Abstract
Objective: To evaluate whether liposomal prostaglandin E1 (lipo-PGE1) can decrease reperfusion no-reflow in a catheter-based porcine model of acute myocardial infarction (AMI). Methods: Twenty-two male Chinese mini-swines were randomized into three groups: six in a sham-operation group, and eight each in the control and lipo-PGE1 groups. The distal part of the left anterior descending coronary artery (LAD) in the latter two groups was completely occluded for 2 h, and then reperfused for 3 h. Lipo-PGE1 (1 μg/kg) was injected 10 min before LAD occlusion until reperfusion for 1 h in the lipo-PGE1 group. Hemodynamic data and proinflammatory cytokines were examined before AMI, 2 h after occlusion, and 1, 2, and 3 h after reperfusion. Myocardial contrast echocardiography (MCE) and double staining were performed to evaluate the myocardial no-reflow area (NRA). Results: Left ventricular systolic pressure and end-diastolic pressure significantly improved in the lipo-PGE1 group after reperfusion compared with the control group and also 2 h after AMI (P<0.05 for both). MCE and double staining both showed that lipo-PGE1 decreased reperfusion NRA after AMI (P<0.05, P<0.01). Lipo-PGE1 decreased serum interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) after myocardial infarction reperfusion (P<0.05 for both). Conclusions: Lipo-PGE1 is cardioprotective in our porcine model of myocardial infarction reperfusion no-reflow, decreasing NRA and attenuating the inflammatory response.
Keywords: Liposomal prostaglandin E1 (lipo-PGE1), Reperfusion no-reflow, Myocardial infarction
1. Introduction
Coronary reperfusion therapy, such as catheter-based percutaneous coronary intervention (PCI), is widely performed in patients with acute myocardial infarction (AMI). However, patency of the infarct-related artery does not always guarantee salvage of myocardium at risk of ischemia. The concept of “no-reflow” refers to a state of myocardial tissue hypoperfusion in the presence of a patent epicardial coronary artery. “Reperfusion no-reflow” occurs after primary PCI for reperfusion of an infarct-related artery in AMI, which may be asymptomatic or present with continued chest pain and ST-segment elevation. Reperfusion no-reflow is preceded by ischemic cell injury, is confined to the irreversibly damaged necrotic zone, and may be exacerbated at the time of reperfusion (Jaffe et al., 2008), which is an independent predictor of adverse clinical outcome after AMI regardless of infarct size and which is associated with heart failure and increased mortality (Morishima et al., 2000).
Prostaglandins reduce free radical production in stimulated human neutrophils and may attenuate reperfusion injury. They also inhibit platelets and are vasodilators. Liposomal delivery of prostaglandin E1 (PGE1) may effectively target the PGE1 to white blood cells, platelets, and endothelial cells, and possibly limits the hemodynamic impact of PGE1 until the liposomal preparation interacts with the target cellular elements. Researchers have previously shown that repetitive administration of bolus doses of liposomal prostaglandin E1 (lipo-PGE1) reduces white blood cell activation and accumulation in ischemic tissue as well as infarct size in a 2-h canine occlusion-reperfusion model (Smalling et al., 1995), which also reduces reperfusion injury of myocardium by inhibiting cytokine production during cardiac surgery (He and Li, 2004).
The aim of the present study is to investigate the preventive effect and mechanism of lipo-PGE1 on a catheter-based porcine model of myocardial infarction reperfusion no-reflow.
2. Materials and methods
2.1. Reperfusion no-reflow models and animal grouping
All animal experiments were approved by the Animal Care and Use Committee of China-Japan Friendship Hospital, Beijing, China and were in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (Publication No. 85-23, revised in 1996). Twenty-two male Chinese mini-swines, (22±3) kg, from China Agricultural University were randomized into three groups: six in the sham-operation group, eight each in the control group and lipo-PGE1 group. Our porcine model of AMI reperfusion no-reflow was modified on the basis of the previous study by Suzuki et al. (2008). The mini-swines were intubated under general anesthesia using 30 mg diazepam, 30 ml of 0.03 g/ml pentobarbital sodium, and 4 mg pipecuronium, and ventilated with a ventilator (Bird). Animals were placed in the right anterior oblique position and had continuous electrocardiograph (ECG) (limb and precordial leads) and hemodynamic monitoring throughout all procedures. Vascular access was obtained using a 6-Fr vascular sheath placed in the right femoral artery. All animals received preprocedural heparin 6 000 U. After advancing a 6-Fr guiding catheter (Judkins Left type) through the ascending aorta into the coronary ostia, baseline coronary angiography (CAG) was performed to identify the subsequent location of the occlusion and coronary artery size. The distal part (about 1/3 to 1/2 site) of the left anterior descending coronary artery (LAD) was completely occluded by dilated balloon (2.0 mm×10.0 mm) for 2 h and a successful AMI model was confirmed by CAG and ECG. Then LAD was reperfused for 3 h and CAG was performed again to ensure LAD with TIMI 3 blood flow. ST-T segment elevation occurred when the LAD was occluded, and returned to baseline when LAD occlusion was relieved. In the sham-operation group, the balloon was just placed in the LAD without dilatation. There was no AMI, reperfusion, or no-reflow. Lipo-PGE1 (Beijing Tide Pharmaceutical Co., Ltd.) at a dosage of 1 μg/kg body weight was injected 10 min before LAD occlusion until reperfusion for 1 h in the lipo-PGE1 group. Saline was used in the control group.
2.2. Hemodynamic assessment
To assess serial cardiac function, left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and heart rate (HR) were obtained by catheter method at five time points: (1) before AMI; (2) 2 h after occlusion; (3) 1 h after reperfusion; (4) 2 h after reperfusion; and, (5) 3 h after reperfusion.
2.3. Myocardial contrast echocardiography
Real-time myocardial contrast echocardiography (MCE) and trigger MCE were performed by an investigator, blind to treatment allocation at three time points: (1) before AMI; (2) 2 h after occlusion; and, (3) 3 h after reperfusion. Short-axis images of the left ventricle at the papillary muscle level were obtained with an echocardiographic system (Philips iE33) equipped with a transducer (S5-1). Real-time MCE was performed by an intravenous injection of SonoVue (25 mg in 5 ml saline; Bracco), and the mechanical index was 0.1. We drew 0.05 ml/kg of SonoVue into a 30-ml syringe and gave a bolus injection from femoral vein in 1 min followed by a 10-ml saline flush. After uniform development of left ventricle, flash triggering (flash mechanical index 1.19, flash frame 8) was performed and dynamic images of at least 20 cardiac cycles were obtained continuously for analysis. In trigger MCE, images were obtained with the fundamental mode and triggering on the R wave of the ECG. We used a mechanical index of 1.02 and a trigger interval of 8–10 cardiac cycles. Dynamic images were recorded when right ventricle developed. Tracing method was used with Q-Lab software to detect myocardial filling defect area and left ventricular area. These measurements were performed three times continuously at R wave in the most clear section. The filling defect areas 2 h after occlusion and 3 h after reperfusion were defined as risk area (RA) and no-reflow area (NRA), respectively. NRA/RA was calculated.
2.4. Pathological staining
Double staining with 0.01 g/ml Evans blue dye and 0.04 g/ml thioflavin-S was performed to delineate RA and NRA. Three hours after reperfusion, 1 ml/kg thioflavin-S was injected into the left ventricle. The reperfused region was stained, while the no-reflow region was not stained. After another complete occlusion of LAD by dialted balloon, Evans blue was injected into the left ventricle and normal myocardium was stained. Animals were then sacrificed with an intravenous bolus injection of potassium chloride after staining. The hearts were then excised and the left ventricles were sectioned into a 10 mm-thick cross-sectional myocardial slice. The cross-sectional myocardial slice at the papillary muscle level was chosen. In room light, normal myocardium, reperfused myocardium, and NRA were stained blue, yellow, and without staining, respectively. Sections similar to MCE were photographed. RA, NRA, and left ventricular wall area (LVWA) were measured using an image processing software IPP 6.0. RA/LVWA and NRA/LVWA were calculated.
2.5. Serum IL-6 and TNF-α levels by radioimmunoassay
To assess serum interleukin (IL)-6 and tumor necrosis factor (TNF)-α by radioimmunoassay, the blood was obtained from the femoral vein at five time points: (1) before AMI; (2) 2 h after occlusion; (3) 1 h after reperfusion; (4) 2 h after reperfusion; and, (5) 3 h after reperfusion. For radioimmunoassay, serum samples were stored at −80 °C until final processing. Concentrations of IL-6 and TNF-α were determined by 125I radioimmunoassay kits (East Asia Radioimmunoassay Center of Chinese PLA General Hospital) according to the manuals. The detection limit was 1000 pg/ml for both IL-6 and TNF-α.
2.6. Statistical analysis
Numeric values were expressed as mean±standard deviation (SD). Comparisons of parameters between two groups were performed with unpaired Student’s t-test. Comparisons of parameters among three groups were performed with a one-way analysis of variance (ANOVA). A value of P<0.05 was considered statistically significant.
3. Results
3.1. Procedure success and mortality
Twenty-six male Chinese mini-swines were used and four died during the procedure. Causes of death included: (1) laryngeal edema by intubation failure; (2) too deep anesthesia; (3) thrombosis in left main coronary artery due to coronary injury by excessive balloon inflation; and, (4) recurrent ventricular tachycardia and fibrillation after reperfusion. Except for the six swines of the sham-operation group, complete occlusion of LAD by dilated balloon was confirmed by CAG, and CAG was performed again to confirm LAD with TIMI 3 blood flow after the balloon deflated in the sixteen swines of the other two groups, which simulated reperfusion after AMI successfully.
3.2. Effect of lipo-PGE1 on hemodynamics
Hemodynamic parameters were similar in each group before AMI as shown in Table 1. LVSP decreased significantly in the control group 2 h after AMI and 1, 2, and 3 h after reperfusion, compared with that before AMI (P<0.05 in all cases), while LVEDP was elevated significantly (P<0.05 in all cases). LVSP and LVEDP were similar in the control and lipo-PGE1 groups 2 h after AMI, while LVSP and LVEDP significantly improved in the lipo-PGE1 group after reperfusion compared with the control group (P<0.05) and 2 h after AMI (P<0.05).
Table 1.
Hemodynamics at different time of each group
| Time | Group | HR (beat/min) | LVSP (mmHg) | LVEDP (mmHg) |
| Before AMI | Sham | 115±5 | 128±3.5 | 4.5±1.3 |
| Control | 122±8 | 126±5.6 | 4.1±1.1 | |
| Lipo-PGE1 | 113±9 | 127±2.8 | 4.2±2.1 | |
| AMI 2 h | Sham | 115±5 | 128±3.5 | 4.5±1.3 |
| Control | 127±7 | 103±2.5# | 7.2±2.2# | |
| Lipo-PGE1 | 110±3 | 109±2.3# | 7.8±1.3# | |
| Reperfusion 1 h | Sham | 115±5 | 128±3.5 | 4.5±1.3 |
| Control | 122±6 | 105±3.0# | 6.6±1.6# | |
| Lipo-PGE1 | 112±3 | 124±8.3*## | 5.6±1.0*## | |
| Reperfusion 2 h | Sham | 115±5 | 128±3.5 | 4.5±1.3 |
| Control | 121±9 | 108±3.5# | 6.4±2.2# | |
| Lipo-PGE1 | 114±2 | 131±8.9*## | 4.9±1.4*## | |
| Reperfusion 3 h | Sham | 115±5 | 128±3.5 | 4.5±1.3 |
| Control | 125±5 | 110±7.8# | 6.3±2.6# | |
| Lipo-PGE1 | 113±6 | 130±3.3*## | 4.3±2.3*## |
LVSP, LVEDP and HR were obtained by catheter method at five time points: (1) before AMI; (2) 2 h after occlusion; (3) 1 h after reperfusion; (4) 2 h after reperfusion; and, (5) 3 h after reperfusion.
P<0.05 vs. the control group
P<0.05 vs. before AMI
P<0.05 vs. 2 h after AMI
3.3. Myocardial contrast echocardiography
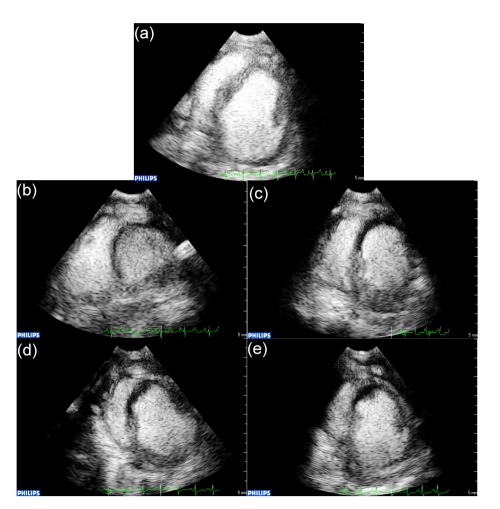
Myocardial perfusion at the left ventricular short-axis papillary muscle level in different groups was evaluated by real-time MCE and trigger MCE, as shown in Fig. 1. There were no differences between LVWA measured by real-time MCE and that by trigger MCE 2 h after LAD occlusion and 3 h after reperfusion (P>0.05 for both), indicating that the sections selected at the same level were of good comparability. The correlation coefficient of trigger MCE and real-time MCE was 0.864, which consistently reflected the effect of lipo-PGE1 on myocardial reperfusion and no-reflow. As shown in Table 2, RA/LVWA was similar in the control and lipo-PGE1 groups when calculated by the two methods (P>0.05). NRA/RA by trigger MCE was 32.4% in the lipo-PGE1 group, which reached statistical significance (P<0.05) compared with the control group (58.2%); NRA/RA by real-time MCE was 59.5% in the lipo-PGE1 group, which also reached statistical significance (P<0.05) compared with the control group (73.5%).
Fig. 1.
Representative MCE of each group
(a) MCE of the sham-operation group; (b, d) MCE of the control and lipo-PGE1 groups 2 h after AMI, respectively; (c, e) MCE of the control and lipo-PGE1 groups 3 h after reperfusion, respectively
Table 2.
MCE results assessing RA and NRA
| Group | NRA/RA (%) |
RA/LVWA (%) |
||
| Trigger | Real-time | Trigger | Real-time | |
| Control | 58.2±24.7 | 73.5±24.8 | 15.2±3.0 | 15.1±3.8 |
| Lipo-PGE1 | 32.4±18.1* | 59.5±23.4* | 13.3±2.1 | 13.1±1.2 |
P<0.05 vs. the control group
3.4. Pathological staining
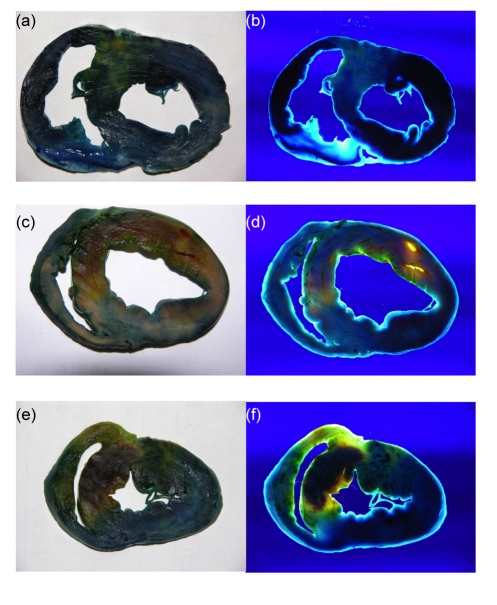
Three hours after reperfusion, normal myocardium, reperfusion area, and NRA could be recognized by double staining with Evans blue dye and thioflavin-S. Normal myocardium, reperfusion area, and no-reflow areas were dark blue, yellow, and dark red, respectively, in room light; while black, bright yellow, and deep red, respectively, in UV light (as shown in Fig. 2). As shown in Table 3, RA/LVWA was 50.73% and 54.37% in the control and lipo-PGE1 groups, respectively, and no significant differences were found between the two groups. NRA/RA in the lipo-PGE1 group was 27.13%, significantly lower than 49.84% in the control group (P<0.01).
Fig. 2.
Excised heart stained with Evans blue dye and thioflavin-S 3 h after reperfusion
Normal myocardium, reperfusion area, and no-reflow area were dark blue, yellow, and dark red, respectively in room light (a, c, e), while black, bright yellow, and deep red, respectively, in UV light (b, d, f). (a, b) Sham-operation group; (c, d) Control group; (e, f) Lipo-PGE1 group
Table 3.
Pathological staining assessing RA and NRA
| Group | No. | RA/LVWA (%) | NRA/RA (%) | NRA/LVWA (%) | (RA-NRA)/LVWA (%) |
| Control | 8 | 50.73±3.93 | 49.84±5.04 | 25.39±3.49 | 25.34±2.68 |
| Lipo-PGE1 | 8 | 54.37±8.72 | 27.13±8.71* | 14.83±5.51* | 39.54±7.55* |
P<0.01 vs. the control group
3.5. Serum IL-6 and TNF-α after myocardial reperfusion
By radioimmunoassay, serum IL-6 and TNF-α did not change significantly at different time points in the sham-operation group. They were also similar in each group 5 min before occlusion. As shown in Tables 4 and 5, serum IL-6 and TNF-α increased significantly 2 h after occlusion, and 1, 2, and 3 h after reperfusion, compared with those before occlusion in the control group (P<0.05 for all). In the lipo-PGE1 group, IL-6 and TNF-α increased significantly 2 h after occlusion compared with that before occlusion (P<0.05), while IL-6 decreased 1, 2, and 3 h after reperfusion compared with 2 h after occlusion (P<0.05 for all). TNF-α increased 1, 2, and 3 h after reperfusion compared with 2 h after occlusion (P<0.05 for all), while they were still lower than those of the control group (P<0.05 for all). Lipo-PGE1 decreased serum IL-6 and TNF-α after myocardial reperfusion.
Table 4.
Serum IL-6 at different time of each group
| Time | IL-6 concentration (pg/ml) |
||
| Sham-operation | Control | Lipo-PGE1 | |
| Before AMI | 114.5±3.6 | 112.5±9.8 | 113.9±2.9 |
| AMI 2 h | 111.2±4.6 | 133.1±2.7# | 127.3±5.1# |
| Reperfusion 1 h | 113.5±1.6 | 124.8±6.2# | 107.5±4.4#*+ |
| Reperfusion 2 h | 112.7±5.6 | 129.9±6.7# | 103.1±2.9#*+ |
| Reperfusion 3 h | 113.2±4.9 | 136.6±8.4# | 103.6±3.9#*+ |
P<0.05 vs. before AMI
P<0.05 vs. AMI 2 h
P<0.05 vs. the control group
Table 5.
Serum TNF-α at different time of each group
| Time | TNF-α concentration (ng/ml) |
||
| Sham-operation | Control | Lipo-PGE1 | |
| Before AMI | 0.51±0.10 | 0.53±0.11 | 0.51±0.20 |
| AMI 2 h | 0.52±0.11 | 0.71±0.13# | 0.61±0.06# |
| Reperfusion 1 h | 0.51±0.06 | 0.93±0.30#* | 0.69±0.13#*+ |
| Reperfusion 2 h | 0.51±0.05 | 1.20±0.30#* | 0.73±0.15#*+ |
| Reperfusion 3 h | 0.51±0.09 | 1.31±0.27#* | 0.85±0.14#*+ |
P<0.05 vs. before AMI
P<0.05 vs. AMI 2 h
P<0.05 vs. the control group
4. Discussion
In our study, LVSP decreased significantly in the control group 2 h after AMI, and 1, 2 and 3 h after reperfusion, compared with that before AMI, while LVEDP was significantly elevated. When the LAD was occluded, systolic function decreased in the ischemic myocardium, usually with a lower LVSP; and at the same time, cardiac diastolic function also decreased, showing a higher LVEDP. After reperfusion, systolic and diastolic functions of ischemic myocardium were restored to some extent, while reperfusion no-reflow might happen. Myocardial ischemia-reperfusion injury and endothelial damage underlie the development of reperfusion no-reflow. Infarct size and microvascular hypoperfusion may increase at the time of coronary reperfusion, beyond that observed during the ischemic period. Endothelial injury is induced by an acute inflammatory response, generation of reactive oxygen species, intracellular calcium overload, and opening of the mitochondrial permeability transition pore (Jaffe et al., 2008).
AMI animal models using dogs or pigs are often developed by coronary ligation after surgical thoracotomy (Fozzard, 1975; Wang et al., 2006). However, left ventricular remodeling of this open-chest model is influenced by opening both the chest and pericardium. Resultant trauma may also cause infection, unsuccessful recovery from anesthesia, and increased mortality. An intracoronary balloon inflation technique allows for the control of both the location and duration of coronary artery occlusion, which has been reported in previous studies (Reffelmann et al., 2004; Krombach et al., 2005). Selective catheterization and placement of the balloon catheter at the desired site of the LAD can be achieved within 10 min. In addition, the duration of occlusion can be controlled exactly because the LAD can be reperfused immediately after balloon deflation.
LVSP and LVEDP were significantly improved in the lipo-PGE1 group after reperfusion compared with the control group and 2 h after AMI, which demonstrated that lipo-PGE1 could improve hemodynamic index after reperfusion. The double staining method with Evans blue dye and thioflavin-S presented here can accurately delineate NRA, RA, and normal myocardium. Lipo-PGE1 decreased NRA after reperfusion in AMI, which was consistent with the result of MCE. However, the specific values of NRA by pathological staining and MCE were not the same. MCE is a new imaging technique with an ability to assess microvascular perfusion, but also has limitations. Thus far, MCE cannot delineate NRA, RA, and normal myocardium as accurately as pathological staining.
Although experimental models and clinical trials have established that prompt, effective, and sustained restoration of blood flow after coronary occlusion can limit infarct size, improve ventricular function, and enhance survival, reperfusion triggers an active inflammatory response associated with an intense neutrophilic infiltration of ischemic myocardium that may produce further tissue injury. Kawamura et al. (2000) reported that PGE1 reduced myocardial reperfusion injury by inhibiting proinflammatory cytokine productions, including IL-6 and IL-8, during cardiac surgery. Here we demonstrated that lipo-PGE1 suppressed the productions of serum IL-6 and TNF-α after myocardial infarction reperfusion. An anti-inflammatory effect may be one of the most important cytoprotective mechanisms of PGE1.
Footnotes
Project (No. 03III02) supported by the Capital Medical Development Research Fund of China
References
- 1.Fozzard HA. Validity of myocardial infarction models. Circulation. 1975;52(6 Suppl.):III131–III146. [PubMed] [Google Scholar]
- 2.He ZF, Li ZJ. Prostaglandin E1 reduces reperfusion injury of myocardium by inhibiting cytokines production during cardiac surgery. J Zhejiang Univ (Med Sci) 2004;33(4):353–356. doi: 10.3785/j.issn.1008-9292.2004.04.017. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 3.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117(24):3152–3156. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura T, Nara N, Kadosaki M, Inada K, Endo S. Prostaglandin E1 reduces myocardial reperfusion injury by inhibiting proinflammatory cytokines production during cardiac surgery. Crit Care Med. 2000;28(7):2201–2208. doi: 10.1097/00003246-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Krombach GA, Kinzel S, Mahnken AH, Gunther RW, Buecker A. Minimally invasive close-chest method for creating reperfused or occlusive myocardial infarction in swine. Invest Radiol. 2005;40(1):14–18. [PubMed] [Google Scholar]
- 6.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36(4):1202–1209. doi: 10.1016/S0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 7.Reffelmann T, Sensebat O, Birnbaum Y, Stroemer E, Hanrath P, Uretsky BF, Schwarz ER. A novel minimal-invasive model of chronic myocardial infarction in swine. Coronary Artery Dis. 2004;15(1):7–12. doi: 10.1097/00019501-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Smalling RW, Feld S, Ramanna N, Amirian J, Felli P, Vaughn WK, Swenson C, Janoff A. Infarct salvage with liposomal prostaglandin E1 administered by intravenous bolus immediately before reperfusion in a canine infarction-reperfusion model. Circulation. 1995;92(4):935–943. doi: 10.1161/01.cir.92.4.935. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Lyons JK, Yeung AC, Ikeno F. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area/area at risk. Catheter Cardiovasc Interv. 2008;71(1):100–107. doi: 10.1002/ccd.21329. [DOI] [PubMed] [Google Scholar]
- 10.Wang JA, Luo RH, Zhang X, Xie XJ, Hu XY, He AN, Chen J, Li JH. Bone marrow mesenchymal stem cell transplantation combined with perindopril treatment attenuates infarction remodeling in a rat model of acute myocardial infarction. J Zhejiang Univ-Sci B. 2006;7(8):641–647. doi: 10.1631/jzus.2006.B0641. [DOI] [PMC free article] [PubMed] [Google Scholar]