Abstract
As life expectancy increases, valvular heart disease is becoming more common. Management of heart disease and primarily valvular heart disease is expected to represent a significant proportion of healthcare provided to the elderly population. Recent years have brought a progression of surgical treatments toward less invasive strategies. This has given rise to percutaneous approaches for the correction of valvular heart disease. Percutaneous mitral valve repair using the MitraClip system (Abbott Vascular, Santa Clara, CA, USA) creates a double orifice and has been successfully used in selected patients with mitral regurgitation. We review the rationale, procedural aspects, and clinical data thus far available for the MitraClip approach to mitral regurgitation.
Keywords: MitraClip, Mitral valve, Mitral regurgitation
1. Introduction
Mitral regurgitation (MR) is common worldwide and is increasing in prevalence (Fedak et al., 2008). The standard of care for the treatment of significant MR is presently surgery. If feasible, mitral valve repair, rather than replacement, is the preferred treatment, offering lower rates of thromboembolism and infection, excellent durability, and increased survival (Fedak et al., 2008). Surgery, even when effective, can be associated with significant morbidity and mortality, especially in the presence of advanced age or significant comorbidity. There is, therefore, a clear need for less invasive approaches. In response to this need, novel percutaneous mitral valve repair techniques have been recently developed, modeled on established surgical repair concepts. One technique, using the MitraClip device, has been recently studied in a randomized controlled trial (Feldman et al., 2011) and employs the concept of edge-to-edge repair developed by surgeons over 10 years previously (Alfieri et al., 2001). The MitraClip is the most widely applied percutaneous approach worldwide (Kar et al., 2008; Hussaini and Kar, 2010) and has been applied clinically in over 3 000 patients since 2003. Percutaneous mitral valve repair procedure using the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA) is the focus of this review.
2. Anatomy of the mitral valve apparatus
The mitral valve is a highly intricate structure with several coordinated components. The functional anatomy of this structure includes the myocardium of the left ventricle, the subvalvular apparatus (including papillary muscles and chordae tendineae), the mitral annulus, the mitral leaflets, and the left atrium (LA). Intrinsic abnormalities or disruption of the coordinated function of these individual parts can result in MR. The mitral valve has two leaflets: the larger (by surface area) anterior leaflet and the smaller posterior leaflet. The anterior and posterior leaflets consist of three pairs of corresponding scallops or segments: lateral (A1/P1), middle (A2/P2), and medial (A3/P3). Each of the leaflets inserts into the mitral annulus and is also connected to two papillary muscles by a web of strong chordae tendineae. The subvalvular apparatus withstands strong retrograde forces from the left ventricle in systole, preventing the leaflets from being pushed back into the LA, and helps to maintain ventricular shape and contractility. Its preservation by surgical therapies is preferred.
3. Causes and mechanisms of mitral regurgitation
MR is due to the failure of anterior and posterior leaflet coaptation, leading to a regurgitation of left ventricular blood into the left atrium in systole. MR is classified according to a number of pathophysiologic mechanisms (Enriquez-Sarano et al., 2009). The causes are broadly classified as ischemic (due to consequences of ischemic heart disease) and non-ischemic. Non-ischemic causes include degenerative (myxomatous disease, leaflet degeneration, and annular calcification), endocarditic, rheumatic, and less common miscellaneous causes (congenital, cardiomyopathy-related, inflammatory, drug-induced, and traumatic).
Mechanisms are anatomic descriptors and are classified as functional (the mitral valve is structurally normal, and regurgitation is caused by extrinsic valve deformation, such as annular dilatation associated with LV remodeling) or organic (there is an abnormality intrinsic to the leaflets such as flail, prolapse, or restriction). The same organic/functional dichotomy for mechanisms of MR is often expressed as primary/secondary (Carabello, 2008).
4. MitraClip device and delivery system
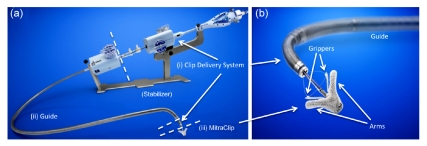
The Evalve MitraClip system (Abbott Vascular, Santa Clara, CA) consists of two major parts: a MitraClip attached to the clip delivery system (CDS) and a steerable guide catheter (Fig. 1). The CDS is advanced through the guide into the left atrium toward the mitral valve. There is also a stabilizer that keeps the system precisely in position. The MitraClip is constructed from cobalt-chromium and covered with polyester. It has two arms that are roughly 8 mm long and 4 mm wide. These measurements approximate the surgical Alfieri stitch and allow adequate vertical coaptation of the leaflets. The arms are opened and closed by a control mechanism on the CDS handle. On the inner aspect of the arms are two corresponding “grippers” that help secure the leaflets. Each leaflet is grasped between an arm and a gripper. The clip can be locked in final position and then deployed in this state.
Fig. 1.
Evalve MitraClip system (a) and MitraClip device (b)
The system consists of two principal components: (i) a clip delivery system (CDS) used to position and deploy the clip and (ii) a steerable guide catheter. The MitraClip device (iii) is attached to the CDS. A stabilizer keeps the system precisely in position
The 24-Fr steerable guide catheter allows the introduction of the CDS into the left atrium. The tip of the guide catheter has a radiopaque marker. The steerable properties of both the guide catheter and the CDS allow precise orientation and positioning of the MitraClip.
5. Case selection
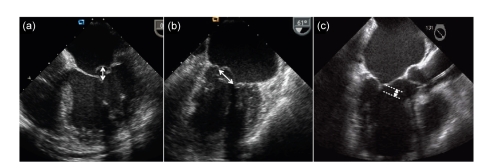
Case selection is probably the most important determinant of success for the MitraClip procedure. All patients should have moderate (3+) or severe (4+) MR, as assessed formally by quantitative and qualitative two-(2D) and three-dimensional (3D) echocardiography. Anatomical suitability is determined by transesophageal echocardiography (TEE). 3D TEE is preferred for all cases, offering a rapid and clear anatomicopathologic visualization of mitral valve, and to confirm the origin of the regurgitant jet, clearly defining multiple and/or eccentric trajectories. The origin of the MR jet should ideally be from the central portion of the valve, although there have been case reports of successful MitraClip implants with MR arising from the commissures. Regardless of underlying aetiology, degenerative or functional, important anatomical prerequisites for MitraClip are a sufficient leaflet tissue for mechanical coaptation and resting mitral valve effective orifice area over 4 cm2, as there is an inevitable but small reduction in valve area on the transformation to a double orifice (Herrmann et al., 2009). In cases of degenerative MR with flail segments, the flail gap should be <10 mm and the flail width <15 mm, in line with the entry criteria for the randomized trial (Fig. 2). In cases of ischemic functional MR, where one or more leaflets may be tethered, a coaptation length of at least 2 mm is required (Fig. 2). Important exclusions include rheumatic MR and calcified leaflets.
Fig. 2.
Anatomical inclusion criteria for the MitraClip device
Failure to meet any of these is an important exclusion. (a) Four-chamber view: flail gap is shown and should be <10 mm; (b) Bicommissural view: flail width is shown and should be <15 mm; (c) Three-chamber view: coaptation length should be ≥2 mm
6. Procedural technique
The MitraClip procedure is a catheterization lab procedure using fluoroscopy and echocardiographic guidance. Online TEE, especially 3D TEE is the most important imaging tool to guide the procedure. The integration of 3D TEE into our usual practice has dramatically shortened procedure time and increased efficiency. The multiplane capability of 3D TEE allows two orthogonal planes to be shown at one time, which allows precise manipulation of catheters during the procedure.
The transseptal puncture is a crucial early step in the procedure. A good transseptal puncture will facilitate a straightforward procedure whereas a suboptimal puncture will prolong the procedure unnecessarily. Optimal imaging is fundamental to this. The bicaval and short axis views are used in combination with the four-chamber view (at the mid esophageal level), which determines the “height” (which also incorporates an anterior-posterior dimension given the axis of the heart) from the mitral valve plane. The transseptal puncture is most optimally 3.5–4.0 cm above the line of coaptation of the leaflets. Accurate localization of transseptal puncture can be achieved with the multiplane TEE. In cases of degenerative mitral valve disease, where the line of coaptation is at or above the plane of the mitral annulus, the transseptal puncture should be posterior (and superior). In contrast, in cases of functional mitral valve disease, where the line of coaptation is below the plane of the mitral annulus, the transseptal puncture should be more anterior (and inferior).
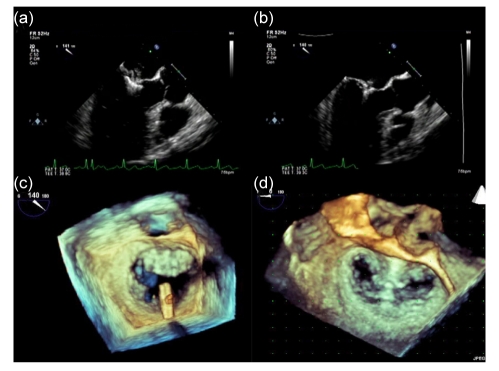
Following successful transseptal puncture, intravenous heparin is administered and activated clotting time (ACT) is monitored throughout the procedure, maintaining a level at around 250 s. A 0.035-inch Super Stiff exchange length guidewire is advanced through the transseptal catheter to the left upper pulmonary vein. The transseptal catheter is then removed and exchanged for the guide catheter. The MitraClip attached to the CDS is then advanced through the guide catheter into the left atrium. With the help of multiplane TEE, the MitraClip is then orientated appropriately over the mitral valve. The clip is then opened and the arms are positioned perpendicularly to the leaflets using the en face 3D TEE projection (Fig. 3). Once properly oriented, the clip is advanced to the left ventricle, and the CDS is then pulled back and the leaflets are grasped by dropping the grippers. After confirmation of adequate grasping of leaflets, the arms are closed and reduction in MR is assessed. If there is no significant change in MR, the clip is repositioned. On the other hand, if the reduction is adequate, the clip is deployed. In cases of some residual MR on one side, a second clip can be deployed along side the first. In addition to assessment of MR, mitral valve gradients are checked periodically throughout the procedure to ensure that there is no iatrogenic mitral stenosis.
Fig. 3.
MitraClip procedure with two- and three-dimensional TEE guidance
(a, b) Two-dimensional TEE (long axis/three-chamber view). (a) Positioning clip; (b) Deployed clip with leaflets grasped; (c, d) Three-dimensional TEE (3D en face view). (c) Correctly orientating the clip in the 3D en face view; (d) Deployed clip with creation of the double orifice
Groin hemostasis is achieved by manual compression after the activated clotting time has decreased appropriately. Preclosure with Perclose/ProGlide devices (Abbott Vascular, Santa Clara, CA) or the “figure of 8” suture technique can also be employed. After repair, it is recommended to give aspirin for six months and some operators also administer clopidogrel for one month. Infective endocarditis prophylaxis is recommended.
During the procedure, it is ideal to have cardiac anesthesiologist monitoring and experienced echocardiographic imaging. In many high volume centers, the cardiac anesthesiologist is trained to facilitate both monitoring and echocardiographic guidance. It is important to note that the accurate assessment of MR pre- and post-procedure must be in the presence of normotension, and the anesthesiologist must therefore manipulate the blood pressure accordingly.
7. Clinical studies
The device remains investigational in the United States and has attained Conformité Européenne (CE) mark approval in 2008. The sum of clinical data currently available for this device includes that from the EVEREST (endovascular valve edge-to-edge repair study, I and II) clinical trials performed in North America and from the clinical studies following commercial approval in Europe and some countries in Asia (Table 1).
Table 1.
Studies evaluating the MitraClip device
| Study | Population | n |
| EVEREST I1 (feasibility) | Non-randomized | 55 |
| EVEREST II1 | Pre-randomization | 60 |
| EVEREST II2 | High risk registry | 78 |
| EVEREST II3 (pivotal) | Randomized patients (2:1 MitraClip to surgery) | 279, 184 (MitraClip) |
| REALISM (continued access) | High risk & non-high risk | 549 |
| European experience | Commercial | 2 113 |
| Total | 3 039 (MitraClip) |
The initial EVEREST cohort included anatomically suitable symptomatic subjects with grade 3+/4+ MR from the initial pilot study and roll-in patients from the subsequent EVEREST II randomized study. Data evaluating the safety and midterm durability of this study was reported by Feldman et al. (2009). The primary success rate was 74% with freedom from death and surgery being 90.1% and 76.3%, respectively, at a median follow-up of 3.2 years. These encouraging results and low complication rate in this early experience affirm the safety of this procedure.
The pivotal randomized controlled clinical trial, EVEREST II (Feldman et al., 2011), compared the percutaneous MitraClip therapy to mitral valve surgery in 279 subjects in a randomized fashion. Eligible subjects were prospectively randomized to the MitraClip® therapy or mitral valve surgery in a 2:1 ratio. Percutaneous repair was associated with superior safety and similar improvements in clinical outcomes compared to conventional surgery, despite being less effective at reducing MR. This landmark study was unique in that it was the first prospective randomized trial comparing a percutaneous mitral repair technique to conventional surgery. The EVEREST II trial also incorporated a non-randomized high-risk arm evaluating subjects at elevated surgical risk. In this arm, the MitraClip procedure was attempted in 78 subjects. The observed mortality in this group was 7.7% at 30 d and this compared favorably to a mean predicted mortality by STS score of 18.2%.
Despite the rapid growth of the MitraClip experience in Europe (Table 1), the data available from this thus far consists of a limited number of reports from a few non-randomized registries (Tamburino et al., 2010; Franzen et al., 2010; 2011). Amongst the patients treated, a significant proportion were those at high surgical risk, with congestive cardiac failure and depressed ejection fractions (Franzen et al., 2010; 2011; Tamburino et al., 2010). The data confirmed favorable outcomes in those treated, with an extremely low frequency of adverse events. Improvement in measures of MR, left ventricular dimensions, 6-min walk distances, and NT pro-BNP plasma levels has been reported (Franzen et al., 2011). This provides further support for safety and efficacy in high-risk patients.
8. Future developments
The MitraClip is the only percutaneous technology effective in both functional and degenerative MR. The procedure is performed via a venous route and the device is removable and repositionable. These important attributes contribute to the safety of this procedure. Of note, in spite of the fact that the degree of reduction in MR is lower than that of surgery, the clinical benefits with respect to LV remodeling were observed to be similar to the surgical group (Feldman et al., 2011). There are ongoing modifications in the device to allow a greater primary success rate and possibly an expansion of suitable candidates. There are other percutaneous devices in development that may have synergistic effects. It is likely that combinations of these devices may present a further therapeutic option.
9. Conclusions
MitraClip percutaneous repair is a safe and effective technique that will be particularly applicable to high surgical risk patients or younger patients seeking a less invasive approach.
References
- 1.Alfieri O, Maisano F, de Bonis M, Stefano PL, Torracca L, Oppizzi M, La Canna G. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001;122(4):674–681. doi: 10.1067/mtc.2001.117277. [DOI] [PubMed] [Google Scholar]
- 2.Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol. 2008;52(5):319–326. doi: 10.1016/j.jacc.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 3.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373(9672):1382–1394. doi: 10.1016/S0140-6736(09)60692-9. [DOI] [PubMed] [Google Scholar]
- 4.Fedak PW, McCarthy PM, Bonow RO. Evolving concepts and technologies in mitral valve repair. Circulation. 2008;117(7):963–974. doi: 10.1161/CIRCULATIONAHA.107.702035. [DOI] [PubMed] [Google Scholar]
- 5.Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54(8):686–694. doi: 10.1016/j.jacc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 6.Feldman T, Foster E, Glower DG, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364(15):1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 7.Franzen O, Baldus S, Rudolph V, Meyer S, Knap M, Koschyk D, Treede H, Barmeyer A, Schofer J, Costard-Jäckle A, et al. Acute outcomes of MitraClip therapy for mitral regurgitation in high-surgical-risk patients: emphasis on adverse valve morphology and severe left ventricular dysfunction. Eur Heart J. 2010;31(11):1373–1381. doi: 10.1093/eurheartj/ehq050. [DOI] [PubMed] [Google Scholar]
- 8.Franzen O, van der Heyden J, Baldus S, Schlüter M, Schillinger W, Butter C, Hoffmann R, Corti R, Pedrazzini G, Swaans ML, et al. MitraClip® therapy in patients with end-stage systolic heart failure. Eur J Heart Fail. 2011;13(5):569–576. doi: 10.1093/eurjhf/hfr029. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann HC, Kar S, Siegel R, Fail P, Loghin C, Lim S, Hahn R, Rogers JH, Bommer WJ, Wang A, et al. Effect of percutaneous mitral repair with the MitraClip device on mitral valve area and gradient. EuroIntervention. 2009;4(4):437–442. doi: 10.4244/EIJV4I4A76. [DOI] [PubMed] [Google Scholar]
- 10.Hussaini A, Kar S. Percutaneous mitral valve repair: potential in heart failure management. Curr Heart Fail Rep. 2010;7:22–26. doi: 10.1007/s11897-010-0006-8. [DOI] [PubMed] [Google Scholar]
- 11.Kar S, Feldman TE, St. Goar F. Mitral valve repair using the MitraClip: from concept to reality. Cardiac Interventions Today. 2008:39–45. [Google Scholar]
- 12.Kar S, Foster E, Glower D, Feldman T. MitraClip therapy demonstrates continued clinical benefit and favorable left ventricular remodeling at two years in high risk surgical patients with significant mitral regurgitation: analysis of the everest II high risk registry. J Am Coll Cardiol. 2011;57:1308. doi: 10.1016/S0735-1097(11)61308-9. [DOI] [Google Scholar]
- 13.Tamburino C, Ussia GP, Maisano F, Capodanno D, La Canna G, Scandura S, Colombo A, Giacomini A, Michev I, Mangiafico S, et al. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J. 2010;31(11):1382–1389. doi: 10.1093/eurheartj/ehq051. [DOI] [PMC free article] [PubMed] [Google Scholar]