIB) above baseline, and was elevated up to 1 min poststimulation (see also table S1) (repeated ANOVA, P = 0.005).
Fig. 2.
VTA DA neurons were excited by theta stimulation of dorsal CA3, and non-DA neurons were inhibited. (A) Representative neuron excited (E-E) by theta stimulation. (Top) Spike train and mean frequency. Red bar indicates time of stimulation. Digitized waveform (bottom left), and expanded view (bottom right) of spike train from top. Red rectangle corresponds to the same time period as red bar in top graph. (B) Representative neuron inhibited (I-I) by theta stimulation. Descriptions are the same as in (A). Stimulation artifacts have been digitally removed from (A) and (B) for clarity. (C) Time course of excitatory and inhibitory responses in E-E and I-I neurons, respectively (Dunnett’s post hoc test, *P < 0.001, **P < 0.0001). Red bar indicates time of stimulation. (D) Photomicrographs of representative juxtacellularly labeled E-E and I-I neurons processed to reveal neurobiotin fills, and of tyrosine hydroxylase immunoreactivity to identify dopaminergic and nondopaminergic neurons. Midline is to the left for E-E photomicrographs and to the right for I-I photomicrographs. Dorsal is up for all images. Scale bars, 50 μm. Biotin, neurobiotin; TH, tyrosine hydroxylase.
I-I cells had electrophysiological characteristics of VTA γ-aminobutyric acid (GABA) neurons (table S1) and all six juxtacellularly labeled neurons were tyrosine hydroxylase–negative, which confirmed that they were not dopaminergic. I-I neurons often showed a brief, nearly immediate inhibition that recovered by 1 min poststimulation (Fig. 2, B and C, and table S1).
Chemical stimulation of dorsal CA3 with local microinjections of d,l-homocysteic acid elicited lasting excitation or inhibition in VTA neurons (fig. S7 and SOM). Therefore, fibers of passage were not necessary for the above responses elicited by electrical stimulation.
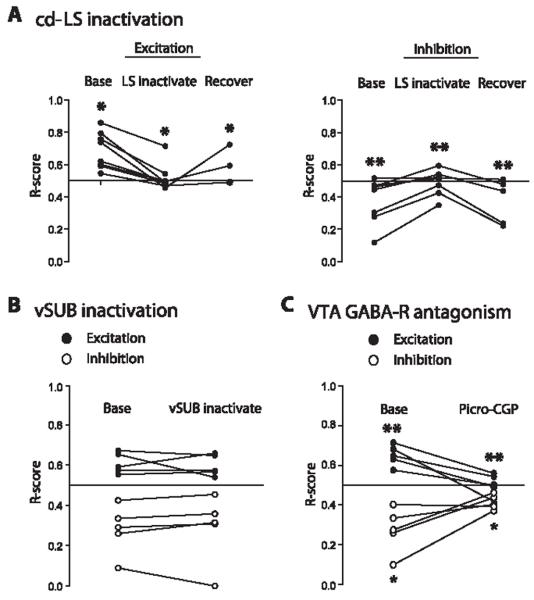
To determine the role of cd-LS in mediating VTA neuron responses, we transiently in-activated cd-LS with a local microinjection of GABA during dorsal CA3 theta stimulation (Fig. 3A). Unilateral GABA infusions into cd-LS reversibly blocked excitatory (repeated ANOVA, P = 0.02) and inhibitory responses (P = 0.005) of VTA neurons to ipsilateral CA3 stimulation (Fig. 3A). Responses in all tested cell categories (11), including E-E and I-I neurons, were blocked. In contrast, neither VTA excitation (paired t test, P = 0.72) nor inhibition (P = 0.79) were blocked by microinjections of GABA into vSUB (Fig. 3B).
Fig. 3.
VTA responses to dorsal CA3 theta stimulation depend on a functional cd-LS and are blocked by local antagonism of GABA receptors. (A) Response scores (R-scores) for excited (left), or inhibited (right) VTA cells to CA3 theta stimulation, before (base), during (LS inactivate), and after (recover) cd-LS was inactivated by a GABA microinjection (Newman-Keuls post hoc test, *P < 0.05, **P ≤ 0.01). Inactivation of cd-LS blocked excitatory and inhibitory responses to theta frequency stimulation. (B) R-scores for excited and inhibited cells to theta stimulation before (base) and after (vSUB inactivate) GABA was microinjected into vSUB. Inactivation of vSUB failed to block VTA excitatory and inhibitory responses. (C) R-scores for excited and inhibited VTA cells before (base) and after (picro-CGP) picrotoxin-CGP 55845 was locally microinfused near the recorded VTA neuron. Microinjection of the GABA receptor antagonists blocked excitatory and inhibitory responses (paired t test, *P < 0.05, **P = 0.01). GABA-R, GABA receptor.
VTA GABA neurons normally hold their DA neighbors under tonic inhibition (12). Therefore, we hypothesized that the excitation of DA neurons was a secondary consequence of the inhibition of non-DA (presumed GABA) VTA neurons (see also single-pulse stimulation studies in SOM for additional rationale). To test this hypothesis, a mixture of GABA receptor antagonists (picrotoxin-CGP55845) was microinjected onto the recorded VTA neuron during CA3 stimulation (11). Excitation (paired t test, P = 0.01) and inhibition (P = 0.04) of VTA neurons by CA3 theta stimulation were each blocked (Fig. 3C and SOM). This blockade occurred for responses in all tested cell categories (11), including E-E and I-I neurons.
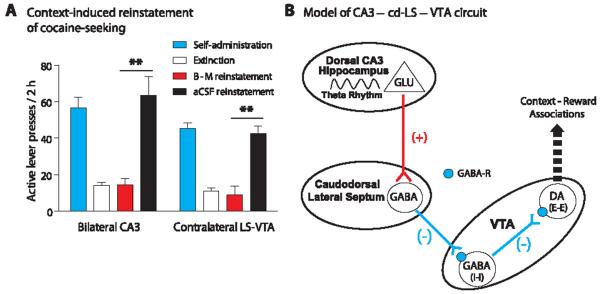
The hippocampus is important for its ability to use contextual information to regulate behavior, including drug-seeking (13, 14). Furthermore, VTA DA neurons are critical for the rewarding effects of cocaine and the reinstatement of cocaine-seeking after abstinence or extinction training (15). Therefore, we tested whether inactivating components of this pathway would interfere with context-induced reinstatement of extinguished cocaine-seeking (11). Inactivation of dorsal CA3 via bilateral infusion of the GABA agonists baclofen and muscimol (B-M) (fig. S3A) blocked context-induced reinstatement of active lever pressing (ANOVA, P < 0.001) (Fig. 4A). We also tested the role of the cd-LS–to–VTA component of the pathway. Injections of B-M into cd-LS in one hemisphere and into VTA in the contralateral hemisphere of the same animal (fig. S3B) also blocked reinstatement (ANOVA, P < 0.001) (Fig. 4A).
Fig. 4.
The dorsal CA3–cd-LS–VTA circuit in context-induced reinstatement of cocaine-seeking and a proposed diagram of the circuit’s mechanisms. (A) Animals were trained to press a lever to self-administer cocaine (fig. S2). Extinction training occurred in a different context from the self-administration context. The GABA agonists baclofen and muscimol (B-M) were infused into dorsal CA3 bilaterally, or LS in one hemisphere and VTA in the opposite hemisphere, just before reexposure to the original cocaine-paired context. Animals with vehicle (aCSF) infusions reinstated cocaine-seeking; however, animals with B-M infusions failed to reinstate (Bonferroni post hoc test, **P < 0.001). (B) Activation of glutamatergic pyramidal cells of dorsal hippocampal CA3 excites GABAergic cd-LS neurons (16) that project onto VTA GABA (I-I) neurons. The release of GABA from cd-LS neurons acts on GABAergic receptors to inhibit local VTA GABA neurons that project to VTA DA neurons. No longer under tonic inhibition from these neurons, DA (E-E) neurons show robust activation via disinhibition. In turn, the influence of the CA3–cd-LS–VTA pathway, together with other brain regions, regulates behaviors that depend on context-reward associations, such as context-induced reinstatement of cocaine-seeking. GABA-R, GABA receptor.
Our working model of the dorsal CA3–cd-LS–VTA pathway response to hippocampal theta rhythm is outlined in Fig. 4B. We propose that a population cd-LS GABA neurons project to VTA GABA neurons and that stimulation of CA3 glutamatergic pyramidal neurons excites these GABAergic cd-LS neurons (16), which results in inhibition of VTA GABA (I-I) neurons. Under basal conditions, VTA GABA neurons tonically inhibit VTA DA neurons (12); therefore, removal of local GABA inhibition by cd-LS activity would be expected to disinhibit VTA DA (E-E) neurons. This effect is proposed to contribute to context-induced modulation of motivated behaviors (Fig. 4B). Although the specific relation between CA3 theta rhythm, VTA dopamine neuron excitation, and context-dependent regulation of behavior remains unknown, our results establish fundamental characteristics of this circuit and its significance in physiology and behavior. On the basis of our anatomical (Fig. 1) and electrophysiological data (Fig. 3), our dorsal CA3-to-VTA pathway appears to be independent of the previously reported hippocampus-to-VTA pathway originating in vSUB (7, 8, 14).
Our results show that this dorsal CA3–cd-LS–VTA pathway is an important conduit by which contextual information reaches the mid-brain DA system for regulation of motivational behaviors. Our pathway may be involved in the processing of context-reward associations, in both normative function and disease states. Recent work has shown that CA3 place cells react more to global, rather than local, changes in the environment (17), and the role of midbrain DA neurons in salience attribution is increasingly appreciated (18). It is possible that dorsal CA3 conveys information to VTA about the current context as a whole, which allows rapid activation of DA neurons to promote salience attribution to conditioned contexts. Such processing is important for cognitive function by providing adjustments in behavior in response to changing real-world environments.
Supplementary Material
Acknowledgments
We thank B. Bingham and J. See for assistance with the initial anatomical experiments; R. Fallon, G. Sartor, L. Zhang, S. Mahler, K. Moussawi, R. Smith, and T. Smith for assistance with behavioral studies; C. Bäckman and W. Freed for use of their microscopy equipment; and C. Mejias-Aponte for his programming expertise and thoughtful comments. This work was supported by U.S. Public Health Service grant awards R37-DA006214 and F31-MH071093, and the National Institute on Drug Abuse Intramural Research Program. The anatomical data were gathered at the University of Pennsylvania.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/333/6040/353/DC1 Materials and Methods
References and Notes
- 1.Tobler PN, Fiorillo CD, Schultz W. Science. 2005;307:1642. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 2.Eichenbaum H. Neuron. 2004;44:109. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Lisman JE, Grace AA. Neuron. 2005;46:703. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Card JP, et al. J. Neurosci. 1993;13:2515. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For simplicity, the region containing CA2 and CA3 will be referred to as CA3.
- 6.Luo AH, Aston-Jones G. Eur. J. Neurosci. 2009;29:748. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legault M, Rompré PP, Wise RA. J. Neurosci. 2000;20:1635. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floresco SB, Todd CL, Grace AA. J. Neurosci. 2001;21:4915. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson LW, Sawchenko PE, Cowan WM. J. Neurosci. 1981;1:548. doi: 10.1523/JNEUROSCI.01-05-00548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasselmo ME. Hippocampus. 2005;15:936. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- 11.Materials and methods are available as supporting material on Science Online.
- 12.Johnson SW, North RA. J. Neurosci. 1992;12:483. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs RA, Eaddy JL, Su ZI, Bell GH. Eur. J. Neurosci. 2007;26:487. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 14.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Science. 2001;292:1175. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 15.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Eur. J. Pharmacol. 2005;526:36. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 16.DeFrance JF, Kitai ST, Shimono T. Exp. Brain Res. 1973;17:447. doi: 10.1007/BF00234861. [DOI] [PubMed] [Google Scholar]
- 17.Alvernhe A, Van Cauter T, Save E, Poucet B. J. Neurosci. 2008;28:7324. doi: 10.1523/JNEUROSCI.1909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Neuron. 2010;68:815. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisler S, Zahm DS. J. Comp. Neurol. 2005;490:270. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.